Abstract
Ecosystems provide a variety of ecosystem services (ES), which act as key linkages between social and ecological systems. ES respond spatially and temporally to abiotic and biotic variation, and to management. Thus, resistant and resilient ES provision is expected to remain within a stable range when facing disturbances. In this study, generic indicators to evaluate resistance, potential resilience and capacity for transformation of ES provision are developed and their relevance demonstrated for a mountain grassland system. Indicators are based on plant trait composition (i.e. functional composition) and abiotic parameters determining ES provision at community, meta-community and landscape scales. First the resistance of an ES is indicated by its normal operating range characterized by observed values under current conditions. Second its resilience is assessed by its potential operating range – under hypotheses of reassembly from the community’s species pool. Third its transformation potential is assessed for reassembly at meta-community and landscape scales. Using a state-and-transition model, possible management-related transitions between mountain grassland states were identified, and indicators calculated for two provisioning and two regulating ES. Overall, resilience properties varied across individual ES, supporting a focus on resilience of specific ES. The resilience potential of the two provisioning services was greater than for the two regulating services, both being linked to functional complementarity within communities. We also found high transformation potential reflecting functional redundancy among communities within each meta-community, and across meta-communities in the landscape. Presented indicators are promising for the projection of future ES provision and the identification of management options under environmental change.
Keywords: Community assembly, Meta-community, Landscape, Functional diversity, Mountain grasslands
1. Introduction
Terrestrial and aquatic ecosystems deliver multiple, interrelated provisioning, regulating, and cultural services that benefit human well-being (Díaz et al., 2015). Ecosystem services (ES) are thereby one of the key linkages between social and ecological systems (Díaz et al., 2015; Reyers et al., 2013), and their steady provision needs to be preserved into the future to sustain societies. However, under increasing anthropogenic pressures threatening ecosystem integrity, the sustainability of ES provision will be determined by ecosystem resilience to combined pressures from land use, changing climate, nitrogen deposition or species invasions (Carpenter and Folke, 2006; Leadley et al., 2014), making the notion of resilience central to forecasting and managing for future human well-being (Spears et al., 2015).
Ecological resilience is defined as the amount of disturbance a system can cope with without shifting to another state (Holling, 1973; Walker et al., 2004). To address social-ecological resilience, which considers interactions between ecosystem properties and social dynamics (Biggs et al., 2012), the definition of resilience can be expanded as the ability of an ecosystem to provide a stable amount of ES while facing management or environmental changes (Carpenter and Folke, 2006; Elmqvist et al., 2003). Considering that the resilience of an ES is maintained through constant dynamics and change (Walker and Salt, 2006), a resilient system will adapt its structure to change while keeping the same dynamic set of states and associated ES (Standish et al., 2014; Walker et al., 2004). While a holistic, conceptual assessment of resilience needs to integrate social and ecological dynamics, for the purpose of measurement and indicator development a simplification, and specification of the term ‘resilience’ is required (Quinlan et al., 2016). In particular, indicators focusing explicitly on the resilience of ES are still missing.
Acknowledging that resistance and resilience are intrinsic properties of all ecosystems, in this paper we propose a conceptual approach that targets the ecological underpinnings of the resilience of ecosystem functions based on ecosystem dynamics (Oliver et al., 2015), and focuses on a measurement approach (sensu Quinlan et al., 2016). More specifically, we linked the concept of resilience to the concept of community functional dynamics (Suding et al., 2008) in order to propose quantifiable indicators of ES resilience. Focusing on the resilience of ES requires assessing specific resilience, defined as the resilience of a specific part of the social-ecosystem to a particular disturbance type (Walker and Salt, 2006; Quinlan et al., 2016), rather than system-level, generic resilience (e.g. Carpenter and Brock, 2006; Scheffer et al., 2009). Indeed, we hypothesize that individual ES may have different sensitivities to disturbances and therefore different resilience (Oliver et al., 2015; Scheffer et al., 2001) due to specific critical changes in their underpinning ecological characteristics. We further refer to ‘potential’ resilience of ES as our approach does not consider the transient dynamics and time lag of returning to the pre-disturbed state which follows once the range of resilience might be exceeded. The ‘realised’ resilience will depend on additional properties such as species regeneration traits and local contingencies. Our approach is positioned within the broad field of social-ecological resilience; however, we apply a purely ecological perspective to the measurement of ES resilience.
Our framework proposes to assess the specific resilience of an ES by distinguishing the three phases of resilience (Walker and Salt, 2006): first, the initial resistance to change which we define as the range of ES provision under observed environmental fluctuations; second, the maintenance of the current range of ES provision, defined more strictly as resilience, given reversible variations in ecosystem state and processes (Standish et al., 2014); and finally transformation, which implies a shift in system state and associated ecosystem processes (Carpenter and Folke, 2006; Oliver et al., 2015). ES resilience is then quantified by: (1) the observed range of values for an ES, indicating resistance, (2) the potential range of values for the ES within the same ecosystem state, indicating potential resilience sensu stricto, and (3) the new potential range of provision after ecosystem transformation to an alternative state.
In the following, we first present the conceptual framework for ES resilience assessment, and associated indicators based on community functional composition. We then illustrate this concept through an application to grassland ecosystems using data from differently managed grassland states in the central French Alps (Lavorel et al., 2011). Subsequently, we analyse the implementation of indicators using quantitative criteria. We end by discussing challenges we faced when using the concept in practice, general restrictions and implications for future research.
2. Conceptual approach
As ecological processes supporting ES provision are determined by land cover and by specific management (Bennett et al., 2009; Allan et al., 2015), an increasing number of studies have attempted to quantify ES provision by considering changes in ecological parameters in response to management in grasslands (Lavorel et al., 2011), agricultural areas and forests (Raudsepp-Hearne et al., 2010), or aquatic systems (Barbier et al., 2011). Each ES provided by a given ecosystem state then varies spatially (and temporally) according to local environmental (e.g. topography, soil characteristics), biotic (community composition), and management characteristics (e.g. nitrogen input, disturbance regime) (Bennett et al., 2009; Díaz et al., 2007b; Lavorel et al., 2011; Quétier et al., 2007). Our concept for quantifying ES resilience is based on the notion of operating ranges (OR) of an ES, defined as its range of values in a given ecosystem state (Pereira e Silva et al., 2013), for instance certain management conditions, given environmental and biotic variation. Further, changes in climate, management, species invasion, or species extinctions can lead successively to variation in environmental and biotic parameters within the same ecosystem state and to transformation to another state.
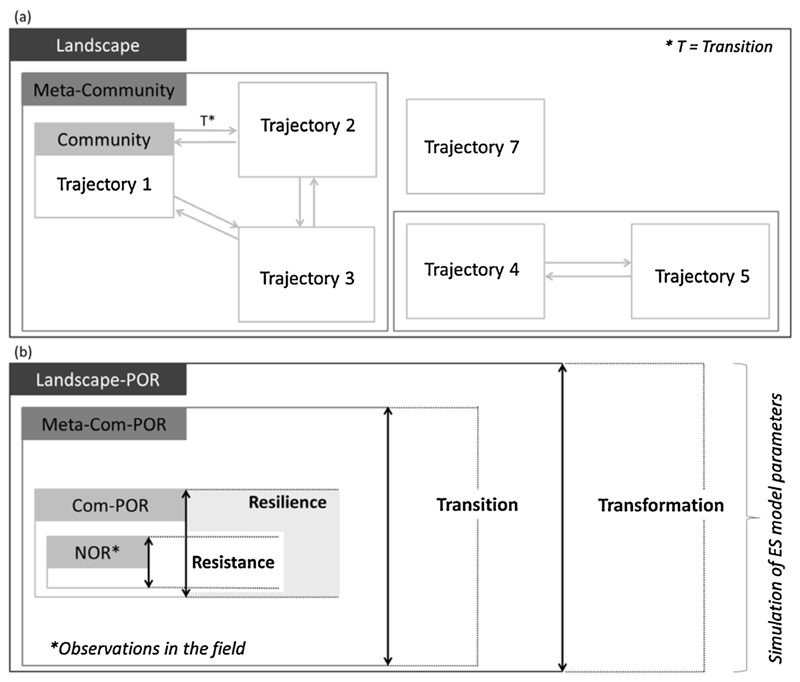
Among available conceptual models describing ecosystem dynamics, and specifically resilience, state-and-transition models (STM) (Fig. 1a) have proven particularly successful in capturing linear and nonlinear changes in ecosystem structure and function and their causal mechanisms (e.g. Lavorel et al., 2015; Prober et al., 2014). They can be used to characterize alternative states depending on land use and drivers of specific transitions such as climate, natural disturbance regimes, management, and their interactions. STM are also one of the tools that might be specifically suitable for the identification of changes and resilience of ES under uncertain futures such as climate change (Lavorel et al., 2015). We therefore believe that they are a possible tool to advance the conceptualization and quantification of ES resilience by analysing OR and transitions in biodiversity and ecosystem functioning.
Fig. 1.
Illustration of the conceptual approach. (a) State-and-transition model (STM) with the three scales (Community, Meta-Community, and Landscape) for the Lautaret study site. Trajectories refer to land use states characterized by past and present management: three on previously cultivated terraces (T1: currently mown and fertilized, T2: mown, T3: grazed in spring and autumn by sheep and cattle), and three on never cultivated grassland (T4: mown, T5: previously mown and currently grazed in summer by sheep and cattle, T7: never mown and grazed in summer by sheep and cattle since the Middle Ages, above 2000 m), (b) Corresponding operating ranges (OR) of ES provision linked to the three different scales of the STM.
Different approaches, based for instance on taxonomic units (e.g. species) or functional traits exist to model community dynamics. Here, we focus on the re-assembly of functional trait composition (Suding et al., 2008), as ecosystem processes, and thus ES, are primarily influenced by species functional roles, i.e. traits (Cardinale et al., 2012; Díaz et al., 2007b; Lavorel et al., 2011; Oliver et al., 2015). ES resilience indicators are therefore linked to community dynamics within the functional trait pool. Specifically, following Quétier et al. (2007) and Lavorel et al. (2015) we propose to use STM that are formulated in terms of functional composition (FC), i.e. the presence and abundance distribution of plant functional trait values (Díaz et al., 2007a), so as to link ES resilience and transitions to specific mechanisms and to gain predictive power (Standish et al., 2014). The focus on the functional rather than the taxonomic composition of communities provides the ability to explain current ES provision based on functional effect traits, as well as to project future ES provision depending on functional responses and community assembly (Allan et al., 2015; Díaz et al., 2007b).
Combining the concepts of OR, STM, and FC for characterizing resilience, we define our indicators of resilience as OR which can be evaluated at different scales according to dynamic relations between ecosystem states. Consistent with hierarchy theory (O’Neill et al., 1989) ecological systems are structured as nested levels of organisation, each associated with specific spatial and temporal scales of states and processes. Each hierarchical level is linked to certain environmental characteristics (e.g. nutrient availability, pH) constraining the OR of community composition, ecosystem functioning and thus ES provision. However, environmental limits may alter over time inducing a shift to an altered OR (O’Neill et al., 1989). We consider the scaled structure of ecological systems by determining four indicators of resilience for individual ES within a landscape (Fig. 1b): The Normal Operating Range (NOR) and the Community Potential Operating Range (Com-POR) are applied to the scale of the community (i.e. ecosystem state). The Meta-Community Potential Operating Range (Meta-Com-POR) encompasses ecosystem states linked by possible management- or environmentally-driven transitions (Leibold et al., 2004). The highest hierarchical level is considered by the Landscape Potential Operating Range (Landscape-POR) representing the functional pool and environmental characteristics of the entire landscape. Hereafter, when comparing two operating ranges, we refer to the lower hierarchical level n as OR (n) and to the higher level n + 1 as OR (n + 1).
NOR represents current ES provision by an ecosystem state and is defined as the stable, realised functioning range assumed to represent resistance of an ES under current conditions. It is estimated from ES values observed in the field across replicate plots for each ecosystem state. Com-POR, Meta-Com-POR, and Landscape-POR represent the potential ranges of ES provision, considering different species pools associated with each scale, along with the associated ranges of abiotic conditions.
We define the Com-POR as extending the NOR by representing ES provision for all possible realizations of community composition from current community structure (and associated trait values) and abiotic conditions. The boundaries of the Com-POR represent the resilience of ES provision. If an ES exceeds or falls below the NOR (i.e. resistance), it remains resilient as long as it stays within the Com-POR. The underlying hypothesis is that as long as FC and abiotic conditions remain within the potential assemblages defined around the observed range, the opportunity exists that ES provision can return to the NOR.
The Meta-Com-POR is applied to ecosystem states which are linked by possible transitions and represent a meta-community characterized by an extended pool of species (traits) and abiotic conditions. The rationale of the Meta-Com-POR is to estimate the potential range of ES provision by integrating the functional structures and abiotic conditions of linked ecosystem states, which may shift among each other as a result of management or environmental change (Quétier et al., 2007). We assume that once ES provision exceeds the Com-POR it is no longer resilient and enters a transition as a result of distinct changes in FC and abiotic conditions from the wider meta-community pool. As such the Meta-Com-POR defines a point of reference for ES provision once resilience is exceeded, but remaining within current possible transitions between ecosystem states.
The Landscape-POR estimates the total potential range of ES provision across the pool of functional traits and abiotic conditions at the landscape scale. It incorporates all possible transformations, i.e. combinations of FC and abiotic conditions within the current functional envelope, which can only be exceeded by changing the ecosystem type (e.g. from grassland ecosystems into forest ecosystems).
3. Case study: implementation of the conceptual approach for managed subalpine/mountain grasslands
3.1. Trait based models of ecosystem services
We quantified the four indicators of resilience using plant trait-based models for four ES. Ecosystem services can be related to underlying biophysical components (e.g. abundance of species), structures or processes (e.g. nutrient cycling) (Lamarque et al., 2011). These ecosystem properties (e.g. green biomass production, total soil C) can be seen as the ecological potentiality to provide ES when used by humans (Lamarque et al., 2011). When modelling ES provision at ecosystem scale a method accounting for landscape heterogeneity is required (Eigenbrod et al., 2010), which captures variability in management (e.g. nitrogen inputs, frequency of mowing) as well as environmental and biophysical characteristics (e.g. topography, aspect, soil pH) (Bennett et al., 2009; Díaz et al., 2007b; Quétier et al., 2007). Plant community functional composition at a specific location is determined by these characteristics (Garnier et al., 2007), and also impacts on ecosystem functioning (Cadotte et al., 2011). Ecosystem processes are strongly affected by plant FC (Díaz et al., 2007a). In particular, following the biomass ratio hypothesis (Grime, 1998), the traits of species with the highest contribution to total plant biomass have the greatest effect on ecosystem processes. Quantitative models developed by Lavorel et al. (2011) and Grigulis et al. (2013) combine abiotic characteristics, plant traits and the contribution of plant species to standing biomass at ecosystem scale to quantify ES for mountain grasslands, and their responses to management (see below).
3.2. Study site
We applied the conceptual framework to analyse the resilience of four ecosystem services in subalpine grasslands at the Lautaret site within the Central French Alps long-term socio-ecological research (LTSER) (Lavorel et al., 2013; Fig. 1a). The study site is located on the south facing slopes of the Romanche valley above the village of Villar d’Arène (N45.03°, E6.24°). The area covers 13 km2 and is dominated by grasslands ranging between 1552 and 2442 m a.s.l. For a more detailed site description see Lavorel et al. (2011). Different ‘trajectories’ of grassland management were identified based on historical and current land use as well as management practices (Lavorel et al., 2011) and linkages between them represented as a STM (Quétier et al., 2007) (Fig. 1a). Indeed Quétier et al. (2007) showed that grasslands on former cultivated land have distinct floristic composition and mean community plant trait values compared to never ploughed grasslands, and thus here we considered six trajectories (T): three on previously cultivated terraces (T1: currently mown and fertilized, T2: mown, T3: grazed in spring and autumn by sheep and cattle), and three on never cultivated grassland (T4: mown, T5: previously mown and currently grazed in summer by sheep and cattle, T7: never mown and grazed in summer by sheep and cattle since the Middle Ages, above 2000 m) (Lavorel et al., 2011) (Fig. 1a). For each trajectory and ES, resilience indicators were calculated. Each trajectory was subsequently considered as an ecosystem state.
3.3. Field measurements
Within each trajectory soil abiotic parameters, plant community composition, and plant functional traits were measured for between four and thirteen replicate plots within each trajectory. The abundance of individual species was determined as a proportion of dry biomass within the plot using the BOTANAL method, which estimates species ranks and biomass by dry-weight-ranking, and was previously shown to adequately capture community functional composition (Lavorel et al., 2008). Subsequently, plant functional traits for identified species were measured (Lavorel et al., 2011, 2008). Plant traits and abiotic parameters (e.g. soil bulk density, soil texture, field capacity (WHC), soil organic matter, total soil carbon and nitrogen) were measured using standardised protocols (Lavorel et al., 2011). Plant vegetative traits (vegetative height – VegHt; leaf dry matter content – LDMC; and leaf nitrogen concentration – LNC), assumed to be relevant to ecosystem processes and the provision of ecosystem services (Lavorel et al., 2011; Quétier et al., 2007), were measured following standard protocols for each of the species that collectively made up 80% of the cumulated biomass (Garnier et al., 2007). For each plant trait we then calculated the community-weighted mean (CWM; Garnier et al., 2004) as the sum across plant species of the product between their biomass relative abundance (%) and their trait values. For detailed information on field methods and measurements see Grigulis et al. (2013) and Lavorel et al. (2011).
3.4. Application of resilience indicators for ES provision
3.4.1. Ecosystem services and associated ecosystem processes
Four ES were selected for the calculation of the resilience indicators. They all are important services provided by grasslands and differently sensitive to changes in management or climate (Lamarque et al., 2014): annual biomass production, forage quality, carbon storage and soil fertility. While green biomass production is a direct measure (g m−2), the others use the following proxies as surrogates for the ES. Forage quality is indicated by its quality to livestock nutrition, expressed as digestible crude protein (g kg−1). Carbon storage is represented by soil organic matter (SOM, i.e. % Corg). The proxy for soil fertility is soil nitrogen mineralization potential (NH4-N μg g−1 d−1), which provides information about potential for sustaining agronomic use.
Each ES was estimated using quantitative models based on field data. Correlative modelling using linear mixed models with residual maximum likelihood (REML) estimations was used to quantify the respective contributions of CWM plant traits (VegHt, LNC, LDMC) and abiotic characteristics (e.g. soil fertility, soil water holding capacity) for different management types to variations in ES, with the optimal model being chosen based on the highest R2 and lowest Akaike criterion of all possible models in an all-subsets regression procedure (see Lavorel et al. (2011), Grigulis et al. (2013) for details). Table 1 summarises the results of this analysis with those CWM plant trait and abiotic characteristics being retained as explanatory factors for each ES together with their coefficient effects.
Table 1.
Equations used for the estimation of ES using CWM plant traits and abiotic parameters retained in the analysis of Grigulis et al. (2013). Presented are the effect coefficients for each predictive parameter, together with the overall equation constant.
| Constant | Coefficient | ||||||
|---|---|---|---|---|---|---|---|
| CWM LNC (mg g−1) | CWM LDMC (g dry g−1 fresh) | CWM VegHt (cm) | WHC (%) | Log LDMC Log (g dry g−1 fresh) | Log DEA Log (N-N2O μg g−1 h−1) | ||
| Green Biomass (g m−2) | −2 | 7.53 | 6.566 | 7.83 | |||
| Crude Protein Content (g kg−1) | 201.9 | 0.2691 | 2.013 | 4.6 | |||
| Log N Mineralisation (NH4-N μg g−1 d−1) | 2.012 | 1.916 | 1.024 | ||||
| Log Soil Organic Matter (%) | 1.697 | 1.494 | 0.4402 | ||||
In the absence of field measured values for the variable DEA (Denitrifying enzyme activity) in this subsequent study, this was estimated from the following model, also developed by Grigulis et al. (2013):
3.4.2. Resilience indicators
Resilience indicators are based on the comparison of the current NOR of an ES to its potential operating range. In the following we detail how operating ranges were estimated for each scale.
NOR: Based on representative field sampling in each trajectory covering the range of FC and abiotic conditions, the current envelope of ES provision (NOR) was calculated by applying actual trait- and abiotic parameter values to the individual ES models. The range of the NOR for each trajectory is presented as the minimum to maximum of the obtained ES provision values in the respective trajectory.
To assess the potential operating ranges we used simulations of parameters underlying ES provision, using simple models of community dynamics. Simulations generate all possible combinations of observed parameters under stated community assembly rules and so allow us to model the potential range of ES provision at each scale.
Com-POR: We simulated the envelope of possible values of each ES, given the range of FC in the species pool of each trajectory. Simulated communities were created by generating stochastic species abundances, and using these abundances together with field measured values for plant traits to calculate simulated CWM trait values. Values for abiotic parameters used for the estimation of ES were based on the range of measured values. These values were entered into the ES estimation models, and repeated iterations of simulated CWM’s and of abiotic values, resulted in probabilistic distributions of possible ES values for each trajectory.
In this study, basic assembly rules of community composition were applied in order to generate realistic species abundance distributions, in particular to ensure that currently observed dominant species had a greater chance of having high abundances compared to currently subordinate species. Following Jaillard et al. (2014), we assigned species as dominant, subdominant or subordinate based on their observed median abundances in the field (Grime, 1998). Dominant species were classified as those having a percentage of biomass in each trajectory greater than 10%, subdominants as those having a still considerable percentage of biomass of less than 10%, with remaining species (median close to zero) being classified as subordinates. Individual studies may choose more advanced community assembly rules under specific hypotheses for biotic and abiotic processes.
Community simulations were carried out using the Monte Carlo simulation software @RISK 6 (Palisade DTools 2014, http://www.palisade.com/risk/), with in each iteration a random number drawn for each species in the species pool from a lognormal distribution whose location parameter (μ) and scale parameter (σ) were dependent on whether the species was dominant, subdominant or subordinate. For each trajectory μ for dominant and subdominant species was based on their median abundance value and σ was based on their maximum abundance. For subordinate species the location parameter (μ) was fixed at 1, and the scale parameter (σ) at 10 to provide for consistent minor representation of these species in the simulated communities. The random number for each species in the trajectory was standardised to a value between 0 and 1, representing its abundance in the simulated community, based on its proportion of the sum of the random numbers for all species in the trajectory. These simulated abundances together with trait values for each species were used to calculate values of CWM for each trait for each iteration. Abiotic parameters were simulated for each iteration using a uniform distribution with real field measured minimum and maximum values from that trajectory defining the boundaries. Repeated iterations (20,000) of simulated CWM in the different ES models resulted in potential ES provision for each trajectory. The range of the Com-POR for each trajectory is presented as the minimum to maximum of the simulated ES provision values in that trajectory.
Meta-Com-POR: Based on the STM of Quétier et al. (2007) developed from an analysis of coexisting historical land-use trajectories at Lautaret (Fig. 1a), the Meta-Com-POR was calculated including all of the species existing in connected trajectories. In more detail, trajectories 1, 2 and 3 were combined into Meta-Com-POR ‘T123′, and trajectories 4 and 5 were combined into Meta-Com-POR ‘T45′. As no transition was identified for T7 which has remained under summer grazing since the Middle Ages no Meta-Com-POR exists for this trajectory. Field abundance data for these new meta-community species pools was used to classify species into three categories of dominance as for the Com-POR (dominant, subdominant, and subordinate). Abiotic parameters were simulated using a uniform distribution, with the minimum value of all combined trajectories and the maximum value of all combined trajectories as lower and upper boundaries. Community assembly simulations and the stochastic simulation of plant trait CWM, abiotic parameters, and ultimately envelopes of possible ES provision were carried out as for the Com-POR calculations. The range of the Meta-Com-POR for each combined trajectory is presented as the minimum to maximum of the simulated ES provision values in that combined trajectory.
Landscape-POR: Species abundance data and upper and lower field observed values of abiotic parameters from all trajectories were combined to depict landscape-level conditions. Assembly rules were not applied in the modelling process as the Landscape-POR only represents a reference of the functional pool and abiotic conditions across the landscape. Therefore, for all species a lognormal curve was used. The location parameter μ was set to 1 and the scale parameter σ was set to 100 to allow high abundances for each species, thereby covering the full range of possible CWM trait values, and thus of ES provision. Abiotic parameters were simulated using a uniform distribution, with the minimum value of all combined trajectories and the maximum value of all combined trajectories as lower and upper boundaries. Stochastic simulations of plant trait CWM’s, abiotic parameters and ultimate envelopes of possible ES provision were carried out as previously. The range of the Landscape-POR is presented as the minimum to maximum of the simulated ES provision values for the landscape species pool.
We applied three different criteria to describe quantitatively and to test significant differences between NOR, Com-POR, Meta-Com-POR, and Landscape-POR. First, we applied a Mann Whitney U test (as NOR data are not normally distributed) in order to test whether the simulated Com-POR adequately captures the distribution of observed variation (NOR). Second, we analysed the nesting of ranges, i.e. whether the operating range (OR) at a given hierarchical level n is within values of the OR at the next hierarchical level n + 1. For this, and considering potential variation across community simulations, we accepted OR (n) as nested within OR (n + 1) if its minimum and maximum values was within five percent variation of the corresponding minimum and maximum values for OR (n + 1). Under this hypothesis our basic assembly rules would be adequate to represent the extension of the range of community functional composition across successive hierarchical levels. Third, we analysed the percentage of overlap between adjacent operating ranges in cases when this nesting criterion was verified. A lower percentage of overlap indicates a greater potential of resilience, i.e. if the ES exceeds its NOR there is high potential to return to predisturbed conditions; vice versa a high percentage range nesting of OR (n) with OR (n + 1) reveals a lower potential of resilience, and a higher potential for transformation. Statistical analyses were carried out using the software R (https://www.r-project.org/).
4. Results
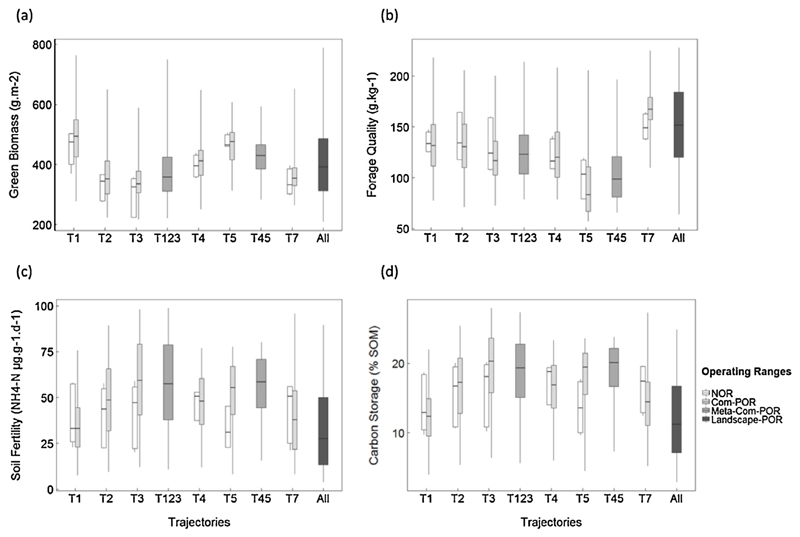
In general, results of the implementation of resistance and resilience indicators to ES provision in managed mountain grasslands supported our conceptual approach. Results for green biomass production (GB), forage quality (FQ), soil fertility (SF) and carbon storage (CS) are shown in Fig. 2(a–d).
Fig. 2.
NOR, Com-POR, Meta-Com-POR and Landscape-POR for (a) green biomass production (GB), (b) forage quality (FQ), (c) soil fertility (SF), and (d) carbon storage (CS) for all trajectories and groups of trajectories at meta-community and landscape scale.
We applied the Mann Whitney U test to the six trajectories and four ES, resulting in 24 tests. In 17 out of 24 cases there was no significant difference (p > 0.05) between NOR and Com-POR calculations, confirming the ability of our community assembly simulations to capture the observed range of functional variation and resulting ranges in ES variation. Examination of the trait distributions for the seven cases in which significant differences (p < 0.05) were found: SF (T2, T3, T5), CS (T3, T5), FQ (T5, T7), revealed that they represented configurations when the trait values of hyper-dominant species (e.g. Festuca paniculata in T5 for height and leaf traits, Festuca laevigata in T3 for LNC) strongly deviated from other abundant species.
The analysis of nesting of operating ranges revealed that the NOR was embedded within the Com-POR for all ES and trajectories (cf. Fig. 2). In contrast, the Meta-Com-POR did not incorporate the Com-POR with regard to either the minimum and/or the maximum value in 9 out of 20 cases, predominantly found for T4 and T5, and for FQ and CS (Table 2).
Table 2.
Range nesting of the Com-POR within the Meta-Com-POR. (T = true/F = false, if Min/Max of Com-POR is nested/not nested within Meta-Com-POR; % = Deviation of Min/Max Com-POR to Min/Max Meta-Com-POR; 1<5% outside of Meta-Com-POR considered as nested).
| Green biomass (GB) | Forage quality (FQ) | Soil fertility (SF) | Carbon storage (CS) | |||||
|---|---|---|---|---|---|---|---|---|
| T1 | T/F | % | T/F | % | T/F | % | T/F | % |
| Min | T | 10.72 | T1 | 0.79 | T1 | 3.70 | F | 7.43 |
| Max | T1 | 2.65 | T1 | 3.39 | T | 26.31 | T | 24.48 |
| T2 | ||||||||
| Min | T | 0.47 | F | 5.41 | T1 | 1.61 | T1 | 1.25 |
| Max | T | 18.84 | T | 5.93 | T | 10.76 | T | 8.97 |
| T3 | ||||||||
| Min | T1 | 0.84 | T1 | 4.38 | T | 1.29 | T | 3.48 |
| Max | T | 30.33 | T | 9.84 | T | 0.89 | T1 | 2.78 |
| T4 | ||||||||
| Min | F | 10.13 | T | 9.99 | F | 5.99 | F | 8.01 |
| Max | F | 17.41 | F | 8.86 | T | 5.39 | T | 2.95 |
| T5 | ||||||||
| Min | T | 9.83 | F | 6.27 | F | 11.66 | F | 17.19 |
| Max | T1 | 4.63 | F | 6.62 | T | 4.05 | T | 1.36 |
In 8 out of 12 cases the Meta-Com-POR was nested within the Landscape-POR, however, in 4 cases the Meta-Com-POR exceeded the Landscape-POR for SF and CS (Table 3). In this analysis, as no Meta-Com-POR was available for T7 due to missing transition options, we considered Com-POR as the surrogate for Meta-Com-POR. In summary, range nesting was fulfilled in 77% of analysed cases.
Table 3.
Range nesting of the Meta-Com-POR within the Landscape-POR (for T7: Com-POR within the Landscape-POR). (T = true/F = false, if Min/Max of Meta-Com-POR is nested/not nested within Landscape-POR; % = Deviation of Min/Max Meta-Com-POR to Min/Max Landscape-POR; 1<5% outside of Landscape-POR considered as nested).
| Green biomass (GB) | Forage quality (FQ) | Soil fertility (SF) | Carbon storage (CS) | |||||
|---|---|---|---|---|---|---|---|---|
| T123 | T/F | % | T/F | % | T/F | % | T/F | % |
| Min | T | 1.90 | T | 8.89 | T | 8.18 | T | 12.27 |
| Max | T | 6.86 | T | 8.69 | F | 11.04 | F | 11.07 |
| T45 | ||||||||
| Min | T | 12.50 | T | 0.89 | T | 13.77 | T | 19.99 |
| Max | T | 33.76 | T | 19.06 | T | 10.64 | T | 4.98 |
| T7 | ||||||||
| Min | T | 9.16 | T | 27.93 | T | 4.98 | T | 10.22 |
| Max | T | 23.44 | T | 1.83 | F | 7.28 | F | 10.68 |
Following the analysis of range nesting, we estimated the proportion of overlapping values for all cases in which OR (n) was embedded in OR (n + 1), including the cases in which overlapping was within a 5% margin of error (Table 4). The overlap between NOR and Com-POR ranged between 15% and 52%, showing that overall the range of possible variation beyond the NOR, and thus potential resilience, was quite high, especially for GB and FQ. Discounting those 9 cases when either the minimum or the maximum values for Com-POR exceeded those for Meta-Com-POR, the percentage of nesting could be calculated for 11 out of 20 Com-POR – Meta-Com-POR combinations, and ranged from 70 to 100%, suggesting more limited transformation potential. The overlap of Meta-Com-POR and Landscape-POR, calculated for 8 out of 12 cases, ranged between 54% and 91%.
Table 4.
Percentage (%) of nesting between nested OR (n) within OR (n + 1), based on minimum and maximum values as boundaries of OR; adding percentage of deviation when nesting was within a 5% margin of error of OR (n + 1).
| NOR − Com-POR (%) | Com-POR − Meta-Com-POR (%) | Meta-Com-POR − Landscape-POR (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GB | FQ | SF | CS | GB | FQ | SF | CS | GB | FQ | SF | CS | |
| T1 | 27.65 | 16.03 | 51.78 | 49.97 | 89.55 | 100 | 74.62 | – | ||||
| T2 | 21.76 | 35.01 | 44.35 | 47.12 | 80.68 | – | 89.40 | 91.13 | 91.22 | 82.41 | – | – |
| T3 | 35.82 | 41.65 | 45.15 | 46.37 | 69.91 | 90.56 | 97.83 | 96.61 | ||||
| T4 | 21.12 | 24.98 | 24.15 | 31.26 | – | – | – | – | 53.73 | 80.06 | 75.57 | 75.01 |
| T5 | 14.73 | 27.41 | 32.76 | 41.89 | 90.59 | – | – | – | ||||
| T7 | 25.30 | 23.77 | 39.92 | 32.56 | 67.39 | 70.23 | – | – | ||||
5. Discussion
Ecosystems undergo constant dynamics and change, with consequent variability in ES provision (Walker and Salt, 2006). As long as the ecosystem retains the functional structure to return to pre-disturbed conditions, keeping ES values within a stable range, we consider the ES as potentially resilient (Biggs et al., 2012). In an attempt to advance the measurement of ES resilience (sensu Quinlan et al., 2016), we linked community functional dynamics to ES operating ranges, used as indicators of the resistance and resilience potentials of an ES. As specific ecosystem processes and services are determined by different functional drivers, our approach focuses on the unique behavior of individual ES, i.e. on specific resilience (Walker and Salt, 2006). We validated the applicability of our conceptual approach for two provisioning and two regulating ES provided in managed mountain grasslands, for which we found different ranges of resistance and resilience between individual ES and grassland states. In the following we address the implications for resilience of differences in OR between ES and between grassland states. We end by discussing how our results relate to concepts of functional diversity, redundancy or complementarity known to control ecosystem functioning (Cadotte et al., 2011; Cardinale et al., 2012).
Our conceptual approach, and its method for the quantification of ES specific resilience, was supported by the nesting of the NOR within the respective Com-POR for the four ES analysed. This indicates that the Com-POR simulations led to plausible results, but partly reflects the simplicity of the applied community assembly rules. Assembly rules did not attempt to describe complex community assembly processes, and specifically did not account for functionally distinct hyper-dominant species. Such assembly patterns can be linked to biotic interactions such as allelopathy (Bais et al., 2003; Hierro and Callaway, 2003) or resource competition (Baptist et al., 2013). For instance, Festuca paniculata, which was dominant in trajectories 4 and 5, is often hyper-dominant because of its allelopathic and competitive characteristics (e.g. Baptist et al., 2013). The presence of this (or any other) competitive species with highly distinct trait values will lead to an offset as compared to real potential species compositions, i.e. NOR and Com-POR. This limitation could be overcome by applying more sophisticated assembly rules. Evaluating the range nesting between Com-POR and Meta-Com-POR reveals another important criterion when simulating potential OR (n + 1). When combining species lists from related trajectories (T1, T2, T3 into T123; T4, T5 into T45) classification of species into dominant, subdominant, and subordinate species was performed based on the extended species list and mean abundance across measured plots in the considered trajectory combinations (Jaillard et al., 2014). Consequently, particular species compositions (i.e. combinations of plant traits) that were observed (NOR) or simulated at community level (Com-POR) might be excluded using this new dominance classification. This fact could also offset the position of the Meta-Com-POR as compared to NOR or Com-POR. A further refinement for Meta-Com-POR and Landscape-POR calculation might be achieved by considering species dispersal traits. Whereas we assume that in the case of resilience the re-assembly is within the community at the site (i.e. no need for dispersal), species regeneration or dispersal performance could be a selection criterion for dominant and subdominant species for Meta-Com-POR or Landscape-POR. This would potentially lead to a restricted set of species for meta-community and landscape re-assembly.
The percentage range nesting between NOR and Com-POR was between 15 and 52% and lower than the range nesting for the Com-POR within the Meta-Com-POR (70–100%), and Meta-Com-POR within the Landscape-POR respectively (54–91%). The overlap between NOR and Com-POR of the two provisioning services, green biomass and forage quality, was generally lower than for the two regulating services, soil fertility and carbon storage, at the same scale. Following our conceptual approach, a lower percentage range nesting between NOR and Com-POR signals a greater potential of resilience. Disturbances, e.g. human or environmental induced perturbations, can cause a transient alteration of functional composition (Díaz et al., 2007a; Griffiths and Philippot, 2013). Thus, the ES can exceed its range of resistance (i.e. NOR), but still remain resilient as long as it stays within its Com-POR. The functional pool remains unchanged and ES provision is able to re-establish its previous NOR. A smaller overlap between NOR and Com-POR for green biomass and forage quality compared to soil fertility and carbon storage thus indicates a higher potential of resilience of these two provisioning services and, therefore greater management flexibility while retaining the potential to return to pre-disturbed levels of provision. Conversely, a high percentage range nesting between NOR and Com-POR is linked to a smaller potential of resilience, i.e. less potential to recover, and therefore limited options to manage soil fertility and carbon storage outside their current range. Overall, these results confirm our hypothesis of different resilience across individual ES.
A small potential of resilience consequently represents a higher potential of transition, and vice versa. Disruptions can no longer be buffered within the Com-POR, but induce a shift to another ecosystem state. We implemented the Meta-Com-POR as a point of reference as to what range of ES provision will be obtained when resilience is exceeded, i.e. the ES passes a transition to an alternative state and loses/gains functional traits from the meta-community. A high percentage nesting between Com-POR and Meta-Com-POR in our case study suggests small potential changes of ES provision after transition to a new state. The high overlap of forage quality in T1 (100%), and soil fertility and carbon storage in T3 (98% and 97%, respectively) with the respective Meta-Com-POR (T123) values for these ES reflects high functional overlap of traits due to functionally redundant species (Griffiths and Philippot, 2013) and stable abiotic parameters. In contrast, a smaller overlap (70%) between green biomass in T3 and its transition state (Meta-Com-POR T123) reveals differences in the functional pool for VegHt and LNC between grazed (T3) and mown (T1, T2) terraces, with complementary trait values, and, therefore, a large potential change in green biomass production when mowing is ceased (transition from T1 or T2 to T3) or resumed (transition from T3 to T1 or T2). The same principle can be applied when focusing on a potential transformation of the Meta-Com-POR and Landscape-POR. As for resilience, transformation potential can thus differ across individual ES, even within a single ecosystem state.
In addition to the percentage range nesting described above, the position of the OR (n) within the OR (n + 1) provides useful information on the status of ES provision. Given the position of the OR (n) at either the lower or upper end of the range of OR (n + 1), the potential for resilience or transition/transformation can be inferred (Fig. 2). For example, the NOR of green biomass in T3 occurs at the lower end of its Com-POR, and likewise for its Meta-Com-POR. A shift in the functional composition of parcels currently classified as T3 in response to disturbance thus might induce a marked increase in green biomass production. Soil fertility in T3 shows a similar pattern, contrary to forage quality and carbon storage in the same trajectory.
Complex mechanisms related to biodiversity, and specifically functional divergence, redundancy or complementarity of functional traits influence ecosystem processes, and consequently the provision of ES and their resilience to environmental change (Allan et al., 2015; Cadotte et al., 2011; Cardinale et al., 2012; Griffiths and Philippot, 2013). Based on our approach, resistance and resilience of ES provision would be linked to within-community functional diversity (α-functional diversity) through mechanisms of functional complementarity (Walker et al., 1999). Also, as different species respond individually to disturbances, high functional redundancy within communities or meta-communities may increase the stability of ecosystem functioning by enhancing the chance of functionally redundant species buffering each other’s loss (Allan et al., 2015; Cardinale et al., 2012; Griffiths and Philippot, 2013; Walker et al., 1999). At our study site, changes in functional composition (and thereby CWM) from observed to potential assemblages within the same trait pool and dominance structure (based on our assembly rules) considerably extended values from the NOR to the Com-POR, indicating high functional complementarity within existing communities (Cadotte et al., 2011; de Bello et al., 2010). Conversely, the relatively low increase in the Meta-Com-POR as compared to Com-POR suggests high functional redundancy across communities within each meta-community, and likewise for the three meta-communities within the landscape (Landscape-POR vs. Meta-Com-POR). So while at this study site individual grassland types have complementary functional composition (i.e. high functional divergence) within each community (Gross et al., 2009), there is limited functional differentiation (β- functional diversity) within each meta-community in response to current management, with historical management having shaped the meta-community’s trait pool and filtered towards high trait redundancy. In other terms, considering that transitions across individual community trait pools are facilitated by a smaller distinctiveness across communities as observed here, a lower β-diversity would indicate a greater ability to transform and stability of ES provision at the meta-community level. Further analyses will need to explore patterns and mechanisms of functional complementarity and redundancy within and across communities and to test their relationships to indicators of ES resistance, resilience and transformation.
6. Conclusion
Social-ecological systems are complex adaptive systems. From an ecological perspective the operating ranges of their ecosystem services depend on site-specific properties and biotic community dynamics, which are captured by our indicators. From our application at a mountain grassland site, we conclude that within an ecosystem state individual ES show differences in potential resilience, supporting the analysis of specific ES resilience. Overall, as ecosystem processes and functions are often more strongly driven by the functional composition of communities than by either species richness per se or species identities (Cadotte et al., 2011; Cardinale et al., 2012), we believe that our trait-based approach captures important mechanisms for resistance, resilience and transformation of ES provision. By going beyond species identities our resistance, resilience and transformation indicators also offer the potential for generalization and comparability of resilience across different ecosystems. Currently, the calculation of OR relies on field data, which is time consuming to obtain. However, the recent development of trait databases such as TRY (Kattge et al., 2011) may help to overcome this limitation and allow the concept to be widely applied and further tested. Lastly, our indicators are promising for the prediction and management of future ES provision. Some ecosystems have been managed for the primary supply of certain provisioning ES (e.g. highly productive grasslands, timber production in forests). An understanding of the resilience of their ES provision is vital for social and economic goals, but the implications of specific management decisions for the resilience of other important regulating or cultural services needs to be considered (Spears et al., 2015). If resilience is exceeded under prospective scenarios of change, the indicators presented here could be applied to evaluate causes of changes in ES provision, design adaptive management and sometimes accept irreversible transformation in ES provision (Lavorel et al., 2015).
Acknowledgements
This work was funded by BiodivERsA project REGARDS, with support from the French Agence Nationale pour la Recherche (ANR-12-EBID-004-01) and the FWF Austrian Science Fund (I 1056-B25), and by the RTD project OPERAsFP7-ENV-2012-308393. Thanks to three anonymous reviewers for their constructive comments on this manuscript.
References
- Allan E, Manning P, Alt F, Binkenstein J, Blaser S, Blüthgen N, Böhm S, Grassein F, Hölzel N, Klaus VH, Kleinebecker T, et al. Land use intensification alters ecosystem multifunctionality via loss of biodiversity and changes to functional composition. Ecol Lett. 2015;18(8):834–843. doi: 10.1111/ele.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bais HP, Vepachedu R, Gilroy S, Callaway RM, Vivanco JM. Allelopathy and exotic plant invasion: from molecules and genes to species interactions. Science. 2003;301(5638):1377–1380. doi: 10.1126/science.1083245. [DOI] [PubMed] [Google Scholar]
- Baptist F, Secher-Fromell H, Viard-Cretat F, Aranjuelo I, Clement J-C, Creme A, Desclos M, Laine P, Nogues S, Lavorel S. Carbohydrate and nitrogen stores in Festuca paniculata under mowing explain dominance in subalpine grasslands. Plant Biol. 2013;15(2):395–404. doi: 10.1111/j.1438-8677.2012.00652.x. [DOI] [PubMed] [Google Scholar]
- Barbier EB, Hacker Sally D, Kennedy C, Koch EW, Stier AC, Silliman BR. The value of estuarine and coastal ecosystem services. Ecol Monogr. 2011;82(2):169–193. [Google Scholar]
- Bennett EM, Peterson GD, Gordon LJ. Understanding relationships among multiple ecosystem services. Ecol Lett. 2009;12(12):1394–1404. doi: 10.1111/j.1461-0248.2009.01387.x. [DOI] [PubMed] [Google Scholar]
- Biggs R, Schlüter M, Biggs D, Bohensky EL, BurnSilver S, Cundill G, Dakos V, Daw TM, Evans LS, Kotschy K, Leitch AM, et al. Toward principles for enhancing the resilience of ecosystem services. Annu Rev Environ Resour. 2012;37(1):421–448. [Google Scholar]
- Cadotte MW, Carscadden K, Mirotchnick N. Beyond species: functional diversity and the maintenance of ecological processes and services. J Appl Ecol. 2011;48(5):1079–1087. [Google Scholar]
- Cardinale BJ, Duffy JE, Gonzalez A, Hooper DU, Perrings C, Venail P, Narwani A, Mace GM, Tilman D, Wardle DA, Kinzig AP, et al. Biodiversity loss and its impact on humanity. Nature. 2012;486(7401):59–67. doi: 10.1038/nature11148. [DOI] [PubMed] [Google Scholar]
- Carpenter SR, Brock WA. Rising variance: a leading indicator of ecological transition. Ecol Lett. 2006;9:311–318. doi: 10.1111/j.1461-0248.2005.00877.x. [DOI] [PubMed] [Google Scholar]
- Carpenter SR, Folke C. Ecology for transformation. Trends Ecol Evol. 2006;21(6):309–315. doi: 10.1016/j.tree.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Díaz S, Lavorel S, Chapin III, Stuart F, Tecco PA, Gurvich DE, Grigulis K. Functional diversity – at the crossroads between ecosystem functioning and environmental filters. In: Canadell JG, Pataki DE, Pitelka LF, editors. Terrestrial Ecosystem in a Changing World. Springer; Berlin: 2007a. pp. 79–91. [Google Scholar]
- de Bello F, Lavorel S, Díaz S, Harrington R, Cornelissen Johannes HC, Bardgett RD, Berg MP, Cipriotti P, Feld CK, Hering D, Martins da Silva P, et al. Towards an assessment of multiple ecosystem processes and services via functional traits. Biodivers Conserv. 2010;19(10):2873–2893. [Google Scholar]
- Díaz S, Lavorel S, de Bello F, Quétier F, Grigulis K, Robson MT. Incorporating plant functional diversity effects in ecosystem service assessments. Proc Natl Acad Sci U S A. 2007b;104(52):20684–20689. doi: 10.1073/pnas.0704716104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz S, Demissew S, Carabias J, Joly C, Lonsdale M, Ash N, Larigauderie A, Adhikari JR, Arico S, Báldi A, Bartuska A, et al. The IPBES conceptual framework—connecting nature and people. Curr Opin Environ Sustain. 2015;14:1–16. [Google Scholar]
- Eigenbrod F, Armsworth PR, Anderson BJ, Heinemeyer A, Gillings S, Roy DB, Thomas CD, Gaston KJ. The impact of proxy-based methods on mapping the distribution of ecosystem services. J Appl Ecol. 2010;47(2):377–385. [Google Scholar]
- Elmqvist T, Folke C, Nyström M, Peterson G, Begtsson J, Walker B, Norberg J. Response diversity, ecosystem change, and resilience. Front Ecol Environ. 2003;1(9):488–494. [Google Scholar]
- Garnier E, Cortez J, Billès G, Navas M-L, Roumet C, Debussche M, Laurent G, Blanchard A, Aubry D, Bellmann A, Neill C, et al. Plant functional markers capture ecosystem properties during secondary succession. Ecology. 2004;85(9):2630–2637. [Google Scholar]
- Garnier E, Lavorel S, Ansquer P, Castro H, Cruz P, Dolezal J, Eriksson O, Fortunel C, Freitas H, Golodets C, Grigulis K, et al. Assessing the effects of land-use change on plant traits, communities and ecosystem functioning in grasslands: a standardized methodology and lessons from an application to 11 european sites. Ann Bot. 2007;99(5):967–985. doi: 10.1093/aob/mcl215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths BS, Philippot L. Insights into the resistance and resilience of the soil microbial community. FEMS Microbiol Rev. 2013;37(2):112–129. doi: 10.1111/j.1574-6976.2012.00343.x. [DOI] [PubMed] [Google Scholar]
- Grigulis K, Lavorel S, Krainer U, Legay N, Baxendale C, Dumont M, Kastl E, Arnoldi C, Bardgett RD, Poly F, Pommier T, et al. Relative contributions of plant traits and soil microbial properties to mountain grassland ecosystem services. J Ecol. 2013;101(1):47–57. [Google Scholar]
- Grime JP. Benefits of plant diversity to ecosystems: immediate, filter and founder effects. J Ecol. 1998;86:902–910. [Google Scholar]
- Gross N, Kunstler G, Liancourt P, de Bello F, Suding KN, Lavorel S. Linking individual response to biotic interactions with community structure: a trait-based framework. Funct Ecol. 2009;23(6):1167–1178. [Google Scholar]
- Hierro JL, Callaway RM. Allelopathy and exotic plant invasion. Plant Soil. 2003;256:29–39. [Google Scholar]
- Holling C. Resilience and stability of ecological systems. Annu Rev Ecol Syst. 1973;4:1–23. [Google Scholar]
- Jaillard B, Rapaport A, Harmand J, Brauman A, Nunan N, Poorter L. Community assembly effects shape the biodiversity-ecosystem functioning relationships. Funct Ecol. 2014;28(6):1523–1533. [Google Scholar]
- Kattge J, Díaz S, Lavorel S, Prentice IC, Leadley P, Bönisch G, Garnier E, Westoby M, Reich PB, Wright IJ, Cornelissen JHC, et al. TRY – a global database of plant traits. Global Change Biol. 2011;17(9):2905–2935. [Google Scholar]
- Lamarque P, Quétier F, Lavorel S. The diversity of the ecosystem services concept and its implications for their assessment and management. C R Biol. 2011;334(5–6):441–449. doi: 10.1016/j.crvi.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Lamarque P, Lavorel S, Mouchet M, Quetier F. Plant trait-based models identify direct and indirect effects of climate change on bundles of grassland ecosystem services. Proc Natl Acad Sci. 2014;111(38):13751–13756. doi: 10.1073/pnas.1216051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavorel S, Grigulis K, McIntyre S, Williams, Nick SG, Garden D, Dorrough J, Berman S, Quétier F, Thébault A, Bonis A. Assessing functional diversity in the field – methodology matters! Funct Ecol. 2008;22(0):134–147. [Google Scholar]
- Lavorel S, Grigulis K, Lamarque P, Colace M-P, Garden D, Girel J, Pellet G, Douzet R. Using plant functional traits to understand the landscape distribution of multiple ecosystem services. J Ecol. 2011;99(1):135–147. [Google Scholar]
- Lavorel S, Spiegelberger T, Mauz I, Bigot S, Granjou C, Dobremez L, Nettier B, Thuiller W, Brun J-J, Cozic P. Fostering research into coupled long-term dynamics of climate, land use, ecosystems and ecosystem services in the central French Alps. In: Singh SJ, Haberl H, Chertow M, Mirtl M, Schmid M, editors. Long Term Socio-Ecological Research. Springer; Netherlands, Dordrecht: 2013. pp. 485–504. [Google Scholar]
- Lavorel S, Colloff MJ, McIntyre S, Doherty MD, Murphy HT, Metcalfe DJ, Dunlop M, Williams RJ, Wise RM, Williams KJ. Ecological mechanisms underpinning climate adaptation services. Glob Change Biol. 2015;21(1):12–31. doi: 10.1111/gcb.12689. [DOI] [PubMed] [Google Scholar]
- Leadley P, Proenca V, Fernandez-Manjarres J, Pereira HM, Alkemade R, Biggs R, Bruley E, Cheung W, Cooper D, Figueiredo J, Gilman E, et al. Interacting regional-Scale regime shifts for biodiversity and ecosystem services. Bioscience. 2014;64(8):665–679. [Google Scholar]
- Leibold MA, Holyoak M, Mouquet N, Amarasekare P, Chase JM, Hoopes MF, Holt RD, Shurin JB, Law R, Tilman D, Loreau M, Gonzalez A. The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett. 2004;7(7):601–613. [Google Scholar]
- O’Neill RV, Johnson AR, King AW. A hierarchical framework for the analysis of scale. Landscape Ecol. 1989;3(3–4):193–205. [Google Scholar]
- Oliver TH, Heard MS, Isaac NJ, Roy DB, Procter D, Eigenbrod F, Freckleton R, Hector A, Orme C, David L, Petchey OL, et al. Biodiversity and resilience of ecosystem functions. Trends Ecol Evol. 2015;30(11):673–684. doi: 10.1016/j.tree.2015.08.009. [DOI] [PubMed] [Google Scholar]
- Pereira e Silva, Michele C, Semenov AV, Schmitt H, van Elsas Jan Dirk Salles JF. Microbe-mediated processes as indicators to establish the normal operating range of soil functioning. Soil Biol Biochem. 2013;57:995–1002. [Google Scholar]
- Prober SM, Stol J, Piper M, Gupta V, Cunningham SA. Towards climate-resilient restoration in mesic eucalypt woodlands: characterizing topsoil biophysical condition in different degradation states. Plant Soil. 2014;383(1–2):231–244. [Google Scholar]
- Quétier F, Thébault A, Lavorel S. Plant traits in a state and transition framework as markers of ecosystem response to land-use change. Ecol Monogr. 2007;77(1):33–52. [Google Scholar]
- Quinlan AE, Berbés-Blázquez M, Haider LJ, Peterson GD, Allen C. Measuring and assessing resilience: broadening understanding through multiple disciplinary perspectives. J Appl Ecol. 2016;53:677–687. [Google Scholar]
- Raudsepp-Hearne C, Peterson GD, Bennett EM. Ecosystem service bundles for analyzing tradeoffs in diverse landscapes. Proc Natl Acad Sci. 2010;107(11):5242–5247. doi: 10.1073/pnas.0907284107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyers B, Biggs R, Cumming GS, Elmqvist T, Hejnowicz AP, Polasky S. Getting the measure of ecosystem services: a social–ecological approach. Front Ecol Environ. 2013;11(5):268–273. [Google Scholar]
- Scheffer M, Carpenter S, Foley JA, Folke C, Walker B. Catastrophic shifts in ecosystems. Nature. 2001;413:591–596. doi: 10.1038/35098000. [DOI] [PubMed] [Google Scholar]
- Scheffer M, Bascompte J, Brock WA, Brovkin V, Carpenter SR, Dakos V, Held H, van Nes Egbert H, Rietkerk M, Sugihara G. Early-warning signals for critical transitions. Nature. 2009;461(7260):53–59. doi: 10.1038/nature08227. [DOI] [PubMed] [Google Scholar]
- Spears BM, Ives SC, Angeler DG, Allen CR, Birk S, Carvalho L, Cavers S, Daunt F, Morton RD, Pocock Michael JO, et al. Effective management of ecological resilience – are we there yet? J Appl Ecol. 2015;52(5):1311–1315. [Google Scholar]
- Standish RJ, Hobbs RJ, Mayfield MM, Bestelmeyer BT, Suding KN, Battaglia LL, Eviner V, Hawkes CV, Temperton VM, Cramer VA, Harris JA, et al. Resilience in ecology: abstraction, distraction, or where the action is? Biol Conserv. 2014;177:43–51. [Google Scholar]
- Suding KN, Lavorel S, Chapin III, Cornellissen FS, Johannes HC, Díaz S, Garnier E, Goldberg D, Hooper DU, Jackson ST, Navas M-L. Scaling environmental change through the community-level: a trait-based response-and-effect framework for plants. Global Change Biol. 2008;14(5):1125–1140. [Google Scholar]
- Walker B, Salt D. How can Landscapes and Communities Absorb Disturbance and Maintain Function. Island Press; Washington: 2006. Resilience Thinking. Sustaining Ecosystems and People in a Changing World. [Google Scholar]
- Walker B, Kinzig A, Langridge J. Plant attribute diversity, resilience, and ecosystem function: the nature and significance of dominant and minor species. Ecosystems. 1999;2(2):95–113. [Google Scholar]
- Walker B, Holling C, Carpenter SR, Kinzig A. Resilience: adaptability and transformability in sociol-ecological systems. Perspect Ecol Soc. 2004;9(2):5. [Google Scholar]