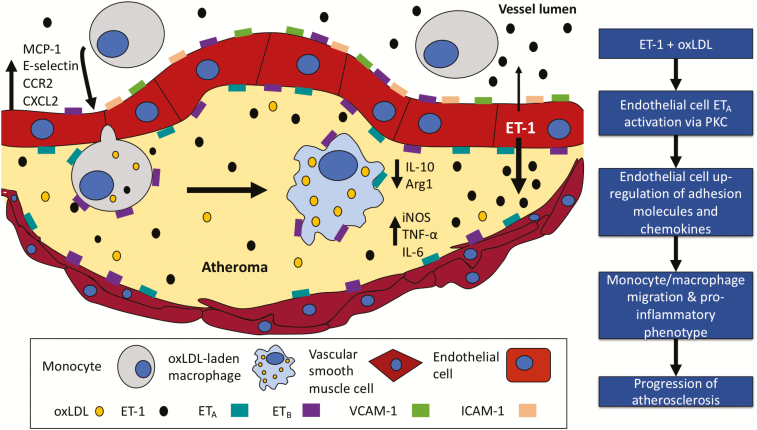
Atherosclerosis is a progressive inflammatory disease of the arterial intima defined by fatty plaques within the vessel wall.1 Plaques are characterized by the subendothelial build-up of low-density lipoprotein (LDL) leading to local inflammation and the accumulation of macrophage-derived foam cells. The development and subsequent rupture of atherosclerotic plaque leads to potentially catastrophic vascular events such as myocardial infarction and stroke. Thus, prevention of this largely asymptomatic condition is vital. Our understanding of the pathogenesis of atherosclerosis has progressed significantly over the last 3 decades, broadening the scope of preclinical studies and producing several exciting potential therapeutic targets. The role of the innate immune system and how it might interact with other key factors involved in atherogenesis have been the subject of much recent interest.2,3
Endothelin-1 (ET-1) may represent a novel therapeutic target for the treatment and prevention of atherosclerosis particularly through its interactions with macrophages.4 ET-1 is the most potent endogenous vasoconstrictor produced primarily by the vascular endothelium. It has been implicated in endothelial dysfunction, inflammation, and vascular remodeling.5,6 It acts through 2 G protein-coupled receptors, the endothelin A (ETA) receptor and the endothelin B (ETB) receptor. Vascular smooth muscle cell ETA receptors mediate vasoconstriction whereas ETB receptors, mostly located on endothelial cells, mediate vasodilation. It is the actions of ET-1 through the ETA receptor that are considered most important in cardiovascular diseases, such as hypertension, chronic kidney disease, and atherosclerosis.6
Circulating ET-1 is increased in patients with atherosclerosis.7 In 1995, Kowala et al. demonstrated that ETA receptor antagonism reduced fatty streak development in hyperlipidemic hamsters.8 These studies and others proposed the rationale that ET-1 may have a role in the early inflammatory phase of atherogenesis and that this may be amenable to treatment. Despite these findings, few clinical trials have attempted to address this. One study randomized 72 patients with non-obstructive coronary artery disease to receive either the selective ETA receptor antagonist, atrasentan, or placebo for 6 months.9 The authors observed no significant difference in the progression of angiographic coronary atheroma, perhaps unsurprising given the relatively short time period of the study. Nevertheless, there was a significant reduction in blood pressure in the treatment group and work from the same group later demonstrated that 6 months of atrasentan improved coronary endothelial function in a similar group of patients. Thus, one might anticipate less atherosclerosis progression if these effects are maintained longer term.
In this issue of the American Journal of Hypertension, Zhang et al. explore the interplay between ET-1, endothelial cells, and macrophages within the context of atherosclerosis.10 They initially make 2 observations. First, that apolipoprotein-E knockout (ApoE–/–) mice fed a high-fat diet have an increase in endothelial cell ET-1 protein expression within atherosclerotic plaques and second, that human umbilical vein endothelial cells (HUVECs) demonstrate an increase in ET-1 production on exposure to oxidized LDL (oxLDL). On the basis of these, the authors hypothesize that oxLDL stimulates endothelial ET-1 production, which then promotes progression of atheromatous plaque.
During a series of in vitro studies, using HUVECs adenovirally transfected to overexpress ET-1 (etHUVECs), Zhang et al. show that a combination of ET-1 overexpression and oxLDL promotes endothelial cell production of a range of adhesion molecules and chemokines. These effects are not seen with excess ET-1 alone and are greater than those seen with oxLDL. Monocyte chemoattractant protein-1 (MCP-1 or CCL2) and its receptor, CCR2, are among the chemokines assessed. These are key players in regulating the migration and infiltration of monocytes and macrophages.11 Thus, this finding provides a natural progression to performing a co-culture of (both mouse and human) macrophages with etHUVECs. Here, the authors find that, whereas ET-1 alone has no effect, a combination of ET-1 and oxLDL promotes macrophage migration. Interestingly, when macrophages are cultured with conditioned medium from etHUVECs treated with oxLDL, they develop a more inflammatory/M1 phenotype and less of an anti-inflammatory/M2 one as demonstrated by upregulation of message encoding interleukin-6 (IL6), tumor necrosis factor-α (TNFα), and inducible nitric oxide synthase (iNOS) and downregulation of IL10 and mannose receptor. These findings are in line with previous work.12 Further experiments demonstrate that all of the inflammatory effects of ET-1 and oxLDL on endothelial cells are dependent on an unblocked ETA receptor and functional protein kinase C (PKC) signaling (Figure 1). PKC has been shown to regulate ET-1 expression in diabetes13,14 and its inhibition is being explored in a range of diseases.15
Figure 1.
Potential role of endothelin-1 (ET-1) and oxidized LDL (oxLDL) on the development and progression of atherosclerosis.
The authors then translate their in vitro findings in vivo using an ApoE–/– mouse with vascular endothelial overexpression of ET-1 (eET-1/ApoE–/–). They find that atherosclerotic plaque size is increased in these mice compared with relevant controls. In addition, plaques contained more cells expressing CD68, a protein highly expressed by cells of the monocyte lineage. Aortic tissue from these animals also showed heightened expression of pro-inflammatory but downregulation of anti-inflammatory cytokines. The authors did not treat these mice with ET receptor blockers, which might have further dissected the role of ETA and/or ETB signaling in the effects seen. An upregulated ET system has been linked to inflammatory cell migration into vascular tissue and reactive oxygen species production and so some assessment of vascular function, e.g., using wire myography, might have been informative.16 Other work has shown that chronically elevated endothelial ET-1 leads to sustained blood pressure elevation and vascular and renal injury and that this is mediated via ETA receptors.17
Recent work has shown that ET-1 is a potent chemoattractant for both mouse and human macrophages and that this is dependent on an unblocked macrophage ETB receptor with some contribution of ETA. This same study showed that ET-1 alone or in combination with either classical or alternative activation methods was unable to alter macrophage phenotype to any measurable extent.4 Zhang et al.’s finding that ET-1 alone does not influence macrophage phenotype is in keeping with these data. However, in their hands, ET-1 alone is insufficient to influence macrophage chemokinesis (unless in the presence of oxLDL), which is out of keeping with this earlier study. This may be due to the different experimental conditions used (e.g., endothelial cell line-derived ET-1 here vs exogenous ET-1).4
It is intriguing that ETA receptor antagonism prevented the inflammatory effects of combined ET-1 and oxLDL on endothelial cells. Healthy vascular endothelial cells are considered to only express ETB receptors,18 although there have been reports of endothelial ETA expression in some disease states and specific cell lines.19 The authors used an ETA receptor antagonist (BMS-182874) that is 1,000-fold selective for ETA over ETB so the effects seen are unlikely to be due to any functional ETB blockade. Also, it is interesting that at baseline they found similar ETA and ETB expression in control HUVECs with etHUVECs expressing greater ETA than ETB. Thus, the findings from this study must be translated with some caution as they may be restricted to the cell lines used.
Both selective ETA and mixed ETA/B receptor antagonists (ERAs) are currently licensed and available in the clinic.6 Thus, the current studies would have benefited from additional investigation of combined ETA/B receptor antagonism. Therapeutic blockade of the ET system has been investigated for several cardiovascular disorders. These agents effectively reduce blood pressure in treatment-resistant hypertension and reduce proteinuria on top of standard care in patients with chronic kidney disease (CKD).4 Indeed, the recently published study of diabetic nephropathy with atrasentan (SONAR) demonstrated a significant reduction in CKD progression with atrasentan compared with placebo in patients with diabetic nephropathy.20 In addition, ERAs are first-line therapy for the management of pulmonary arterial hypertension, a rare but devastating condition. All these conditions are associated with accelerated atherosclerosis and so the authors should be commended for these data, which expand our knowledge with respect to the interaction between ET and innate immune systems in the development of atherosclerosis.
DISCLOSURE
None of the authors have any conflicts of interest.
REFERENCES
- 1. Libby P, Loscalzo J, Ridker PM, Farkouh ME, Hsue PY, Fuster V, Hasan AA, Amar S. Inflammation, immunity, and infection in atherothrombosis: JACC review topic of the week. J Am Coll Cardiol 2018; 72:2071–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Gils JM, Derby MC, Fernandes LR, Ramkhelawon B, Ray TD, Rayner KJ, Parathath S, Distel E, Feig JL, Alvarez-Leite JI, Rayner AJ, McDonald TO, O’Brien KD, Stuart LM, Fisher EA, Lacy-Hulbert A, Moore KJ. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat Immunol 2012; 13:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gerszten RE, Tager AM. The monocyte in atherosclerosis–should I stay or should I go now? N Engl J Med 2012; 366:1734–1736. [DOI] [PubMed] [Google Scholar]
- 4. Czopek A, Moorhouse R, Guyonnet L, Farrah T, Lenoir O, Owen E, van Bragt J, Costello HM, Menolascina F, Baudrie V, Webb DJ, Kluth DC, Bailey MA, Tharaux PL, Dhaun N. A novel role for myeloid endothelin-B receptors in hypertension. Eur Heart J 2019; 40:768–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988; 332:411–415. [DOI] [PubMed] [Google Scholar]
- 6. Dhaun N, Webb DJ. Endothelins in cardiovascular biology and therapeutics. Nat Rev Cardiol 2019. doi:10.1038/s41569-019-0176-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7. Lerman A, Edwards BS, Hallett JW, Heublein DM, Sandberg SM, Burnett JC Jr. Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. N Engl J Med 1991; 325:997–1001. [DOI] [PubMed] [Google Scholar]
- 8. Kowala MC, Rose PM, Stein PD, Goller N, Recce R, Beyer S, Valentine M, Barton D, Durham SK. Selective blockade of the endothelin subtype A receptor decreases early atherosclerosis in hamsters fed cholesterol. Am J Pathol 1995; 146:819–826. [PMC free article] [PubMed] [Google Scholar]
- 9. Raichlin E, Prasad A, Mathew V, Kent B, Holmes DR Jr, Pumper GM, Nelson RE, Lerman LO, Lerman A. Efficacy and safety of atrasentan in patients with cardiovascular risk and early atherosclerosis. Hypertension 2008; 52:522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang J, Wang Y, Wang X, Xu L, Yang X, Zhao W. PKC-mediated Endothelin-1 expression in endothelial cell. Am J Hypertens 2019; 32:880–889. [DOI] [PubMed] [Google Scholar]
- 11. Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol 2010; 7:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen C, Khismatullin DB. Oxidized low-density lipoprotein contributes to atherogenesis via co-activation of macrophages and mast cells. PLoS One 2015; 10:e0123088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park JY, Takahara N, Gabriele A, Chou E, Naruse K, Suzuma K, Yamauchi T, Ha SW, Meier M, Rhodes CJ, King GL. Induction of endothelin-1 expression by glucose: an effect of protein kinase C activation. Diabetes 2000; 49:1239–1248. [DOI] [PubMed] [Google Scholar]
- 14. Li Q, Park K, Li C, Rask-Madsen C, Mima A, Qi W, Mizutani K, Huang P, King GL. Induction of vascular insulin resistance and endothelin-1 expression and acceleration of atherosclerosis by the overexpression of protein kinase C-β isoform in the endothelium. Circ Res 2013; 113:418–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lincoff AM, Roe M, Aylward P, Galla J, Rynkiewicz A, Guetta V, Zelizko M, Kleiman N, White H, McErlean E, Erlinge D, Laine M, Dos Santos Ferreira JM, Goodman S, Mehta S, Atar D, Suryapranata H, Jensen SE, Forster T, Fernandez-Ortiz A, Schoors D, Radke P, Belli G, Brennan D, Bell G, Krucoff M; PROTECTION AMI Investigators Inhibition of delta-protein kinase C by delcasertib as an adjunct to primary percutaneous coronary intervention for acute anterior ST-segment elevation myocardial infarction: results of the PROTECTION AMI randomized controlled trial. Eur Heart J 2014; 35:2516–2523. [DOI] [PubMed] [Google Scholar]
- 16. Li MW, Mian MO, Barhoumi T, Rehman A, Mann K, Paradis P, Schiffrin EL. Endothelin-1 overexpression exacerbates atherosclerosis and induces aortic aneurysms in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol 2013; 33:2306–2315. [DOI] [PubMed] [Google Scholar]
- 17. Coelho SC, Berillo O, Caillon A, Ouerd S, Fraulob-Aquino JC, Barhoumi T, Offermanns S, Paradis P, Schiffrin EL. Three-month endothelial human endothelin-1 overexpression causes blood pressure elevation and vascular and kidney injury. Hypertension 2018; 71:208–216. [DOI] [PubMed] [Google Scholar]
- 18. Davenport AP, Hyndman KA, Dhaun N, Southan C, Kohan DE, Pollock JS, Pollock DM, Webb DJ, Maguire JJ. Endothelin. Pharmacol Rev 2016; 68:357–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sek AC, Xie Z, Terai K, Long LM, Nelson C, Dudek AZ, Druey KM. Endothelial expression of endothelin receptor A in the systemic capillary leak syndrome. PLoS One 2015; 10:e0137373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heerspink HJL, Parving HH, Andress DL, Bakris G, Correa-Rotter R, Hou FF, Kitzman DW, Kohan D, Makino H, McMurray JJV, Melnick JZ, Miller MG, Pergola PE, Perkovic V, Tobe S, Yi T, Wigderson M, de Zeeuw D; SONAR Committees and Investigators Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet 2019; 393:1937–1947. [DOI] [PubMed] [Google Scholar]