Abstract
BACKGROUND
Post-traumatic stress disorder (PTSD) is characterized by a disordered stress response and associated with increased cardiovascular disease risk. The present study investigated whether angiotensin (Ang) II-elicited hypertensive response is sensitized in a model of PTSD and whether inhibition of angiotensin-converting enzyme (ACE) or tumor necrosis factor (TNF)-α prior to PTSD blocks this sensitization of Ang II hypertension.
METHODS
The resident–intruder paradigm was used to model PTSD. Each intruder rat (male Sprague-Dawley) was given normal drinking water or was pretreated with either an ACE inhibitor (captopril) or a TNF-α inhibitor (pentoxifylline) in the drinking water for 2 weeks. Subsequently, they were exposed to a different resident (male Long-Evans) for 2 hours on 3 days with each session separated by 1 day and then received a subcutaneous infusion of Ang II for 2 weeks.
RESULTS
The stressed rats had a significantly enhanced hypertensive response to the Ang II infusion (stressed Δ40.2 ± 3.9 mm Hg vs. unstressed Δ20.5 ± 4.5 mm Hg) and an upregulation of mRNA or protein expression of renin–angiotensin system (RAS) and proinflammatory cytokine (PIC) components and of a microglial marker in the lamina terminalis and hypothalamic paraventricular nucleus when compared with unstressed control rats. Both the sensitized hypertensive response and enhanced gene and protein expression were blocked by pretreatment with either ACE (Δ21.3 ± 3.9 mm Hg) or TNF-α inhibitor (Δ21.4 ± 2.6 mm Hg).
CONCLUSIONS
The results indicate that upregulation of the brain RAS and PICs produced by severe stress contributes to traumatic-induced sensitization of hypertensive response to Ang II, and disorders such as PTSD may predispose individuals to development of hypertension.
Keywords: blood pressure, hypertension, inflammation, rennin–angiotensin system, traumatic stress
Post-traumatic stress disorder (PTSD) is a complex psychiatric disorder characterized by a disordered stress response following a traumatic event.1,2 Multiple epidemiological studies demonstrate that PTSD is independently associated with an increased cardiovascular disease risk, which is manifested by enhanced sympathetic nervous system activity and hemodynamic response, blunted baroreflex sensitivity, and inflammation.3–5 Individuals with PTSD have significantly higher levels of circulating and brain proinflammatory cytokines (PICs) including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6.6 Animal studies confirm these findings by showing that social stress results in activated microglia, oxidative stress, and increased expression of PICs in the central nervous system.1,7,8 Administration of minocycline, a microglial inhibitor, significantly decreased these elevated cytokines and attenuated anxious-like behaviors in stress-exposed rats.9
PTSD also leads to activation of the renin–angiotensin system (RAS), increasing angiotensin (Ang) II synthesis in the periphery and the central nervous system.10 Recent studies demonstrate that the therapeutic actions of both angiotensin-converting enzyme (ACE) inhibitors and Ang II receptor blockers extend beyond blood pressure (BP) reduction and have been associated with lower PTSD symptoms.11,12 In numerous preclinical studies, Ang II receptor blocker treatment has also been shown to have therapeutic and protective effects on the brain, including reductions of the stress response, anxiety, brain inflammation, and ischemia.13–16 The hypothalamic paraventricular nucleus (PVN) is a critical site that integrates stress-related signals and regulates hypothalamic–pituitary–adrenal axis and autonomic responses to stressors. Krause et al. reported that knockdown of Ang II type 1 receptor (AT1-R) in the PVN attenuates the hypothalamic–pituitary–axis neuroendocrine and anxiety stress response in rats.17 These results suggest that the RAS is involved in the regulation of the stress response in individuals with PTSD and that inhibition of brain AT1-R activity improves stress-related behaviors and associated brain dysfunction.
The lamina terminalis (LT) structures include the subfornical organ, the median preoptic nucleus, and the organum vasculosum of the LT. The subfornical organ and organum vasculosum of the LT lack the normal blood–brain barrier and have direct projections into the PVN.18 Both the LT and PVN are essential for the normal control sympathetic activation and behaviors due to their receptive and integrative functions.19 Using an Induction–Delay–Expression experimental protocol, our previous studies demonstrate that activation of RAS and inflammation in the LT and PVN by various challenges sensitizes Ang II-elicited hypertension.20–23
An ethologically relevant animal model of psychosocial defeat is the resident–intruder paradigm. This model involves introducing a smaller male rat, the intruder, into the cage of a larger male, the resident. The resident immediately assumes the role of the dominant animal, and the intruder becomes submissive and displays psychosocial defeat. The resident–intruder paradigm is regarded as one of the most robust models of PTSD.7,24 Given that activation of the RAS and inflammation is involved in both PTSD-induced stress responses and challenge-induced sensitization of Ang II hypertension, the present study investigated whether the Ang II-elicited hypertensive response is also sensitized in a model of PTSD, and if so, whether inhibition of ACE or TNF-α might block traumatic stress-induced sensitization of Ang II hypertension. Furthermore, we examined the central mechanisms, especially in the LT and PVN, that are active in rats that have normal BP but are at increased risk of hypertension by virtue of exposure to traumatic stress.
METHODS
Animals
All animals were maintained in a temperature (23 ± 2 °C) and light (12-h light/dark cycle) controlled facility, with unlimited access to food and water. A total of 70 male Sprague-Dawley rats (SD, 10 weeks old, Harlan) and 24 Long-Evans male rats (12 weeks old, Charles River Laboratories) were used in the present experiments. Body weight, food, and drinking water intakes were measured one time per week.
All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Iowa Animal Care and Use Committee.
Telemetry probe and osmotic pump implantation
Rat BP transmitters (HD-S10, DSI, St. Paul, MN) were used to directly measure arterial pressure. The rats were anesthetized with a ketamine-xylazine mixture (90% ketamine and 10% xylazine), and the femoral artery was accessed with a ventral incision. The right femoral artery was isolated, and the catheter of a telemetry probe was inserted into the vessel. Through the same ventral incision, a pocket along the right flank was formed. The body of the transmitter was slipped into the pocket and secured with tissue adhesive. The ventral incision was then closed with suture. Beginning 7 days after recovery from surgery, BP and heart rate (HR) data collection was initiated.
In a separate procedure under isoflurane anesthesia (0.5–5% inhalation), osmotic pumps (model 2002, ALZET) containing Ang II (120 ng/kg/min, Sigma) were implanted subcutaneously in the back of both the intruder (stressed) rats and nonstressed control rats.
Resident–Intruder Stress Paradigm
The resident–intruder paradigm used to model PTSD was modified from the resident–intruder model originally developed by Miczek.25 At 11 weeks of age, each intruder rat (SD) was exposed to a different resident (Long-Evans) for 2 hours on 3 days with each session separated by 1 day. In this model, social defeat was observed in the intruder with defeat characterized by the intruder surrendering or acquiring a supine position for approximately 3 seconds.26 If the resident showed sustained aggression, a screen partition with holes was placed in the cage to avoid direct physical contact between the resident and intruder. The intruder rat is the “stressed” animal and the subject of these studies. Age-matched SD rats that were not subjected to the resident–intruder paradigm were used as “nonstressed” controls.
Experiment 1
BP and HR were recorded by telemetry for 5 days at baseline and then for the subsequent 34 consecutive days during which some rats were subjected to the resident–intruder stress paradigm and all rats received a slow-pressor Ang II or saline infusion for 2 weeks via osmatic minipump. The Ang II infusion began 3 days after the last stress exposure. Among the stressed rats, some were treated for the 2 weeks prior to initiating the stress paradigm with the ACE inhibitor captopril (Cap; 0.5 mg/ml) or the TNF-α synthesis inhibitor pentoxifylline (PTX; 100 mg/kg/day) in the drinking water. Therefore, the rats comprised 5 groups (6 rats per group): (i) vehicle + nonstressed + saline (BP and HR data not shown); (ii) vehicle + nonstressed+Ang II; (iii) vehicle + stressed + Ang II; (iv) Cap + stressed + Ang II; and (v) PTX + stressed + Ang II. At the conclusion of the Ang II treatment protocol, the animals (n = 6 per group) were euthanized to collect brain tissue to determine mRNA and protein expression of RAS and PIC components and a microglial marker in the LT and the PVN.
Experiment 2
Additional studies were performed to assess the effect of the stress paradigm alone on RAS and PIC components and microglial activity in the LT and PVN. The brains of nonstressed rats, stressed rats, and stressed rats pretreated with Cap or PTX rats (n = 10 per group) were collected on day 3 after the last stress exposure, corresponding to the time at which Ang II infusion was initiated in Experiment 1.
Real-Time RT-PCR analysis
Total RNA was isolated from the LT and PVN using the Trizol method (Invitrogen) and treated with DNase I (Invitrogen, Carlsbad, CA) to remove any genomic DNA contamination. RNA integrity was checked by gel electrophoresis. Total RNA was reverse transcribed following the manufacturer’s instructions (Applied Biosystems, Foster City, CA). Real-time PCR was conducted using 200–300 ng of cDNA and 500 nM of each primer in a 20 μl reaction with iQ SYBR Green Supermix (Bio-Rad, Hercules, CA). Amplification cycles were conducted at 95°C for 3 min, followed by 40 cycles of 95°C for 15 s and annealing/extension at 60°C for 30 s. Reactions were performed in duplicate and analyzed using a C1000 thermocycler system (Bio-Rad). mRNA levels for RAS components (ACE and AT1-R), PICs (TNF-α, IL-1β, and IL-6), microglial marker (CD11b), and GAPDH were analyzed with SYBR Green real-time RT-PCR. The values were corrected by GAPDH, and the final concentration of mRNA was calculated using the formula x = 2(−ΔΔCt), where x = fold difference relative to control. Primers were purchased from Integrated DNA Technologies (Coralville, IA). The sequences of the primers are shown in Table 1.
Table 1.
Primer sequences for real-time PCR
| Gene | Forward primer | Reverse primer | Product size (bp) |
|---|---|---|---|
| GAPDH | TGACTCTACCCACGGCAAGTTCAA | ACGACATACTCAGCACCAGCATCA | 141 |
| ACE | GTGTTGTGGAACGAATACGC | CCTTCTTTATGATCCGCTTGA | 187 |
| AT1-R | CTCAAGCCTGTCTACGAAAATGAG | GTGAATGGTCCTTTGGTCGT | 188 |
| TNF-α | GCCGATTTGCCACTTCATAC | AAGTAGACCTGCCCGGACTC | 209 |
| IL-6 | GCCTATTGAAAATCTGCTCTGG | GGAAGTTGGGGTAGGAAGGA | 160 |
| IL-1β | AGCAACGACAAAATCCCT GT | GAAGACAAACCGCTTTTCCA | 209 |
| CD11b | TTACCGGACTGTGTGGACAA | AGTCTCCCACCACCAAAGTG | 239 |
ACE, angiotensin-converting enzyme 1; AT1-R, angiotensin II type 1 receptor; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; IL-6, interleukin-6; CD11b, cluster of differentiation molecule 11b.
Western blot analysis
The LT tissues were homogenized in lysis buffer, and the protein concentration in the supernatant was measured with the BCA protein assay kit (Pierce, Rockford, IL). Equivalent amounts of protein were separated on 4–15% sodium dodecyl sulfate–polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Millipore Corporation, Bedford, MA). The membranes were blocked in 5% nonfat dry milk for 1 h and then incubated with primary antibodies to ACE (Santa Cruz Biotechnology Inc., Santa Cruz, CA), IL-1β receptor (Abcam, Cambridge, MA), and β-actin (Cell Signaling Inc., Danvers, MA) overnight at 4°C. After 3 washing, the membranes were incubated with horseradish peroxidase-conjugated second antibodies (Abcam) for 1 h at room temperature. The signal was visualized using an enhanced chemiluminescence detection system (Amersham, Arlington Heights, IL), and densities of the immunobands were quantitated using NIH ImageJ software (Bethesda, MD). All data were corrected by β-actin.
Data analysis
Mean arterial pressure (MAP) and HR, obtained from the telemetry recordings, are presented as mean daily values. Differences for MAP and HR were calculated for each animal based on the mean of a 5-day baseline subtracted from the mean of the final 5 days of Ang II treatment. Two-way ANOVA for the experimental groups was then conducted on daily MAP, HR, or the means of calculated differences. (The factors were stress and Ang II treatment.) After establishing a significant ANOVA, post hoc analyses were performed with Tukey multiple comparison tests between pairs of mean changes. One-way ANOVAs and post hoc Tukey analyses were used to test for the differences in body weight, drinking water intake, mRNA or protein expression of the RAS and PIC components, and microglial marker in the LT and PVN. All data are expressed as means ± SE. Statistical significance was set at P < 0.05.
RESULTS
Traumatic stress sensitizes the hypertensive response to systemic Ang II infusion
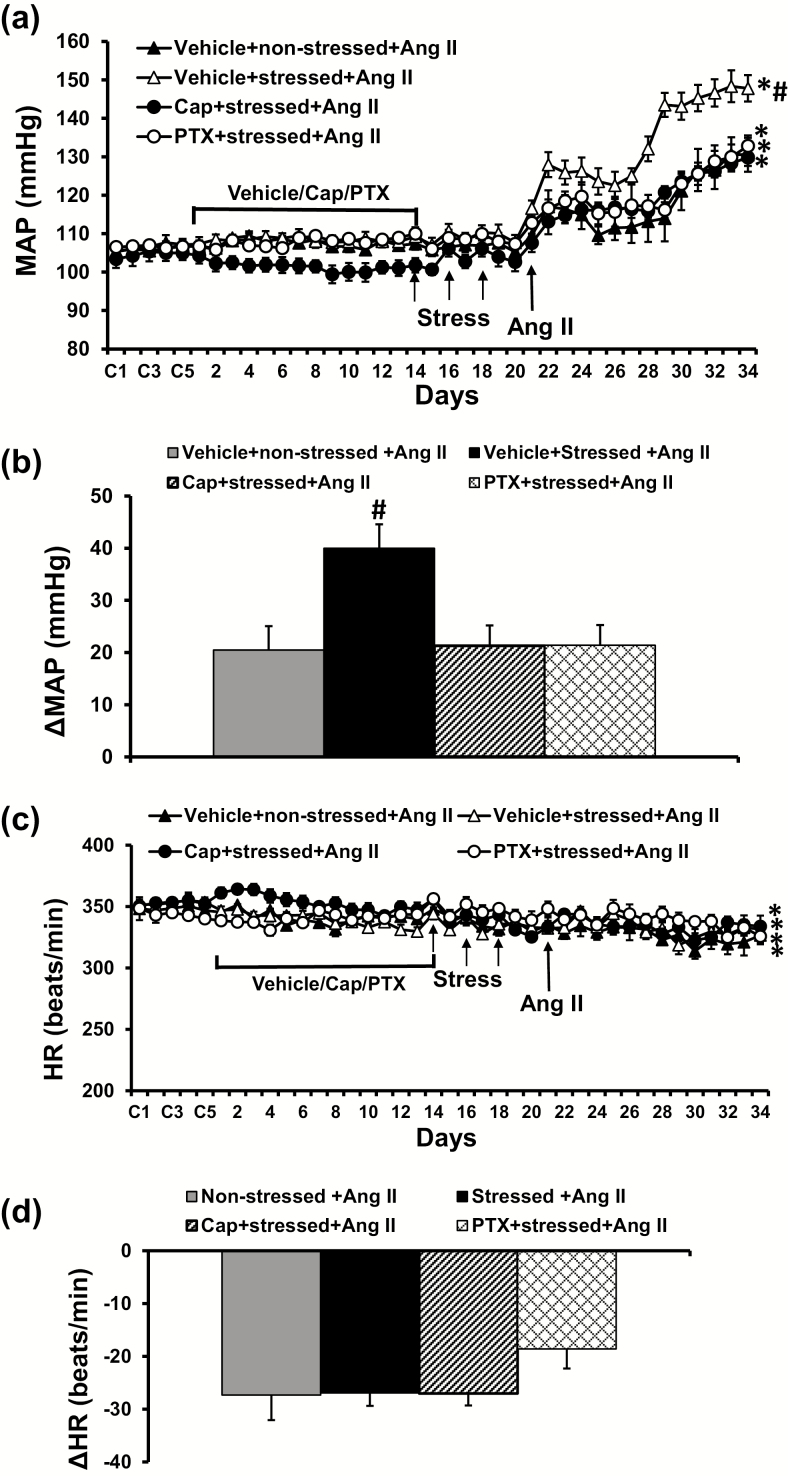
At the end of resident–intruder paradigm induction, there were no significant differences in MAP and HR in all groups of rats although pretreatment with ACE inhibitor tended to reduce basal MAP, but this was not statistically different. However, during infusion of the slow-pressor dose of Ang II, the stressed rats showed a significantly enhanced hypertensive response (Δ40.2 ± 3.9 mm Hg) compared with nonstressed control rats (Δ20.5 ± 4.5 mm Hg, P < 0.05, Figure 1a,b).
Figure 1.
Pressor effects (a and b) and heart rate (HR) (c and d) changes induced by angiotensin (Ang) II in nonstressed, stressed, stressed with pretreatment with either captopril (Cap) or pentoxifylline (PTX) rats. The enhanced pressor effect in stressed rats was attenuated by either Cap or PTX pretreatment (n = 6/group; *P < 0.05 vs. baseline; #P < 0.05 vs. nonstressed or stressed rats with Cap or PTX pretreatment).
Effects of pretreatment with Cap or PTX on traumatic stress-induced sensitization of Ang II hypertension
Pretreatment with either Cap or PTX significantly reduced the Ang II-elicited pressor response including daily MAP and change from baseline MAP in the stressed rats (Cap, Δ21.3 ± 3.9 mm Hg; PTX, Δ21.4 ± 2.6 mm Hg, P < 0.05; Figure 1a,b). Chronic Ang II infusion produced significant, but comparable decreases in HR in all groups (P > 0.05, Figure 1c,d).
Effect of Cap or PTX on traumatic stress-induced RAS, PIC, and microglial activity in the brain
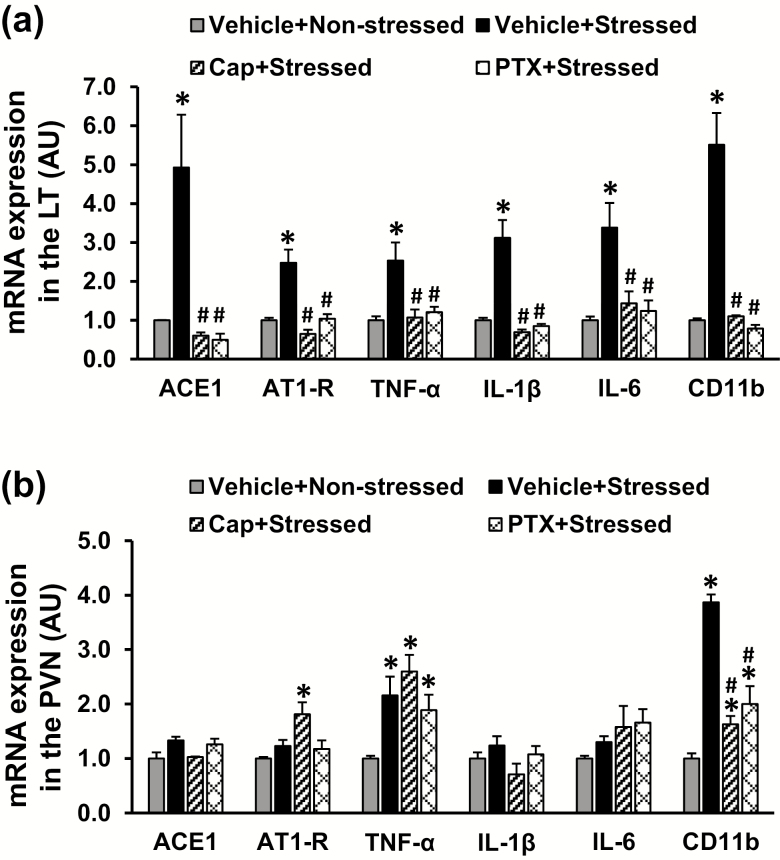
In LT tissues collected from rats on day 3 after the last stress exposure, stressed rats exhibited increased mRNA expression of RAS components (ACE and AT1-R), the PICs (TNF-α, IL-6, and IL-1β), and the microglial marker (CD11b) when compared with nonstressed rats (P < 0.05, Figure 2a). Pretreatment with either ACE inhibitor or TNF-α inhibitor blocked these stress-induced increases in gene expression. In contrast, in PVN tissues, the stressed rats only showed upregulation of mRNA expression of TNF-α and CD11b, and only increased CD11b expression was reversed by pretreatment with Cap or PTX (P < 0.05, Figure 2b).
Figure 2.
Quantitative comparison of the mRNA expression of renin–angiotensin system components, proinflammatory cytokines, and microglial marker in the lamina terminalis (LT) and paraventricular nucleus (PVN) in nonstressed, stressed, stressed with pretreatment with either captopril (Cap) or pentoxifylline (PTX) rats before angiotensin II infusion (a and b) (n = 5 per group; *P < 0.05 vs. nonstressed rats; #P < 0.05 vs. stressed rats without pretreatment).
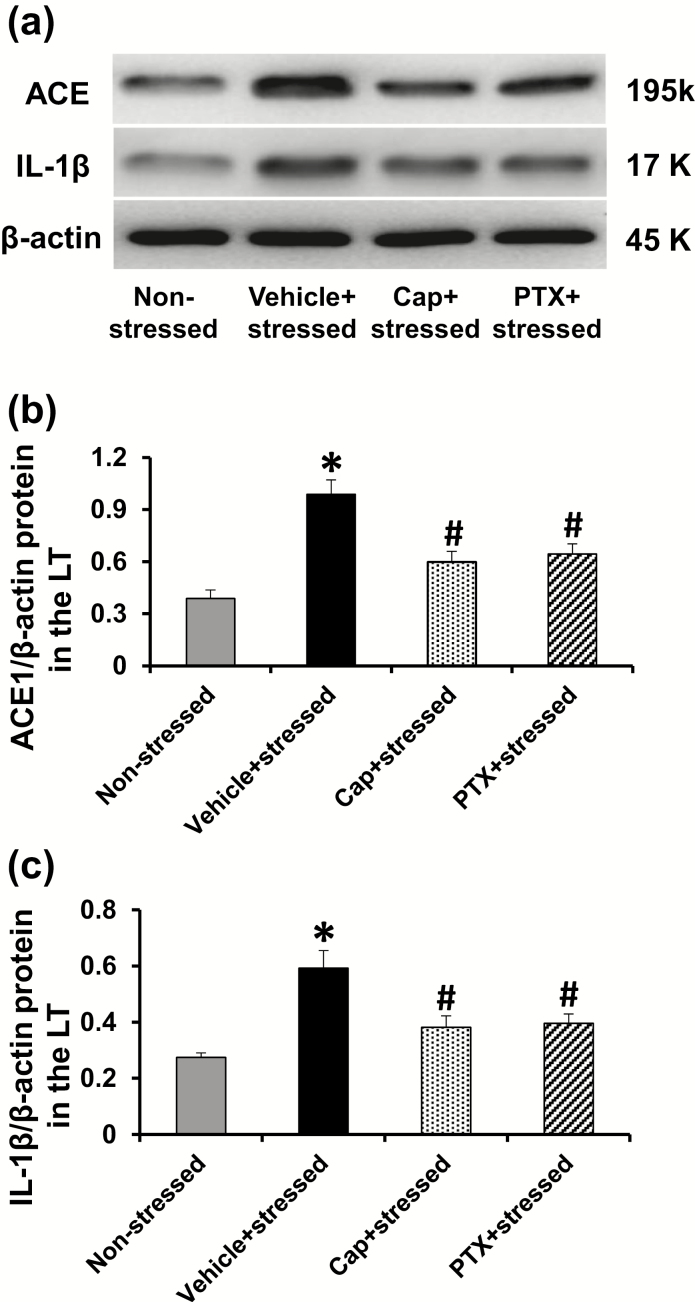
Western blot analysis to confirm the effects of traumatic stress on genomic expression revealed increased ACE and IL-1β protein in the LT of the stressed rats, both of which were significantly attenuated by pretreatment with either Cap or PTX (P < 0.05, Figure 3).
Figure 3.
Representative western blots and quantitative comparison of protein levels for ACE (a and b) and IL-1β (a and c) in the LT of nonstressed, stressed, stressed with pretreatment with either captopril (Cap) or pentoxifylline (PTX) rats before angiotensin II infusion. Values are corrected by β-actin and expressed as mean ± SEM (n = 5 per group; *p < .05 vs. nonstressed rats; #p < 0.05 vs. stressed rats without pretreatment).
Effect of Cap or PTX on stress-induced plus Ang II-induced RAS, PIC, and microglial activity
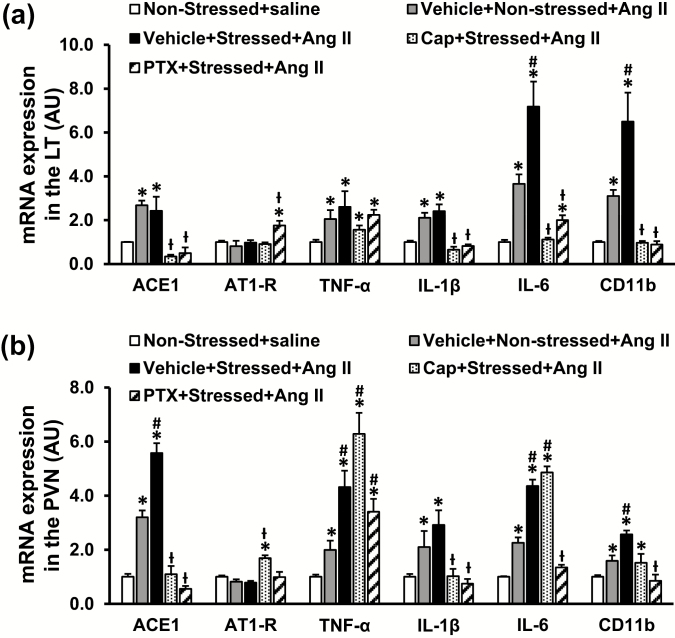
In LT tissues, the slow-pressor Ang II infusion resulted in a significant increase in mRNA expression of ACE, TNF-α, IL-6, IL-1β, and CD11b in both nonstressed and stressed rats when compared with the saline group (P < 0.05, Figure 4a). The mRNA expression of AT1-R was not higher after Ang II (P > 0.05). Compared with the nonstressed rats treated with Ang II, the stressed rats exhibited enhanced expression of IL-6 and CD11b (P < 0.05), but not ACE, TNF-α, and IL-1β (P > 0.05) after Ang II treatment (Figure 4a). Either Cap or PTX significantly attenuated the increased mRNA expression of ACE, IL-1β, and IL-6 (P < 0.05), but TNF-α expression remained high in stressed rats following Ang II administration (P > 0.05). mRNA expression of AT1-R was upregulated in PTX-pretreated rats when compared with other rats (P < 0.05, Figure 4a).
Figure 4.
Quantitative comparison of the mRNA expression of renin–angiotensin–aldosterone system components, proinflammatory cytokines, and microglial marker in the lamina terminalis (LT; a) and paraventricular nucleus (PVN; b) of nonstressed, stressed, stressed with pretreatment with either captopril (Cap) or pentoxifylline (PTX) rats after angiotensin (Ang) II administration (n = 6 per group; *P < 0.05 vs. nonstressed + saline; #P < 0.05 vs. nonstressed+Ang II; ƚP < 0.05 vs. stressed + Ang II).
In PVN tissues, Ang II infusion elicited a significant increase in the mRNA expression of ACE, TNF-α, IL-1β, IL-6, and CD11b in both nonstressed and stressed rats when compared with the saline group (P < 0.05, Figure 4b). The mRNA expression of AT1-R was not higher after Ang II (P > 0.05). The stressed rats showed enhanced mRNA expression of ACE, TNF-α, IL-6, and CD11b after Ang II treatment (P < 0.05, Figure 4b). Cap pretreatment reversed the increased gene expression of ACE, IL-1β, but TNF-α, IL-6, and CD11b remained high in stressed rats after Ang II infusion (P < 0.05, Figure 4b). mRNA expression of AT1-R was upregulated in Cap-pretreated rats (P < 0.05, Figure 4b). In contrast, PTX pretreatment significantly attenuated most of the increased mRNA expression including ACE, IL-1β, IL-6, and CD11b (P < 0.05, Figure 4b), and TNF-α expression remained high in stressed rats after Ang II infusion (P > 0.05, Figure 4b).
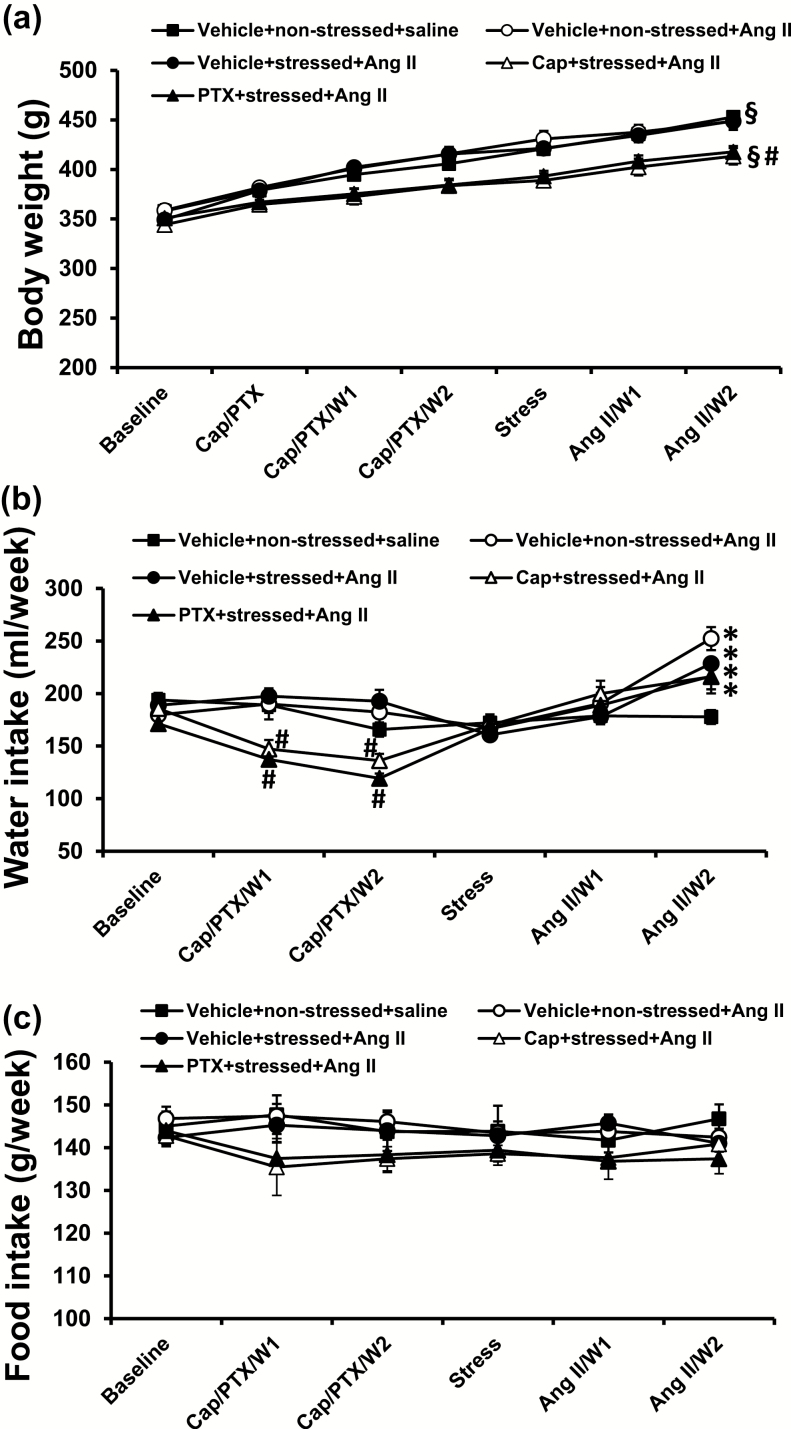
Effects of the PTSD paradigm and Ang II infusion on body weight and food and water intakes
There were no differences in basal body weight among groups. The stress induction and Ang II infusion had no effect on rat growth: body weight gradually increased and there was no difference between groups (nonstressed + saline, 354.6.0 ± 3.9 to 453.0 ± 2.1 g; nonstressed + Ang II, 358.6 ± 6.1 to 448.6 ± 8.7 g; stressed + Ang II 349.4 ± 3.1 to 449.0 ± 7.9 g). However, pretreatment with either Cap or PTX induced a slower increase in body weight when compared with nonpretreated rats (Cap + stressed + Ang II, 344.2 ± 4.4 to 413.0 ± 8.3 g; PTX + stressed + Ang II, 351.0 ± 5.8 to 417.7 ± 5.9 g, P < 0.05; Figure 5a).
Figure 5.
Changes in body weight (a) and in drinking water intake (b) during pretreatment with either captopril (Cap) or pentoxifylline (PTX), stress induction, and angiotensin (Ang II) administration (W = week; n = 6 per group; §P < 0.05 vs. basal body weights; *P < 0.05 vs. water intake before Ang II infusion; #P < 0.05 vs. body weight or water intake in nonpretreatment groups).
Pretreatment with either Cap or PTX for 2 weeks decreased the water intake (Cap, 185.6 ± 5.6 to 141.7 ± 7.5 ml/week; PTX, 171.3 ± 5.7 to 128.3 ± 3.7 ml/week; P < 0.05), whereas the stress induction had no effects. Two weeks of Ang II treatment resulted in a significant and similar increase in water intake in all groups (nonstressed + Ang II, 245.3 ± 10.8 ml/week; stressed + Ang II, 228.7 ± 5.9 ml/week; Cap + stressed + Ang II, 216.0 ± 11.2 ml/week; PTX + stressed + Ang II, 216.7 ± 12.7 ml/week; P < 0.05; Figure 5b) when compared with saline treatment (177.7 ± 6.7 ml/week).
There were no significant differences in food intake among groups during the entire experimental period (P > 0.05, Figure 5c).
DISCUSSION
The major findings of the present study are as follows: (i) Ang II administration resulted in an enhanced hypertensive response in stressed rats, which was reversed by either Cap or PTX pretreatment, and (ii) prior to challenging rats with a pressor dose of Ang II, the stressed rats showed upregulated expression of RAS components, PICs, and a microglial marker mainly in the LT when compared with the nonstressed controls. The stress paradigm further enhanced Ang II-induced changes in some of the genes expressed in both the LT and PVN, and some of these changes were prevented by pretreatment with either Cap or PTX. These results demonstrate that upregulation of the brain RAS and PICs produced by a severe stress can sensitize the hypertensive response to Ang II. These findings suggest a potential mechanism for the increased susceptibility to hypertension among patients with PTSD.
Stressors that elicit a stress response are of 2 types: physiological stressors and psychosocial stressors. Physiological stressors are challenges that result from an immediate disruption in homeostasis (e.g., hypoxia, hypovolemia, extracellular hypertonicity, hypoglycemia, etc.).27–29 In contrast, psychosocial stressors, such as the resident–intruder paradigm, do not immediately disrupt homeostasis but are perceived as posing a potential threat that may eventuate in compromising physical or psychological integrity (e.g., restraint, psychosocial defeat).27–29 Psychosocial stressors eliciting sustained or repeated activation of the defense reaction and sympathetic nervous system activation have been demonstrated to be important causes of hypertension.30–33
In the previous studies, we used an Induction–Delay–Expression experimental design to study physiological stressor-induced sensitization of the hypertensive response. In these studies, during Induction rats were exposed over a relatively short period of time to various types of challenges, such as low doses of Ang II or aldosterone, leptin, high-fat diet feeding that did not produce any sustained effect on BP. Then following a period of Delay, a sensitized hypertensive response to a slow-pressor dose of Ang II was observed in these rats when challenged during a period of Expression.20–23
The present study tested the hypothesis that a psychosocial stressor, in this case social defeat in a resident–intruder experimental model of PTSD, might induce sensitization of a hypertensive response. We demonstrated that this post-traumatic stress paradigm, which alone did not alter basal BP and HR, led to an enhanced hypertensive response to Ang II treatment. This finding is consistent with many studies on PTSD in humans, showing that PTSD is independently associated with an increased cardiovascular disease risk including hypertension.34 It has been shown that PTSD patients from military and general populations have decreased HR variability, suggestive of increased sympathetic and decreased parasympathetic tone to the heart.35,36 The resting systolic BP, diastolic BP and HR, as well as BP reactivity to anxiety were all significantly higher in the PTSD group.37,38 Likewise, the present study revealed the sensitizing effect of stress on the hypertensive response in PTSD animal model, suggesting that PTSD can predispose individuals to exacerbation of renin–angiotensin-elicited hypertension.
Numerous studies have demonstrated microglial activation and enhanced PIC expression in a variety of stress models.39 Increased levels of PICs such as TNF-α, IL-1β, and IL-6 are observed in PTSD patients and animal models that exhibit increased risk for cardiovascular disease.2,40,41 Furthermore, augmented inflammation prior to a traumatic event may increase risk for subsequent development of PTSD symptoms following trauma, supporting a potential role of inflammation in the pathophysiology of PTSD.42 In general, the microglial activation and increased PIC expression occur particularly within brain regions involved in the stress response including the frontal cortex, hypothalamus, amygdala, and hippocampus.6,8,9 Inhibition of microglia by systemic minocycline administration significantly decreased the elevated cytokines in these nuclei and attenuated anxiety-like behaviors in the stress-exposed animals.9,43,44 Central blockade of IL-1β also blunts the stress-induced pressor response.45 The present study extends the above studies by showing that traumatic stress not only resulted in microglial activation and upregulation of PIC expression, but also enhanced Ang II-induced changes in expression of these genes in the LT and PVN, two critical nuclei involved in BP regulation. Similar to the brain inflammatory response to post-traumatic stress, hypertension and sensitization of hypertensive response are also characterized by activation of microglia and chronic inflammation in the central nervous system that contribute to enhanced sympathetic activity and blunted baroreflex sensitivity, and anti-inflammatory treatments have been shown to affect autonomic, baroreceptor function, and hypertensive sensitization response.20,21,46,47 Based on the role of microglial activation and inflammation in both PTSD symptom severity and development of hypertension,20,21,48–50 it can be speculated that the traumatic stress-induced activation of microglia and increased expression of PICs in the LT and PVN may account, at least in part, for sensitization of Ang II hypertension in the present study. This is due to inhibition of TNF-α production not only reversed traumatic stress-induced microglial activation and PIC expression, but also significantly diminished the sensitization of Ang II-induced hypertension.
It has been shown that many types of stress promote RAS activity including increased brain Ang II synthesis and upregulated ACE and AT1-R expression, especially in the LT structures (subfornical organ and organum vasculosum of the LT) and downstream in the PVN.10,51 Variants of the ACE gene associated with higher enzyme activity and Ang II synthesis are associated with post-traumatic stress symptoms and affect the outcomes of angiotensin-pathway medications on PTSD.11 Many studies have demonstrated that treatment with either Ang II receptor blockers or ACE inhibitors not only reduces BP levels, but also decreases anxiety and depression in hypertensive and diabetic patients and in animal PTSD models.15,16,52,53 In the present study, we found that the traumatic stress condition upregulated expression of ACE and AT1-R in the LT and enhanced Ang II-induced upregulation of ACE expression in the PVN, which were blocked by pretreatment with ACE inhibitor. It is well established that RAS hyperactivity in the central nervous system is associated with increased BP and sensitization of hypertensive response.20–23 Therefore, the upregulated RAS components induced by traumatic stress are also likely to contribute to the sensitized hypertensive response to Ang II in the present study.
It should be noticed that first, in stressed rats without Ang II treatment, the mRNA expression of all RAS and PIC components and the microglial marker assessed in this study were upregulated in the LT. Only TNF-a and the microglial marker expression were increased in the PVN, whereas for most of these genes, expression was upregulated in both the LT and PVN after Ang II infusion. The results may reflect the differences in the brain location between these 2 structures (outside vs. inside blood–brain barrier) and the nature of the conditions involving the 2 structures (stress alone vs. stress + Ang II). Therefore, besides the direct activation of these 2 structures involved in BP regulation through neural network connections with stress-related nuclei such as hippocampus, amygdala,19 the LT and PVN activation initiated by peripherally heightened RAS and inflammation induced by stress and systemic Ang II infusion cannot be ruled out in the present study. Second, blockade of either ACE or TNF-α production reversed all of stress-induced upregulation of RAS and PIC components and a microglial marker, indicative of a mutually necessary cooperative action between the RAS and PICs that contributes to traumatic stress-induced sensitization of Ang II hypertension. This is consistent with the established notion that the crosstalk between RAS and inflammatory signaling within the brain increases sympathetic output and cardiovascular dysfunction in a positive feedback manner, which contributes to the development and progression of hypertension.54,55 Third, we found that administration of either ACE inhibitor or TNF-α inhibitor in drinking water reduced the water intake during the treatment period, but drinking behavior was restored to normal as the protocol progressed. As a result, the body weights for those animals were less than for those without Cap or PTX treatment although they had similar food intake. It has been shown that high doses of Cap or PTX in the drinking water or food induce a taste aversion and a side effect of stomach upset that make animals drink less and gain less body weight.56,57 Therefore, the changes in water intake and body weight induced by Cap or PTX may be attributed to an aversion for the drinking water. It seems unlikely that these changes would have significantly affected our results.
In summary, epidemiological studies and animal experiments demonstrate a link between PTSD and cardiovascular disease and hypertension, in which RAS activation and inflammation contribute to compromised cardiovascular health in PTSD. The present study further demonstrates that upregulation of the brain RAS and PICs produced by severe stressors in this model of PTSD contributes to sensitization of the hypertensive response to Ang II. Our studies provide insight into the potential central mechanisms by which the PTSD induces hypertensive response sensitization, and may be useful in generating new therapeutic approaches (i.e., targeting central RAS and inflammatory signaling) to the treatment of hypertension in those suffering from both mental and cardiovascular diseases.
FUNDING
This work was supported by the National Institutes of Health grants HL-14388, HL-98207 (A.K.J. and B.X.), HL-139575 (A.K.J. and B.X.), HL-139521 (S.G.W.), HL-073986 (R.B.F.).
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1. Finnell JE, Wood SK. Neuroinflammation at the interface of depression and cardiovascular disease: evidence from rodent models of social stress. Neurobiol Stress 2016; 4:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Michopoulos V, Powers A, Gillespie CF, Ressler KJ, Jovanovic T. Inflammation in fear- and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology 2017; 42:254–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kibler JL, Joshi K, Ma M. Hypertension in relation to posttraumatic stress disorder and depression in the US National Comorbidity Survey. Behav Med 2009; 34:125–132. [DOI] [PubMed] [Google Scholar]
- 4. Park J, Marvar PJ, Liao P, Kankam ML, Norrholm SD, Downey RM, McCullough SA, Le NA, Rothbaum BO. Baroreflex dysfunction and augmented sympathetic nerve responses during mental stress in veterans with post-traumatic stress disorder. J Physiol 2017; 595:4893–4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roy SS, Foraker RE, Girton RA, Mansfield AJ. Posttraumatic stress disorder and incident heart failure among a community-based sample of US veterans. Am J Public Health 2015; 105:757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zass LJ, Hart SA, Seedat S, Hemmings SM, Malan-Müller S. Neuroinflammatory genes associated with post-traumatic stress disorder: implications for comorbidity. Psychiatr Genet 2017; 27:1–16. [DOI] [PubMed] [Google Scholar]
- 7. Patki G, Solanki N, Atrooz F, Allam F, Salim S. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res 2013; 1539:73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wilson CB, McLaughlin LD, Nair A, Ebenezer PJ, Dange R, Francis J. Inflammation and oxidative stress are elevated in the brain, blood, and adrenal glands during the progression of post-traumatic stress disorder in a predator exposure animal model. PLoS One 2013; 8:e76146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levkovitz Y, Fenchel D, Kaplan Z, Zohar J, Cohen H. Early post-stressor intervention with minocycline, a second-generation tetracycline, attenuates post-traumatic stress response in an animal model of PTSD. Eur Neuropsychopharmacol 2015; 25:124–132. [DOI] [PubMed] [Google Scholar]
- 10. Saavedra JM, Sánchez-Lemus E, Benicky J. Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation and ischemia: therapeutic implications. Psychoneuroendocrinology 2011; 36:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nylocks KM, Michopoulos V, Rothbaum AO, Almli L, Gillespie CF, Wingo A, Schwartz AC, Habib L, Gamwell KL, Marvar PJ, Bradley B, Ressler KJ. An angiotensin-converting enzyme (ACE) polymorphism may mitigate the effects of angiotensin-pathway medications on posttraumatic stress symptoms. Am J Med Genet B Neuropsychiatr Genet 2015; 168B:307–315. [DOI] [PubMed] [Google Scholar]
- 12. Khoury NM, Marvar PJ, Gillespie CF, Wingo A, Schwartz A, Bradley B, Kramer M, Ressler KJ. The renin-angiotensin pathway in posttraumatic stress disorder: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with fewer traumatic stress symptoms. J Clin Psychiatry 2012; 73:849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen D, La Greca L, Head GA, Walther T, Mayorov DN. Blood pressure reactivity to emotional stress is reduced in AT1A-receptor knockout mice on normal, but not high salt intake. Hypertens Res 2009; 32:559–564. [DOI] [PubMed] [Google Scholar]
- 14. Dandona P, Kumar V, Aljada A, Ghanim H, Syed T, Hofmayer D, Mohanty P, Tripathy D, Garg R. Angiotensin II receptor blocker valsartan suppresses reactive oxygen species generation in leukocytes, nuclear factor-kappa B, in mononuclear cells of normal subjects: evidence of an antiinflammatory action. J Clin Endocrinol Metab 2003; 88:4496–4501. [DOI] [PubMed] [Google Scholar]
- 15. Matsumoto S, Shimodozono M, Miyata R, Kawahira K. The angiotensin II type 1 receptor antagonist olmesartan preserves cerebral blood flow and cerebrovascular reserve capacity, and accelerates rehabilitative outcomes in hypertensive patients with a history of stroke. Int J Neurosci 2010; 120:372–380. [DOI] [PubMed] [Google Scholar]
- 16. Papademetriou V, Farsang C, Elmfeldt D, Hofman A, Lithell H, Olofsson B, Skoog I, Trenkwalder P, Zanchetti A; Study on Cognition and Prognosis in the Elderly study group . Stroke prevention with the angiotensin II type 1-receptor blocker candesartan in elderly patients with isolated systolic hypertension: the Study on Cognition and Prognosis in the Elderly (SCOPE). J Am Coll Cardiol 2004; 44:1175–1180. [DOI] [PubMed] [Google Scholar]
- 17. Krause EG, de Kloet AD, Scott KA, Flak JN, Jones K, Smeltzer MD, Ulrich-Lai YM, Woods SC, Wilson SP, Reagan LP, Herman JP, Sakai RR. Blood-borne angiotensin II acts in the brain to influence behavioral and endocrine responses to psychogenic stress. J Neurosci 2011; 31:15009–15015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J 1993; 7:678–686. [DOI] [PubMed] [Google Scholar]
- 19. Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 2009; 10:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xue B, Thunhorst RL, Yu Y, Guo F, Beltz TG, Felder RB, Johnson AK. Central renin-angiotensin system activation and inflammation induced by high-fat diet sensitize angiotensin II-elicited hypertension. Hypertension 2016; 67:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xue B, Yu Y, Zhang Z, Guo F, Beltz TG, Thunhorst RL, Felder RB, Johnson AK. Leptin mediates high-fat diet sensitization of angiotensin II-elicited hypertension by upregulating the brain renin-angiotensin system and inflammation. Hypertension 2016; 67:970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xue B, Zhang Z, Johnson RF, Johnson AK. Sensitization of slow pressor angiotensin II (Ang II)-initiated hypertension: induction of sensitization by prior Ang II treatment. Hypertension 2012; 59:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xue B, Zhang Z, Roncari CF, Guo F, Johnson AK. Aldosterone acting through the central nervous system sensitizes angiotensin II-induced hypertension. Hypertension 2012; 60:1023–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zoladz PR, Diamond DM. Predator-based psychosocial stress animal model of PTSD: preclinical assessment of traumatic stress at cognitive, hormonal, pharmacological, cardiovascular and epigenetic levels of analysis. Exp Neurol 2016; 284:211–219. [DOI] [PubMed] [Google Scholar]
- 25. Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology (Berl) 1979; 60:253–259. [DOI] [PubMed] [Google Scholar]
- 26. Golden SA, Covington HE III, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc 2011; 6:1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo–pituitary–adrenocortical axis. Trends Neurosci 1997; 20:78–84. [DOI] [PubMed] [Google Scholar]
- 28. Pacák K, Palkovits M. Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev 2001; 22:502–548. [DOI] [PubMed] [Google Scholar]
- 29. Sawchenko PE, Li HY, Ericsson A. Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. Prog Brain Res 2000; 122:61–78. [DOI] [PubMed] [Google Scholar]
- 30. Esler M, Eikelis N, Schlaich M, Lambert G, Alvarenga M, Dawood T, Kaye D, Barton D, Pier C, Guo L, Brenchley C, Jennings G, Lambert E. Chronic mental stress is a cause of essential hypertension: presence of biological markers of stress. Clin Exp Pharmacol Physiol 2008; 35:498–502. [DOI] [PubMed] [Google Scholar]
- 31. Folkow B. Psychosocial and central nervous influences in primary hypertension. Circulation 1987; 76:I10–I19. [PubMed] [Google Scholar]
- 32. Folkow B. Mental “stress” and hypertension. Evidence from animal and experimental studies. Integr Physiol Behav Sci 1991; 26:305–308. [DOI] [PubMed] [Google Scholar]
- 33. Folkow B. Physiological aspects of primary hypertension. Physiol Rev 1982; 62:347–504. [DOI] [PubMed] [Google Scholar]
- 34. Brudey C, Park J, Wiaderkiewicz J, Kobayashi I, Mellman TA, Marvar PJ. Autonomic and inflammatory consequences of posttraumatic stress disorder and the link to cardiovascular disease. Am J Physiol Regul Integr Comp Physiol 2015; 309:R315–R321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chang HA, Chang CC, Tzeng NS, Kuo TB, Lu RB, Huang SY. Decreased cardiac vagal control in drug-naïve patients with posttraumatic stress disorder. Psychiatry Investig 2013; 10:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shah AJ, Lampert R, Goldberg J, Veledar E, Bremner JD, Vaccarino V. Posttraumatic stress disorder and impaired autonomic modulation in male twins. Biol Psychiatry 2013; 73:1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Edmondson D, Sumner JA, Kronish IM, Burg MM, Oyesiku L, Schwartz JE. The association of posttraumatic stress disorder with clinic and ambulatory blood pressure in healthy adults. Psychosom Med 2018; 80:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Paulus EJ, Argo TR, Egge JA. The impact of posttraumatic stress disorder on blood pressure and heart rate in a veteran population. J Trauma Stress 2013; 26:169–172. [DOI] [PubMed] [Google Scholar]
- 39. Weber MD, Godbout JP, Sheridan JF. Repeated social defeat, neuroinflammation, and behavior: monocytes carry the signal. Neuropsychopharmacology 2017; 42:46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baker DG, Nievergelt CM, O’Connor DT. Biomarkers of PTSD: neuropeptides and immune signaling. Neuropharmacology 2012; 62:663–673. [DOI] [PubMed] [Google Scholar]
- 41. Pace TW, Heim CM. A short review on the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain Behav Immun 2011; 25:6–13. [DOI] [PubMed] [Google Scholar]
- 42. Eraly SA, Nievergelt CM, Maihofer AX, Barkauskas DA, Biswas N, Agorastos A, O’Connor DT, Baker DG; Marine Resiliency Study Team . Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry 2014; 71:423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, Maier SF, Yirmiya R. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatry 2014; 19:699–709. [DOI] [PubMed] [Google Scholar]
- 44. McKim DB, Niraula A, Tarr AJ, Wohleb ES, Sheridan JF, Godbout JP. Neuroinflammatory dynamics underlie memory impairments after repeated social defeat. J Neurosci 2016; 36:2590–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ufnal M, Sikora M, Szczepanska-Sadowska E. Interleukin-1 receptor antagonist reduces the magnitude of the pressor response to acute stress. Neurosci Lett 2008; 448:47–51. [DOI] [PubMed] [Google Scholar]
- 46. Marvar PJ, Hendy EB, Cruise TD, Walas D, DeCicco D, Vadigepalli R, Schwaber JS, Waki H, Murphy D, Paton JF. Systemic leukotriene B4 receptor antagonism lowers arterial blood pressure and improves autonomic function in the spontaneously hypertensive rat. J Physiol 2016; 594:5975–5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zera T, Ufnal M, Szczepanska-Sadowska E. Central TNF-alpha elevates blood pressure and sensitizes to central pressor action of angiotensin II in the infarcted rats. J Physiol Pharmacol 2008; 59(Suppl 8):117–121. [PubMed] [Google Scholar]
- 48. Burg MM, Soufer R. Post-traumatic stress disorder and cardiovascular disease. Curr Cardiol Rep 2016; 18:94. [DOI] [PubMed] [Google Scholar]
- 49. von Känel R, Begré S, Abbas CC, Saner H, Gander ML, Schmid JP. Inflammatory biomarkers in patients with posttraumatic stress disorder caused by myocardial infarction and the role of depressive symptoms. Neuroimmunomodulation 2010; 17:39–46. [DOI] [PubMed] [Google Scholar]
- 50. von Känel R, Hepp U, Kraemer B, Traber R, Keel M, Mica L, Schnyder U. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res 2007; 41:744–752. [DOI] [PubMed] [Google Scholar]
- 51. Castren E, Saavedra JM. Repeated stress increases the density of angiotensin II binding sites in rat paraventricular nucleus and subfornical organ. Endocrinology 1988; 122:370–372. [DOI] [PubMed] [Google Scholar]
- 52. Braszko JJ, Karwowska-Polecka W, Halicka D, Gard PR. Captopril and enalapril improve cognition and depressed mood in hypertensive patients. J Basic Clin Physiol Pharmacol 2003; 14:323–343. [DOI] [PubMed] [Google Scholar]
- 53. Pavlatou MG, Mastorakos G, Lekakis I, Liatis S, Vamvakou G, Zoumakis E, Papassotiriou I, Rabavilas AD, Katsilambros N, Chrousos GP. Chronic administration of an angiotensin II receptor antagonist resets the hypothalamic-pituitary-adrenal (HPA) axis and improves the affect of patients with diabetes mellitus type 2: preliminary results. Stress 2008; 11:62–72. [DOI] [PubMed] [Google Scholar]
- 54. Cardinale JP, Sriramula S, Mariappan N, Agarwal D, Francis J. Angiotensin II-induced hypertension is modulated by nuclear factor-κBin the paraventricular nucleus. Hypertension 2012; 59:113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kang YM, Ma Y, Zheng JP, Elks C, Sriramula S, Yang ZM, Francis J. Brain nuclear factor-kappa B activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovasc Res 2009; 82:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Evered MD, Robinson MM, Richardson MA. Captopril given intracerebroventricularly, subcutaneously or by gavage inhibits angiotensin-converting enzyme activity in the rat brain. Eur J Pharmacol 1980; 68:443–449. [DOI] [PubMed] [Google Scholar]
- 57. Fregly MJ. Effect of the angiotensin converting enzyme inhibitor, captopril, on NaCl appetite of rats. J Pharmacol Exp Ther 1980; 215:407–412. [PubMed] [Google Scholar]