Abstract
PURPOSE:
Patients hospitalized outside the intensive care unit (ICU) frequently experience clinical deterioration. Little has been done to describe the landscape of clinical deterioration among inpatients with cancer. We aimed to describe the frequency of clinical deterioration among patients with cancer hospitalized on the wards at a major academic hospital and to identify independent risk factors for clinical deterioration among these patients.
METHODS:
This was a retrospective cohort study at a 1,300-bed urban academic hospital with a 138-bed inpatient cancer center. We included consecutive admissions to the oncology wards between January 1, 2014, and June 30, 2017. We defined clinical deterioration as the composite of ward death and transfer to the ICU.
RESULTS:
We evaluated 21,219 admissions from 9,058 patients. The composite outcome occurred during 1,945 admissions (9.2%): 1,365 (6.4%) had at least one ICU transfer, and 580 (2.7%) involved ward death. Logistic regression identified several independent risk factors for clinical deterioration, including the following: age (odds ratio [OR], 1.33 per decade; 95% CI, 1.07 to 1.67), male sex (OR, 1.15; 95% CI, 1.05 to 1.33), comorbidities, illness severity (OR, 1.11; 95% CI, 1.10 to 1.13), emergency admission (OR, 1.45; 95% CI, 1.26 to 1.67), hospitalization on particular wards (OR, 1.525; 95% CI, 1.326 to 1.67), bacteremia (OR, 1.24; 95% CI, 1.01 to 1.52), fungemia (OR, 3.76; 95% CI, 1.90 to 7.41), tumor lysis syndrome (OR, 3.01; 95% CI, 2.41 to 3.76), and receipt of antimicrobials (OR, 2.04; 95% CI, 1.72 to 2.42) and transfusions (OR, 1.65; 95% CI, 1.42 to 1.92).
CONCLUSION:
Clinical deterioration was common; it occurred in more than 9% of admissions. Factors independently associated with deterioration included comorbidities, admission source, infections, and blood product transfusion.
INTRODUCTION
Cancer incidence and prevalence are expected to increase as a result of aging populations,1 widespread screening, and treatment improvements.2 Simultaneously, improved care of seriously ill patients and changes to guidelines3,4 and triage practices have led more patients with cancer to be treated in intensive care units (ICUs) upon clinical deterioration.5,6 In some cohorts, up to 20% of critical care admissions involve active malignancy.7 These numbers are increasing as novel therapies introduce new complications and syndromes that require intensive medical care.8,9 Among survivors of clinical deterioration, postrecovery morbidity and mortality remain notable.10,11
Identification of patients on the wards before deterioration may offer the opportunity for interventions aimed to prevent ICU transfer, cardiopulmonary arrest, and death.12-14 Early intervention has been associated with improved short-term15,16 and long-term17 outcomes among patients with cancer whose health is deteriorating. Patients with malignancy who are on wards may be at risk for deterioration from both treatment adverse effects (eg, neutropenic sepsis, cytokine release syndrome) and cancer-related complications (eg, respiratory failure from pulmonary embolism).
Although current guidelines recommend screening patients on wards for common deterioration syndromes,3 no studies clearly describe the landscape of deterioration among patients with cancer on wards. Prior work mostly has been limited to patients already recognized as having critical illness18,19 or to subpopulations of patients, such as those with specific cancer types.20-23 We aimed to describe the frequency of clinical deterioration among patients hospitalized with cancer at a major academic hospital and to identify independent risk factors for clinical deterioration among this unique cohort.
METHODS
Setting and Study Population
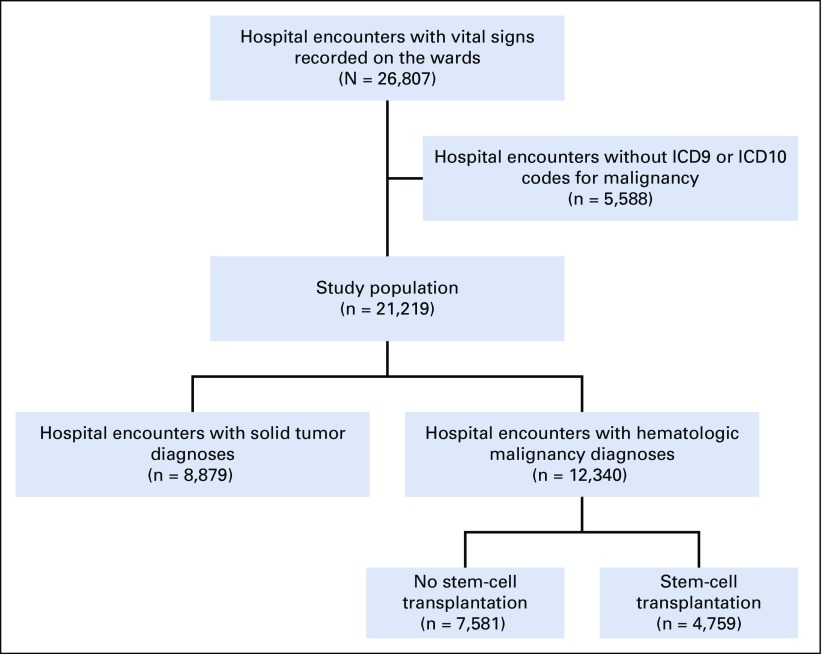
We conducted an observational cohort study at Barnes-Jewish Hospital, an urban teaching hospital and National Cancer Institute (NCI)–designated Comprehensive Cancer Center. Barnes-Jewish Hospital has 1,300 beds, of which 138 are in a geographically distinct cancer hospital (Siteman Cancer Center). We collected de-identified data on all consecutive adult patient admissions in the cancer hospital between January 1, 2014, and June 30, 2017, from the Clinical Research Data Warehouse, which is maintained by the Center for Research Informatics at Washington University School of Medicine. We included patients with an International Classification of Diseases, 9th revision (ICD9) or 10th revision (ICD10), code for cancer in any position (Appendix Fig A1, online only). The study protocol was approved by the Institutional Review Board at Washington University (No. 201707080).
Data Collection and Definitions
Patient age, sex, and ethnicity data were collected from the electronic health record, as were date- and time-stamped location information. Because patients with similar diagnoses (eg, solid tumors; hematologic malignancies, including stem-cell transplantations) are cordoned within particular areas in the hospital, we collected the location for all patients. Patient comorbidities were extracted using the Elixhauser comorbidity index, which has shown to perform well in populations with cancer.24,25 In addition, because obstructive sleep apnea may influence clinical deterioration,26 it was identified using ICD9 and ICD10 clinical modification (CM) codes. Cancer diagnoses and some cancer-related complications (eg, tumor lysis syndrome [TLS]) were collected using ICD9 and ICD10 CM codes.27,28 We extracted oncologic diagnoses using individual diagnosis codes separately from the Elixhauser index to achieve greater specificity with diagnostic categories. We obtained insurance information from hospital billing data.
Vital signs and laboratory values (including microbiology cultures and orders for antibiotics) were extracted from the electronic health record to identify neutropenia, bacteremia, fungemia, and other complications. For severity of illness, we calculated the Acute Physiology and Chronic Health Evaluation (APACHE) II score within 24 hours on the wards for each inpatient.29 Procedures, including blood product administration and central line insertion, were identified.
Outcomes
The primary outcome of the study was the composite of ward death and ICU transfer. Secondary outcomes included these individual outcomes as well as hospital and ICU length of stay. Because cardiopulmonary arrest on the wards results in death or transfer to the ICU after return of spontaneous circulation, this outcome was included indirectly.
Statistics
Continuous variables are expressed as means and standard deviations (SDs) or medians and interquartile ranges (IQRs) when appropriate. The t test and one-way analysis of variance tests were used to analyze normally distributed continuous variables, and the Mann-Whitney U and Kruskal-Wallis tests were used to analyze non-normally distributed continuous variables. Categoric data were reported as frequency distributions and analyzed using the χ2 test or McNemar test.
We fit logistic regression models for each outcome to evaluate patient characteristics (age, sex, ethnicity, body mass index [BMI], comorbidities, severity of illness) and hospitalization variables (prior hospital admission, admission source [emergency department {ED}, ICU, operating room or procedure area, or direct admission from home or clinic], year of admission, season within academic year [modeled in tertiles: July to October, November to February, March to June], antibiotic orders, receipt of blood products, bacteremia, fungemia, and TLS).
In the adjusted model, APACHE II score and age were entered linearly, with the addition of a squared term for age to account for nonlinear effects. All other variables were modeled categorically. Comorbidities were modeled without a summary score, because models that involved individual Elixhauser comorbidities have superior performance to summary scores in patients with cancer.25
Because repeat hospitalization could confound the results of the study, we performed a sensitivity analysis that included only one randomly selected hospital admission per patient. In addition, we performed a sensitivity analysis test to evaluate the categoric effects of age 65 years or older versus age younger than 65 years. A final sensitivity analysis tested for interaction among the cancer type, neutropenia, and transfusion variables by including interaction terms for each pairwise combination of these variables.
We also performed subgroup analysis of logistic models across malignancy type (solid tumor v hematologic malignancy [lymphoma, leukemia, multiple myeloma, and stem-cell transplantations]), insurance status, age quartiles, and quartiles of initial severity of illness. All tests of significance used a two-sided P value of less than .05. Statistical analyses and data visualization30 were completed using STATA, version 15 (STATA, College Station, TX) and R, version 3.4.3.
RESULTS
The study cohort included 21,219 hospital admissions from 9,058 unique patients. In total, 1,945 patient admissions (9.2%) were involved the composite outcome: 1,365 (6.4%) admissions had at least one ICU transfer, and 580 (2.7%) admissions died on the wards.
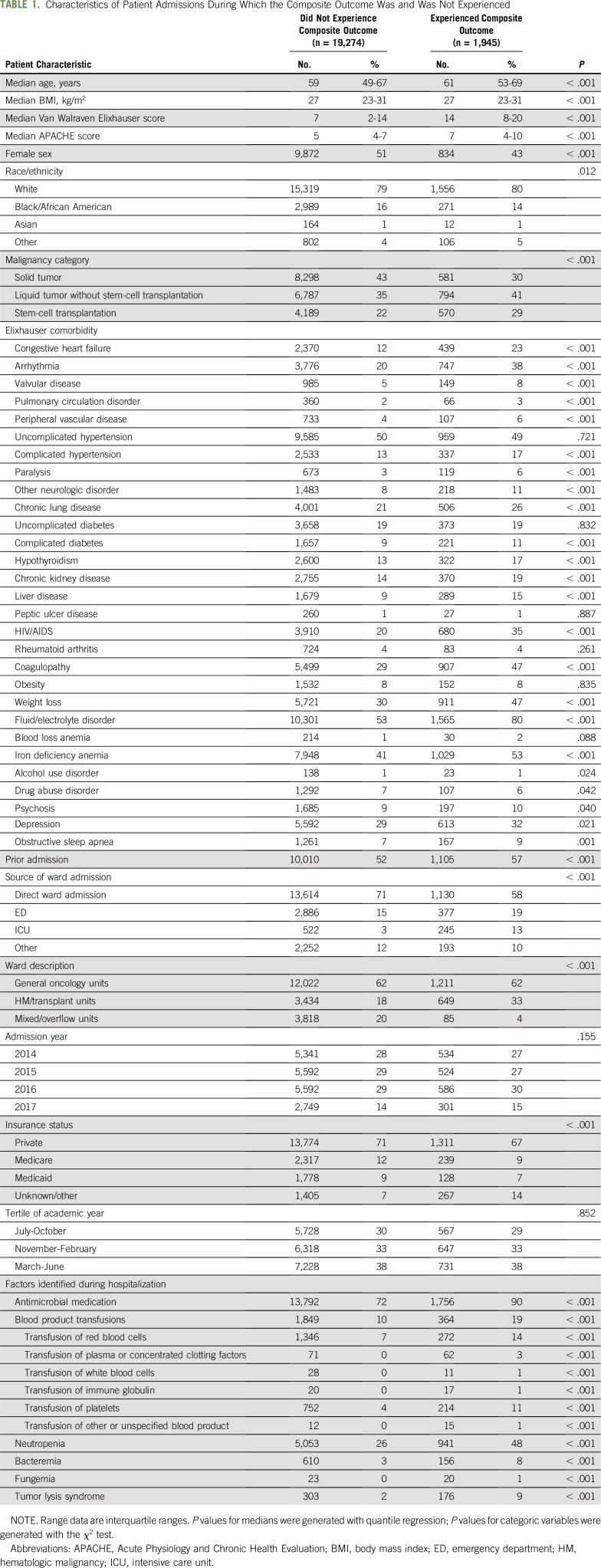
Patients who experienced clinical deterioration were older (median [IQR] age, 61 years [53 to 69 years] v 59 years [49 to 67 years]; P < .001), were more likely to be male (57% v 49%; P < .001), carried more comorbidities (median [IQR] van Walraven Elixhauser burden,31 14 [8 to 20] v 7 [2 to 14]; P < .001), and had higher initial severity of illness (median [IQR] APACHE score, 7 [4 to 10] v 5 [4 to 7]; P < .001) than patients who did not deteriorate on the wards (Table 1). In particular, patients who experienced deterioration were more likely to have hematologic malignancy (41% v 35%) or a hematopoietic stem-cell transplantation (HSCT; 29% v 22%) and were less likely to have solid malignancy (30% v 43%; P < .001 for all comparisons). They were also more likely to have cardiac (eg, 23% v 12% congestive heart failure; 38% v 20% arrhythmia; P < .001 for both comparisons), renal (eg, 19% v 14% chronic kidney disease; P < .001), and fluid or electrolyte (80% v 53%; P < .001) pathologies. Patients who experienced deterioration ultimately had significantly longer hospital length of stay and higher overall hospital mortality than patients who never experienced deterioration (Table 2, P < .001 for both outcomes).
TABLE 1.
Characteristics of Patient Admissions During Which the Composite Outcome Was and Was Not Experienced
TABLE 2.
Unadjusted Secondary Outcome Rates for Patient Admissions With and Without the Composite Outcome
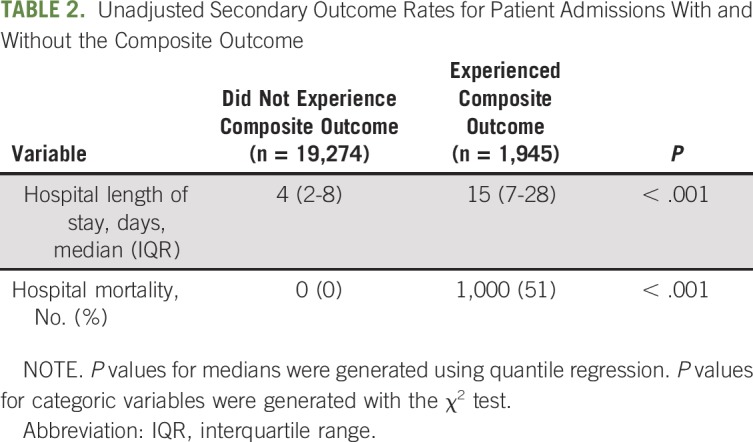
With the exception of time variables (year and academic season), all hospitalization characteristics varied between patients who did versus did not experience deterioration. Patients whose health deteriorated were more frequently admitted to the wards from the ICU or the ED than as a direct admission from home, a clinic, or another hospital. The patients whose health deteriorated more frequently received antibiotics and blood products and more frequently developed neutropenia, bacteremia, fungemia, and tumor lysis syndrome (P < .001 for all comparisons).
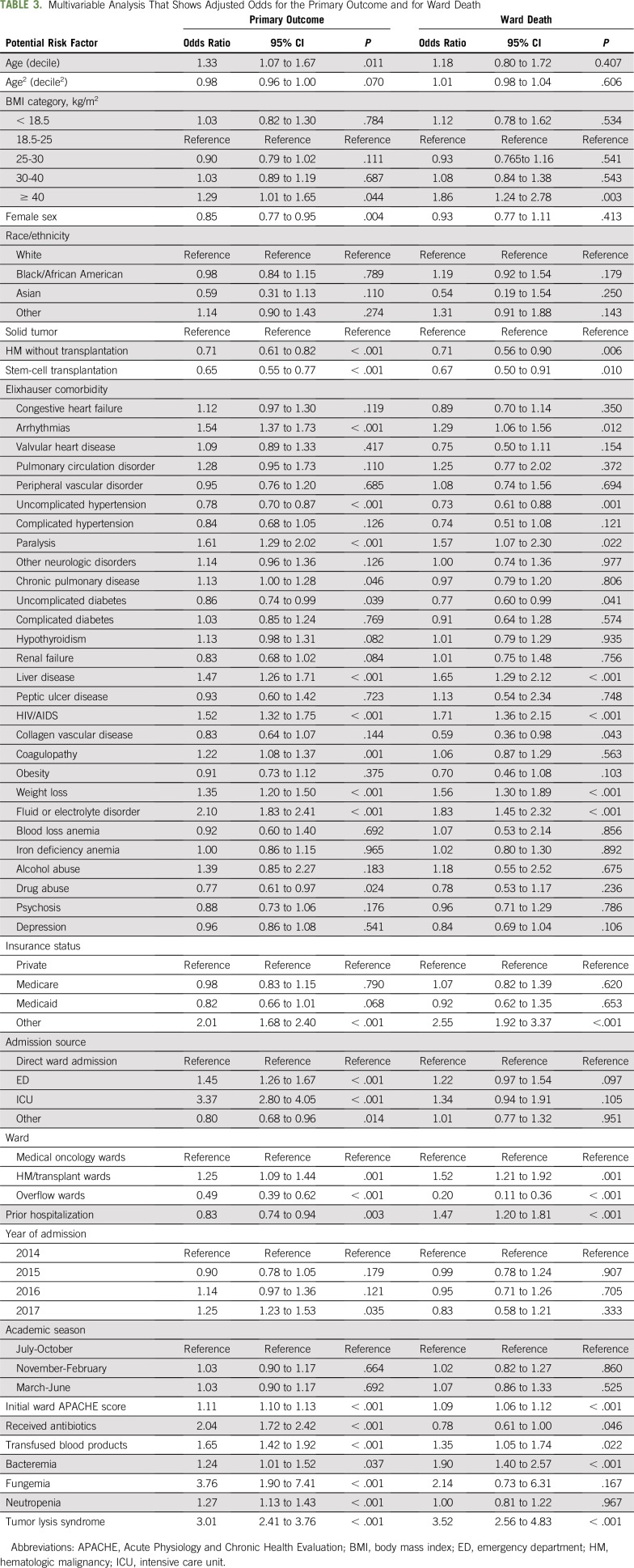
Logistic regression identified several patient characteristics as independent risk factors for clinical deterioration, including the following: age; male sex; unknown or other insurance status; and comorbidities, such as electrolyte disturbances, liver disease, and cardiac arrhythmias (Table 3). The initial APACHE II score also predicted deterioration. In addition, hospitalization factors, such as emergency admission to the wards or admission via the ICU, hospitalization on particular hematologic malignancy wards, and identification of expected or unexpected complications during hospitalization (eg, development of neutropenia, bacteremia, fungemia, or TLS and receipt of antimicrobials and blood transfusions), were also independent predictors of the composite outcome. Of these risk factors, severity of illness, source of ward admission, ward location, bacteremia, and tumor lysis also predicted death on the wards (Table 3). Notably, neutropenia was independently associated with clinical deterioration but not with ward death in the overall cohort. In contrast, neutropenia was associated with ward death among patients with stem-cell transplantation (odds ratio [OR], 2.45; 95% CI, 1.74 to 3.67; P for interaction < .001). Prior hospital admission during the study period was associated with decreased odds for the composite end point but was associated with increased risk for ward death.
TABLE 3.
Multivariable Analysis That Shows Adjusted Odds for the Primary Outcome and for Ward Death
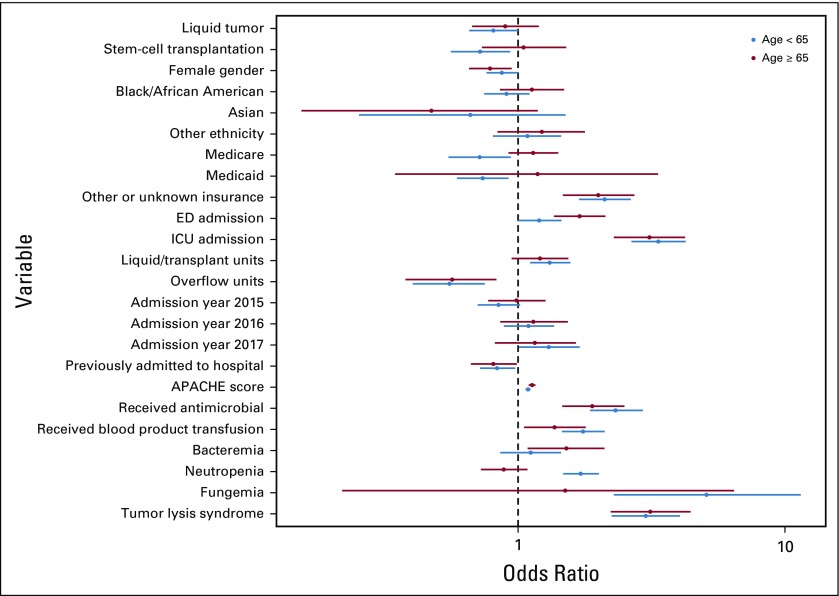
A sensitivity analysis that included one randomly selected admission per patient (n = 10,104) found similar results to the primary analysis except that age and neutropenia were not associated with risk for deterioration. Similarly, a sensitivity analysis that modeled age as a binary categoric variable found that age 65 years or older was associated with increased adjusted odds for deterioration, although this association did not reach statistical significance (OR, 1.12; 95% CI, 1.00 to 1.26; P = .058; Appendix Fig A2, online only). There was no interaction between cancer type and blood transfusion, but there was an interaction between cancer type and neutropenia that showed decreased odds for deterioration in patients with hematologic malignancy (OR, 0.68; 95% CI, 0.50 to 0.94; P = .018) and stem-cell transplantation (OR, 0.39; 95% CI, 0.28 to 0.54; P < .001) who did not experience neutropenia.
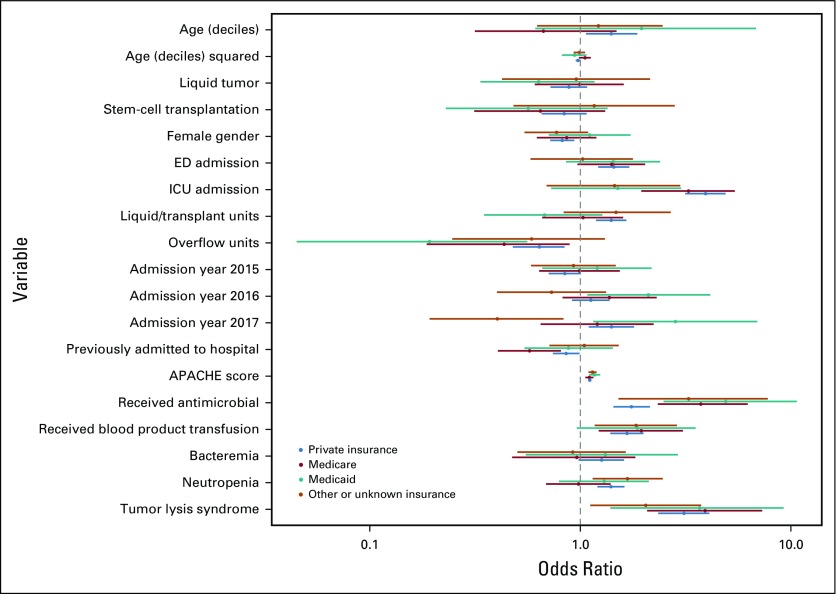
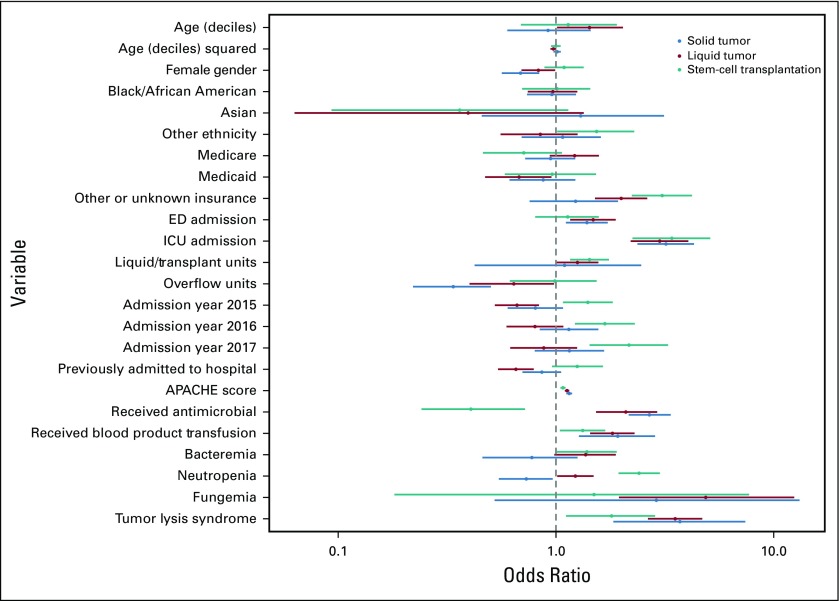
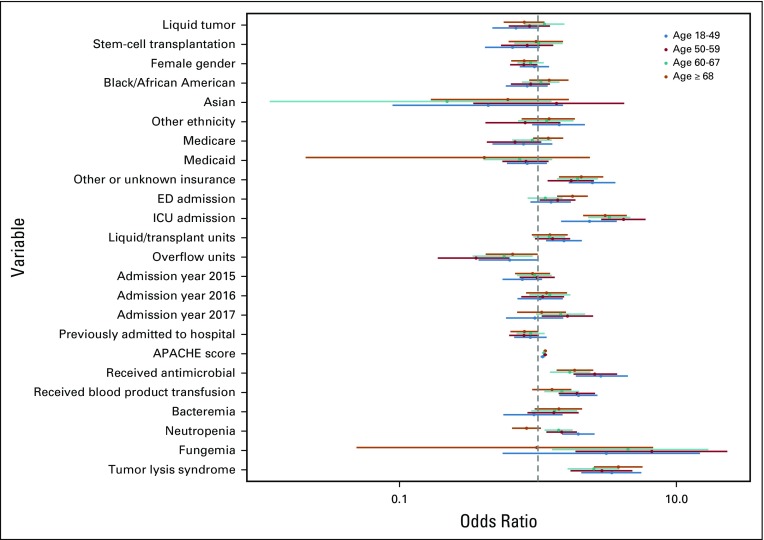
Subgroup analysis across cancer diagnoses (Appendix Fig A3, online only) identified similar findings to the primary analysis, except that neutropenia was associated with significantly increased odds for deterioration among patients with HSCT recipients (P < .001) but decreased odds for deterioration among patients with solid tumor (P = .016), fungemia was only significantly associated with deterioration among patients with hematologic malignancy who did not undergo transplantation (P = .001), and receipt of antimicrobial medications was associated with decreased odds for deterioration among recipients of HSCT (P = .004). Subgroup analysis across insurance types identified statistical differences between insurance types for patients initially admitted to the ICU, those hospitalized on particular ward units, and those hospitalized during the years 2016 and 2017 (Appendix Fig A4, online only). Notable differential effects were not seen across quartiles of age (Appendix Fig A5, online only) or initial ward severity of illness (not shown).
DISCUSSION
In this large evaluation of clinical deterioration among patients with cancer on inpatient wards at an NCI-designated Comprehensive Cancer Center, we found that more than 9% of ward admissions involved transfer to the ICU or death on the wards. We also found that unclear insurance status; patient comorbidity burden and cancer diagnosis; as well as hospitalization factors, such as location on particular wards, positive blood cultures, and receipt of antibiotics, were associated with deterioration.
This rate is higher than that of nonselected inpatients in prior studies,12,14,32 so our findings suggest that inpatients with active cancer are at increased risk for clinical deterioration. This risk is particularly important, because prior work has shown that patients with cancer who develop critical illness may have worse outcomes than patients without cancer whose health deteriorates similarly.33,34
The increased rate of deterioration among patients with cancer suggests that they may be a population likely to benefit from inpatient monitoring and use of early warning systems (EWS).33 Although prior applications of EWS have not consistently found benefit with regard to patient outcomes—potentially related to high rates of false-positive alerts—the use of an EWS for a cohort with a higher prevalence of deterioration would improve its positive predictive values (on the basis of Bayes’ rule). Given that we found differential risk across categories of cancer diagnoses and specific ward locations, such a system could be applied at each of these levels on the basis of geographic location, type of cancer, or both.
Moreover, it is possible that patients with hematologic malignancies, in particular, could benefit from an EWS. First, subjective triage of these patients is difficult: in one study, many patients deemed not sick enough for the ICU died before hospital discharge, whereas even more patients felt to be too sick to glean benefit from critical care ultimately survived.35 Second, critically ill patients with hematologic malignancy have relatively high survival and postdischarge functional status,36 which continue to improve over time37 and potentially increase the magnitude of benefit for patients rescued from deterioration. Third, because the most common causes38 of critical illness in these patients (eg, neutropenic sepsis) are related to transient, reversible factors (eg, neutropenia pre-engraftment), the number of patients with hematologic malignancy who have potentially preventable or treatable critical illness may be relatively high.
In this study, we found decreased rates of deterioration among patients with hematologic malignancy or HSCT recipients, which may reflect common hospital admissions for lower-risk scenarios—such as the transplant itself; chemotherapy administration; or management of symptoms, such as graft-versus-host disease. To this point, most hospitalizations for patients who underwent HSCT in our cohort came without ED contact, which suggests that many were elective admissions. Also, it is important to note that our center performed all autologous HSCTs, which are particularly low risk for hospital mortality,39 during hospital admission.
We also found strong associations between individual wards and clinical deterioration, which may be evidence of cohorting on the basis of specific cancer, admission diagnosis, or expected prognosis. For example, the highest-risk wards in our study contained the majority of patients who had received, or are receiving, allogenic stem-cell transplantations. Beyond serving as a surrogate marker for high-risk malignancy status, ward location may actually confer risk. Ward effects have been shown to be strong predictors of outcome in cohorts of general patients on wards, and deterioration events on particular units are associated with increased short-term risk for deterioration in neighboring patients.40 Attention to resource allocation may be particularly important on wards with high-risk populations.
Our work differs from prior studies in several important ways. First, we analyzed a specific cohort of inpatients whose cancer diagnoses were associated with increased risk of deterioration compared with all patients on wards. Among this unique population, however, we evaluated a heterogeneous group of patients with cancer—one that included both allogeneic and autologous stem-cell transplantation recipients—rather than a specialized oncology population, such as hematologic malignancies or solid tumors of a particular organ. This population may increase the generalizability of our results, especially because many hospital wards include heterogeneous populations. Second, we evaluated patients on wards at risk for deterioration rather than patients already in the ICU; prior work to describe deterioration among inpatients with malignancy mostly has been limited to patients already recognized as critically ill.18,19 This previously used approach is limited, in that the time of ICU admission may be too late to rescue patients whose deterioration may have been reversible. In addition, this approach is subject to survivorship bias by omitting patients who die on the wards.
Strengths of our study include its large cohort, which allowed evaluation of a number of potential risk factors, even across subgroups of specific diagnoses. Such potential risk factors included those suggested as markers of high-risk status by prior work (eg, blood product transfusion41 and neutropenia42) as well as variables that, to our knowledge, have not previously been investigated in this cohort (eg, academic season).
Limitations of this study include its single center. Thus, our results may not be generalizable to other Comprehensive Cancer Centers. Indeed, outcome differences among different cancer hospitals have been described.43 Moreover, because ICU-specific factors, such as bed availability and admission criteria, may influence the outcome of ICU transfer, our results may not translate to centers with different structures or practice patterns. Relatedly, our institution’s status as an NCI-designated Comprehensive Cancer Center may draw patients with more severe or atypical malignancy presentations compared with other hospitals. Also, we were unable to identify patients who received comfort care, to ascertain cancer staging or treatment histories, to differentiate the source of stem cells for transplantation and conditioning regimens, and to obtain time-stamps or locations for blood transfusions. Although we used clinical data to identify some risk factors, we identified most comorbid conditions, as well as TLS, from ICD9 and ICD10 codes. Use of these codes facilitated this proof-of-principle study, but EWS should use clinical data that are available in real time. Another disadvantage of ICD codes is that mild conditions (eg, hypertension) are more likely to be coded among patients who do not have more serious diagnoses and therefore can appear protective, as seen in this study and our prior work.44 Also, ICD codes have not been validated to identify TLS during hospitalization45; given that our rates of TLS (approximately 2% of all admissions, and approximately 4% of hematologic malignancy admissions) are lower than in other reports,46 our reported incidence of TLS may be an underestimation. Finally, the majority of patients in this cancer center were receiving medical, not surgical, treatment.
In conclusion, we found that clinical deterioration is common among inpatients with cancer. Our results also suggest that patient characteristics (eg, comorbidities) and factors associated with hospitalizations (eg, positive blood cultures, receipt of antibiotics and blood product transfusions) are independent risk factors for deterioration. These findings have important implications for monitoring of patients with active malignancy on hospital wards.
Appendix
Fig A1.
Flow diagram of patient inclusion into the study cohort. ICD, International Classification of Diseases.
Fig A2.
Subgroup analysis comparing odds ratios for clinical deterioration between patients age 65 years or older and patients age younger than 65 years. APACHE, Acute Physiology and Chronic Health Evaluation; ED, emergency department; ICU, intensive care unit.
Fig A3.
Subgroup analysis comparing odds ratios for clinical deterioration across malignancy categories. APACHE, Acute Physiology and Chronic Health Evaluation; ED, emergency department; ICU, intensive care unit.
Fig A4.
Subgroup analysis comparing odds ratios for clinical deterioration across primary insurance status. APACHE, Acute Physiology and Chronic Health Evaluation; ED, emergency department; ICU, intensive care unit.
Fig A5.
Subgroup analysis comparing odds ratios for clinical deterioration across quartiles of age. APACHE, Acute Physiology and Chronic Health Evaluation; ED, emergency department; ICU, intensive care unit.
Footnotes
Supported by National Institutes of Health Training Grants No. 5T32 HL007317 and UL1 TR002345 (P.G.L.) and by the Barnes-Jewish Hospital Foundation (M.H.K.).
AUTHOR CONTRIBUTIONS
Conception and design: Patrick G. Lyons, Jeff Klaus, Colleen A. McEvoy, Peter Westervelt, Marin H. Kollef
Collection and assembly of data: Patrick G. Lyons, Colleen A. McEvoy
Data analysis and interpretation: Patrick G. Lyons, Jeff Klaus, Colleen A. McEvoy, Peter Westervelt, Brian F. Gage
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Factors Associated With Clinical Deterioration Among Patients Hospitalized on the Wards at a Tertiary Cancer Hospital
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jop/site/ifc/journal-policies.html.
Jeff Klaus
Consulting or Advisory Role: Juno Therapeutics, Astellas Pharma, Stemline Therapeutics, Spectrum Pharmaceuticals
Speakers’ Bureau: Astellas Pharma, Merck, Jazz Pharmaceuticals
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “silver tsunami”: Prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25:1029–1036. doi: 10.1158/1055-9965.EPI-16-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study global burden. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiehl MG, Beutel G, Böll B, et al. Consensus statement for cancer patients requiring intensive care support. Ann Hematol. 2018;97:1271–1282. doi: 10.1007/s00277-018-3312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guidelines for intensive care unit admission, discharge, and triage: Task Force of the American College of Critical Care Medicine, Society of Critical Care Medicine. Crit Care Med 27:633-638, 1999. [PubMed]
- 5.Azoulay E, Mokart D, Pène F, et al. Outcomes of critically ill patients with hematologic malignancies: Prospective multicenter data from France and Belgium—A groupe de recherche respiratoire en réanimation onco-hématologique study. J Clin Oncol. 2013;31:2810–2818. doi: 10.1200/JCO.2012.47.2365. [DOI] [PubMed] [Google Scholar]
- 6.Bird GT, Farquhar-Smith P, Wigmore T, et al. Outcomes and prognostic factors in patients with haematological malignancy admitted to a specialist cancer intensive care unit: A 5 year study. Br J Anaesth. 2012;108:452–459. doi: 10.1093/bja/aer449. [DOI] [PubMed] [Google Scholar]
- 7.Soares M, Caruso P, Silva E, et al. ) Characteristics and outcomes of patients with cancer requiring admission to intensive care units: A prospective multicenter study. Crit Care Med. 2010;38:9–15. doi: 10.1097/CCM.0b013e3181c0349e. [DOI] [PubMed] [Google Scholar]
- 8.Gutierrez C, McEvoy C, Mead E, et al. Management of the critically ill adult chimeric antigen receptor-T cell therapy patient: A critical care perspective. Crit Care Med. 2018;46:1402–1410. doi: 10.1097/CCM.0000000000003258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bach PB, Giralt SA, Saltz LB. FDA approval of tisagenlecleucel: Promise and complexities of a $475,000 cancer drug. JAMA. 2017;318:1861–1862. doi: 10.1001/jama.2017.15218. [DOI] [PubMed] [Google Scholar]
- 10.Moors I, Benoit DD. Time to look beyond one-year mortality in critically ill hematological patients? Crit Care. 2014;18:107. doi: 10.1186/cc13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernal T, Pardavila EV, Bonastre J, et al. Survival of hematological patients after discharge from the intensive care unit: A prospective observational study. Crit Care. 2013;17:R302. doi: 10.1186/cc13172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kipnis P, Turk BJ, Wulf DA, et al. Development and validation of an electronic medical record-based alert score for detection of inpatient deterioration outside the ICU. J Biomed Inform. 2016;64:10–19. doi: 10.1016/j.jbi.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Churpek MM, Yuen TC, Winslow C, et al. Multicenter comparison of machine learning methods and conventional regression for predicting clinical deterioration on the wards. Crit Care Med. 2016;44:368–374. doi: 10.1097/CCM.0000000000001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailey TC, Chen Y, Mao Y, et al. A trial of a real-time alert for clinical deterioration in patients hospitalized on general medical wards. J Hosp Med. 2013;8:236–242. doi: 10.1002/jhm.2009. [DOI] [PubMed] [Google Scholar]
- 15.Mokart D, Lambert J, Schnell D, et al. Delayed intensive care unit admission is associated with increased mortality in patients with cancer with acute respiratory failure. Leuk Lymphoma. 2013;54:1724–1729. doi: 10.3109/10428194.2012.753446. [DOI] [PubMed] [Google Scholar]
- 16.Song J-U, Suh GY, Park HY, et al. Early intervention on the outcomes in critically ill cancer patients admitted to intensive care units. Intensive Care Med. 2012;38:1505–1513. doi: 10.1007/s00134-012-2594-0. [DOI] [PubMed] [Google Scholar]
- 17.Lee D-S, Suh GY, Ryu J-A, et al. Effect of early intervention on long-term outcomes of critically ill cancer patients admitted to ICUs. Crit Care Med. 2015;43:1439–1448. doi: 10.1097/CCM.0000000000000989. [DOI] [PubMed] [Google Scholar]
- 18.Alp E, Tok T, Kaynar L, et al. Outcomes for haematological cancer patients admitted to an intensive care unit in a university hospital. Aust Crit Care. 2018;31:363–368. doi: 10.1016/j.aucc.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Gordon AC, Oakervee HE, Kaya B, et al. Incidence and outcome of critical illness amongst hospitalised patients with haematological malignancy: A prospective observational study of ward and intensive care unit based care. Anaesthesia. 2005;60:340–347. doi: 10.1111/j.1365-2044.2005.04139.x. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson MK, Celauro AD, Vigneswaran WT. Validation of a modified scoring system for cardiovascular risk associated with major lung resection. Eur J Cardiothorac Surg. 2012;41:598–602. doi: 10.1093/ejcts/ezr081. [DOI] [PubMed] [Google Scholar]
- 21.Epstein AS, Yang A, Colbert LE, et al. Outcomes of ICU admission of patients with progressive metastatic gastrointestinal cancer. J Intensive Care Med. doi: 10.1177/0885066617748874. doi: 10.1177/0885066617748874 [epub ahead of print on January 1, 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gruson D, Vargas F, Hilbert G, et al. Predictive factors of intensive care unit admission in patients with haematological malignancies and pneumonia. Intensive Care Med. 2004;30:965–971. doi: 10.1007/s00134-004-2237-1. [DOI] [PubMed] [Google Scholar]
- 23.Hu SB, Wong DJL, Correa A, et al. Prediction of clinical deterioration in hospitalized adult patients with hematologic malignancies using a neural network model. PLoS One. 2016;11:e0161401–e0161401. doi: 10.1371/journal.pone.0161401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Mehta HB, Sura SD, Adhikari D, et al. Adapting the Elixhauser comorbidity index for cancer patients. Cancer. 2018;124:2018–2025. doi: 10.1002/cncr.31269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyons PG, Zadravecz FJ, Edelson DP, et al. Obstructive sleep apnea and adverse outcomes in surgical and nonsurgical patients on the wards. J Hosp Med. 2015;10:592–598. doi: 10.1002/jhm.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Cancer Institute 2014 https://seer.cancer.gov/tools/casefinding/case2014.html Surveillance, Epidemiology, and End Results Program: ICD-9-CM casefinding list.
- 28.National Cancer Institute https://seer.cancer.gov/tools/casefinding/case2017-icd10cm.html Surveillance, Epidemiology, and End Results Program: Comprehensive ICD-10-CM casefinding code list for reportable tumors.
- 29.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 30.Jann B. Plotting regression coefficients and other estimates in Stata. Stata J. 2014;14:708–737. [Google Scholar]
- 31.van Walraven C, Austin PC, Jennings A, et al. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626–633. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 32.Churpek MM, Yuen TC, Winslow C, et al. Multicenter development and validation of a risk stratification tool for ward patients. Am J Respir Crit Care Med. 2014;190:649–655. doi: 10.1164/rccm.201406-1022OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Austin CA, Hanzaker C, Stafford R, et al. Utilization of rapid response resources and outcomes in a comprehensive cancer center. Crit Care Med. 2014;42:905–909. doi: 10.1097/CCM.0000000000000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruckel JT, Wong SL, Chan PS, et al. Patterns of resuscitation care and survival after in-hospital cardiac arrest in patients with advanced cancer. J Oncol Pract. 2017;13:e821–e830. doi: 10.1200/JOP.2016.020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thiéry G, Azoulay E, Darmon M, et al. Outcome of cancer patients considered for intensive care unit admission: A hospital-wide prospective study. J Clin Oncol. 2005;23:4406–4413. doi: 10.1200/JCO.2005.01.487. [DOI] [PubMed] [Google Scholar]
- 36.de Vries VA, Müller MCA, Arbous MS, et al. Long-term outcome of patients with a hematologic malignancy and multiple organ failure admitted at the intensive care. Crit Care Med. 2019;47:e120–e128. doi: 10.1097/CCM.0000000000003526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Vliet M, Verburg IWM, van den Boogaard M, et al. Trends in admission prevalence, illness severity, and survival of haematological patients treated in Dutch intensive care units. Intensive Care Med. 2014;40:1275–1284. doi: 10.1007/s00134-014-3373-x. [DOI] [PubMed] [Google Scholar]
- 38.Pène F, Soares M. Can we still refuse ICU admission of patients with hematological malignancies? Intensive Care Med. 2008;34:790–792. doi: 10.1007/s00134-008-1003-1. [DOI] [PubMed] [Google Scholar]
- 39.Jones JA, Qazilbash MH, Shih Y-CT, et al. In-hospital complications of autologous hematopoietic stem cell transplantation for lymphoid malignancies: Clinical and economic outcomes from the Nationwide Inpatient Sample. Cancer. 2008;112:1096–1105. doi: 10.1002/cncr.23281. [DOI] [PubMed] [Google Scholar]
- 40.Volchenboum SL, Mayampurath A, Göksu-Gürsoy G, et al. Association between in-hospital critical illness events and outcomes in patients on the same ward. JAMA. 2016;316:2674–2675. doi: 10.1001/jama.2016.15505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mirouse A, Resche-Rigon M, Lemiale V, et al. Red blood cell transfusion in the resuscitation of septic patients with hematological malignancies. Ann Intensive Care. 2017;7:62–72. doi: 10.1186/s13613-017-0292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng Q, Tang Y, Yang Q, et al. The prognostic factors for patients with hematological malignancies admitted to the intensive care unit. Springerplus. 2016;5:2038. doi: 10.1186/s40064-016-3714-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfister DG, Rubin DM, Elkin EB, et al. Risk adjusting survival outcomes in hospitals that treat patients with cancer without information on cancer stage. JAMA Oncol. 2015;1:1303–1310. doi: 10.1001/jamaoncol.2015.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan Y, Birman-Deych E, Radford MJ, et al. Comorbidity indices to predict mortality from Medicare data: Results from the national registry of atrial fibrillation. Med Care. 2005;43:1073–1077. doi: 10.1097/01.mlr.0000182477.29129.86. [DOI] [PubMed] [Google Scholar]
- 45.Durani U, Shah ND, Go RS. In‐hospital outcomes of tumor lysis syndrome: A population‐based study using the National Inpatient Sample. Oncologist. 2017;22:1506–1509. doi: 10.1634/theoncologist.2017-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364:1844–1854. doi: 10.1056/NEJMra0904569. [DOI] [PMC free article] [PubMed] [Google Scholar]