Abstract
AIM
To investigate whether the gene variants in MYOC and ABCA1 are associated with primary angle-closure glaucoma (PACG) and anterior chamber depth (ACD) and axial length (AL) in samples from northern China.
METHODS
The present case-control association study consisted of 500 PACG patients and 720 unrelated controls. Each participant was genotyped for eleven single nucleotide polymorphisms (SNPs) in MYOC and ABCA1 genes (rs12076134, rs183532, rs235875 and rs235913 in MYOC, rs2422493, rs2487042, rs2472496, rs2472493, rs2487032, rs2472459 and rs2472519 near ABCA1) using an improved multiplex ligation detection reaction (iMLDR) technique. The genetic association analyses were performed by PLINK using a logistic regression model. The association between genotypes and ocular biometric parameters was performed by SPSS using generalized estimation equation. Bonferroni corrections were implemented and the statistical power was calculated by the Power and Sample Size Calculation.
RESULTS
Two SNPs rs183532 and rs235875 as well as a haplotype TTC in MYOC were nominally associated with PACG despite the significance was lost after Bonferroni correction. No association was observed between ABCA1 and PACG, neither did the association between these variants and ACD as well as AL.
CONCLUSION
The present study suggests MYOC and ABCA1 do not play a part in the pathogenesis of PACG as well as the regulation of ocular biometric parameters in a northern Chinese population. Further investigations with large sample size are needed to verify this consequence.
Keywords: MYOC, ABCA1, primary angle-closure glaucoma, anterior chamber depth, axial length, single nucleotide polymorphisms
INTRODUCTION
Glaucoma is a common eye disease characterized by the progressive degeneration of retinal ganglion cells and optic nerve axons, for which, intraocular pressure (IOP) is the primary modifiable risk factor[1]. Meanwhile, glaucoma is considered to be the most common disease leading to irreversible blindness worldwide[2]. In 2013, the glaucoma patients in the elderly population (aged 40-80y) worldwide was estimated to be 64.3 million, which will increase to 76.0 million in 2020 and 111.8 million in 2040[3]. According to the anatomical features, glaucoma is mainly subdivided into two major forms: primary open angle glaucoma (POAG) and primary angle-closure glaucoma (PACG). The prevalence of POAG is highest in Africa while most PACG cases are in Asia[4]–[5], especially in China[6]. Epidemiological studies have found that PACG is the most common cause of bilateral glaucoma blindness worldwide[7].
Glaucoma is a multifactorial disease, genetic factors take an important part in its pathogenesis[1], many disease-causing mutations and multiple susceptibility loci have been found to be associated with various forms of glaucoma. What's interesting is some genetic risks are shared for the two main types of glaucoma PACG and POAG, for example polymorphisms within the ARHGEF12 and GAS7 gene are found to be associated with both POAG and PACG[8]. Since MYOC is preferentially expressed in trabecular meshwork (TM)[9], which is thought to affect the outflow of aqueous humor resulting in elevation of IOP[10], while ABCA1 has been proved to be associated with POAG and IOP by GWAS[11], in addition, mutations in MYOC gene as well as variants near ABCA1 gene have been confirmed to be associated with POAG[12]–[15]. In view of this, this study aimed to investigate whether the POAG related genes MYOC and ABCA1 are associated with PACG in a northern Chinese cohort. Meanwhile, the associations between these single nucleotide polymorphisms (SNPs) and anterior chamber depth (ACD) and axial length (AL) were also evaluated.
SUBJECTS AND METHODS
Ethical Approval
The present study was approved by the Ethics Committee of Ningxia People's Hospital and its implementation process strictly complied with the standards of the Declaration of Helsinki. Each participant was informed in detail of the purpose of the study and signed a written informed consent prior to the study.
Subjects
It consisted of 500 PACG cases and 720 unrelated control subjects recruited from Ningxia Eye Hospital from the northern regions of China. The detailed ophthalmic examinations for every participant as well as the inclusion and exclusion criteria were identical to our previous study[16].
DNA Extraction
Peripheral venous blood was drawn from all subjects, genomic DNA was extracted utilizing the Simgen DNA Blood Mini Kit (Simgen, Hangzhou, China) according to the manufacturer's protocol. The extracted DNA was eluted in TE buffer (10 mmol/L Tris-HCl, 0.5 mmol/L EDTA, pH 9.0) and was measured for the A260/A280 optical density by Nanodrop2000. DNA was then stored at -80° until use.
Single Nucleotide Polymorphism Selection and Genotyping
Drew on the experiences of previous studies[17]–[18], eleven SNPs were chosen as candidate SNPs including rs12076134, rs183532, rs235875 and rs235913 in MYOC, rs2422493, rs2487042, rs2472496, rs2472493, rs2487032, rs2472459 and rs2472519 near ABCA1. Genotyping was conducted using an improved multiplex ligation detection reaction (iMLDR) technique by Genesky Biotechnologies Inc (Shanghai, China).
Statistical Analysis
The comparison of demographic characteristics between cases and controls and the correlation analysis between genotypes and ocular biometric parameters were implemented by SPSS software (version 17.5: SPSS Science, Chicago, IL, USA). Linkage disequilibrium (LD) patterns were generated using Haploview 4.2 software (Daly Lab at the Broad Institute, Cambridge, MA). The genetic association analyses were performed by PLINK (version 1.07; http://pngu.mgh.harvard.edu/-purcell/plink/, in the public domain) using a logistic regression model. Bonferroni correction was used for multiple comparisons and the statistical power was evaluated by the Power and Sample Size Calculation (PS; version 3.1.232).
RESULTS
We enrolled 500 PACG patients and 720 control subjects in this study. The general demographic characteristics of the participants are listed in Table 1. The control subjects were significantly older and included less women than the case group.
Table 1. Demographic characteristic of study participants.
| Parameters | PACG | Controls | P |
| Number | 500 | 720 | |
| Age, y, mean±SD | 63.77±9.576 | 71.82±7.2 | 0.000 |
| Gender, n (%) | 0.000 | ||
| Male | 147 (29.4) | 332 (46.1) | |
| Female | 353 (70.6) | 388 (53.9) |
PACG: Primary angle-closure glaucoma.
All eleven SNPs conformed to the Hardy-Weinburg equilibrium (HWE; P>0.05; Table 2), in which, rs183532 in MYOC was nominally associated with PACG under the allelic model as well as the dominant genetic model (P=0.025, 0.029, respectively; Tables 2 and 3). The frequency of the minor T allele of rs183532 was less in the PACG group than in the control group. In addition, rs235875 in MYOC was also nominally associated with PACG under the recessive genetic model with a P-value of 0.032. The frequency of the TT genotype of rs235875 was higher in PACG group than in the control group (Table 3). However, none of these SNPs remained significant after Bonferroni correction. In LD analysis, three LD blocks were identified in strong linkage disequilibrium (Figure 1). A haplotype TTC in MYOC (including rs12076134, rs183532, rs235875) was found to be significantly different between the two groups (P=0.018), but the significance was lost after 10000 permutations (P=0.157; Table 4). There were no differences between the two groups in the distribution of genotype and allele frequencies for the remaining 9 SNPs. Furthermore, no association between the eleven SNP genotypes and the ocular biometric parameters of AL and ACD was found using generalized estimation equation tests (Table 5).
Table 2. Allele frequencies and the association between SNPs and PACG under the allelic model.
| Gene | SNP | CHR | BP | Minor allele | Genotype (AA/Aa/aa)a |
MAF |
HWE-P |
OR (95%CI) | P | |||
| Case | Control | Case | Control | Case | Control | |||||||
| MYOC | rs12076134 | 1 | 171606054 | G | 331/150/19 | 468/218/33 | 0.188 | 0.198 | 0.662 | 0.2401 | 0.954 (0.759-1.198) | 0.6828 |
| MYOC | rs183532 | 1 | 171609481 | T | 295/181/24 | 402/271/47 | 0.229 | 0.254 | 0.7037 | 0.9215 | 0.781 (0.629-0.969) | 0.025 |
| MYOC | rs235875 | 1 | 171613756 | T | 239/204/57 | 363/300/57 | 0.318 | 0.287 | 0.1813 | 0.716 | 1.171 (0.964-1.423) | 0.1124 |
| MYOC | rs235913 | 1 | 171618656 | T | 193/225/82 | 237/363/120 | 0.389 | 0.419 | 0.2586 | 0.3589 | 0.883 (0.733-1.063) | 0.1895 |
| ABCA1 | rs2422493 | 9 | 107690995 | A | 175/242/83 | 232/374/114 | 0.408 | 0.418 | 1 | 0.0782 | 0.968 (0.801-1.171) | 0.7397 |
| ABCA1 | rs2487042 | 9 | 107694522 | T | 262/192/46 | 366/296/58 | 0.284 | 0.286 | 0.2262 | 0.9274 | 0.959 (0.783-1.173) | 0.6817 |
| ABCA1 | rs2472496 | 9 | 107695353 | A | 169/237/94 | 223/372/125 | 0.425 | 0.433 | 0.5219 | 0.1718 | 0.979 (0.812-1.181) | 0.8267 |
| ABCA1 | rs2472493 | 9 | 107695848 | A | 149/245/106 | 195/382/142 | 0.457 | 0.464 | 0.7871 | 0.0722 | 0.992 (0.822-1.197) | 0.9303 |
| ABCA1 | rs2487032 | 9 | 107703934 | A | 156/239/105 | 202/374/143 | 0.449 | 0.459 | 0.4697 | 0.2295 | 0.942 (0.782-1.135) | 0.5304 |
| ABCA1 | rs2472459 | 9 | 107710562 | T | 250/196/54 | 350/306/64 | 0.304 | 0.302 | 0.1124 | 0.8596 | 0.981 (0.804-1.196) | 0.8476 |
| ABCA1 | rs2472519 | 9 | 107715878 | G | 236/202/62 | 333/314/71 | 0.326 | 0.318 | 0.0832 | 0.8634 | 0.984 (0.809-1.196) | 0.8691 |
SNP: Single nucleotide polymorphisms; PACG: Primary angle-closure glaucoma; CHR: Chromosome; BP: Base pair position; MAF: Minor allele frequency; HWE-P: The P-value of Hardy-Weinburg equilibrium; OR: Odds ratio; CI: Confidence intervals. P, OR, and CI were calculated with logistic regression model by adjusting for age and gender under the allelic model with the major allele as reference. aA represents the wild allele and a represents the minor allele.
Table 3. Genotype distribution of target SNPs in cases and controls.
| Gene | SNP | Dominant model |
Recessive model |
||||||||||
| Aa+aaa | AAa | Aa+aab | AAb | OR (95%CI) | P | aaa | AA+Aaa | aab | AA+Aab | OR (95%CI) | P | ||
| MYOC | rs12076134 | 169 | 331 | 251 | 468 | 0.960 (0.732-1.26) | 0.769 | 19 | 481 | 33 | 686 | 0.858 (0.447-1.649) | 0.647 |
| MYOC | rs183532 | 205 | 295 | 318 | 402 | 0.748 (0.575-0.972) | 0.029 | 24 | 476 | 47 | 673 | 0.707 (0.403-1.242) | 0.228 |
| MYOC | rs235875 | 261 | 239 | 357 | 363 | 1.114 (0.861-1.441) | 0.412 | 57 | 443 | 57 | 663 | 1.595 (1.04-2.446) | 0.032 |
| MYOC | rs235913 | 307 | 193 | 483 | 237 | 0.776 (0.593-1.015) | 0.064 | 82 | 418 | 120 | 600 | 0.987 (0.698-1.397) | 0.942 |
| ABCA1 | rs2422493 | 325 | 175 | 488 | 232 | 0.852 (0.649-1.12) | 0.251 | 83 | 417 | 114 | 606 | 1.168 (0.822-1.661) | 0.386 |
| ABCA1 | rs2487042 | 238 | 262 | 354 | 366 | 0.858 (0.663-1.11) | 0.243 | 46 | 454 | 58 | 662 | 1.324 (0.831-2.112) | 0.238 |
| ABCA1 | rs2472496 | 331 | 169 | 497 | 223 | 0.836 (0.635-1.101) | 0.203 | 94 | 406 | 125 | 595 | 1.221 (0.873-1.71) | 0.244 |
| ABCA1 | rs2472493 | 351 | 149 | 524 | 195 | 0.861 (0.647-1.146) | 0.305 | 106 | 394 | 142 | 577 | 1.18 (0.855-1.628) | 0.314 |
| ABCA1 | rs2487032 | 344 | 156 | 517 | 202 | 0.795 (0.599-1.055) | 0.112 | 105 | 395 | 143 | 576 | 1.126 (0.816-1.554) | 0.471 |
| ABCA1 | rs2472459 | 250 | 250 | 370 | 350 | 0.865 (0.668-1.119) | 0.270 | 54 | 446 | 64 | 656 | 1.401 (0.899-2.183) | 0.137 |
| ABCA1 | rs2472519 | 264 | 236 | 385 | 333 | 0.846 (0.653-1.095) | 0.204 | 62 | 438 | 71 | 647 | 1.443 (0.95-2.192) | 0.086 |
SNP: Single nucleotide polymorphisms; OR: Odds ratio; CI: Confidence intervals. aThe genotype counts in cases; bThe genotype counts in controls; a represents the minor allele, A represents the wild allele; Dominant model: Aa+aa compared with AA; Recessive model: aa compared with AA+Aa. P, OR, and CI were calculated with logistic regression model by adjusting for age and gender.
Figure 1. The patterns of linkage disequilibrium of the eleven target SNPs with their D' value.
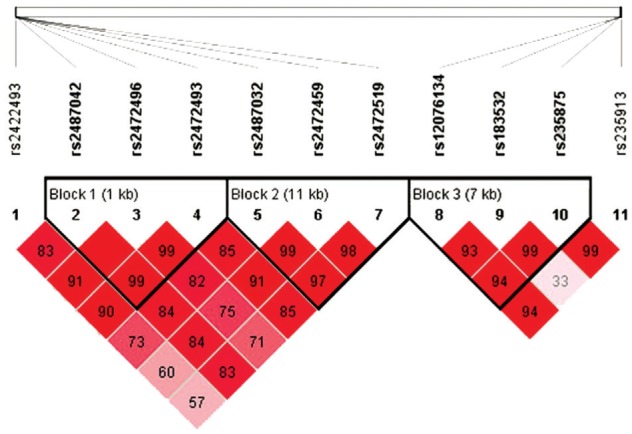
Three haplotype blocks are presented in HapMap CHB cohort combined of PACG cases and controls.
Table 4. Haplotype frequencies in PACG and control cohorts.
| Block | SNPs | Haplotype | f1 (%) | f2 (%) | ORa | Pa | P-permutationb |
| Block 1 | rs2487042, rs2472496, rs2472493 | TAA | 0.283 | 0.286 | 0.958 | 0.679 | 0.9998 |
| CAA | 0.14 | 0.146 | 1.01 | 0.913 | 1 | ||
| CGA | 0.034 | 0.031 | 1.2 | 0.493 | 0.9967 | ||
| CGG | 0.541 | 0.537 | 0.998 | 0.98 | 1 | ||
| Block 2 | rs2487032, rs2472459, rs2472519 | ATG | 0.303 | 0.293 | 1.01 | 0.905 | 1 |
| ACG | 0.018 | 0.019 | 0.72 | 0.334 | 0.9671 | ||
| ACA | 0.127 | 0.141 | 0.948 | 0.691 | 1 | ||
| GCA | 0.546 | 0.534 | 1.06 | 0.536 | 0.9981 | ||
| Block 3 | rs12076134, rs183532, rs235875 | TCT | 0.315 | 0.284 | 1.18 | 0.104 | 0.6279 |
| TTC | 0.223 | 0.253 | 0.77 | 0.018 | 0.1576 | ||
| GCC | 0.179 | 0.196 | 0.94 | 0.598 | 0.999 | ||
| TCC | 0.274 | 0.264 | 1.1 | 0.34 | 0.9693 |
OR: Odds ratio; f1: Haplotype frequencies in cases; f2: Haplotype frequencies in controls. aOR and P value were calculated with logistic regression model by adjusting for age and gender. bA total of 10 000 permutations were performed.
Table 5. Association between SNPs and AL and ACD.
| Gene | SNP | Minor allele | ACD (2.74±0.474; 0.67-3.51)a |
AL (22.92±0.891; 20.01-25.51)a |
||||
| β | SE | P | β | SE | P | |||
| MYOC | rs12076134 | G | 0.008 | 0.0194 | 0.674 | -0.009 | 0.037 | 0.817 |
| MYOC | rs183532 | T | 0.027 | 0.0178 | 0.132 | -0.015 | 0.0345 | 0.667 |
| MYOC | rs235875 | T | -0.017 | 0.0175 | 0.330 | -0.010 | 0.0328 | 0.757 |
| MYOC | rs235913 | T | 0.008 | 0.0158 | 0.617 | 0.014 | 0.0290 | 0.635 |
| ABCA1 | rs2422493 | A | 0.009 | 0.0162 | 0.598 | 0.017 | 0.0319 | 0.592 |
| ABCA1 | rs2487042 | T | 0.009 | 0.0174 | 0.594 | -0.014 | 0.0338 | 0.688 |
| ABCA1 | rs2472496 | A | 0.014 | 0.0161 | 0.395 | 0.006 | 0.0314 | 0.85 |
| ABCA1 | rs2472493 | A | 0.013 | 0.0160 | 0.405 | -0.011 | 0.0313 | 0.725 |
| ABCA1 | rs2487032 | A | -0.005 | 0.0161 | 0.758 | 0.025 | 0.0301 | 0.407 |
| ABCA1 | rs2472459 | T | -0.0001 | 0.0178 | 0.996 | -0.013 | 0.0342 | 0.714 |
| ABCA1 | rs2472519 | G | -0.003 | 0.0173 | 0.844 | 0.010 | 0.0337 | 0.770 |
SNP: Single nucleotide polymorphisms; AL: Axial length; ACD: Anterior chamber depth; β: Per-allele effect of the minor allele; SE: Standard error; P: P-value for association adjusting for age and gender. aThe mean±SD and the range of measured values for AL or ACD.
Due to the differences of the minor allele frequencies (MAF), the power varies between the eleven SNPs. Therefore, assuming an allelic odds ratio (OR) of 1.5, our sample size provides more than 98% of statistical power to detect a significant association at an α level of 0.05.
DISCUSSION
The present study evaluated the association of two POAG related genes MYOC and ABCA1 with PACG in a northern China cohort and found rs183532 and rs235875 as well as a haplotype TTC in MYOC were nominally associated with PACG, moreover, there was no correlation between ABCA1 and PACG. Glaucoma is a multifactorial disease, genetic factors have been found to be significant for its progression[1]. Studies have shown that PACG partly shared the genetic risks with POAG[8]. MYOC is the first and the most significant gene found to be associated with POAG[9]–[10]. About 3% to 5% of adult POAG cases worldwide are caused by MYOC[19]–[23]. MYOC is preferentially expressed in the anterior segment of eye, especially in the TM[13],[24]. Transgenic mouse models of POAG indicated mutant MYOC accumulated in the endoplasmic reticulum of TM, thereby inducing endoplasmic reticulum stress in TM, which was found to be associated with TM cell death and elevation of IOP[25]–[26]. The mechanism of action of MYOC working in POAG is still being researched deeply, since PACG and POAG share the same basic characteristics: elevated IOP and progressive degeneration of retinal ganglion cells and optic nerve axons[1]. Whether or not MYOC is associated with PACG is also worth exploring. Faucher et al[27] founded a mutation in MYOC was associated with PACG in the Quebec population. The association between MYOC gene mutation and PACG was also reported in Chinese population[28]–[29]. Even so, there are also some studies that did not support the association between MYOC mutation and PACG[30]–[31]. Hence. The relationship of MYOC gene variants with PACG is still unclear. In 2015, Jin et al[17] identified rs183532 in MYOC was associated with PACG in samples from south of China. The OR value of the A allele of rs183532 in their study was 1.541. In present study, we found rs183532 and rs235875 as well as a haplotype TTC in MYOC were nominally associated with PACG (uncorrected P=0.025, 0.032, 0.018; OR=0.781, 1.595, 0.77, respectively). The OR value of the two SNPs were in line with the haplotype, but contrary to previous Jin et al's[17] finding. The explanation for this consequence is that our finding did not survive multiple testing corrections and may represent a false positive result. In addition, in view of the fact that Jin et al's[17] study is relatively small sample size (212 patients and 255 controls), hence, the relationship between MYOC and PACG still need further study.
ABCA1 gene encodes a membrane protein that is a major regulator of cellular cholesterol and phospholipid homeostasis[32]. It is expressed in many ocular tissues especially in the ganglion cell layer of retina[11]. Recent GWAS have indicated that genetic variants near ABCA1 gene were significantly associated with POAG[11]–[12]. Variants near ABCA1 gene were also associated with IOP in normal populations[15]. Previous studies using the DBA/2J glaucoma mouse model identified ABCA1 was related to ganglion cell death[33]. Degeneration of retinal ganglion cells is a common sign of both PACG and POAG[1]. It also prompts us for the possible association between ABCA1 and PACG. However, Luo et al[18] evaluated the correlation between ABCA1 and PACG in a Chinese population and did not find a certain association between them, although they found two haplotypes in ABCA1 were associated with PACG/PAC. In present study, the association between ABCA1 and PACG has not been confirmed, thus, further research is necessary to validate the role of ABCA1 in the progress of PACG.
Moreover, seeing that shallow ACD and short AL have been reported to be strong risk factors for PACG[34]–[36], the associations between these eleven SNP genotypes and the ocular biometric parameters of AL and ACD were also evaluated in our study. We found that none of the target SNPs showed significant association with ocular biometric parameters of AL and ACD. Our result suggests these POAG related genes MYOC and ABCA1 do not have a role in the regulation of ocular biometric parameters of AL and ACD.
The limitation of the study is also obvious. The SNPs were chosen on the experiences of previous studies, which may not represent the gene completely in our cohort. Therefore, further study utilizing the tagger program based on our cohort should be done in the future.
In conclusion, our study investigated the association of two POAG related genes MYOC and ABCA1 with PACG as well as ocular biometric parameters of AL and ACD in a northern Chinese cohort. Our result suggests these POAG related genes MYOC and ABCA1 do not play a part in the pathogenesis of PACG as well as the regulation of ocular biometric parameters of AL and ACD. Additional studies are necessary to confirm this conclusion.
Acknowledgments
The authors thank all the patients and participants.
Foundations: Supported by the National Natural Science Foundation of China (No.81460093); the Ningxia Nature Science Funding from the Department of Science and Technology of Ningxia Hui Autonomous Region (No.NZ16194).
Conflicts of Interest: Wang SL, None; Piao SY, None; Xu MY, None; Zhang W, None; Ma JQ, None; Hao J, None; Chi H, None; Xue ZQ, None; Ha SP, None; Zhuang WJ, None.
REFERENCES
- 1.Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet. 2017;390(10108):2183–2193. doi: 10.1016/S0140-6736(17)31469-1. [DOI] [PubMed] [Google Scholar]
- 2.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and Meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Kwon YH, Fingert JH, Kuehn MH, Alward WLM. Primary open-angle glaucoma. N Engl J Med. 2009;360(11):1113–1124. doi: 10.1056/NEJMra0804630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Congdon N, Wang F, Tielsch JM. Issues in the epidemiology and population-based screening of primary angle-closure glaucoma. Surv Ophthalmol. 1992;36(6):411–423. doi: 10.1016/s0039-6257(05)80022-0. [DOI] [PubMed] [Google Scholar]
- 6.Foster PJ, Baasanhu J, Alsbirk PH, Munkhbayar D, Uranchimeg D, Johnson GJ. Glaucoma in Mongolia. A population-based survey in Hövsgöl province, northern Mongolia. Arch Ophthalmol. 1996;114(10):1235–1241. doi: 10.1001/archopht.1996.01100140435011. [DOI] [PubMed] [Google Scholar]
- 7.Quigley HA, Congdon NG, Friedman DS. Glaucoma in China (and worldwide): changes in established thinking will decrease preventable blindness. Br J Ophthalmol. 2001;85(11):1271–1272. doi: 10.1136/bjo.85.11.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khor CC, Do T, Jia H, et al. Genome-wide association study identifies five new susceptibility loci for primary angle closure glaucoma. Nat Genet. 2016;48(5):556–562. doi: 10.1038/ng.3540. [DOI] [PubMed] [Google Scholar]
- 9.Tamm ER, Russell P, Epstein DL, Johnson DH, Piatigorsky J. Modulation of myocilin/TIGR expression in human trabecular meshwork. Invest Ophthalmol Vis Sci. 1999;40(11):2577–2582. [PubMed] [Google Scholar]
- 10.Jacobson N, Andrews M, Shepard AR, Nishimura D, Searby C, Fingert JH, Hageman G, Mullins R, Davidson BL, Kwon YH, Alward WL, Stone EM, Clark AF, Sheffield VC. Non-secretion of mutant proteins of the glaucoma gene myocilin in cultured trabecular meshwork cells and in aqueous humor. Hum Mol Genet. 2001;10(2):117–125. doi: 10.1093/hmg/10.2.117. [DOI] [PubMed] [Google Scholar]
- 11.Hysi PG, Cheng CY, Springelkamp H, Macgregor S, Bailey JNC, Wojciechowski R, Vitart V, Nag A, Hewitt AW. Genome-wide analysis of multi-ancestry cohorts identifies new loci influencing intraocular pressure and susceptibility to glaucoma. Nat Genet. 2014;46(10):1126–1130. doi: 10.1038/ng.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheffield VC, Stone EM, Alward WL, Drack AV, Johnson AT, Streb LM, Nichols BE. Genetic linkage of familial open angle glaucoma to chromosome 1q21-q31. Nat Genet. 1993;4(1):47–50. doi: 10.1038/ng0593-47. [DOI] [PubMed] [Google Scholar]
- 13.Stone EM, Fingert JH, Alward WL, Nguyen TD, Polansky JR, Sunden SL, Nishimura D, Clark AF, Nystuen A, Nichols BE, Mackey DA, Ritch R, Kalenak JW, Craven ER, Sheffield VC. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275(5300):668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Lin Y, Vithana EN, et al. Common variants near ABCA1 and in PMM2 are associated with primary open-angle glaucoma. Nat Genet. 2014;46(10):1115–1119. doi: 10.1038/ng.3078. [DOI] [PubMed] [Google Scholar]
- 15.Gharahkhani P, Burdon KP, Fogarty R, Sharma S, Hewitt AW, Martin S, Law MH, Cremin K, Bailey JNC, Loomis SJ. Common variants near ABCA1, AFAP1 and GMDS confer risk of primary open angle glaucoma. Nat Genet. 2014;46(10):1120–1125. doi: 10.1038/ng.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S, Zhuang W, Ma J, Xu M, Piao S, Hao J, Zhang W, Chi H, Xue Z, Ha S. Association of Genes implicated in primary angle-closure Glaucoma and the ocular biometric parameters of anterior chamber depth and axial length in a northern Chinese population. BMC Ophthalmol. 2018;18(1):271. doi: 10.1186/s12886-018-0934-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin X, Wang DJ, Qu LH, Hou BK, Gong Y, Xu WW. Haplotype analysis of association of the MYOC gene with primary angle-closure glaucoma in a Han Chinese population. Genet Test Mol Biomarkers. 2015;19(1):3–8. doi: 10.1089/gtmb.2014.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo H, Chen Y, Ye Z, et al. Evaluation of the association between common genetic variants near the ABCA1 gene and primary angle closure glaucoma in a Han Chinese population. Invest Ophthalmol Vis Sci. 2015;56(11):6248–6254. doi: 10.1167/iovs.15-16741. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki Y, Shirato S, Taniguchi F, Ohara K, Nishimaki K, Ohta S. Mutations in the TIGR gene in familial primary open-angle glaucoma in Japan. Am J Hum Genet. 1997;61(5):1202–1204. doi: 10.1086/301612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alward WL, Fingert JH, Coote MA, Johnson AT, Lerner SF, Junqua D, Durcan FJ, McCartney PJ, Mackey DA, Sheffield VC, Stone EM. Clinical features associated with mutations in the chromosome 1 open-angle glaucoma gene (GLC1A) N Engl J Med. 1998;338(15):1022–1027. doi: 10.1056/NEJM199804093381503. [DOI] [PubMed] [Google Scholar]
- 21.Wiggs JL, Allingham RR, Vollrath D, Jones KH, De La Paz M, Kern J, Patterson K, Babb VL, Del Bono EA, Broomer BW, Pericak-Vance MA, Haines JL. Prevalence of mutations in TIGR/Myocilin in patients with adult and juvenile primary open-angle glaucoma. Am J Hum Genet. 1998;63(5):1549–1552. doi: 10.1086/302098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alward WL, Kwon YH, Khanna CL, Johnson AT, Hayreh SS, Zimmerman MB, Narkiewicz J, Andorf JL, Moore PA, Fingert JH, Sheffield VC, Stone EM. Variations in the myocilin gene in patients with open-angle glaucoma. Arch Ophthalmol. 2002;120(9):1189–1197. doi: 10.1001/archopht.120.9.1189. [DOI] [PubMed] [Google Scholar]
- 23.Fingert JH, Heon E, Liebmann JM, et al. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum Mol Genet. 1999;8(5):899–905. doi: 10.1093/hmg/8.5.899. [DOI] [PubMed] [Google Scholar]
- 24.Ortego J, Escribano J, Coca-Prados M. Cloning and characterization of subtracted cDNAs from a human ciliary body library encoding TIGR, a protein involved in juvenile open angle glaucoma with homology to myosin and olfactomedin. FEBS Lett. 1997;413(2):349–353. doi: 10.1016/s0014-5793(97)00934-4. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y, Grinchuk O, Tomarev SI. Transgenic mice expressing the Tyr437His mutant of human myocilin protein develop glaucoma. Invest Ophthalmol Vis Sci. 2008;49(5):1932–1939. doi: 10.1167/iovs.07-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zode GS, Kuehn MH, Nishimura DY, Searby CC, Mohan K, Grozdanic SD, Bugge K, Anderson MG, Clark AF, Stone EM, Sheffield VC. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J Clin Invest. 2015;125(8):3303. doi: 10.1172/JCI82799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faucher M, Anctil JL, Rodrigue MA, et al. Founder TIGR/myocilin mutations for glaucoma in the Québec population. Hum Mol Genet. 2002;11(18):2077–2090. doi: 10.1093/hmg/11.18.2077. [DOI] [PubMed] [Google Scholar]
- 28.Dai X, Nie S, Ke T, Liu J, Wang Q, Liu M. Two variants in MYOC and CYP1B1 genes in a Chinese family with primary angle closure glaucoma. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2008;25(5):493–496. [PubMed] [Google Scholar]
- 29.Huang X, Li M, Guo X, Li S, Xiao X, Jia X, Liu X, Zhang Q. Mutation analysis of seven known glaucoma-associated genes in Chinese patients with glaucoma. Invest Ophthalmol Vis Sci. 2014;55(6):3594–3602. doi: 10.1167/iovs.14-13927. [DOI] [PubMed] [Google Scholar]
- 30.Aung T, Yong VH, Chew PT, Seah SK, Gazzard G, Foster PJ, Vithana EN. Molecular analysis of the myocilin gene in Chinese subjects with chronic primary-angle closure glaucoma. Invest Ophthalmol Vis Sci. 2005;46(4):1303–1306. doi: 10.1167/iovs.04-1163. [DOI] [PubMed] [Google Scholar]
- 31.Abu-Amero KK, Morales J, Osman MN, Bosley TM. Nuclear and mitochondrial analysis of patients with primary angle-closure glaucoma. Invest Ophthalmol Vis Sci. 2007;48(12):5591–5596. doi: 10.1167/iovs.07-0780. [DOI] [PubMed] [Google Scholar]
- 32.Santamarina-Fojo S, Peterson K, Knapper C, Qiu Y, Freeman L, Cheng JF, Osorio J, Remaley A, Yang XP, Haudenschild C, Prades C, Chimini G, Blackmon E, Francois T, Duverger N, Rubin EM, Rosier M, Denèfle P, Fredrickson DS, Brewer HB., Jr Complete genomic sequence of the human ABCA1 gene: analysis of the human and mouse ATP-binding cassette A promoter. Proc Natl Acad Sci U S A. 2000;97(14):7987–7992. doi: 10.1073/pnas.97.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howell GR, Macalinao DG, Sousa GL, Walden M, Soto I, Kneeland SC, Barbay JM, King BL, Marchant JK, Hibbs M, Stevens B, Barres BA, Clark AF, Libby RT, John SW. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J Clin Invest. 2011;121(4):1429–1444. doi: 10.1172/JCI44646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alsbirk PH. Primary angle-closure glaucoma. Oculometry, epidemiology, and genetics in a high risk population. Acta Ophthalmol Suppl. 1976;(127):5–31. [PubMed] [Google Scholar]
- 35.Lowe RF. Aetiology of the anatomical basis for primary angle-closure glaucoma. Biometrical comparisons between normal eyes and eyes with primary angle-closure glaucoma. Br J Ophthalmol. 1970;54(3):161–169. doi: 10.1136/bjo.54.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lavanya R, Wong TY, Friedman DS, Aung HT, Alfred T, Gao H, Seah SK, Kashiwagi K, Foster PJ, Aung T. Determinants of angle closure in older Singaporeans. Arch Ophthalmol. 2008;126(5):686–691. doi: 10.1001/archopht.126.5.686. [DOI] [PubMed] [Google Scholar]


