Abstract
AIM
To establish a model of retinal neurodegeneration induced by N-Methyl-D-aspartic acid (NMDA) in adult zebrafish.
METHODS
We compared the effects of three different NMDA delivery methods on retinal neurodegeneration in adult zebrafish: immersion (I.M.), intravitreal injection (I.V.), and intraperitoneal injection (I.P.), and examined retinal pathology and degeneration by hematoxylin and eosin and TUNEL staining in the treated zebrafish. Effects of the NMDA receptor antagonist MK-801 and the natural product resveratrol on NMDA-induced retinal neurodegeneration were also assessed.
RESULTS
The thickened inner retina was seen in histology with 100 µmol/L NMDA by I.M. administration. Significant apoptosis in the retinal ganglion cell layer and retinal thickness reduction occurred in 0.5 mol/L NMDA I.P. administration group.Seizure-like behavioral changes, but no retinal histological alteration occurred in 16 mg/kg NMDA I.P. administration group. Resveratrol and MK-801 prevented NMDA-induced retinal neurodegeneration in the zebrafish.
CONCLUSION
Among the three drug administration methods, I.V. injection of NMDA is the most suitable for establishment of an acute retinal damage model in zebrafish. I.M. with NMDA is likely the best for use as a chronic retinal damage model. I.P. treatment with NMDA causes brain damage. Resveratrol and MK801 may be a clinically valuable treatment for retinal neurodegeneration.
Keywords: zebrafish, NMDA, administration method, retinal ganglion cell, glaucomatous animal model, resveratrol
INTRODUCTION
Glaucoma is the second leading cause of blindness worldwide, with over 60 million people (and over 10 million in China) suffering from this disease[1]. Pathologically, glaucoma is characterized by the progressive loss of retinal ganglion cells (RGC) and their axons, leading to visual field defects and optic nerve atrophy[2]–[5]. An elevated intraocular N-Methyl-D-aspartic acid (NMDA) concentration plays an important role in retinal ganglion cell loss[6]. Currently, glaucoma drug discovery is focused on visual nerve protection, and the discovery of any treatments that can prevent retinal ganglion cell death would have a major clinical impact[7]. Many glaucoma models have been developed in rats, rabbits, rhesus macaques, and dogs. Some of these glaucoma models have used direct injection of NMDA to the vitreous chamber of rat eyes, which leads to death of retinal ganglion cells[8]–[10]. Compared with mammals, less glaucoma studies have utilized zebrafish despite its usefulness in eye research.
Zebrafish has become increasing popular as a vertebrate model for developmental biology and genetics research over the past 20y[11]–[12]. There are many reasons for its prevalence. Maintaining zebrafish is relatively easy and they can quickly and effortlessly be bred in large numbers. Moreover, many zebrafish genes have been highly conserved throughout animal evolution, with 70%-80% of its genes having homologs in humans[13]. These advantages have facilitated the adoption of zebrafish as a valuable preclinical drug screening system in pharmaceutical research[14]–[15].
Importantly, the visual system of zebrafish is highly similar to the human visual system but develops in just 5d after birth, which is much faster than most other animal models[16]–[17]. Moreover, the central nervous system of zebrafish has similar structural properties with the mammalian system (including the fore-, mid-, and hind-brain, diencephalon, telencephalon, and cerebellum), and the noradrenergic, serotonergic, GABAergic, and histaminergic signaling systems are also highly similar[11],[18]. This has made zebrafish a valuable model for studying brain diseases. Establishment of a better glaucoma model in zebrafish would be valuable not only for understanding more about its molecular pathology but may also be a useful model system to screen for drugs that can protect the visual nerves damaged in glaucoma. Both directions will help to produce superior clinical treatments for glaucoma.
NMDA, an analog of L-glutamate and an important excitatory neurotransmitter in the mammalian central nervous system, has been used in many neuronal diseases model, such as Alzheimer's disease, Huntington disease, Parkinson's disease, epilepsy, and glaucoma[19]–[24]. NMDA-induced cellular excitotoxicity can eventually cause cell death[25]–[26]. The primary administration methods for drugs in adult zebrafish that we utilized are immersion (I.M.), intravitreal (I.V.) injection, and intraperitoneal (I.P.) injection. In previous studies, I.M. allowed for drug absorption directly through zebrafish skin from the aqueous environment[27], while I.P. drug injection was used in zebrafish to cause seizure-like behavior[28]. I.V. has been used to inject ouabain into the eyes of zebrafish to cause retinal damage[29]. Although additional methods, such as intraperitoneal perfusion, have been used in some studies, they have been excluded from this study.
In this study, we investigated two important issues related to the establishment of a glaucomatous zebrafish model for drug screening: whether the three NMDA delivery methods can cause retinal damage in adult zebrafish and if resveratrol or MK-801 can provide protection against this retinal neurodegeneration. MK-801, a noncompetitive antagonist of NMDA receptors, was included to serve as a positive control as it should directly prevent NMDA-induced excitotoxicity. Resveratrol, a natural product found in many plants, was studied given its many documented health-promoting effects, including its ability to increase lifespan[30] and prevent age-related diseases such as inflammation[31], neurodegeneration[32]–[33], epilepsy[34], heart disease, metabolic disorders, and autoimmune diseases[35]. Moreover, we have previously found resveratrol to exert positive effects in models of retinal neurodegeneration[36].
MATERIALS AND METHODS
Ethical Approval
The study was approved by the Ethical Review Committee of Nanchang University (Nanchang, China). We confirm adherence to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Animals
Adult male and female wild-type zebrafish (Danio Rerio, AB strain) were obtained from the China Zebrafish Resource Center (Wuhan, China). All adult zebrafish were raised in a zebrafish breeding system (HAISHENG Biotech, China) at 28°C with 14h/10h light/dark controlled environment and were fed brine shrimp twice daily (WUDi, Shandong Province, China). The zebrafish used in each experiment were 3-6mo old with body weights between 0.3-0.4 g and vitreous volumes of 300-500 nL; therefore, 100-200 nL drug injections should not cause any significant retinal damage.
Drug Preparations
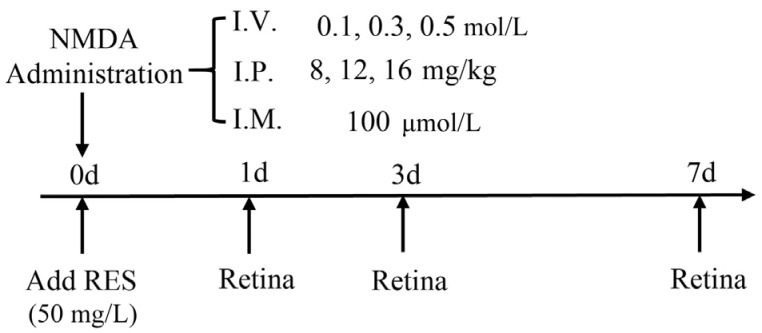
NMDA (M3262, Sigma, USA) was dissolved in phosphate-buffered saline (PBS; I.M.: 100 µmol/L; I.P.: 8, 12 and 16 mg/kg; I.V.: 0.1, 0.3 and 0.5 mol/L; Figure 1). MK-801 (M107, Sigma, USA) was dissolved in 50% ethanol/50% PBS (I.P.: 3 mg/kg, I.V.: 0.05 mol/L). Resveratrol (R5010, Sigma, USA) was dissolved in 100% ethanol and kept in the dark while stored and throughout the entire experiment (50 mg/L). MS-222 (A5040, Sigma, USA) was dissolved in distilled water (0.2 g/L).
Figure 1. Experimental protocols.
I.V.: Intravitreal injection; I.P.: Intraperitoneal injection; I.M.: Immersion.
Intravitreal Injection
Prior to I.V., zebrafish were anaesthetized with MS-222. The approximate volume of the vitreous cavity was calculated to be approximately 200-500 nL by measurements taken with digital calipers as described previously[37]. In a preliminary experiment to determine a suitable injection volume we found 100 nL PBS would not cause any retinal damage or behavioral change. Subsequently, 100 nL freshly prepared NMDA solutions were delivered into the right eye of treated zebrafish through a small incision between the vitreous body and the retina made by a very thin acupuncture needle pin (0.20 mm). Delivery of the appropriate volume of NMDA was accomplished using syringe pumps (HARVARD, C-14171). The injected volume was always set to 100 nL (inject the liquid bead 6 times with radius 1.5 nm, Volume=(4/3)πr3=98.5 nL). After injection, we did not pull out the needle immediately but waited for 30s to allow the drug to spread around the zebrafish eye equally. Subsequently, the fish were kept out for at least for 1min to allow for drug absorption and then returned into the normal living environment until collection for dissection. Drug treatment times were 0h, 1, 3 and 7d. Control groups were injected with 1×PBS by the same method.
Intraperitoneal Injection
Adult zebrafish were first anaesthetized with MS-222 and then injected intraperitoneally (in the middle of the abdomen as with rodents) with NMDA (dosages were selected by comparison with those typically used in rodents[28]). Treatment times were 1, 3, and 7d. Injections of 10 µL drug (NMDA or MK-801) or 1×PBS (control) were then performed in one side of the fish.
Immersion
For NMDA I.M. treatment, we exposed the adult zebrafish (n=6 for each small group) to a solution of 100 µmol/L NMDA for different time points (0h, 1, 3, 7d; solution was prepared under 28°C). NMDA was dissolved in 200 mL distilled water for 1h for complete solubilization in a 1 L cylindroid water tank. For resveratrol treatment, the fish were exposed to solutions with resveratrol for 1d. Resveratrol was dissolved in 10 µL ethanol and then mixed in 200 mL distilled water for 1h for complete solubilization. The aquariums were completely covered during resveratrol treatment to avoid degradation of the compound by ultraviolet light. The NMDA and resveratrol treatment began at the same time point, so the first day of drug treatment was controlled strictly without light influence. After the first day, all the zebrafish were returned to the normal daylight environment. Upon completion of treatments, zebrafish retinas were harvested and immediately stored in PBS at 4°C for subsequent analysis.
Cardiac Perfusion, Eye Dissection and Storage
Zebrafish were euthanized, set on a custom operating table, and then a T-shape lesion was cut into the thorax of each zebrafish. A microinjector was then used to inject 2 mL PBS followed by 2 mL 2% PFA/2% glutaraldehyde (G5882, Sigma, USA) into the heart. Subsequently, the whole eyes from each zebrafish were harvested, put into 10 mL 1×PBS for several minutes, and then fixed with 2 mL 2% PFA/2% glutaraldehyde at 4°C for 5h. Next, the eye tissues were washed three times in 1× PBS on a rack at 4°C for 30min per cycle. After washing, the eyes were dehydrated stepwise in 30%, 50%, and 70% ethanol, each for 30min. Finally, the dehydrated eyes were stored in 70% ethanol at 4°C for long-term storage until embedding.
Hematoxylin and Eosin Staining and Histological Evaluation
Briefly, eyes were dehydrated stepwise in 70%, 80% (2×), 95% (2×), and 100% (3×) ethanol for 30min per step. Then they were processed with xylene (3×) for 20min per step and embedded in paraffin. Next, the embedded eyes were cut with a paraffin slicing machine (LEICA RM2235) into 4 µm thick sections (horizontal with the optic nerve head). Each section contained the whole retina from both superior and inferior hemisphere because the eyes were aligned vertically to the ground and sections were made along the vertical meridian. Hematoxylin and eosin (H&E) staining of sections was then performed and their morphology determined by light microscopy (Optiphot-2, Nikon, Tokyo, Japan). The total retinal ganglion cell number in the retinal ganglion cell layer (GCL) was manually counted in a region at the middle of one side of the retina between the center of the optic nerve head and ending. Thickness measurements were then determined for the nerve fiber layer (NFL), the GCL and the whole retina by software analysis (Image-pro Plus 6.0). Pictures were captured using an IX71 camera (OLYMPUS, Japan).
TUNEL Staining
Animals were euthanized with ice water after treatment with drugs. Retinal horizontal sections were obtained as described in the histological evaluation step. Following the manufacturer's instructions, TUNEL staining was performed to detect apoptotic cells using the In Situ Apoptosis Detection Kit (11684817910, Roche, Germany). Images of TUNEL staining were collected with an LSM800 microscope (ZEISS, Gottingen, Germany).
Statistical Analysis
Graphpad PRISM 7.00 was used for data analysis, with values presented as the mean±SEM. Student's t-test was used to compare the means of different groups with a P<0.05 considered significant.
RESULTS
NMDA-Induced Inner Retinal Thickening with Immersion Treatment
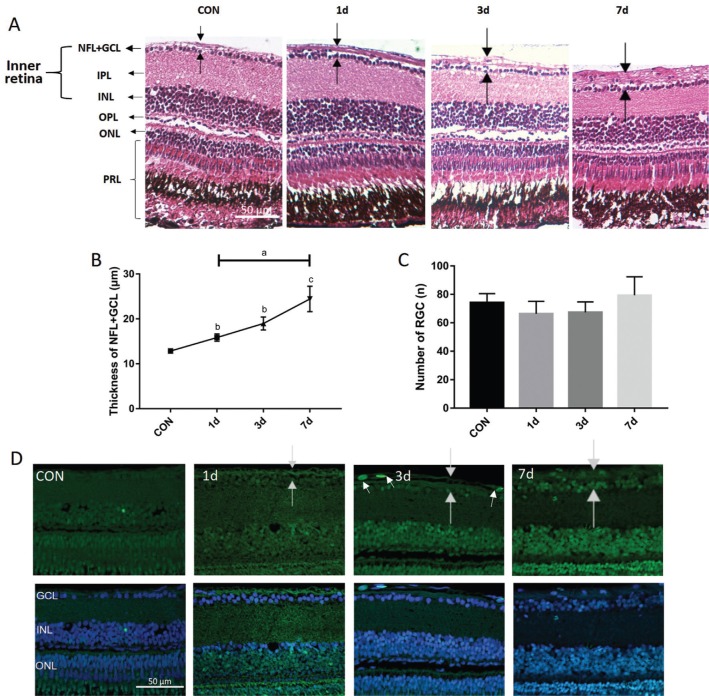
We first assessed the effectiveness of the I.M. method on the retina. In order to induce retinal neurodegeneration, the adult zebrafish were immersed into 100 µmol/L NMDA for various times (0-7d). H&E staining showed that the mean thickness NFL+GCL increased significantly after 1d and gradually reached approximately 2× larger by 7d compared with the control group (P=0.0021; Figure 2A, 2B), which indicates that NMDA caused structural damage to the retina. However, no significant differences were evident between the retinal ganglion cell numbers of each group even after 7d treatment as shown by H&E and TUNEL staining (Figure 2C, 2D), suggesting that the detrimental effect of 100 µmol/L NMDA was not sufficient to cause significant retinal ganglion cell death within one week. However, several apoptotic cells could be seen in the NFL of the 3d treatment group, indicating that retinal damage did cause a small amount of apoptosis (Figure 2D).
Figure 2. Histology of NMDA-induced thicker NFL (time gradient).
A-D: The zebrafish were treated by I.M. in NMDA and divided into 4 distinct groups with different treatment times. A: H&E staining shows representative paraffin sections (4 µm) from a wild-type control retina immersed in distilled water along with retinas treated with 100 µmol/L NMDA for 1, 3, and 7d. The dark arrow points out the thickness of each layer. B: The thickness of the NFL+GCL (y-axis) plotted against the NMDA treatment time; C: The number of GCL (y-axis) plotted against the NMDA treatment time. Error bars represent standard error of the mean (±SEM); n=6. (unpaired t-test, aP<0.05, bP<0.01, and cP<0.001 compared with control); D: TUNEL staining of NMDA treatment time points and control retinas. White arrows point to apoptotic cells. NFL: Nerve fiber layer; GCL: Ganglion cell layer; IPL: Inner plexiform layer; INL: Inner nuclear layer; OPL: Outer plexiform layer; ONL: Outer nuclear layer; PRL: Photoreceptor layer. Magnification is 40×. Scale bar, 50 µm.
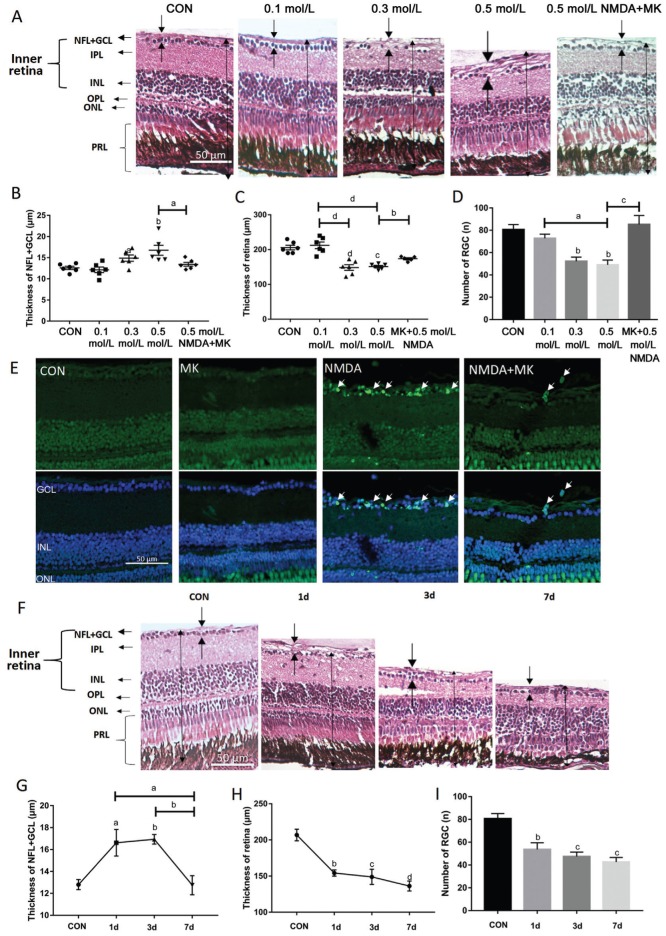
NMDA-Induced Thicker NFL, Retinal Thickness Reduction, and Retinal Ganglion Cell Apoptosis with Intravitreal Injection
We tested different drug concentrations with a treatment time of 1d to determine the optimal intravitreal dose of each drug. The 100 nL solution was microinjected into each eye of all zebrafish treatment groups. Preliminary experiments indicated that a suitable dose range of NMDA was 0-0.5 mol/L, from which we found the most effective concentration to be 0.5 mol/L (0.6 mol/L was found to be lethal for zebrafish, causing seizure-like symptoms and death in just several minutes). We also determined that 1d was a suitable time point to induce significant retinal damage. Therefore, 100 nL 0.5mol/L NMDA injection for 1d was used for subsequent testing.
We found that the NFL+GCL thickness increased while the retinal thickness was decreased significantly after NMDA treatment (retinal thickness slightly increased in the 0.1 mol/L NMDA-treated group but decreased considerably in the 0.3 and 0.5 mol/L NMDA-treated groups, P<0.0001; Figure 3A-3C). Moreover, the retinal ganglion cell number decreased with increasing NMDA concentration (approximately 40% decrease in the 0.5 mol/L NMDA treatment group compared to control, P<0.01; Figure 3D). TUNEL staining showed that apoptosis was induced in the GCL and inner nuclear layer (INL) with 0.5 mol/L NMDA treatment (Figure 3E). Together these data indicate that I.V. injection of 100 nL 0.5 mol/L NMDA can cause considerable damage to the zebrafish retina. Importantly, MK-801 treatment prevented all of the NMDA-induced changes (Figure 3A-3E), demonstrating that our positive control NMDA antagonist works to prevent the detrimental effects of NMDA and suggests that this system can be used for novel glaucoma drug discovery.
Figure 3. NMDA-induced alterations in retinal histology, thickness and retinal ganglion cell apoptosis.
A-D: The zebrafish were treated by I.V. of NMDA at different doses for 1d. A: H&E staining of paraffin sections (4 µm) from a control retina treated with 100 nL PBS and retinas treated with 0.1, 0.3, 0.5 mol/L NMDA, and 0.05 mol/L MK-801+0.5 mol/L NMDA. The dark arrows show the thickness of each layer. B: The thickness of the NFL+GCL from different treatment groups; C: The thickness of retinas from different treatment groups; D: The retinal ganglion cell number of retinas from different treatment groups. Error bars represent standard error of the mean (±SEM); n=6 (unpaired t-test, aP<0.05, bP<0.01, cP<0.001, and dP<0.0001 compared with control); E: TUNEL staining of representative control retinas and retinas treated with 50 mmol/L MK-801, 0.5 mol/L NMDA, and MK-801+NMDA. The white arrows point to apoptosis cells. F: H&E staining of paraffin sections (4 µm) from retinas treated intravitreally with 1× PBS (control) or with 0.5 mol/L NMDA for various times; G: The thickness of the NFL+GCL from different treatment groups; H: The thickness of retinas from different treatment groups; I: The retinal ganglion cell number from retinas of different treatment groups. Error bars represent standard error of the mean (±SEM); n=6 (unpaired t-test, aP<0.05, bP<0.01, cP<0.001, and dP<0.0001 compared with control). Original magnification is 40×. Scale bar, 50 µm.
A time gradient from 0-7d was then set up to find the optimal NMDA treatment time. Although already significantly perturbed after only 1d of treatment, we observed a continued degeneration of the retina throughout the week of NMDA treatment (Figure 3F). Interestingly, the NFL+GCL became thicker and the cell distribution scattered after 1 and 3d of treatment, but then decreased back to baseline at 7d (Figure 3F, 3G). We also found that the retinal ganglion cell number and retinal thickness was already significantly reduced after only 1d, but continued to gradually decrease further during the week of treatment and reached their lowest levels after 7d treatment (Figure 3H, 3I). At this point, mean thickness was reduced by approximately 33% (Figure 3H) while the mean retinal ganglion cell number decreased by approximately 48% (Figure 3I). Together these data demonstrate that the most significant retinal damage induced by I.V. NMDA administration occurred during the first day after treatment, but further degeneration occurred in the following 6d.
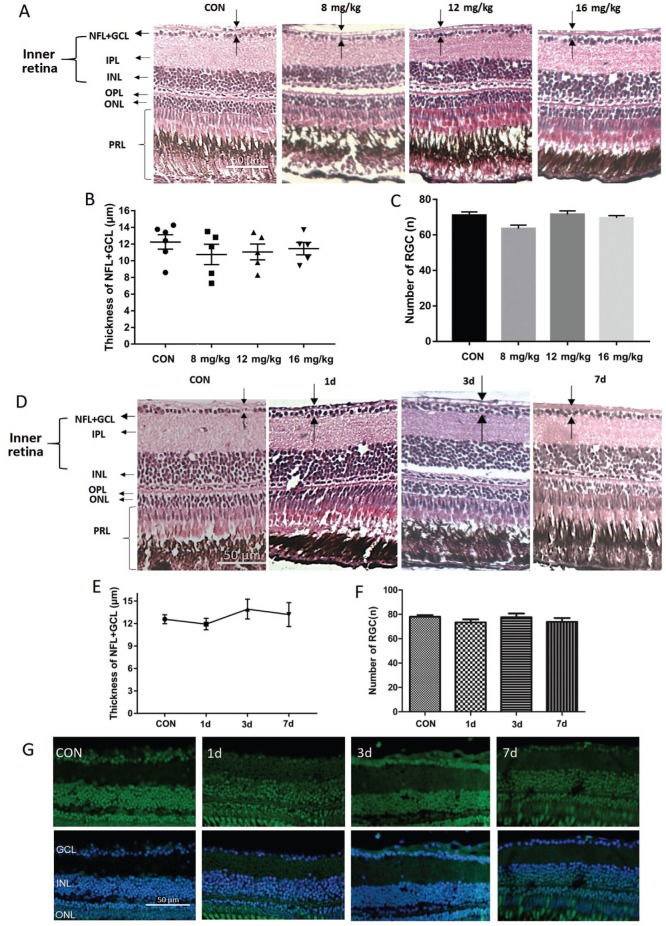
No Histologic Change in the Retina after NMDA Intraperitoneal Injection
For I.P. injection, zebrafish were injected with different NMDA concentrations on one side of the abdomen. For reference we referred to the concentrations used in other studies and set up a dose range of 0-24 mg/kg NMDA for 1d treatment. Zebrafish injected with 24 mg/kg NMDA died immediately and were not analyzed further. At the non-lethal doses of NMDA (8, 12, and 16 mg/kg), zebrafish presented seizure-like behavior, however, no changes to the retinal histology were observed (Figure 4A), nor was there a significant change in the thickness of the NFL+GCL (Figure 4B) or in the retinal ganglion cell number (Figure 4C). To further test for potential retinal damage upon I.P. injection, we also performed a time gradient using the highest tolerated dose of 16 mg/kg NMDA. H&E and TUNEL staining showed that no morphologic changes or induction of apoptosis occurred even 7d post-treatment (Figure 4D-4G). Therefore, I.P. injection of NMDA is not a sufficient route of administration for studies of zebrafish retinal damage and was not pursued further.
Figure 4. Analysis of intraperitoneal NMDA injection for retinal damage.
A: H&E staining of paraffin sections (4 µm) from retinas treated intraperitoneally for one day with 10 µL PBS (control) or 8, 12, or 16 mg/kg NMDA; B: The thickness of the NFL+GCL of retinas from each treatment group; C: The retinal ganglion cell number in retinas from each treatment group. Error bars represent standard error of the mean (SEM); n=6 (unpaired t-test showed no significant differences). D: H&E staining of paraffin sections (4 µm) from a control retina treated intraperitoneally with 10 µL PBS for 1d and retinas treated intraperitoneally with 16 mg/kg NMDA for 1, 3, and 7d. The dark arrow points out the thickness of each layer. E: The thickness of the NFL+GCL of retinas from each time point; F: The retinal ganglion cell number in retinas from each time point. Error bars represent standard error of the mean (±SEM); n=6. G: TUNEL staining of retinas from zebrafish treated intraperitoneally with PBS (control) or with 16 mg/kg NMDA for 1, 3, or 7d. Original magnification is 40×. Scale bar, 50 µm.
Resveratrol Prevented the NMDA-Induced Thickened Inner Retina by Immersion
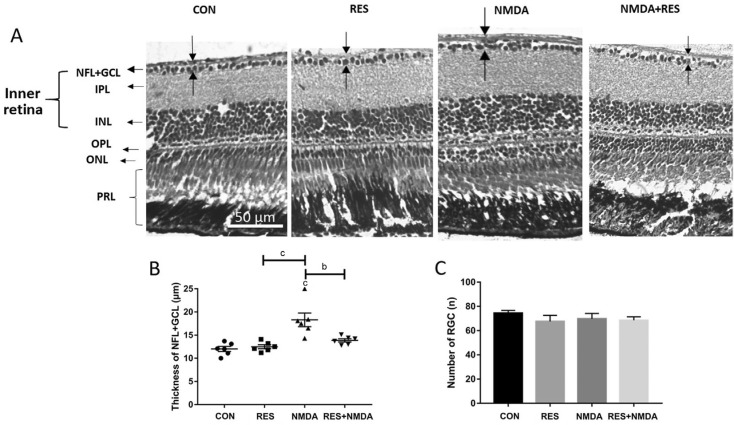
To assess the effects of resveratrol on retinal damage, two pairs of treatment and control groups were set up: 100 µmol/L NMDA (treatment groups) versus water (control groups), each one with or without 50 mg/L resveratrol. All groups were treated for 1d and were kept in the dark to prevent light-induced degradation of resveratrol. The thickness of the NFL+GCL was increased in the NMDA-only treated group, whereas it was not significantly altered in the resveratrol-only group or the NMDA+resveratrol group (Figure 5A, 5B). This indicates that resveratrol may exert a neuroprotective effect against NMDA-caused retinal damage. Consistent with our prior results for NMDA I.M., we did not observe any change in the retinal ganglion cell number upon I.M. treatment (Figure 5C). This indicates that resveratrol also does not alter the retinal ganglion cell number at a concentration at which it can prevent NMDA-induced thickening of the NFL+GCL, suggesting its potential for safe usage to prevent retinal damage.
Figure 5. Resveratrol prevents from NMDA-induced thicker NFL.
A: H&E staining of paraffin sections (4 µm) from representative retinas treated by I.M. in distilled water (control), 50 mg/L resveratrol, 100 µmol/L NMDA, or 50 mg/L resveratrol+100 µmol/L NMDA. The dark arrows point out the thickness of each layer. B: The thickness of the NFL+GCL for each treatment group; C: The retinal ganglion cell number for each treatment group. Error bars represent standard error of the mean (±SEM); n=6 (unpaired t-test, bP<0.01 and cP<0.001). Original magnification is 40×. Scale bar, 50 µm.
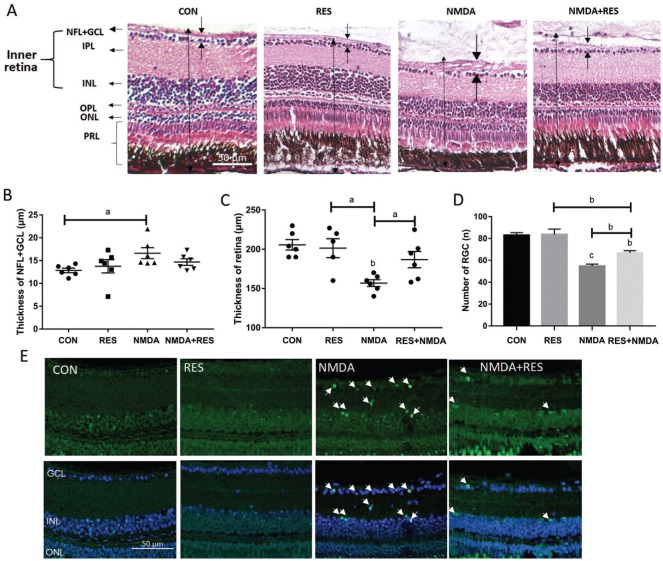
Resveratrol Protected from I.V. NMDA-Induced Retinal Damage
To further assess resveratrol's ability to prevent retinal damage, zebrafish eyes were first injected intravitreally with 100 nL 0.5 mol/L NMDA, and then the zebrafish were immediately immersed into 50 mg/L resveratrol for 1d. Whereas NMDA I.V. treatment caused serious retinal neurodegeneration, and treatment with resveratrol largely prevented retinal damage caused by NMDA treatment (Figure 6A). Moreover, resveratrol had a significant preventative effect on the NMDA-induced changes in NFL+GCL thickness (Figure 6B), retina thickness (Figure 6C), and retinal ganglion cell number (Figure 6D), while resveratrol treatment alone was not significantly different from the control group (Figure 6B-6D). TUNEL staining also showed that NMDA induced apoptosis in many cells in the GCL and INL, which could be prevented by resveratrol treatment (Figure 6E). These data indicate that resveratrol is able to protect the retina from NMDA-induced damage but itself does not cause any significant changes to the zebrafish retina.
Figure 6. Resveratrol protects against NMDA-induced retinal damage.
A-E: Zebrafish were treated intravitreally with NMDA and by I.M. in resveratrol. A: H&E staining of paraffin sections (4 µm) from retinas treated with I.V. of 100 nL PBS (control), or 0.5 mol/L NMDA, each plus or minus I.M. in 50 mg/L resveratrol. The dark arrows point out the thickness of each layer. B: The thickness of the NFL+GCL from each treatment group; C: The thickness of the retinas from each treatment group; D: The retinal ganglion cell number from each treatment group. Error bars represent standard error of the mean (SEM); n=6 (unpaired t-test, aP<0.05, bP<0.01, and cP<0.0001 compared with control); E: Representative TUNEL staining images of each treatment group as in A. The white arrows point to apoptotic cells. Original magnification is 40×. Scale bar, 50 µm.
DISCUSSION
Good preclinical animal models are crucial for successful drug screening. In this study, we performed three different methods of drug delivery to establish a retinal neurodegeneration model in adult zebrafish. We found that I.V. injection of NMDA was the most effective delivery method as it caused considerable damage to the zebrafish retina in just one day. Additionally, NMDA administrated by I.M. resulted in a thicker retinal NFL, showing that this delivery method may also have potential use for an eye-related disease model. Importantly, we demonstrated that resveratrol and MK-801 both exerted protective effects in our zebrafish models and significantly reduced NMDA-induced retinal neurodegeneration caused by two different administration routes (I.M. and I.V. injection).
Prior zebrafish glaucoma models utilized genetic methods such as gene knockout to affect the zebrafish eye structure and cause glaucomatous symptoms[38]–[39]. Genetic disease models have many advantages for studying the relevant homologous genes and the underling molecular mechanisms of the disease. However, development of genetic glaucoma models in zebrafish is slow and costly, which is not ideal for drug screening due to the large number of fish needed and the slow time necessary to develop the disease pathology. In contrast, our retinal neurodegeneration zebrafish model uses drug treatment of wild-type animals to very quickly induce a glaucoma-like phenotype in a large number of animals, making this system ideal for drug screening.
NMDA, which is considered to play an important role in the process of glaucoma, has not been previously used in zebrafish glaucoma research. Although prior studies have found neuroprotective substances using NMDA treatment in mice and rats[40]–[43], this study is the first to identify neuroprotective compounds using NMDA-induced neurotoxicity in zebrafish. The NMDA model is convenient and has been widely used in many glaucomatous animal studies, providing reproducible outcomes with a simple operation[29].
The I.M. method, which has been used for several eye-related models, such as low-oxygen water leading to hypoxia-induced retinopathy[44], has not been used in a glaucomatous zebrafish model. When NMDA is added to the zebrafish's water, it is rapidly absorbed by the blood vessels in the skin and the gills. The compound then diffuses through the systemic circulation system and reaches the target tissue to bind NMDA receptors and produce a response. In the process of getting into the retina, NMDA should penetrate two barriers [the blood-retinal barrier (BRB) and the blood-aqueous barrier (BAB)][45]–[46]. In a recent study, the small molecule cadmium chloride (Mr=183.32) was utilized to cause retinal damage[47], demonstrating the ability of small molecules to effectively pass through both barriers to cause retinal damage.
Therefore, we hypothesized that the small molecule NMDA (Mr=147.13), a homolog of L-glutamate which is naturally abundant in the brain and retina[48], should also be able to easily pass through the BRB and BAB to reach the retina. In our NMDA I.M. experiment we observed a small number of apoptotic cells in the RNFL as well as inner retinal thickening, demonstrating that a significant amount of NMDA did make it into the retina (Figure 3). We believe this phenomenon was the early stage of retinal neurodegeneration caused by NMDA. Previous studies have shown that inner retinal thickening was due to swelling of the massive retinal cells and their axons caused by NMDA-induced excitatory neurotoxicity[49]–[51]. However, in zebrafish there was no loss of retinal ganglion cells and only a small amount of apoptosis occurred in the NFL (data not shown) compared to the analogous rat model where much more apoptosis occurred[41],[52]. We believe that the apoptotic cells in the NFL are myelin cells because myelin cells exist in the NFL in lower vertebrates to protect and support the retinal ganglion cells[53].
According to other studies of established eye disease models, I.M. required a long time to cause significant symptoms of cellular damage (i.e. apoptotic retinal cells). For example, I.M. of adult zebrafish in high concentrations of glucose or cadmium to induce eye damage took 14 and 29d respectively to see significant levels of retinal apoptosis[47],[54]. Furthermore, prior studies have shown that I.M. often is not as effective as injection to cause high levels of small molecule accumulation[55]. Therefore, we suspect that the treatment time and accumulated concentration of NMDA in our I.M. experiment is likely not sufficient to cause significant damage and induce considerable retinal cell apoptosis.
Importantly, we found that resveratrol was able to reduce inner retinal thickening caused by NMDA treatment, indicating that resveratrol possesses retinal protective properties. These results are consistent with our recent report that resveratrol increases the expression of Sirtuin genes in the retina to regulate mitochondria function and produce an anti-excitotoxicity effect[36],[56]. Additionally, another recent report showed that resveratrol delivered via drinking water could protect rats from the retinal neurodegeneration caused by acute bright light exposure[57]. These data show that resveratrol can be effectively delivered into the eye via the circulatory system, which is in agreement with a prior study showing resveratrol could enter into the systemic circulation and thereby be delivered to the aqueous humor and vitreous humor[58]. It should be noted that the distribution pathway of resveratrol is partly different from that of NMDA, as resveratrol is not delivered through the BRB like NMDA[58].
The I.V. injection method, which delivers drug directly into the vitreous cavity to act on the inner retina, has been widely employed in eye research. In our study, we showed that I.V. injection of NMDA could cause a massive loss of retinal ganglion cells and that both MK-801 and resveratrol provided a protective effect against this treatment, demonstrating this method can be used to establish a physiologically relevant glaucomatous zebrafish model that could be successfully employed for drug discovery. This model was established in reference to similar models in rats by comparing the volume of the vitreous cavity of rats with that of zebrafish[59] to estimate a suitable NMDA concentration for zebrafish. However, the equivalent concentration of NMDA in rats was ineffective for zebrafish, and a relatively larger dose was necessary in our study. This may be due to drug leakage from the zebrafish upon being returned to water, which is not a problem with mammalian models. We also found that 1d was the best time point for our assay, as it was sufficient to see massive retinal ganglion cell loss, while later at day 7 the level of retinal ganglion cell loss became much slower, which may be due to retinal ganglion cell regeneration in zebrafish that begins 3d after retinal damage[60]–[61]. Based on these preliminary studies, I.V. injection of 100 nL 0.5 mol/L NMDA and a treatment time of 1d were chosen as the optimal parameters to establish our retinal neurodegeneration zebrafish model for further drug screening.
Administration of kainic acid by I.P. was used successfully in zebrafish to induce seizure-like behaviors[28],[62]. In contrast, I.P. injection of drugs has not been previously used in glaucomatous models. Since NMDA can be delivered throughout the body including the eye through systemic circulation, we sought to determine if I.P. administration of NMDA could also induce retinal damage. However, although NMDA administration by I.P. was sufficient to induce as much seizure-like behavior as the previous study, there was no significant change in the histology of the retina, the number of retinal ganglion cells, or any induction of apoptosis in the retina of zebrafish treated with NMDA by I.P. This could be due to a lower level of NMDA reaching the retina versus the brain when administered by I.P. Alternatively, the level of NMDA needed to cause retinal damage in zebrafish may be significantly higher than the level necessary to induce seizure-like behaviors.
Comparing the three drug administration routes, all were able to effectively deliver treatments to the brain or eye as deduced by their ability to induce pathological and behavioral alterations. Although I.P. and I.V. injection of NMDA had a much more rapid effect on zebrafish, I.M. treatment had a longer lasting effect. We imagine that I.P. and I.V. administration result in an immediate surge in the NMDA concentration in the blood and target cells to cause the rapid, acute changes observed, but the NMDA concentration would then quickly decrease back to baseline levels and may not be sufficient to cause significant target tissue damage. However, compared with I.V. administration, I.P. injection of NMDA is less target-specific, and cannot cause accumulation of a sufficient concentration in the retina but leads to uncontrollable damage to other organs such as the brain. I.M. administration, on the contrary, could maintain a relatively stably increased concentration of NMDA in the blood sufficient to cause long-term NMDA-induced damage, making it more useful as a model of chronic disease. However, I.M. requires a relatively long time to cause retinal neurodegeneration, which makes it unsuitable for large-scale drug screening. At the operational level, I.M is the easiest and most convenient method of drug administration, whereas I.V. administration is relatively complicated but most effective for establishing eye disease models in zebrafish. I.P. administration does not seem suitable for specific models of eye disease, but it is an easy and effective method for establishing models of brain disease.
It should be noted that a major flaw of this NMDA-induced neurotoxicity model is that it focuses on only a single mechanism (glutamate excitotoxicity) of glaucoma pathobiology. Given the more complex pathogenesis of glaucoma in humans, these models may not fully represent the glaucoma disease process and therefore could fail to detect some treatments that would be effective against glaucoma clinically[61]. Another limitation of this study is that the NMDA I.M. method did not cause significant damage to the zebrafish retina, so this model is not sufficient for neuroprotective drug screening and requires further optimization for it to be a useful model system. Moreover, even though I.V. administration of NMDA causes the same effect on the zebrafish as in other animal models, the microinjection procedure can be difficult to perform, which could hinder its application to drug screening. Therefore, improvements to each administration procedure are likely required to achieve the full potential of this model system.
In summary, the data presented in this study demonstrate that intravitreal NMDA injection is an effective model of retinal neurodegeneration in zebrafish, while NMDA I.M. may serve as a suitable model of chronic eye diseases. By comparing the three different drug administration methods, we discovered the best means to establish effective NMDA-induced models of retinal damage in zebrafish. These models will be valuable for drug screening campaigns and studies to identify the underlying mechanisms of glaucomatous retinal neurodegeneration. Moreover, our study provides further evidence for the potential utility of resveratrol, a common natural product with a well-established safety profile, in the treatment of glaucoma.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81271425; No.81260148; No.31400988; No.81160144; No.31171044); the Jiangxi Provincial Natural Science Foundation (No.20181ACG70010); the Natural Science Foundation of Jiangxi (No.20151BBG70243; No.20122BCB23007).
Conflicts of Interest: Luo ZW, None; Wang HT, None; Wang N, None; Sheng WW, None; Jin M, None; Lu Y, None; Bai YJ, None; Zou SQ, None; Pang YL, None; Xu H, None; Zhang X, None.
REFERENCES
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley HA, Hohman RM, Addicks EM, Massof RW, Green WR. Morphologic changes in the Lamina cribrosa correlated with neural loss in open-angle glaucoma. Am J Ophthalmol. 1983;95(5):673–691. doi: 10.1016/0002-9394(83)90389-6. [DOI] [PubMed] [Google Scholar]
- 3.Danesh-Meyer HV, Boland MV, Savino PJ, Miller NR, Subramanian PS, Girkin CA, Quigley HA. Optic disc morphology in open-angle glaucoma compared with anterior ischemic optic neuropathies. Invest Ophthalmol Vis Sci. 2010;51(4):2003–2010. doi: 10.1167/iovs.09-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerrigan LA, Zack DJ, Quigley HA, Smith SD, Pease ME. TUNEL-positive ganglion cells in human primary open-angle glaucoma. Arch Ophthalmol. 1997;115(8):1031–1035. doi: 10.1001/archopht.1997.01100160201010. [DOI] [PubMed] [Google Scholar]
- 5.Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995;36(5):774–786. [PubMed] [Google Scholar]
- 6.Dreyer EB, Zurakowski D, Schumer RA, Podos SM, Lipton SA. Elevated glutamate levels in the vitreous body of humans and monkeys with glaucoma. Arch Ophthalmol. 1996;114(3):299–305. doi: 10.1001/archopht.1996.01100130295012. [DOI] [PubMed] [Google Scholar]
- 7.Munemasa Y, Kim SH, Ahn JH, Kwong JM, Caprioli J, Piri N. Protective effect of thioredoxins 1 and 2 in retinal ganglion cells after optic nerve transection and oxidative stress. Invest Ophthalmol Vis Sci. 2008;49(8):3535–3543. doi: 10.1167/iovs.08-1716. [DOI] [PubMed] [Google Scholar]
- 8.Vorwerk CK, Kreutz MR, Böckers TM, Brosz M, Dreyer EB, Sabel BA. Susceptibility of retinal ganglion cells to excitotoxicity depends on soma size and retinal eccentricity. Curr Eye Res. 1999;19(1):59–65. doi: 10.1076/ceyr.19.1.59.5336. [DOI] [PubMed] [Google Scholar]
- 9.Kokona D, Charalampopoulos I, Pediaditakis I, Gravanis A, Thermos K. The neurosteroid dehydroepiandrosterone (DHEA) protects the retina from AMPA-induced excitotoxicity: NGF TrkA receptor involvement. Neuropharmacology. 2012;62(5-6):2106–2117. doi: 10.1016/j.neuropharm.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Asami Y, Nakahara T, Asano D, Kurauchi Y, Mori A, Sakamoto K, Ishii K. Age-dependent changes in the severity of capillary degeneration in rat retina following N-methyl-D-aspartate-induced neurotoxicity. Curr Eye Res. 2015;40(5):549–553. doi: 10.3109/02713683.2014.933851. [DOI] [PubMed] [Google Scholar]
- 11.Bibliowicz J, Tittle RK, Gross JM. Toward a better understanding of human eye disease insights from the zebrafish, Danio rerio. Prog Mol Biol Transl Sci. 2011;100:287–330. doi: 10.1016/B978-0-12-384878-9.00007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang XY, Yang LZ, Luo Y. Animal models of diabetic retinopathy. Curr Eye Res. 2015;40(8):761–771. doi: 10.3109/02713683.2014.964415. [DOI] [PubMed] [Google Scholar]
- 13.Barbazuk WB, Korf I, Kadavi C, Heyen J, Tate S, Wun E, Bedell JA, McPherson JD, Johnson SL. The synthetic relationship of the zebrafish and human genomes. Genome Research. 2000;10(9):1351–1358. doi: 10.1101/gr.144700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakraborty C, Hsu CH, Wen ZH, Lin CS, Agoramoorthy G. Zebrafish: a complete animal model for in vivo drug discovery and development. Curr Drug Metab. 2009;10(2):116–124. doi: 10.2174/138920009787522197. [DOI] [PubMed] [Google Scholar]
- 15.Kari G, Rodeck U, Dicker AP. Zebrafish: an emerging model system for human disease and drug discovery. Clin Pharmacol Ther. 2007;82(1):70–80. doi: 10.1038/sj.clpt.6100223. [DOI] [PubMed] [Google Scholar]
- 16.Kitambi SS, Chandrasekar G, Addanki VK. Teleost fish-powerful models for studying development, function and diseases of the human eye. Current Science. 2011;100(12):1815–1823. [Google Scholar]
- 17.Renninger SL, Schonthaler HB, Neuhauss SC, Dahm R. Investigating the genetics of visual processing, function and behaviour in zebrafish. Neurogenetics. 2011;12(2):97–116. doi: 10.1007/s10048-011-0273-x. [DOI] [PubMed] [Google Scholar]
- 18.Bouhenni RA, Dunmire J, Sewell A, Edward DP. Animal models of glaucoma. J Biomed Biotechnol. 2012;2012:692609. doi: 10.1155/2012/692609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen HS, Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem. 2006;97(6):1611–1626. doi: 10.1111/j.1471-4159.2006.03991.x. [DOI] [PubMed] [Google Scholar]
- 20.Kemp JA, McKernan RM. NMDA receptor pathways as drug targets. Nat Neurosci. 2002;5(Suppl):1039–1042. doi: 10.1038/nn936. [DOI] [PubMed] [Google Scholar]
- 21.Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat Rev Drug Discov. 2006;5(2):160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- 22.Koch HJ, Uyanik G, Fischer-Barnicol D. Memantine: a therapeutic approach in treating Alzheimer's and vascular dementia. Curr Drug Targets CNS Neurol Disord. 2005;4(5):499–506. doi: 10.2174/156800705774322021. [DOI] [PubMed] [Google Scholar]
- 23.Namekata K, Kimura A, Kawamura K, Guo X, Harada C, Tanaka K, Harada T. Dock3 attenuates neural cell death due to NMDA neurotoxicity and oxidative stress in a mouse model of normal tension glaucoma. Cell Death Differ. 2013;20(9):1250–1256. doi: 10.1038/cdd.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ElGohary AA, Elshazly LH. Photopic negative response in diagnosis of glaucoma: an experimental study in glaucomatous rabbit model. Int J Ophthalmol. 2015;8(3):459–464. doi: 10.3980/j.issn.2222-3959.2015.03.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soriano FX, Papadia S, Hofmann F, Hardingham NR, Bading H, Hardingham GE. Preconditioning doses of NMDA promote neuroprotection by enhancing neuronal excitability. J Neurosci. 2006;26(17):4509–4518. doi: 10.1523/JNEUROSCI.0455-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothman SM, Olney JW. Excitotoxity and the NMDA receptor. Trends Neurosci. 1987;10(7):299–302. [Google Scholar]
- 27.Schirmer H, Pereira TC, Rico EP, Rosemberg DB, Bonan CD, Bogo MR, Souto AA. Modulatory effect of resveratrol on SIRT1, SIRT3, SIRT4, PGC1α and NAMPT gene expression profiles in wild-type adult zebrafish liver. Mol Biol Rep. 2012;39(3):3281–3289. doi: 10.1007/s11033-011-1096-4. [DOI] [PubMed] [Google Scholar]
- 28.Alfaro JM, Ripoll-Gómez J, Burgos JS. Kainate administered to adult zebrafish causes seizures similar to those in rodent models. Eur J Neurosci. 2011;33(7):1252–1255. doi: 10.1111/j.1460-9568.2011.07622.x. [DOI] [PubMed] [Google Scholar]
- 29.Fimbel SM, Montgomery JE, Burket CT, Hyde DR. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J Neurosci. 2007;27(7):1712–1724. doi: 10.1523/JNEUROSCI.5317-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao XN, Wang WJ, Chen J, Zhou Q, Qu YX, Liu XY, Xu W. Effects of resveratrol on ARPE-19 cell proliferation and migration via regulating the expression of proliferating cell nuclear antigen, P21, P27 and p38MAPK/MMP-9. Int J Ophthalmol. 2016;9(12):1725–1731. doi: 10.18240/ijo.2016.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yar AS, Menevse S, Alp E, Helvacioglu F, Take G. The effects of resveratrol on cyclooxygenase-1 and cyclooxygenase-2 mRNA and protein levels in diabetic rat kidneys. Mol Biol Rep. 2010;37(5):2323–2331. doi: 10.1007/s11033-009-9737-6. [DOI] [PubMed] [Google Scholar]
- 32.Marques FZ, Markus MA, Morris BJ. Resveratrol: cellular actions of a potent natural chemical that confers a diversity of health benefits. Int J Biochem Cell Biol. 2009;41(11):2125–2128. doi: 10.1016/j.biocel.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Luo HD, Zhou M, Ji KB, Zhuang JJ, Dang WJ, Fu SY, Sun T, Zhang X. Expression of sirtuins in the retinal neurons of mice, rats, and humans. Front Aging Neurosci. 2017;9:366. doi: 10.3389/fnagi.2017.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ethemoglu MS, Seker FB, Akkaya H, Kilic E, Aslan I, Erdogan CS, Yilmaz B. Anticonvulsant activity of resveratrol-loaded liposomes in vivo. Neuroscience. 2017;357:12–19. doi: 10.1016/j.neuroscience.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 35.Szkudelska K, Szkudelski T. Resveratrol, obesity and diabetes. Eur J Pharmacol. 2010;635(1-3):1–8. doi: 10.1016/j.ejphar.2010.02.054. [DOI] [PubMed] [Google Scholar]
- 36.Sheng WW, Lu Y, Mei F, Wang N, Liu ZZ, Han YY, Wang HT, Zou SQ, Xu H, Zhang X. Effect of resveratrol on sirtuins, OPA1, and fis1 expression in adult zebrafish retina. Invest Ophthalmol Vis Sci. 2018;59(11):4542–4551. doi: 10.1167/iovs.18-24539. [DOI] [PubMed] [Google Scholar]
- 37.Reymond L. Spatial visual acuity of the falcon, Falco berigora: a behavioural, optical and anatomical investigation. Vision Res. 1987;27(10):1859–1874. doi: 10.1016/0042-6989(87)90114-3. [DOI] [PubMed] [Google Scholar]
- 38.Berry FB, Skarie JM, Mirzayans F, Fortin Y, Hudson TJ, Raymond V, Link BA, Walter MA. FOXC1 is required for cell viability and resistance to oxidative stress in the eye through the transcriptional regulation of FOXO1A. Hum Mol Genet. 2008;17(4):490–505. doi: 10.1093/hmg/ddm326. [DOI] [PubMed] [Google Scholar]
- 39.Stujenske JM, Dowling JE, Emran F. The bugeye mutant zebrafish exhibits visual deficits that arise with the onset of an enlarged eye phenotype. Invest Ophthalmol Vis Sci. 2011;52(7):4200–4207. doi: 10.1167/iovs.10-6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yum MS, Lee M, Woo DC, Kim DW, Ko TS, Velíšek L. Β-Hydroxybutyrate attenuates NMDA-induced spasms in rats with evidence of neuronal stabilization on MR spectroscopy. Epilepsy Res. 2015;117:125–132. doi: 10.1016/j.eplepsyres.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Lambuk L, Jafri AJ, Arfuzir NN, Iezhitsa I, Agarwal R, Rozali KN, Agarwal P, Bakar NS, Kutty MK, Yusof AP, Krasilnikova A, Spasov A, Ozerov A, Ismail NM. Neuroprotective effect of magnesium acetyltaurate against NMDA-induced excitotoxicity in rat retina. Neurotox Res. 2017;31(1):31–45. doi: 10.1007/s12640-016-9658-9. [DOI] [PubMed] [Google Scholar]
- 42.Maekawa S, Sato K, Fujita K, Daigaku R, Tawarayama H, Murayama N, Moritoh S, Yabana T, Shiga Y, Omodaka K, Maruyama K, Nishiguchi KM, Nakazawa T. The neuroprotective effect of hesperidin in NMDA-induced retinal injury acts by suppressing oxidative stress and excessive calpain activation. Sci Rep. 2017;7(1):6885. doi: 10.1038/s41598-017-06969-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aksar AT, Yuksel N, Gok M, Cekmen M, Caglar Y. Neuroprotective effect of edaravone in experimental glaucoma model in rats: a immunofluorescence and biochemical analysis. Int J Ophthalmol. 2015;8(2):239–244. doi: 10.3980/j.issn.2222-3959.2015.02.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jensen LD, Rouhi P, Cao ZQ, Länne T, Wahlberg E, Cao YH. Zebrafish models to study hypoxia-induced pathological angiogenesis in malignant and nonmalignant diseases. Birth Defects Res C Embryo Today. 2011;93(2):182–193. doi: 10.1002/bdrc.20203. [DOI] [PubMed] [Google Scholar]
- 45.Cunha-Vaz J, Bernardes R, Lobo C. Blood-retinal barrier. Eur J Ophthalmol. 2011;21(Suppl 6):S3–S9. doi: 10.5301/EJO.2010.6049. [DOI] [PubMed] [Google Scholar]
- 46.Toda R, Kawazu K, Oyabu M, Miyazaki T, Kiuchi Y. Comparison of drug permeabilities across the blood-retinal barrier, blood-aqueous humor barrier, and blood-brain barrier. J Pharm Sci. 2011;100(9):3904–3911. doi: 10.1002/jps.22610. [DOI] [PubMed] [Google Scholar]
- 47.Avallone B, Crispino R, Cerciello R, Simoniello P, Panzuto R, Maria Motta C. Cadmium effects on the retina of adult Danio rerio. C R Biol. 2015;338(1):40–47. doi: 10.1016/j.crvi.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 48.Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130(4S Suppl):1007S–1015S. doi: 10.1093/jn/130.4.1007S. [DOI] [PubMed] [Google Scholar]
- 49.Chen JJ, Chiang CW, Zhang HY, Song SK. Cell swelling contributes to thickening of low-dose N-methyl-D-aspartate-induced retinal edema. Invest Ophthalmol Vis Sci. 2012;53(6):2777–2785. doi: 10.1167/iovs.11-8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishikawa M, Yoshitomi T, Zorumski CF, Izumi Y. Effects of acutely elevated hydrostatic pressure in a rat ex vivo retinal preparation. Invest Ophthalmol Vis Sci. 2010;51(12):6414–6423. doi: 10.1167/iovs.09-5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parisi V, Oddone F, Ziccardi L, Roberti G, Coppola G, Manni G. Citicoline and retinal ganglion cells: effects on morphology and function. Curr Neuropharmacol. 2018;16(7):919–932. doi: 10.2174/1570159X15666170703111729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qiu XD, Johnson JR, Wilson BS, Gammon ST, Piwnica-Worms D, Barnett EM. Single-cell resolution imaging of retinal ganglion cell apoptosis in vivo using a cell-penetrating caspase-activatable peptide probe. PLoS One. 2014;9(2):e88855. doi: 10.1371/journal.pone.0088855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vihtelic TS, Fadool JM, Gao J, Thornton KA, Hyde DR, Wistow G. Expressed sequence tag analysis of zebrafish eye tissues for NEIBank. Mol Vis. 2005;11:1083–1100. [PubMed] [Google Scholar]
- 54.Capiotti KM, Antonioli R, Jr, Kist LW, Bogo MR, Bonan CD, Da Silva RS. Persistent impaired glucose metabolism in a zebrafish hyperglycemia model. Comp Biochem Physiol B Biochem Mol Biol. 2014;171:58–65. doi: 10.1016/j.cbpb.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 55.Li YB, Han ZH, Zheng XM, Ma ZY, Liu HL, Giesy JP, Xie YW, Yu HX. Comparison of waterborne and in ovo nanoinjection exposures to assess effects of PFOS on zebrafish embryos. Environ Sci Pollut Res Int. 2015;22(3):2303–2310. doi: 10.1007/s11356-014-3527-y. [DOI] [PubMed] [Google Scholar]
- 56.Wang N, Luo ZW, Jin M, Sheng WW, Wang HT, Long XY, Wu Y, Hu PP, Xu H, Zhang X. Exploration of age-related mitochondrial dysfunction and the anti-aging effects of resveratrol in zebrafish retina. Aging (Albany NY) 2019;11(10):3117–3137. doi: 10.18632/aging.101966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu ZR, Wu ZZ, Li J, Marmalidou A, Zhang RF, Yu M. Protective effect of resveratrol against light-induced retinal degeneration in aged SAMP8 mice. Oncotarget. 2017;8(39):65778–65788. doi: 10.18632/oncotarget.19473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang SS, Wang Z, Yang S, Yin TM, Zhang YL, Qin YJ, Weinreb RN, Sun XF. Tissue distribution of trans-resveratrol and its metabolites after oral administration in human eyes. J Ophthalmol. 2017;2017:4052094. doi: 10.1155/2017/4052094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luo XP, Yu YK, Xiang ZQ, Wu HS, Ramakrishna S, Wang YQ, So KF, Zhang ZJ, Xu Y. Tetramethylpyrazine nitrone protects retinal ganglion cells against N-methyl-d-aspartate-induced excitotoxicity. J Neurochem. 2017;141(3):373–386. doi: 10.1111/jnc.13970. [DOI] [PubMed] [Google Scholar]
- 60.Raymond PA, Reifler MJ, Rivlin PK. Regeneration of goldfish retina: rod precursors are a likely source of regenerated cells. J Neurobiol. 1988;19(5):431–463. doi: 10.1002/neu.480190504. [DOI] [PubMed] [Google Scholar]
- 61.Huang WJ, Hu FY, Wang M, Gao FJ, Xu P, Xing C, Sun XH, Zhang SH, Wu JH. Comparative analysis of retinal ganglion cell damage in three glaucomatous rat models. Exp Eye Res. 2018;172:112–122. doi: 10.1016/j.exer.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 62.Zhang X, Cheng M, Chintala SK. Kainic acid-mediated upregulation of matrix metalloproteinase-9 promotes retinal degeneration. Invest Ophthalmol Vis Sci. 2004;45(7):2374–2383. doi: 10.1167/iovs.03-1239. [DOI] [PubMed] [Google Scholar]