Abstract
AIM
To investigate the dynamic changes of activator protein 1 (AP1) and collagen I expression in the sclera of form-deprivation myopic model in guinea pigs.
METHODS
A form-deprivation myopic model in guinea pigs were established with the left eye covered for 2 to 6wk (FDM group). Normal control group (n=25) were untreated. Changes in refractive power and axial length (AL) were measured and recorded at different time points. Expressions of AP1 and collagen 1 of the sclera were measured with Western blotting and reverse transcription-polymerase chain reaction (RT-PCR). The relationship between AP1 and collagen I levels was analyzed.
RESULTS
After 0, 2, 4, 6wk, and 4/-1wk of form-deprivation, the diopter in the FDM group was gradually changed (2.08±0.31, -1.23±0.68, -4.17±0.58, -7.07±0.55, and -2.67±0.59 D, respectively, P<0.05), and the AL was gradually increased (5.90±0.38, 6.62±0.37, 7.30±0.35, 7.99±0.31, and 6.97±0.32 mm, respectively, P<0.05). With the prolongation of covered time, the protein expressions of AP1 and collagen I in the FDM group were gradually down-regulated (all P<0.05); the mRNA expressions of them were also gradually down-regulated (all P<0.05); and there was positive correlation between them. The control group had no obvious change in each index (all P>0.05).
CONCLUSION
AP1 may be an important transcription factor involved in the regulation of collagen I synthesis and degradation during myopic scleral remodeling.
Keywords: sclera remodeling, activator protein 1, collagen I, form deprivation myopia, guinea pig
INTRODUCTION
Myopia is the most common type of refractive error and has a high prevalence in the world. The morbidity rate of myopia in young people in East Asia has reached 80%-90%. In western countries, myopia is the most common disease that needs to be managed, but the morbidity rate is up to 15% to 49%, due to the lack of real treatment. About 2% of the myope has high myopia globally, which results in irreversible visual loss such as retinal detachment, choroidal neovascularization, cataract, glaucoma, myopic maculopathy and so on[1]–[2]. In China, the prevalence of myopia in children and adolescents increases with age, which brings heavy burden to the society and the famillies[3]. Although there are some methods to correct and prevent myopia, they fail to fundamentally reduce its early occurrence and high morbidity, therefore, there are a lot of efforts to explore its etiology and pathogenesis.
Myopia is commonly deemed as a result of multifactor interactions involving genes and the environment[4]. Since Wiesel and Raviola[5] sutured the eyelids of rhesus and established myopia animal models in 1977, researchers have better understood the pathogenesis of myopia. The studies of experimental myopia animal model found that the sclera structure changed during the process of myopia development, the ocular axis was prolonged excessively, the posterior sclera thinned and the scleral extracellular matrix (ECM) metabolized abnormally[6]–[8]. ECM is mainly composed of collagen fibers (especially collagen I, III, and IV), which account for 90% of the total sclera actual weight. Among them, collagen I is the major component and its synthesis and degradation are important features of scleral remodeling[9]–[10]. Previous studies have shown that transforming growth factor-β1(TGF-β1) plays a significant role in sclera remodeling and regulates collagen I synthesis and degradation by virtue of downstream transcription factors[11]–[12]. However, the specific regulatory mechanism is uncertain. Collagen I is also closely related with the expression of activator protein 1 (AP1), a transcription factor that can regulate the synthesis and degradation of collagen I and a downstream transcription factor in various signaling pathways[13]–[14]. However, AP1 whether can express in myopic sclera remodeling and its association with collagen I has not been investigated in ophthalmology. In this study, we adopted the mask method to establish a form-deprivation animal model. The dynamic changes in the expressions of AP1 and collagen I were detected via Western blotting and reverse transcription-polymerase chain reaction (RT-PCR). We explored the role of AP1 and its relationship with collagen I expression in myopia sclera remodeling, which suggests a new mechanism underlying the disease pathogenesis.
MATERIALS AND METHODS
Ethical Approval
All animal experiments and procedures were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision research. The study was examined and approved by the Laboratory Animal Ethical Committee of Anhui Medical University.
Establishing the Form Deprivation Myopia Model and Designing Experiment
As previous description[15], seventy-five healthy guinea pigs (one-week old; 110-140 g; without myopia or systemic diseases) were selected. Then they were randomly divided into two groups: form deprivation myopia (FDM) group (n=50) and normal control group (n=25). The left eyes in the FDM group were covered for 0, 2, 4, 6wk and 4/-1wk respectively; the right eyes of them served as self-control group; eyes of guinea pigs in the normal control group remained untreated.
Diopter and Axial Length
After the eye pupils had been fully enlarged by tropicamide, a streak retinoscope (66 VISION TECH Co., Ltd., China) was adopted for cycloplegic refraction in a dark room (accurate to 0.01 D), and axial length (AL) was measured with A-scan ultrasonography (TOMEYAL-100, Japan) after the eyes were anesthetized by 0.5% proparacaine hydrochloride (accurate to 0.01 mm). The results of diopter and AL were recorded by the same person (Dr. Jian Bao) at different time pionts in the experiment (0, 2, 4, 6wk, and 4/-1wk)[15].
Preparation of Scleral Tissue
The guinea pigs were sacrificed by excess 1% pentobarbital sodium, the eyeballs were removed on the ice. anterior segments of the eyes were discarded, the posterior sclera were excised by a 6-mm-diameter trephine around the head of the optic nerves, then the optic nerves were abandoned and the sclera tissues were frozen by grinding with liquid nitrogen for reserve.
Western Blotting
The frozen scleral tissues were mixed with 100 µL of lysate with PMSF according to the proportion of scleral tissue (20 mg). After pyrolysis, it was centrifuged at 12 000× g for 5min at 4°C. The supernatant after centrifugation was fully mixed with the prepared BCA working solution. the absorbance value was recorded and the standard curve was drawn to determine the protein concentration with the reference of blank control at 562 nm wavelength, then protein samples (50 µg) were separated by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransfer onto polyvinyl difluoride (PVDF) membranes with 300 mA constant current for 2h. The PVDF membranes were combined with the diluted first antibody and incubated overnight at 4°C, and then combined with the diluted second antibody at room temperature for 2h. The prepared chemiluminescent reagent was dripped onto the PVDF membranes for film-pressing exposure. After developed and photographic fixing, the film was scanned and analyzed by the gel imaging system. β-actin served as an internal standard. The gray values of each band were calculated and the recorded data were kept for statistical analysis.
Reverse Transcription-Polymerase Chain Reaction
Extracted total RNA from the scleral tissue prepared above in accordance with the manufacturer's instructions. Total RNA (5 µg), 10 mol/L Oligo (dT) 1 µL and DEPC water 11 µL were added to the RNase-free PCR tube. The samples were gently mixed and centrifuged, then they were heat for 5min at 70°C, the tubes were immediately cold on the ice for 3min. Reverse transcription was performed in a tube containing 10 mmol/L dNTP mix 2 mL, 25 mmol/L MgCl2 2 µL, 0.1 mol/L DTT 2µL, 10×PCR buffer 2 µL at 42°C for 50min and 70°C for 5min. The reaction solution namely the cDNA was taken out and stored at -80°C. The procedure of PCR amplification was as follows: set the thermal cycle parameters of the PCR at 95°C for 5min; then 35 cycles were performed at 95°C for 30s, 55°C for 30s, and 72°C for 40s; annealed at 72°C for 10min and 4°C for 10min respectively. β-actin was used as internal reference. The agarose gel plate was placed in the electrophoresis tank, added the PCR reaction solution 5 µL and 6× loading buffer at 120 V electrophoresis for 25min, and the results were analyzed by using a gel imaging system. Primer sequences used are shown in Table 1.
Table 1. Primer sequences used in RT-PCR.
| Gene name | Forward primer (5′-3′) | Reverse primer (5′-3′) | Length (bp) |
| β-actin | GCTCTATCCTGGCCTCACTC | GGGTGAGGGACTTCCTGTAA | 400 |
| AP1 | AACTCATGCTAACGCAGCAG | GTCAATGCTGAACAGTCCGT | 311 |
| Collagen I | ACAAGCGATTACACACCCAA | TTAGTTTCCTGCCTCTGCCT | 239 |
Statistical Analysis
SPSS statistics 19.0 statistical software was used for statistical analysis. All data were expressed as mean±standard deviation (SD). Paired t-test was used for comparison between eyes, and one-way ANOVA was used for comparison between groups. The results were considered statistically significant at P<0.05. Pearson linear correlation analysis (bilateral) was used to evaluate the relationship between AP1 and collagen I expression.
RESULTS
Diopter and Axial Length
Before covering, the difference between the groups in the diopter and AL was not statistically significant (P>0.05). With the extension of covered time, diopter of guinea pigs in the FDM group gradually changed from hyperopia to myopia, the myopic degree gradually deepened (P<0.05), and their AL also gradually increased (P<0.05). While diopter of the normal control group and the self-control group slightly decreased and their AL slightly increased. In comparison with the self-control group and the normal control group, the difference in diopter and AL in the FDM group was statistically significant at 2, 4, 6wk, and 1wk after 4wk treatment (P<0.05), the results were shown in Table 2.
Table 2. Diopter and axial length between the eyes of the three groups.
| Time (wk) | Diopter (D) |
Axial length (mm) |
||||
| Normal control | Self-control | FDM | Normal control | Self-control | FDM | |
| 0 | 2.18±0.26 | 2.10±0.32 | 2.08±0.31 | 5.86±0.34 | 5.85±0.36 | 5.90±0.38 |
| 2 | 1.38±0.25a | 1.28±0.28a | -1.23±0.68 | 6.22±0.31a | 6.24±0.35a | 6.62±0.37 |
| 4 | 0.90±0.41a | 0.87±0.28a | -4.17±0.58 | 6.36±0.32a | 6.42±0.33a | 7.30±0.35 |
| 4/-1 | 0.62±0.26a | 0.48±0.26a | -2.67±0.59 | 6.50±0.32a | 6.58±0.32a | 6.97±0.32 |
| 6 | -0.14±0.24a | -0.33±0.25a | -7.07±0.55 | 6.80±0.29a | 6.97±0.34a | 7.99±0.31 |
FDM: Form deprivation myopia. aP<0.05 compared with FDM group.
mean±SD
Expression of Activator Protein 1 and Collagen I of the Sclera by Western Blotting
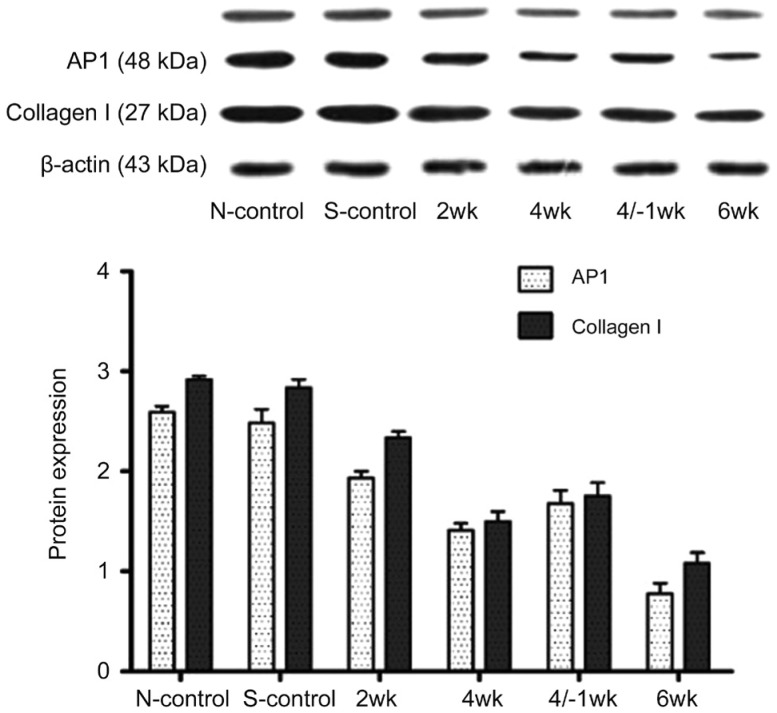
AP1 (c-Jun and c-Fos) and collagen I expression was detected in all groups (Figure 1). With the prolongation of covered time, the protein expressions of AP1 and collagen I in the FDM group were gradually down-regulated (all P<0.05). At 1wk after 4wk treatment, the protein expression of AP1 and collagen I were higher than those at 4 and 6wk, but lower compared with that at 2wk. The difference of AP1 and collagen I between the self-control group and the normal control group was not statistically significant (P>0.05). In the FDM group, the difference between 4/-1wk and 4wk treatment was no statistical significance, while the differences were statistically significant among the other groups (P<0.05; Figure 1).
Figure 1. Expression of activator protein 1 (AP1) and collagen I of the sclera by Western blotting.
Expression of Activator Protein 1 and Collagen I mRNA of the Sclera by RT-PCR
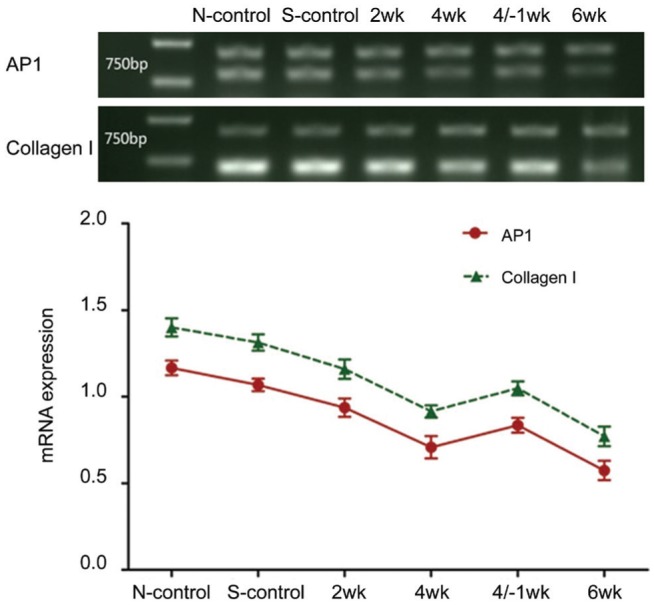
With the extension of the form-deprivation time, the mRNA expression of AP1 and collagen I gradually decreased. However, at 1wk after 4wk treatment, the mRNA expressive quantity of AP1 and collagen I was higher than those at 4wk and 6wk, but lower compared with that at 2wk. The difference in the expression of AP1 and collagen I mRNA between the self-control group and the normal control group was not statistically significant (P>0.05). In the FDM group, the difference between 4/-1wk and 4wk treatment was no statistical significance, while the differences were statistically significant among the other groups (P<0.05; Figure 2).
Figure 2. Expression of activator protein 1 (AP1) and collagen I mRNA of the sclera by RT-PCR.
Correlation Analysis Between AP1 and Collagen I Expression
In the sclera tissue, protein expression of collagen I and AP1 were highly correlated (r=0.980, P<0.05), so were the mRNA expression of collagen I and AP1 (r=0.965, P<0.05).
DISCUSSION
With the myopia development, the main morphological change is the excessive elongation of the ocular AL[16]. Our results showed that the diopter was gradually deepened and the AL of guinea pigs eyes was gradually increased in the FDM group with the prolongation of covering time.
Owing to the advances in molecular biology and the development of FDM animal models, many biological factors were found involved in myopic scleral remodeling. The TGF-β1 is a hotspot in current research, and the polypeptide encoded by TGF-β1 gene plays an important role in regulating cell proliferation, differentiation and apoptosis; the TGF-β1 gene contributes to the genetic predisposition to high myopia and TGF-β1 expression in sclera tissues can promote sclera fibroblasts to secrete collagen fibers[17]–[18]. Previous studies have indicated that TGF-β1 serves as an important transcription factor in sclera remodeling and is positively correlated with collagen I, the expression of which gradually declines with the development of myopic sclera remodeling[15],[19]. Our study shows the expressions of collagen I protein and mRNA gradually decline with the FDM induction. These results indicate that as the eye covering time prolongs and myopia deepens, the collagen I expression gradually declines, suggesting its association with myopia development, which is consistent with the study of Gentle et al[9].
The nuclear transcription factor, AP1 is mainly composed of the fos family (c-fos, FosB, Fra-1, Fra-2) and the Jun family (c-Jun, JunB and JunD) and plays an important role in regulating cell differentiation, proliferation apoptosis and tumor formation[20]. The AP1 signaling transduction pathway is involved in tumor neovascularization, abnormal vascular endothelial growth factor and Basic fibroblastic growth factor expression and malignant tumor metastasis[21]–[23]. AP1 and TGF-β1 are significantly correlated in many fields. JNK (C-Jun N terminal enzyme) is a key phosphatase of AP1, and the JNK signaling pathway is an important pathway downstream of TGF-β1, which can make Smad3 phosphorylation, further activate the TGF receptor on Smad3 and its nuclear translocation, and positively regulate the activation of Smad3[24]. Guo et al[25] discovered that AP1 mediates the TGF-β1-induced expression of plasminogen activator inhibitor 1 in rat mesangial cells. When evaluating the impact of all-trans retinoic acid on hepatic stellate cell, Ye and Dan[26] found the reduction of retinoic acid and the introduction of exogenous trans-retinoic acid increase the expression of AP1 and TGF-β1.
Other researches further illustrate AP1 is the downstream transcription factor of TGF-β1 and is related to the synthesis and degradation of collagen I. Tang et al[27] showed that angiotensin II can activate the AP1-mediated autocrine pathway of TGF-β1 through the AT1 receptor, which up-regulates the collagen I expression and the production of other ECM proteins in skin fibroblasts. This process may be involved in the repair of skin trauma. Hu et al[28] found TGF-β1 can induce the transcription and secretion of collagen I in lung fibroblasts. TGF-β1 can upregulate the DNA-binding activity of AP1, and curcumin (an AP1 inhibitor) can also inhibit the secretion of collagen I, suggesting AP1 may be related to the synthesis of collagen I induced by TGF-β1 in human lung fibroblasts. Li et al[29] found that TGF-β1 could promote the activation of AP1 and other transcription factors in rat lung fibroblasts and upregulate the expression of collagen I. However, the expression and role of AP1 in sclera remodeling have not been reported. Our results showed that both the AP1 protein and mRNA expressions decreased with the prolonging of the covering time. It is noteworthy that at 1wk after the 4wk treatment, the AP1 and collagen I expressions were upregulated compared with those at 4 and 6wk, but lower compared with those at the 2wk. We considered that the induced myopia can recover and reverse the scleral remodeling after the removal of the induction factor. In addition, the protein and mRNA expressions of AP1 were highly correlated with those of collagen I, suggesting AP1 and collagen I expressions were downregulated correspondingly with the myopic development, and they are positively related. In conclusion, AP1 is expressed in the sclera tissues of guinea pigs. With the prolongation of covering, the AP1 and collagen I expressions were both down-regulated, indicating AP1 and collagen I might be related to scleral remodeling in myopia.However, their specific relationship and mechanism need to be further verified and studied. Nevertheless, we provide a new target for myopia prevention and treatment.
Acknowledgments
The authors thank Dr. Jian Bao for assistance in the measurement of refractive power and axial length.
Foundations: Supported by the Natural Science Foundation of Anhui Province (No.1508085MH188); Education and Research Project of Anhui Education Department (No.2016jyxm0546).
Conflicts of Interest: Zhan X, None; Zhu ZC, None; Sun SQ, None; Wen YC, None.
REFERENCES
- 1.Pan CW, Ramamurthy D, Saw SM. Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol Opt. 2012;32(1):3–16. doi: 10.1111/j.1475-1313.2011.00884.x. [DOI] [PubMed] [Google Scholar]
- 2.Wu PC, Huang HM, Yu HJ, Fang PC, Chen CT. Epidemiology of myopia. Asia Pac J Ophthalmol (Phila) 2016;5(6):386–393. doi: 10.1097/APO.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 3.Dong YH, Liu HB, Wang ZH, Xu RB, Yang ZP, Ma J. The epidemic status and secular trends of myopia prevalence for Chinese children and adolescents aged 7-18 years from 2005 to 2014. Zhonghua Yu Fang Yi Xue Za Zhi. 2017;51(4):285–289. doi: 10.3760/cma.j.issn.0253-9624.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Stambolian D. Genetic susceptibility and mechanisms for refractive error. Clin Genet. 2013;84(2):102–108. doi: 10.1111/cge.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiesel TN, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977;266(5597):66–68. doi: 10.1038/266066a0. [DOI] [PubMed] [Google Scholar]
- 6.Heberlein U, Moses K. Mechanisms of Drosophila retinal morphogenesis: the virtues of being progressive. Cell. 1995;81(7):987–990. doi: 10.1016/s0092-8674(05)80003-0. [DOI] [PubMed] [Google Scholar]
- 7.Dunmire JJ, Lagouros E, Bouhenni RA, Jones M, Edward DP. MicroRNA in aqueous humor from patients with cataract. Exp Eye Res. 2013;108:68–71. doi: 10.1016/j.exer.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 8.de Iongh RU, Wederell E, Lovicu FJ, McAvoy JW. Transforming growth factor-β-induced epithelial-mesenchymal transition in the lens: a model for cataract formation. Cells Tissues Organs. 2005;179(1-2):43–55. doi: 10.1159/000084508. [DOI] [PubMed] [Google Scholar]
- 9.Gentle A, Liu YY, Martin JE, Conti GL, McBrien NA. Collagen gene expression and the altered accumulation of scleral collagen during the development of high myopia. J Biol Chem. 2003;278(19):16587–16594. doi: 10.1074/jbc.M300970200. [DOI] [PubMed] [Google Scholar]
- 10.Harper AR, Summers JA. The dynamic sclera: extracellular matrix remodeling in normal ocular growth and myopia development. Exp Eye Res. 2015;133:100–111. doi: 10.1016/j.exer.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M, Yuan Y, Chen Q, Me R, Gu Q, Yu Y, Sheng M, Ke B. Expression of wnt/β-catenin signaling pathway and its regulatory role in type I collagen with TGF-β1 in scleral fibroblasts from an experimentally induced myopia guinea pig model. J Ophthalmol. 2016;2016:5126560. doi: 10.1155/2016/5126560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McBrien NA. Regulation of scleral metabolism in myopia and the role of transforming growth factor-beta. Exp Eye Res. 2013;114:128–140. doi: 10.1016/j.exer.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Lin CH, Yu MC, Tung WH, Chen TT, Yu CC, Weng CM, Tsai YJ, Bai KJ, Hong CY, Chien MH, Chen BC. Connective tissue growth factor induces collagen I expression in human lung fibroblasts through the Rac1/MLK3/JNK/AP-1 pathway. Biochim Biophys Acta. 2013;1833(12):2823–2833. doi: 10.1016/j.bbamcr.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 14.Kook SH, Hwang JM, Park JS, Kim EM, Heo JS, Jeon YM, Lee JC. Mechanical force induces type I collagen expression in human periodontal ligament fibroblasts through activation of ERK/JNK and AP-1. J Cell Biochem. 2009;106(6):1060–1067. doi: 10.1002/jcb.22085. [DOI] [PubMed] [Google Scholar]
- 15.Jiang B, Wu ZY, Zhu ZC, Ke GJ, Wen YC, Sun SQ. Expression and role of specificity protein 1 in the sclera remodeling of experimental myopia in guinea pigs. Int J Ophthalmol. 2017;10(4):550–554. doi: 10.18240/ijo.2017.04.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Xu G, Fan J, Zhang M. Mechanical stretching induces matrix metalloproteinase-2 expression in rat retinal glial (Müller) cells. Neuroreport. 2013;24(5):224–228. doi: 10.1097/WNR.0b013e32835eb9d1. [DOI] [PubMed] [Google Scholar]
- 17.Patel A, Scott WR, Lympany PA, Rippin JD, Gill GV, Barnett AH, Bain SC, Warren 3/UK GoKind Study Group The TGF-beta 1 gene codon 10 polymorphism contributes to the genetic predisposition to nephropathy in Type 1 diabetes. Diabet Med. 2005;22(1):69–73. doi: 10.1111/j.1464-5491.2005.01376.x. [DOI] [PubMed] [Google Scholar]
- 18.Lin HJ, Wan L, Tsai Y, Tsai YY, Fan SS, Tsai CH, Tsai FJ. The TGFbeta1 gene codon 10 polymorphism contributes to the genetic predisposition to high myopia. Mol Vis. 2006;12:698–703. [PubMed] [Google Scholar]
- 19.Wang Q, Xue ML, Zhao GQ, Liu MG, Ma YN, Ma Y. Form-deprivation myopia induces decreased expression of bone morphogenetic protein-2, 5 in guinea pig sclera. Int J Ophthalmol. 2015;8(1):39–45. doi: 10.3980/j.issn.2222-3959.2015.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han R, Wang R, Zhao Q, Han Y, Zong S, Miao S, Song W, Wang L. Trim69 regulates zebrafish brain development by ap-1 pathway. Sci Rep. 2016;6:24034. doi: 10.1038/srep24034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez-Bergami P, Lau E, Ronai Z. Emerging roles of ATF2 and the dynamic AP1 network in cancer. Nat Rev Cancer. 2010;10(1):65–76. doi: 10.1038/nrc2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaulian E. AP-1--the Jun proteins: Oncogenes or tumor suppressors in disguise? Cell Signal. 2010;22(6):894–899. doi: 10.1016/j.cellsig.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Gao J, Yan Q, Wang J, Liu S, Yang X. Epithelial-to-mesenchymal transition induced by TGF-β1 is mediated by AP1-dependent EpCAM expression in MCF-7 cells. J Cell Physiol. 2015;230(4):775–782. doi: 10.1002/jcp.24802. [DOI] [PubMed] [Google Scholar]
- 24.Velden JL, Alcorn JF, Guala AS, Badura EC, Janssen-Heininger YM. C-Jun N-terminal kinase 1 promotes transforming growth Factor-β1-induced epithelial-to-mesenchymal transition via control of linker phosphorylation and transcriptional activity of Smad3. Am J Respir Cell Mol Biol. 2011;44(4):571–581. doi: 10.1165/rcmb.2009-0282OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo B, Inoki K, Isono M, Mori H, Kanasaki K, Sugimoto T, Akiba S, Sato T, Yang BF, Kikkawa R, Kashiwagi A, Haneda M, Koya D. MAPK/AP-1-dependent regulation of PAI-1 gene expression by TGF-beta in rat mesangial cells. Kidney Int. 2005;68(3):972–984. doi: 10.1111/j.1523-1755.2005.00491.x. [DOI] [PubMed] [Google Scholar]
- 26.Ye Y, Dan ZL. All-trans retinoic acid diminishes collagen production in a hepatic stellate cell line via suppression of active protein-1 and c-Jun N-terminal kinase signal. Hua zhong ke ji da xue xue bao. Yi xue Ying De wen ban. 2010;30(6):726–733. doi: 10.1007/s11596-010-0648-5. [DOI] [PubMed] [Google Scholar]
- 27.Tang HT, Cheng DS, Jia YT, Ben DF, Ma B, Lv KY, Wei D, Sheng ZY, Xia ZF. Angiotensin II induces type I collagen gene expression in human dermal fibroblasts through an AP-1/TGF-beta1-dependent pathway. Biochem Biophys Res Commun. 2009;385(3):418–423. doi: 10.1016/j.bbrc.2009.05.081. [DOI] [PubMed] [Google Scholar]
- 28.Hu YB, Zeng QF, Feng DY, Li X, Peng JW. AP-1 regulates TGF-beta1-induced secretion of Type I collagen in human lung fibroblasts. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2007;32(5):776–781. [PubMed] [Google Scholar]
- 29.Li Y, Wang DW, Song LW, Peng RY, Gao YB, Ma JJ. Effects of TGFbeta1 on the transcriptional activity of SP1, AP1 and Smad3-Smad4 in lung fibroblasts. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2005;21(6):679–682. [PubMed] [Google Scholar]