Abstract
AIM
To evaluate the effects of atropine 0.01% on slowing myopia progression.
METHODS
We searched for relevant studies in the Cochrane Library, PubMed, Embase, Ovid, CBM, CNKI, VIP and Wan Fang Data in Chinese. A supplementary search was conducted in OpenGrey (System for Information on Grey Literature in Europe), the ISRCTN registry, ClinicalTrials.gov, and the WHO International Clinical Trials Registry Platform (ICTRP) from the dates of inception to June 30, 2018.
RESULTS
Seven randomized controlled trials (RCTs) with a total of 1079 subjects were included (505 in the atropine 0.01% group and 574 in the control group). The results showed that the atropine 0.01% group exhibited significantly greater control of axial growth than the control group [MD=-0.12, 95%CI (-0.19, -0.06)]. There was also a statistically significant difference between the atropine 0.01% and control groups in the changes in axial length [MD=-0.14, 95%CI (-0.25, -0.03)], but the quality of evidence was low. There were no significant differences between the atropine 0.01% and control groups in the overall effect with respect to diopter value, change in diopter, distance vision and intraocular pressure [MD=0.08, 95%CI (-0.27, 0.42); MD=0.09, 95%CI (-0.17, 0.36); MD= -0.01, 95%CI (-0.02, 0.00); MD=0.08, 95%CI (-0.56,0.40)]. The sensitivity analysis showed that the conclusion of the Meta-analysis is relatively stable. With respect to adverse events, there were significant differences between the atropine 0.01% and control groups [OR=0.26, 95%CI (0.11, 0.61)].
CONCLUSION
Based on the available evidence, atropine 0.01% eye drops offer benefits in controlling axial growth and safety without causing significant differences in diopter values, distance vision and intraocular pressure.
Keywords: atropine 0.01% eye drops, myopia, systematic review, Meta-analysis
INTRODUCTION
In the past two decades, a series of epidemiological studies have demonstrated that the persistently high prevalence of myopia has become a major public health problem[1]–[5]. The factors associated with myopia induction include environment, ethnicity, and inheritance[6]–[9]. The progression of myopia in children and adolescents is gradual. Furthermore, early-onset myopia can be associated with the development of high myopia[10], which could lead to several pathological complications, such as choroidal thinning, posterior scleral staphyloma, cataracts, peripheral retinal tears, myopic choroidal neovascularization, glaucoma, macular degeneration, and even blindness[11]–[12]. Myopia has been considered the sixth major cause of vision loss. Therefore, to reduce the incidence of diseases related to this condition, it is important to identify effective ways to slow the development of myopia[13].
Atropine was first used to prevent myopia in 1920s[14]. Since then, numerous related studies have been conducted[15]–[21]. These studies confirmed that atropine can effectively control the progression of myopia. Atropine 1% eye drops were initially used as a standard dose to control nearsightedness. Although the results of the studies confirmed its efficacy in controlling myopia, its side effects, which include photophobia, poor near vision, dry mouth, flushing, constipation, ciliary muscle paralysis and allergies, should not be underestimated[22]–[23]. The main concerns that deter the use of higher concentrations of atropine are photophobia, risk of cataracts and blurry near vision[15]. The higher the concentration of atropine, the greater the pupillary dilation and incidence of unclear vision, photophobia symptoms, poisoning and eye irritation. In recent years, several studies were performed to compare the effectiveness of different concentrations of atropine in the control of myopia. They demonstrated that atropine 0.01% eye drops can achieve a balance between a higher efficacy and lower incidence of side effects than other concentrations[24]–[25]. As new clinical evidence continues to emerge, it is necessary to systematically evaluate the safety and efficacy of atropine 0.01% eye drops in the prevention and treatment of myopia and provide guidance for clinical practice.
MATERIALS AND METHODS
Inclusion and Exclusion Criteria
We used the following inclusion criteria to identify published studies for this Meta-analysis: 1) the studies were clinical randomized controlled trials (RCTs) for the treatment of myopia with atropine 0.01% eye drops; 2) participants in the trials were people with a confirmed diagnosis of myopia; 3) we included atropine 0.01% eye drops for the treatment of myopia, and the control group was treated with atropine 0.1% or 0.5%, placebo or a blank control; 4) baseline data of the experimental and control groups were well balanced between groups. The exclusion criteria were as follows: 1) self-contrast test before and after; 2) repetitive publications or duplicate data; 3) secondary articles such as review articles; 4) original data could not be extracted and could not be obtained after contacting the author; 5) animal experimental study.
Search Strategy
We searched for relevant studies in the Cochrane Library, PubMed, Embase, Ovid, CBM (http://www.sinomed.ac.cn/), CNKI (http://www.cnki.net/), VIP (http://www.cqvip.com/) and Wang Fang Data (http://www.wanfangdata.com.cn/) in Chinese. A supplementary search was conducted in OpenGrey (System for Information on Grey Literature in Europe; www.opengrey.eu/); the ISRCTN registry (www.isrctn.com/editAdvancedSearch); ClinicalTrials.gov (www.clinicaltrials.gov); and the WHO International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp/search/en), ranging from the dates of inception to 30 June 2018.
Data Extraction
Two reviewers (Zhao Y, Feng K) independently extracted data from the publications and evaluated the risk of bias for all included studies. We also contacted the investigators for further research information and trials with unpublished results. Then, we cross-checked the literature and asked for assistance and guidance from relative experts when different opinions emerged. Pre-established data extraction tables were used to extract information, including the following: 1) basic information included in the study, including the first author, title, and year of publication; 2) basic characteristics of the patients, including the number of cases and age; 3) interventions, including the use of drugs, dosage, and treatment; 4) outcome indicators and measurement results; 5) follow-up time; 6) methodological information.
Qualitative Assessment
Two review authors independently assessed the quality of all included studies. The quality of the selected studies was determined by the Cochrane Handbook 5.3, including 6 items: random sequence generation, allocation concealment, blinding of subjects and intervention providers (blinding of participants and caregivers), blinding of outcome assessments, incomplete outcome data, selective reporting, and other biases. Two reviewers judged whether the risk of bias was low, high, or unclear. When necessary, we contacted the study authors to obtain information to better evaluate the study.
Statistical Analysis
We used Review Manager 5.3 software (Copenhagen: the Nordic Cochrane Centre, the Cochrane Collaboration, 2014) for the statistical analysis. The odds ratio (OR) was used for count data, and the standard mean difference (SMD) was used for measurement data. Both of them used the 95%CI as the effect amount. First, the statistical heterogeneity of the included clinical RCTs was analyzed using the Cochrane I2 test. If I2<30%, the heterogeneity was small, and 30%<I2<50% indicated moderate heterogeneity. A fixed-effects model was used if significant evidence of statistical heterogeneity or clinical diversity was not found (P≥0.10, I2≤50%). If I2>50%, there was a high degree of heterogeneity among the results. In this case, the source of heterogeneity was identified, and a subgroup analysis was performed. If no source of heterogeneity was found, a descriptive analysis was performed.
RESULTS
Search Results
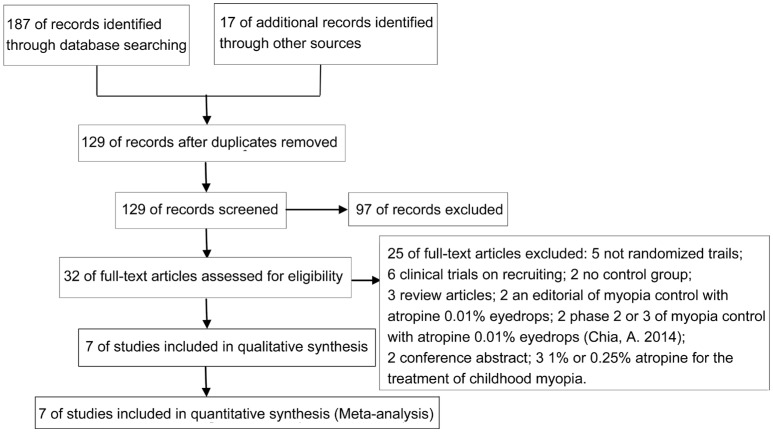
A total of 204 related studies were obtained from the preliminary screening. After excluding 75 duplicate reports of the same studies and 97 unrelated studies, 32 full-text publications were assessed for eligibility. Twenty-five of these studies were excluded for various reasons, and 7 studies were eventually included. The literature screening process and results are shown in Figure 1.
Figure 1. Literature screening process and results.
Description of Studies
A total of 1079 subjects were included (505 in the atropine eye drop group and 574 in the control group). The basic characteristics of each subject are shown in Table 1[25]–[31]. After reading the full-text articles, 25 were excluded.
Table 1. Characteristics of the included studies.
| Literature | Age (y) | Study design | Experimental group | Control group | Treatment time (mo) | Criteria | Total No. of patients (test group/control group) | Outcomes |
| Chia et al, 2012[25] | 6-12 | RCT | 0.01% atropine eye drops | 0.1% atropine eye drops | 12 | Diopter ≥2 D, astigmatism <1.5 D | 75/141 | AL; ALC; D; DC; DV |
| Diaz-Llopis and Pinazo-Durán, 2018[26] | 9-12 | RCT | 0.01% atropine eye drops | blank control | 60 | Diopter -0.5 to -2 D, astigmatism <1.5 D | 100/100 | DC |
| Yam et al, 2019[27] | 4-12 | RCT | 0.01% atropine eye drops | placebo | 12 | Diopter <1.0 D, astigmatism <2.5D | 97/93 | ALC; DC; DV |
| Cui et al, 2017[28] | 7-13 | RCT | 0.01% atropine eye drops | 1% atropine eye drops | 12 | Myopic ametropia | 35/35 | ALC; DC |
| Li et al, 2018[29] | 7-12 | RCT | 0.01% atropine eye drops | blank control | 12 | Corrected visual acuity ≥5.0 | 110/117 | AL; ALC; D; DC; IOP |
| Shi et al, 2017[30] | 13-19 | RCT | 0.01% atropine eye drops+ orthokeratology lens | Orthokeratology lens | 6 | Diopter 1.50 to 6.00 D, astigmatism ≤1.00 D | 47/47 | AL; ALC |
| Zhang and Zhou, 2016[31] | 9-23 | RCT | 0.01% atropine eye drops | 0.1% atropine eye drops | 24 | Myopic ametropia | 41/41 | AL; ALC; D; DC; DV; IOP |
RCT: Randomized controlled trial; AL: Axial length; ALC: Axial length change; D: Diopter; DC: Diopter change; DV: Distance vision; IOP: Intraocular pressure.
Methodological Quality Evaluation
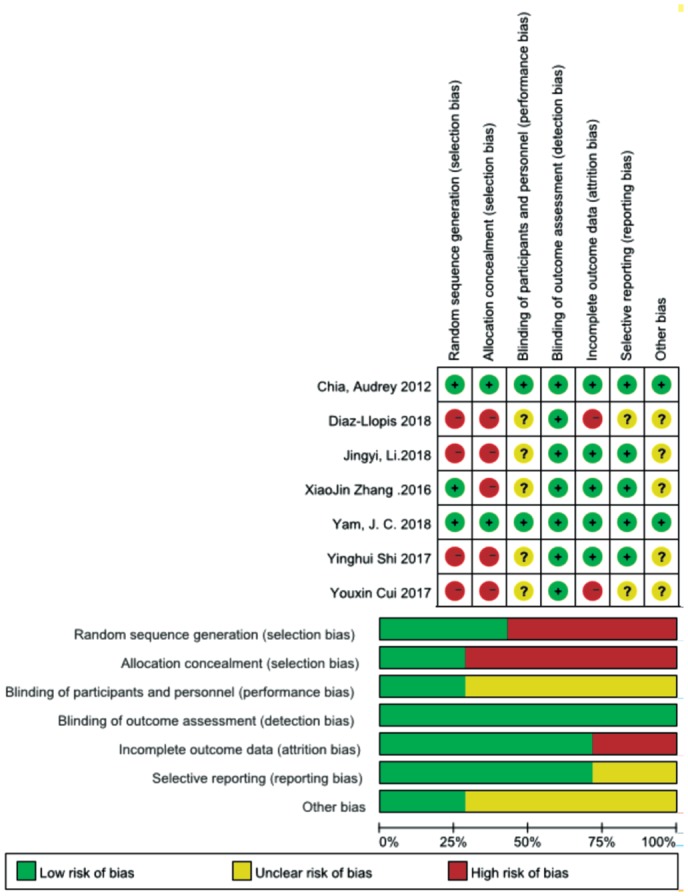
We assessed the risk of bias for seven of the included publications. Most of the RCTs did not report the method of random sequence generation, which is associated with a high risk of selection bias, and they did not mention blinding of participants and personnel. A summary of the risk of bias assessment is provided in Figure 2.
Figure 2. Method quality evaluation.
Efficacy Analysis
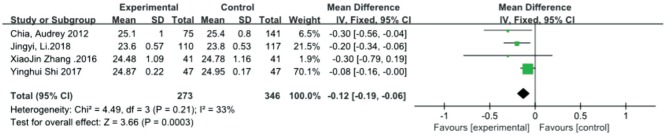
Axial length
Four studies[25],[29]–[31] measured the axial length as the main outcome measure. The results of the axial length showed (Figure 3) that the atropine 0.01% group exhibited significantly better control of axial growth than the control group [MD=-0.12, 95%CI (-0.19, -0.06)]. There was no significant heterogeneity detected between the studies (P=0.21, I2=33%). The combined results demonstrated that atropine 0.01% yielded significantly greater improvement in axial length.
Figure 3. Meta-analysis of axial length.
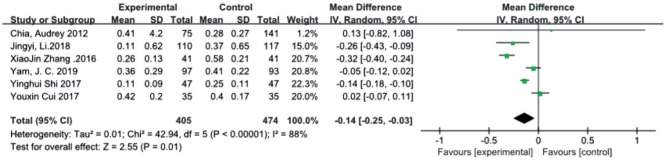
Axial length change
Six studies[25],[27]–[31] evaluated the changes in axial length after treatment. There was a statistically significant difference between the atropine 0.01% group and control group in the overall effect [MD=-0.14, 95%CI (-0.25, -0.03)], and it was characterized by high heterogeneity (I2=88%, τ2=0.01). We performed another analysis after excluding one trial[30] based on the observation period, which was at least one year. Low heterogeneity was not found (I2=91%, τ2=0.03), and the quality of the evidence was low (Figure 4).
Figure 4. Meta-analysis of the changes in axial length.
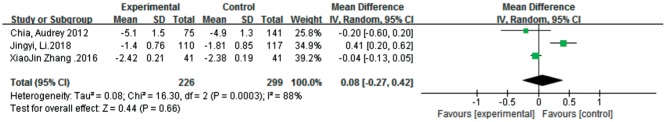
Diopter
Three studies [25],[29],[31] conducted an analysis of diopter after the experiment. There was no significant difference between atropine 0.01% eye drops and the control in the overall effect [MD=0.08, 95%CI (-0.27, 0.42)]. We performed the analysis again after excluding one trial[29] because of the significant problem of comparability of this study with other studies, and low heterogeneity was found (I2=0, τ2=0.00). However, the results still did not show a significant difference between the two groups (Figure 5).
Figure 5. Meta-analysis of diopter.
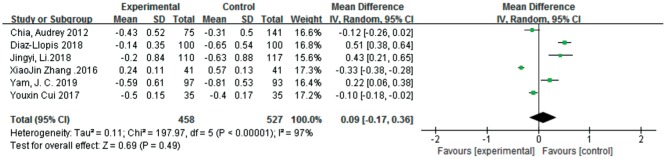
Diopter Change
Six studies[25]–[29],[31] analyzed the changes in diopter after treatment. There was no significant difference between the atropine 0.01% and control groups in the overall effect [MD=0.09, 95%CI (-0.17, 0.36)]. As shown in Figure 6, one study[25] showed that there was no significant difference in the change in diopter between the atropine 0.01% and control groups [MD=-0.12, 95%CI (-0.26, 0.02)], while two studies[28],[31] showed that there was a small but statistically significant difference in the change in diopter that favored atropine 0.01% eye drops [MD=-0.10, 95%CI (-0.18, -0.02); MD=-0.33, 95%CI (-0.38, -0.28)]. Three studies[26]–[27],[29] showed that the changes in diopter due to atropine 0.01% eye drops were greater than those due to the control [MD=0.51, 95%CI (0.38, 0.64); MD=0.22, 95%CI (0.06, 0.38); MD=0.43, 95%CI (0.21, 0.65)]. The quality of the evidence was low because all studies were characterized by high heterogeneity (I2=97%, P<0.01).
Figure 6. Meta-analysis of the changes in diopter.
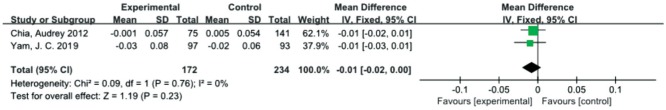
Distance Vision
Two studies[25],[27] included distance vision as an outcome indicator. We recorded the logarithm of the minimum angle of resolution (logMAR) scale as the distance vision measurement indicator. There was no significant difference between the atropine 0.01% and control groups in the overall effect with respect to distance vision [MD=-0.01, 95%CI (-0.02, 0.00)]. There was no statistically significant heterogeneity detected between the studies (P=0.76, I2=0; Figure 7).
Figure 7. Meta-analysis of distance vision.
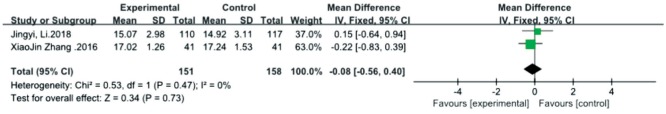
Intraocular Pressure
The results of intraocular pressure (IOP) were included in 2 studies[29],[31]. There was no statistically significant heterogeneity detected between the 2 studies (P=0.47, I2=0), and the fixed effects model was used. The combined results demonstrated that there was no significant difference in IOP between the atropine 0.01% eye drops and the control in the treatment of myopia [MD=-0.08, 95%CI (-0.56, 0.40); Figure 8].
Figure 8. Meta-analysis of intraocular pressure.
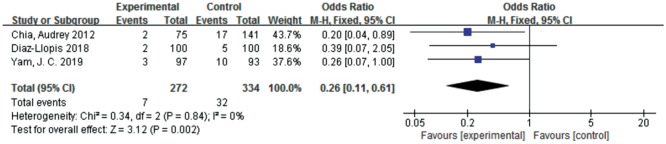
Security Analysis
A total of 3 studies[25]–[27] reported adverse events. There were significant differences between the atropine 0.01% eye drops and the control with respect to adverse events [OR=0.26, 95%CI (0.11, 0.61)]. There was no significant heterogeneity detected between the studies (Figure 9). One study[25] concluded that most of the adverse events were considered unrelated to the research treatment. Regarding the direct cause of the adverse reactions caused by atropine, in the control group, 13 cases had allergic conjunctivitis and 4 cases had orbital allergic dermatitis; in the atropine 0.01% group, 1 patient had eye irritation and 1 patient had blurred vision. One study[26] reported that the incidence of side effects requiring treatment cessation was only 2% (photophobia, difficulty in reading, mydriasis and headache). One study[27] reported that in the atropine 0.01% group, 1 participant had a lip injury requiring surgical repair, 1 participant had influenza, and 1 participant had a distal radial fracture requiring plaster casting. It seemed that these adverse reactions were not significantly related to the experiment, but the experimenter still reported them as adverse events.
Figure 9. Estimated relative risks of adverse events.
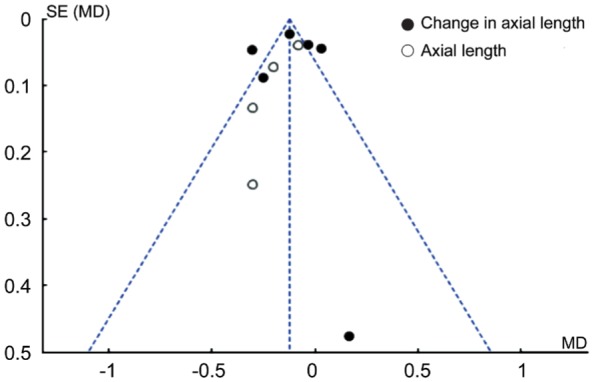
Publication Bias Analysis
Funnel plot analysis was performed to assess the publication bias of the studies. As shown in Figure 10, axial length was asymmetric in four studies, suggesting the possible presence of publication bias. As for change in axial length, the symmetric funnel plots provide no evidence of publication bias.
Figure 10. Funnel plot of the studies.
DISCUSSION
The widespread prevalence and rapidly increasing rates of myopia make it a significant public health concern. The goal of myopia control therapy is to slow myopia progression to reduce the incidence and blinding rate of high myopia. Recently, studies have demonstrated that the optimal concentration of atropine in eye drops should be the one with the best balance between efficacy and safety[25],[32]–[33]. In this Meta-analysis, we compared the results from seven RCTs (505 patients in the atropine eye drop group and 574 patients in the control group) to assess the effect of atropine 0.01% in slowing myopia progression. To our knowledge, this is the first study to systematically evaluate the safety and effectiveness of atropine 0.01% eye drops in the prevention and treatment of myopia. The results showed that, compared with the control group, atropine 0.01% eye drops can effectively control axial length, thus effectively preventing and controlling the progression of myopia. The axial length decrease was 0.12 mm less in the atropine 0.01% group compared with the control group (95%CI, -0.19 to -0.06), and the change in axial length was 0.14 mm less than that in the control group (95%CI, -0.25 to -0.03). There was no significant difference between atropine 0.01% eye drops and the control in the overall effect with respect to diopter, change in diopter, distance vision, and IOP. However, from a safety perspective, three studies reported adverse events, and atropine 0.01% had a lower incidence of adverse events and was well tolerated without adverse effects on vision-related quality of life [OR=0.26, 95%CI (0.11, 0.61)]. Moreover, there was high-quality evidence that atropine 0.01% eye drops provide superior benefit compared with high-concentration atropine in clinical trial populations[25]–[26]. High-concentration atropine also decreases accommodation amplitude and near vision such that children may require bifocal or progressive glasses to read. However, atropine 0.01% exhibited the best balance between efficacy and safety. It is suggested that a nightly dose of 0.01% atropine seems to be a safe and effective regimen for slowing myopia progression in children, with minimal impact on visual function. These existing studies collectively indicate that atropine 0.01% eye drops are safe and well tolerated and could be used as effective interventions to prevent the progression of myopia. However, due to the limited quality of the included studies and the unstable statistical results, the above conclusions need to be further verified by more rigorously designed large-sample RCTs.
It should be noted that all of the included trials were RCTs. Furthermore, to decrease the heterogeneity in the meta-analysis, we conducted a systematic, comprehensive, complete and reasonable literature search and tried to contact the author in the case of missing data. Nevertheless, there are some limitations of this meta-analysis. First, most of the RCTs did not report the method of random sequence generation, and 3 RCTs[28]–[30] adopted the principle of open random allocation. The potential bias of the original study may be misleading in the calculation of a single combined statistic of exposure effects, which may be a source of heterogeneity. The use of atropine 0.01% eye drops is a new direction in recent years, and a greater number of registered clinical trials are currently being performed. This Meta-analysis only filtered out 7 RCTs, all of which were single research center studies, which may lead to insufficient statistical efficiency. Third, we established clear literature inclusion and exclusion criteria, and more than two people completed the screening of the literature independently. We also performed sensitivity analysis of the change in axial length and the diopter. However, the results still did not show significant differences between the two groups. The control groups differed between studies, which in turn led to certain differences in the therapeutic effect. However, the stability of our sensitivity analysis shows that the conclusion of the meta-analysis is relatively stable and consistent. Fourth, atropine 0.01% eye drops are currently only sold in Taiwan, but some of the studies included in this Meta-analysis used self-formulated atropine eye drops. There may be some differences in the concentration of atropine, which may be another reason for the differences in results. Further well-designed studies with uniform standards of atropine 0.01% eye drops are required. It is hoped that more rigorous trials in the future will provide evidence of the efficacy of atropine 0.01% eye drops for myopia, which is a problem that threatens the healthy development of adolescents.
In summary, the present meta-analysis showed that atropine 0.01% eye drops can achieve the best balance between efficacy and safety in the prevention and treatment of myopia and could be used as a clinically feasible method to control the progression of myopia. These eye drops are worth popularizing and applying in clinical practice. Remaining questions that need to be clarified in future studies are “How long can the effect persist after the intervention stops?” and “Is this treatment more appropriate for preschoolers?”. It is expected that more large-sample, multi-center, and high-quality RCTs will provide strong clinical evidence in the future.
Acknowledgments
We are grateful to the Chinese Cochrane Center, Center for Evidence-Based Medicine and Epidemiology, West China Hospital, Sichuan University for advice on the electronic searches and statistics; Jing Li from the Chinese Cochrane Centre for her critical comments on earlier versions of this protocol.
Authors' contributions: Zhao Y, Feng K, Liu RB, Pan JH, Zhang LL collected the data. Zhao Y, Feng K extracted data. All authors were involved in the analysis. Zhao Y wrote the first draft of the manuscript. Feng K and Lu XJ, Xu ZP reviewed and revised the manuscript and produced the final version. All authors read and approved the final manuscript.
Foundations: Supported by National Major Science and Technology Project “Application of Multi-wavelength Structure Functional Retina Imager in Theoretical Research of Traditional Chinese Medicine” (No.2013YQ49085904); Sichuan Science and Technology Department-Research on the basic protection of retinal ganglion cells in traditional Chinese medicine (No.17CXTD0064); Chengdu Science and Technology Bureau-Promotion and demonstration of fundus screening technology for diabetic microangiopathy (No.2015-HM02-00093-SF).
Conflicts of Interest: Zhao Y, None; Feng K, None; Liu RB, None; Pan JH, None; Zhang LL, None; Xu ZP, None; Lu XJ, None.
REFERENCES
- 1.Vitale S, Sperduto RD, Ferris FL., 3rd Increased prevalence of myopia in the United States between 1971-1972 and 1999-2004. Arch Ophthalmol. 2009;127(12):1632–1639. doi: 10.1001/archophthalmol.2009.303. [DOI] [PubMed] [Google Scholar]
- 2.Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, Wong TY, Naduvilath TJ, Resnikoff S. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036–1042. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Zheng YF, Pan CW, Chay J, Wong TY, Finkelstein E, Saw SM. The economic cost of myopia in adults aged over 40 years in Singapore. Invest Ophthalmol Vis Sci. 2013;54(12):7532–7537. doi: 10.1167/iovs.13-12795. [DOI] [PubMed] [Google Scholar]
- 4.French AN, Morgan IG, Burlutsky G, Mitchell P, Rose KA. Prevalence and 5- to 6-year incidence and progression of myopia and hyperopia in Australian schoolchildren. Ophthalmology. 2013;120(7):1482–1491. doi: 10.1016/j.ophtha.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 5.Wu LJ, You QS, Duan JL, et al. Prevalence and associated factors of myopia in high-school students in Beijing. PLoS One. 2015;10(3):e0120764. doi: 10.1371/journal.pone.0120764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rong SS, Chen LJ, Pang CP. Myopia genetics-the Asia-Pacific perspective. Asia Pac J Ophthalmol (Phila) 2016;5(4):236–244. doi: 10.1097/APO.0000000000000224. [DOI] [PubMed] [Google Scholar]
- 7.Jones-Jordan LA, Sinnott LT, Manny RE, Cotter SA, Kleinstein RN, Mutti DO, Twelker JD, Zadnik K, Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study Group Early childhood refractive error and parental history of myopia as predictors of myopia. Invest Ophthalmol Vis Sci. 2010;51(1):115–121. doi: 10.1167/iovs.08-3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee YY, Lo CT, Sheu SJ, Lin JL. What factors are associated with myopia in young adults? A survey study in Taiwan Military Conscripts. Invest Ophthalmol Vis Sci. 2013;54(2):1026–1033. doi: 10.1167/iovs.12-10480. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Zhang Q. Insight into the molecular genetics of myopia. Mol Vis. 2017;23:1048–1080. [PMC free article] [PubMed] [Google Scholar]
- 10.Zadnik K, Sinnott LT, Cotter SA, Jones-Jordan LA, Kleinstein RN, Manny RE, Twelker JD, Mutti DO, Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study Group Prediction of juvenile-onset myopia. JAMA Ophthalmol. 2015;133(6):683–689. doi: 10.1001/jamaophthalmol.2015.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka A, Ohno-Matsui K, Shimada N, Hayashi K, Shibata Y, Yoshida T, Yamashita M, Tokoro T, Mochizuki M. Prevalence of strabismus in patients with pathologic myopia. J Med Dent Sci. 2010;57(1):75–82. [PubMed] [Google Scholar]
- 12.Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Opt. 2005;25(5):381–391. doi: 10.1111/j.1475-1313.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 13.Morgan IG, He M, Rose KA. Epidemic of pathologic myopia: what can laboratory studies and epidemiology tell us? Retina. 2017;37(5):989–997. doi: 10.1097/IAE.0000000000001272. [DOI] [PubMed] [Google Scholar]
- 14.Gimbel HV. The control of myopia with atropine. Can J Ophthalmol. 1973;8(4):527–532. [PubMed] [Google Scholar]
- 15.Chua WH, Balakrishnan V, Chan YH, Tong L, Ling Y, Quah BL, Tan D. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113(12):2285–2291. doi: 10.1016/j.ophtha.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 16.Fan DS, Lam DS, Chan CK, Fan AH, Cheung EY, Rao SK. Topical atropine in retarding myopic progression and axial length growth in children with moderate to severe myopia: a pilot study. Jpn J Ophthalmol. 2007;51(1):27–33. doi: 10.1007/s10384-006-0380-7. [DOI] [PubMed] [Google Scholar]
- 17.Cheng HC, Hsieh YT. The effect of low-concentration atropine combined with auricular acupoint stimulation in myopia control. Complement Ther Med. 2014;22(3):449–455. doi: 10.1016/j.ctim.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Smith MJ, Walline JJ. Controlling myopia progression in children and adolescents. Adolesc Heal Med Ther. 2015;6:133–140. doi: 10.2147/AHMT.S55834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yi S, Huang Y, Yu SZ, Chen XJ, Yi H, Zeng XL. Therapeutic effect of atropine 1% in children with low myopia. J Am Assoc Pediatr Ophthalmol Strabismus. 2015;19(5):426–429. doi: 10.1016/j.jaapos.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Tan D, Tay SA, Loh KL, Chia A. Topical atropine in the control of myopia. Asia Pac J Ophthalmol (Phila) 2016;5(6):424–428. doi: 10.1097/APO.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 21.Lee CY, Sun CC, Lin YF, Lin KK. Effects of topical atropine on intraocular pressure and myopia progression: a prospective comparative study. BMC Ophthalmol. 2016;16:114. doi: 10.1186/s12886-016-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tong L, Huang XL, Koh AL, Zhang X, Tan DT, Chua WH. Atropine for the treatment of childhood myopia: effect on myopia progression after cessation of atropine. Ophthalmology. 2009;116(3):572–579. doi: 10.1016/j.ophtha.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 23.Leo SW, Young TL. An evidence-based update on myopia and interventions to retard its progression. J AAPOS. 2011;15(2):181–189. doi: 10.1016/j.jaapos.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark TY, Clark RA. Atropine 0.01% eyedrops significantly reduce the progression of childhood myopia. J Ocul Pharmacol Ther. 2015;31(9):541–545. doi: 10.1089/jop.2015.0043. [DOI] [PubMed] [Google Scholar]
- 25.Chia A, Chua WH, Cheung YB, Wong WL, Lingham A, Fong A, Tan D. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2) Ophthalmology. 2012;119(2):347–354. doi: 10.1016/j.ophtha.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 26.Diaz-Llopis M, Pinazo-Durán MD. Superdiluted atropine at 0.01% reduces progression in children and adolescents. A 5 year study of safety and effectiveness. Arch Soc Esp Oftalmol. 2018;93(4):182–185. doi: 10.1016/j.oftal.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Yam JC, Jiang Y, Tang SM, Law AKP, Chan JJ, Wong E, Ko ST, Young AL, Tham CC, Chen LJ, Pang CP. Low-concentration atropine for myopia progression (LAMP) study: a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology. 2019;126(1):113–124. doi: 10.1016/j.ophtha.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 28.Cui YX. Clinical progress of 0.01% atropine in delaying children's myopia. Chinese Health Standards Management. 2017;22(8):79–80. [Google Scholar]
- 29.Li JY, Liu FR, Zhou XW, et al. Study on myopia prevention and control of school-age children with 0.01% atropine. Chinese Journal of School Health. 2018;39(03):432–435. [Google Scholar]
- 30.Shi YH, Li YG, Zhang JZ, et al. Observation of the effect of keratoplasty combined with volume fraction of 0.01% atropine on juvenile myopia. J Chin Pract Diagn Ther. 2017;31(11):1102–1103. [Google Scholar]
- 31.Zhang XJ, Zhou L. Efficacy and safety of different concentrations of atropine in the treatment of refractive errors. Chinese Journal of Biochemical Medicine. 2016;36(11):106–108. [Google Scholar]
- 32.Chia A, Chua WH, Wen L, Fong A, Goon YY, Tan D. Atropine for the treatment of childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5% Am J Ophthalmol. 2014;157(2):451–457.e1. doi: 10.1016/j.ajo.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 33.Chia A, Lu QS, Tan D. Five-year clinical trial on atropine for the treatment of myopia 2: myopia control with atropine 0.01% eyedrops. Ophthalmology. 2016;123(2):391–399. doi: 10.1016/j.ophtha.2015.07.004. [DOI] [PubMed] [Google Scholar]