Abstract
Pulmonary complications and a poor clinical outcome are common in response to transthoracic esophagectomy, but their etiology is not well understood. Clinical observation suggests that patients undergoing pulmonary resection, a surgical intervention with similarities to the thoracic part of esophagectomy, fare much better, but this has not been investigated in detail. A retrospective single-center analysis of 181 consecutive patients after right-sided thoracotomy for either Ivor Lewis esophagectomy (n = 83) or major pulmonary resection (n = 98) was performed. An oxygenation index <300 mm Hg was used to indicate respiratory impairment. When starting surgery, respiratory impairment was seen more frequently in patients undergoing major pulmonary resection compared to esophagectomy patients (p = 0.009). On postoperative days one to ten, however, esophagectomy caused higher rates of respiratory impairment (p < 0.05) resulting in a higher cumulative incidence of postoperative respiratory impairment for patients after esophagectomy (p < 0.001). Accordingly, esophagectomy patients were characterized by longer ventilation times (p < 0.0001), intensive care unit and total postoperative hospital stays (both p < 0.0001). In conclusion, the postoperative clinical course including respiratory impairment after Ivor Lewis esophagectomy is significantly worse than that after major pulmonary resection. A detailed investigation of the underlying causes is required to improve the outcome of esophagectomy.
Subject terms: Surgical oncology, Oesophageal cancer, Risk factors, Oesophagus
Introduction
Esophageal cancer is one of the leading cancer diagnoses with a high cancer-related mortality and a rapidly growing incidence over the past years world-wide1. The only curative treatment option in earlier, limited stages (Union internationale contre le cancer (UICC) – stages I and II) as well as in selected cases with a locally advanced disease (UICC stage III) is the subtotal resection of the esophagus, frequently following neoadjuvant and accompanied by adjuvant oncologic treatment modalities1. Nevertheless, the surgical therapy is challenging for the patients since it is associated with high rates of postoperative complications and morbidity. Overall, up to 60% of the patients experience any type of postoperative complication and the mortality rate of up to 14% after transthoracic esophagectomy for esophageal cancer is unacceptably high2,3. Especially pulmonary complications including atelectasis, pneumonia, pulmonary embolism, respiratory failure and acute respiratory distress syndrome (ARDS) are overrepresented after esophagectomy with an incidence ranging between 20% and 40% and consequently contribute to postoperative morbidity and even mortality3–8. After transthoracic esophagectomy, pneumonia, respiratory failure and ARDS rates accounted for 22%, 6% and 1.5%, respectively, in a large patient cohort reported by Zingg et al.3. As published recently, pulmonary complications after esophagectomy negatively impact not only on short-term clinical but also on long-term oncological patient outcome3,9,10. Pulmonary complications increase the time spent on the intensive care unit (ICU) and in the hospital, thus raising the overall health care costs after esophageal resections3,9,11,12. As it has been discussed by Molena et al., development of postoperative respiratory complications after esophagectomy is a multifactorial process9. Different hypotheses have been published on the pathophysiology of exceptional high pulmonary complication rates after esophagectomy with all kinds of consecutive respiratory impairment9,13,14.
However, these factors should resemble the pulmonary complication rates after conventional (open) major pulmonary resection (MPR). Similar to Ivor Lewis transthoracic esophagectomy (ILE), thoracotomy is used to access the thoracic cavity and single-lung ventilation is performed during MPR. Pulmonary complication rates after conventional open MPR are, however, considerably lower than those after esophagectomy15,16.
We conducted a single-center analysis to compare patients after right-sided thoracotomy for ILE with those after MPR, primarily focusing on the pulmonary outcome.
Results
Patients
Patient characteristics of both groups were similar regarding physical status, indicated by the ‘American society of Anesthesiologist’s classification of physical health’ (ASA) score, however, patients from the MPR-group suffered more frequently from chronic lung diseases (19.3% vs. 35.7%, p = 0.020). In contrast to the MPR-group, patients awaiting ILE underwent more frequently induction therapy of any kind (p < 0.0001). Indication for ILE was mainly primary malignancy (carcinoma of the esophagus in 98.8%). MPR was performed for malignancy in 88.8% (predominantly primary lung cancer) and benign diseases in 11.2% of the patients (lung volume reduction for emphysema (n = 1), inflammatory destroyed pulmonary lobe (n = 2), aspergilloma or tuberculoma (n = 4), and sequestration (n = 2), Table 1).
Table 1.
Patient characteristics.
| Variables | ILE | MPR | p-value |
|---|---|---|---|
| Male gender | 68 (81.9%) | 65 (66.3%) | 0.019 |
| Age [years] | 63.0 (41–80) | 62.0 (26–82) | 0.631 |
| BMI [kg/m²] | 24.2 (15.6–41.3) | 26.5 (15.9–39.0) | 0.041 |
| ASA | 0.431 | ||
| 1 | 6 (7.2%) | 9 (9.2%) | |
| 2 | 40 (48.2%) | 37 (37.8%) | |
| 3 | 33 (39.8%) | 49 (50.0%) | |
| 4 | 4 (4.8%) | 3 (3.1%) | |
| Chronic lung disease | 16 (19.3%) | 35 (35.7%) | 0.020 |
| Chronic kidney disease | 6 (7.2%) | 10 (10.2%) | 0.602 |
| Induction therapy | |||
| Chemo | 42 (50.6%) | 8 (8.2%) | <0.0001 |
| Radio | 20 (24.1%) | 2 (2.0%) | <0.0001 |
| Indication | 0.007 | ||
| Malignancy | 82 (98.8%) | 87 (88.8%) | |
| Primary Tumor | 82 (100%) | 81 (93.1%) | |
| Metastasis | 0 | 5 (5.7%) | |
| Lymphoma | 0 | 1 (1.1%) | |
| Benign disease | 1 (1.2%) | 11 (11.2%) | |
ILE = Ivor Lewis esophagectomy. MPR = major pulmonary resection. BMI = body mass index. ASA = American society of Anesthesiologist’s classification of physical health score.
Surgery
The rate of intraoperative lymph node dissection was higher in the ILE-group (98.8% vs. 88.8%, p = 0.007), as was the total duration of the surgical procedure compared with patients from the MPR-group (306 (177–635) min vs. 180.5 (78–356) min, p < 0.0001) – although the rates of extended surgical procedures did not differ between both groups. Of note, the duration of the thoracic part of the two-stage ILE procedure was significantly shorter compared to the total duration of surgery in the MPR-group (142 (48–423) min vs. 180.5 (78–356) min, p < 0.0001, Table 2).
Table 2.
Procedure characteristics.
| Variables | ILE | MPR | p-value |
|---|---|---|---|
| Main procedure |
Laparoscopy: 31 (37.3%) Laparotomy: 52 (62.7%) Gastric tube: 79 (95.2%) Colon interposition: 4 (4.8%) |
Upper lobectomy: 45 (45.9%) Middle lobectomy: 5 (5.1%) Lower lobectomy: 33 (33.7%) Upper bilobectomy: 7 (7.1%) Lower bilobectomy: 8 (8.2%) |
|
| Lymph node dissection | 82 (98.8%) | 87 (88.8%) | 0.007 |
| Relevant abdomino/thoracic extended procedures (additional to main procedure) |
n = 17 (20.5%) Major lung resection: 3# Minor lung resection: 5# Lung decortication: 1 Minor liver resection: 3 Multivisceral resection: 1 Jejunum catheter: 3 Cholezystectomy: 1 Colon resection: 1 Others: 3§ |
n = 26 (26.5%) Sleeve resections, bronchoplasty: 11 Sublobar resection: 12& Pleurectomy: 3 Decortication: 2 Chest wall resection: 1 |
0.3836 |
| Duration of the thoracic part of Ivor Lewis procedure [min] | 142 (48–423)$ | <0.0001 | |
| Total duration of surgery [min] | 306 (177–635) | 180.5 (78–356) | <0.0001 |
| Blood loss [ml] | 600 (50–4800)* | 500 (50–3000) | 0.221 |
| Peridural anesthesia | 57 (68.7%) | 49 (50%) | 0.015 |
ILE = Ivor Lewis esophagectomy. MPR = major pulmonary resection. #Including 3 lobectomies and 5 wedge resections. &Including 3 segmentectomies and 9 wedge resections. §Including appendectomy, resection of a soft tissue tumor and hemithyroidectomy. $Not available retrospectively in 9 patients. *Not available retrospectively in 2 patients.
Inflammation
Blood leukocytes on POD 0–2 were higher in patients of the MPR-group compared to the ILE-group. Later on POD 8-9, there was a tendency towards slightly higher leukocyte counts in ILE patients (p < 0.1). No differences in serum C-reactive protein (CRP) values were observed on POD 0-1. CRP levels tended higher after ILE compared to MPR on POD 2 and POD 5 (p < 0.1), whereas on POD 3-4 and POD 6-10 these differences between both patient groups were statistically significant (Table 3).
Table 3.
Perioperative leukocyte counts and C-reactive protein levels.
| Variables | ILE | MPR | p-value | ||
|---|---|---|---|---|---|
| Leukocytes [giga/l] | missing values | missing values | |||
| POD 0 (on arrival at ICU) | 8.6 (2.9–29.6) | 1 | 13.1 (0.9–31.9) | 3 | <0.0001 |
| POD 1 | 10.1 (3.6–71.0) | 1 | 12.3 (4.2–30.3) | 0 | 0.0001 |
| POD 2 | 11.3 (1.8–24.6) | 0 | 12.2 (3.8–114.0) | 33 | 0.0488 |
| POD 3 | 9.7 (1.9–34.1) | 8 | 10.3 (3.6–21.3) | 34 | 0.271 |
| POD 4 | 8.3 (1.0–106.0) | 14* | 9.8 (2.5–22.9) | 42 | 0.3052 |
| POD 5 | 7.8 (3.9–21.5) | 22* | 9.5 (2.5–25.0) | 52* | 0.135 |
| POD 6 | 9.3 (3.6–26.10) | 26# | 10.0 (3.8–21.5) | 53* | 0.992 |
| POD 7 | 10.2 (2.9–29.0) | 24# | 10.4 (3.4–74.0) | 47* | 0.6968 |
| POD 8 | 11.3 (3.4–33.3) | 30# | 9.7 (3.6–28.5) | 61* | 0.0927 |
| POD 9 | 12.5 (4.5–49.7) | 30# | 10.8 (3.4–31.5) | 68# | 0.0747 |
| POD 10 | 13.6 (4.2–38.7) | 38# | 11.65 (5.7–45.7) | 74# | 0.4021 |
| C-reactive protein [mg/l] | missing values | missing values | |||
| POD 0 (on arrival at ICU) | 6.35 (0–80.1) | 3 | 6.6 (0–238.7) | 9 | 0.592 |
| POD 1 | 92.79 (31.6–226.2) | 1 | 87.25 (15.4–300.2) | 0 | 0.24 |
| POD 2 | 211.3 (82.7–359.4) | 0 | 194.5 (46.8–390.7) | 33 | 0.098 |
| POD 3 | 219.2 (68.5–403.9) | 8 | 166.7 (31.5–453.6) | 34 | 0.001 |
| POD 4 | 196.2 (30.1–410.0) | 14* | 153.4 (37.1–348.7) | 43 | 0.019 |
| POD 5 | 158.8 (37.1–539.1) | 22* | 109.1 (33.7–413.0) | 52* | 0.0776 |
| POD 6 | 137.7 (24.1–423.2) | 25# | 74.4 (17.6–338.2) | 53* | 0.004 |
| POD 7 | 147.7 (12.1–445.1) | 24# | 66.2 (3.9–408.8) | 48* | 0.0002 |
| POD 8 | 152.8 (12.0–491.9) | 31# | 85.37 (22.9–270.2) | 61* | 0.002 |
| POD 9 | 182.1 (19.0–446.9) | 32# | 73.1 (8.5–385.1) | 68# | 0.0008 |
| POD 10 | 184.3 (4.9–387.3) | 38# | 97.8 (20.4–239.4) | 74# | 0.0111 |
ILE = Ivor Lewis esophagectomy. MPR = major pulmonary resection. *Including 1 death. #Including 2 deaths.
Respiratory impairment
The cumulative duration of perioperative mechanical ventilation (invasively and non-invasively) and cumulative postoperative stay on the ICU was longer in patients after ILE (both p < 0.0001). Accordingly, the rates of postoperative ventilation (p < 0.05 at POD 1-10), re-intubation (p = 0.0058) (independently from re-do surgery making re-intubation necessary), therapy with nitric oxide (p = 0.095), and of tracheotomy (p = 0.013) were higher in patients of the ILE-group indicating higher rates of severe respiratory impairment (Tables 4 and 5). Even higher rates of pneumonia defined by the ‘Uniform Pneumonia Score’17 (39.8% vs. 20.4%, p = 0.0053) and aspiration (10.8% vs. 1%, p = 0.006) were found in patients from the ILE-group (Table 5). These factors lead to higher rates of mechanical ventilation on POD 1-10 as well as longer cumulative duration of mechanical ventilation in patients after ILE compared to MPR (ILE-group: 17.93 (5–2280) h vs. MPR-group: 9.16 (3–701) h, p < 0.0001). Markedly, there were no differences in the rates of early postoperative mechanical ventilation (POD 0) as well as initial extubation success (during initial 12 h after surgery) between both groups (Tables 4, 5). The same holds true for intra- and early postoperative OI, where no obvious differences were found between both groups. However, since the rate of reduced OI (<300 mm Hg) was significantly higher initially in patients awaiting right-sided MPR (ILE: 17 patients vs. MPR: 38 patients, p = 0.009), the rates of reduced OI vice versa were significantly higher on all POD 1-10 in patients after ILE (overall 59 patients (71.1%) from the ILE-group and 35 patients (35.7%) from the MPR-group suffered from an OI < 300 mm Hg at least once during the observational period on POD 1-10, p < 0.0001, Table 5). Nevertheless, there was no difference concerning the clinical diagnosis of ARDS between both groups (ILE: 3 patients vs. MPR: 1 patient, p = 0.334).
Table 4.
Perioperative results.
| Variables | ILE | MPR | p-value |
|---|---|---|---|
| Total postoperative hospital stay [d]$ | 16 (9–68) | 10 (6–87) | <0.0001 |
| Cumulative postoperative stay on ICU [d] | 4.64 (1–140) | 0.95 (0–66) | <0.0001 |
| Cumulative perioperative mechanical ventilation [h] | 17.93 (5–2280) | 9.16 (3–701) | <0.0001 |
| Rate of postoperative ventilation & | |||
| POD 0 (on arrival at ICU) | 66 (79.5%) | 66 (67.3%) | 0.093 |
| Invasive | 64 | 66 | |
| Non-invasive | 2 | 0 | |
| POD 1 | 45 (54.2%) | 23 (23.5%) | <0.0001 |
| Invasive | 41 | 22 | |
| Non-invasive | 4 | 1 | |
| POD 2 | 17 (20.5%) | 7 (7.1%) | 0.0142 |
| Invasive | 9 | 5 | |
| Non-invasive | 8 | 2 | |
| POD 3 | 19 (22.9%) | 8 (8.2%) | 0.0065 |
| Invasive | 13 | 7 | |
| Non-invasive | 6 | 1 | |
| POD 4 | 20 (24.4%) | 9 (9.2%) | 0.0077 |
| Invasive | 16 | 7 | |
| Non-invasive | 4 | 2 | |
| POD 5 | 19 (23.2%) | 6 (6.2%) | 0.0019 |
| Invasive | 16 | 4 | |
| Non-invasive | 3 | 2 | |
| POD 6 | 20 (24.7%) | 7 (7.2%) | 0.0015 |
| Invasive | 18 | 5 | |
| Non-invasive | 2 | 2 | |
| POD 7 | 24 (29.6%) | 7 (7.2%) | 0.0001 |
| Invasive | 20 | 5 | |
| Non-invasive | 4 | 2 | |
| POD 8 | 22 (27.2%) | 6 (6.2%) | 0.0001 |
| Invasive | 20 | 5 | |
| Non-invasive | 2 | 1 | |
| POD 9 | 19 (23.5%) | 4 (4.2%) | 0.0002 |
| Invasive | 18 | 3 | |
| Non-invasive | 1 | 1 | |
| POD 10 | 21 (25.9%) | 4 (4.2%) | <0.0001 |
| Invasive | 17 | 3 | |
| Non-invasive | 4 | 1 | |
| Blood transfusion* | 18 (21.7%) | 18 (18.4%) | 0.582 |
| Postoperative catecholamine therapy §,& | |||
| POD 0 | 29 (34.9%) | 17 (17.3%) | 0.0098 |
| POD 1 | 30 (36.1%) | 14 (14.3%) | 0.0009 |
| POD 2 | 21 (25.3%) | 6 (6.1%) | 0.0003 |
| POD 3 | 22 (26.5%) | 8 (8.2%) | 0.0012 |
| POD 4 | 17 (20.7%) | 7 (7.1%) | 0.0086 |
| POD 5 | 14 (17.1%) | 4 (4.1%) | 0.0053 |
| POD 6 | 13 (16.0%) | 5 (5.2%) | 0.0232 |
| POD 7 | 17 (21.0%) | 4 (4.1%) | 0.0007 |
| POD 8 | 15 (18.5%) | 4 (4.1%) | 0.0027 |
| POD 9 | 16 (19.8%) | 2 (2.1%) | <0.0001 |
| POD 10 | 17 (21.0%) | 1 (1.0%) | <0.0001 |
| Return to ICU | 16 (19.3%) | 12 (12.2%) | 0.22 |
| Re-do (revision) surgery | 12 (14.5%) | 6 (6.1%) | 0.0811 |
| Bronchial stump insufficiency after MPR/Anastomotic complications after ILEß | 13 (15.7%) | 1 (1.0%) | |
| Organ Failure | |||
| Liver | 4 (4.8%) | 1 (1.0%) | 0.181 |
| Kidney | 5 (6.0%) | 5 (5.2%) | 1 |
| Dialysis | 5 (6.0%) | 1 (1.0%) | 0.095 |
| In-hospital mortality§ | 11 (13.3%) | 5 (5.1%) | 0.07 |
ILE = Ivor Lewis esophagectomy. MPR = major pulmonary resection. POD = postoperative day. ICU = intensive care unit. $ excluding patients, who suffered from in-hospital mortality. *Within the first 24 hours after surgery, including blood transfusions, thrombocyte concentrates and fresh frozen plasma. §Including arterenol and/or dobutamine. & Patients who died during POD 0–10 (n = 4, two in each group) were excluded from further analysis after their death. ß anastomotic complications, i.e. insufficiency and/or gastric tube necrosis requiring therapy (i.e. stent, endo-vacuum therapy or re-do surgery). § even exceeding 30-day mortality. The 30-day in-hospital mortality was 8.4% in the ILE-group and 5.1% in the MPR-group.
Table 5.
Pulmonary outcome.
| Variables | ILE | MPR | p-value | ||
|---|---|---|---|---|---|
| Pneumonia rate | 33 (39.8%) | 20 (20.4%) | 0.0053 | ||
| Pneumonia - diagnosis on POD | 6 (0–25)§ | 3 (1–17) | 0.0052 | ||
| Tracheotomy | 10 (12.0%) | 2 (2.0%) | 0.013 | ||
| ECMO | 4 (4.8%) | 1 (1.0%) | 0.181 | ||
| NO therapy | 3 (3.6%) | 0 | 0.095 | ||
| Aspiration | 9 (10.8%) | 1 (1.0%) | 0.006 | ||
| Initial extubation during first 12 h postoperatively | 66 (79.5%) | 88 (89.8%) | 0.0614 | ||
| Initial extubation ≥ POD 10 | 4 (4.8%) | 1 (1.0%) | 0.1809 | ||
| Re-intubation$ | 22 (26.5%) | 10 (10.2%) | 0.0058 | ||
| Oxygenation Index | missing values | missing values | |||
| First intraoperative | 416.8 (76.32–749.2) | 1 | 425.8 (68.35–1219) | 2 | 0.572 |
| <300 mm Hg [n patients] | 17 (20.7%) | 1 | 38 (39.6%) | 0.009 | |
| PEEP [mm Hg] | 5 (1–12) | 5 (0–13) | 0.3 | ||
| Last intraoperative | 290.6 (55.79–655.7) | 252.6 (43.33–637.9) | 0.84 | ||
| <300 mm Hg [n patients] | 46 (55.4%) | 56 (57.1%) | 0.8808 | ||
| PEEP [mm Hg] | 5 (0–12) | 5 (0–8) | 0.207 | ||
| POD 0 (on arrival at ICU) | 381.6 (87.33–870.0) | 0 | 401.4 (103.0–828.9) | 5 | 0.188 |
| <300 mm Hg [n patients] | 27 (32.5) | 18 (18.4%) | 0.0379 | ||
| POD 1 | 325.3 (113.8–843.3) | 0 | 395.0 (128.0–1020) | 14 | 0.0003 |
| <300 mm Hg [n patients] | 30 (36.1%) | 15 (15.3%) | 0.0017 | ||
| POD 2 | 264.7 (83.17–685.0) | 20 | 297.2 (112.3–483.3) | 74 | 0.195 |
| <300 mm Hg [n patients] | 39 (47.0%) | 13 (13.3%) | <0.0001 | ||
| POD 3 | 259.9 (65.5–523.3) | 38 | 263.3 (160.8–556.7) | 80 | 0.784 |
| <300 mm Hg [n patients] | 29 (34.9%) | 12 (12.2%) | 0.0003 | ||
| POD 4 | 236.0 (65.8–642.9) | 44* | 260.7 (110.7–646.7) | 78 | 0.671 |
| <300 mm Hg [n patients] | 26 (31.7%) | 17 (17.3%) | 0.0346 | ||
| POD 5 | 236.1 (101.9–642.0) | 50* | 284.1 (106.3–560.0) | 83* | 0.392 |
| <300 mm Hg [n patients] | 22 (26.8%) | 10 (10.3%) | 0.0057 | ||
| POD 6 | 235.0 (106.6–419.5) | 54# | 317.7 (193.7–476.7) | 84* | 0.002 |
| <300 mm Hg [n patients] | 25 (30.9%) | 6 (6.2%) | <0.0001 | ||
| POD 7 | 227.1 (108.9–666.7) | 59# | 320.0 (158.0–790.5) | 85* | 0.0095 |
| <300 mm Hg [n patients] | 20 (24.7%) | 6 (6.2%) | 0.0006 | ||
| POD 8 | 189.6 (95.33–685.0) | 59# | 420.0 (120.3–642.9) | 87* | 0.0042 |
| <300 mm Hg [n patients] | 22 (27.2%) | 5 (5.2%) | <0.0001 | ||
| POD 9 | 218.1 (75.29–533.2) | 60# | 219.9 (115.1–350.0) | 91# | 0.883 |
| <300 mm Hg [n patients] | 19 (23.5%) | 6 (6.3%) | 0.0019 | ||
| POD 10 | 218.8 (80.32–523.3) | 60# | 238.7 (104.9–389.2) | 91# | 0.922 |
| <300 mm Hg [n patients] | 19 (23.5%) | 6 (6.3%) | 0.0019 | ||
ILE = Ivor Lewis esophagectomy. MPR = major pulmonary resection. POD = postoperative day. ECMO = extracorporal membrane oxygenation. NO = nitric oxide. §One patient suffered from preoperative pneumonia. $Independently from re-do (revision) surgery. Missing values for the postoperative oxygenation index come into account through discharge from ICU or death. The oxygenation index of patients intraoperatively as well as staying postoperatively on ICU is given in median(range). Patients not staying postoperatively on ICU are considered not to have any respiratory impairment (oxygenation index ≥300 mm Hg). *Including 1 death. #Including 2 deaths.
As revealed by the Spearman’s Rho test, a correlation between total duration of surgery and early postoperative OI (POD 1-3), as well as cumulative duration of perioperative mechanical ventilation can be excluded (Table 6).
Table 6.
Results of Spearman’s Rho rank correlation.
| Cumulative perioperative mechanical ventilation [h] | Oxygenation index | |||||
|---|---|---|---|---|---|---|
| First intraoperative [mm Hg] | Last intraoperative [mm Hg] | POD 1 [mm Hg] | POD 2 [mm Hg] | POD 3 [mm Hg] | ||
| Total duration of surgery [min] | ||||||
| ILE-group | ||||||
| Correlation coefficient | 0.186 | 0.118 | 0.084 | −0.095 | −0.171 | −0.089 |
| p-value (two-sided) | 0.092 | 0.29 | 0.45 | 0.394 | 0.182 | 0.563 |
| missing data | 0 | 1 | 0 | 0 | 20 | 38 |
| Total duration of surgery [min] | ||||||
| MPR-group | ||||||
| Correlation coefficient | 0.141 | 0.02 | 0.94 | −0.041 | 0.295 | −0.053 |
| p-value (two-sided) | 0.166 | 0.849 | 0.358 | 0.712 | 0.162 | 0.836 |
| missing data | 0 | 2 | 0 | 14 | 74 | 80 |
ILE = Ivor Lewis esophagectomy. MPR = major pulmonary resection. The cumulative duration of mechanical ventilation as well as perioperative oxygenation indexes were independent from the duration of surgery.
Kaplan-Meier analysis was performed to compare the cumulative incidences of postoperative pneumonia as well as postoperative respiratory impairment (OI < 300 mm Hg) over time. Pneumonia was more prevalent after ILE during POD 0–30 compared to right-sided thoracotomy for MPR (p = 0.042). This difference occurs beyond POD 10 (Fig. 1). In line with the differences observed in postoperative rates of reduced OI between both groups (Table 5), the cumulative incidence of respiratory impairment (OI < 300 mm Hg) differed significantly among the groups (p < 0.001) from POD 3 onwards (Fig. 2).
Figure 1.
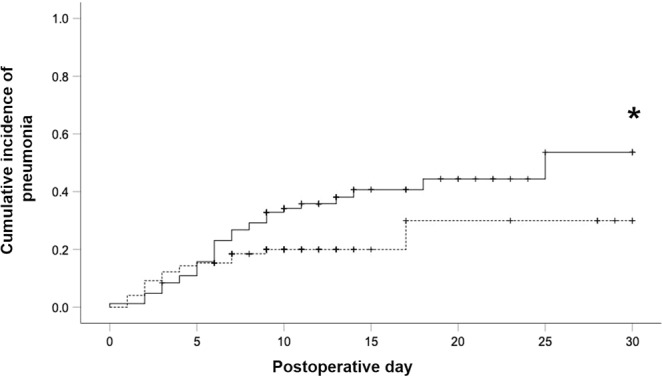
Kaplan-Meier estimation of cumulative incidence of postoperative pneumonia. Black line: Ivor Lewis esophagectomy (ILE)-group, n = 83 patients. Dashed line: Major pulmonary resection (MPR)-group, n = 98 patients. Patients who were discharged or died were censored from the analysis of cumulative incidence of postoperative pneumonia since the day of the event. Censored data are indicated in the figure by vertical ticks. *Indicates differences in the cumulative incidence of postoperative pneumonia between both groups at postoperative day 30 (p = 0.042).
Figure 2.
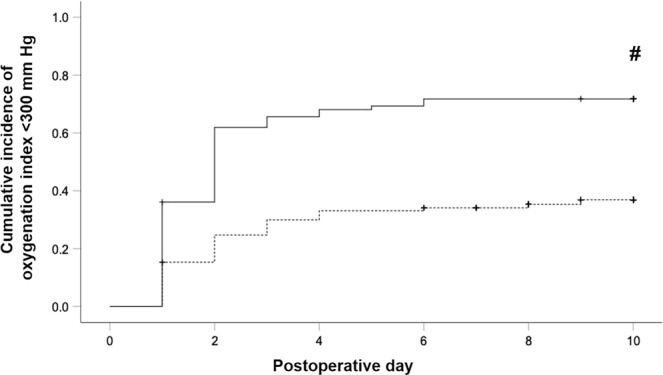
Kaplan-Meier estimation of cumulative incidence of postoperative oxygenation index <300 mm Hg indicating respiratory impairment. Black line: Ivor Lewis esophagectomy (ILE)-group, n = 83 patients. Dashed line: Major pulmonary resection (MPR)-group, n = 98 patients. Patients who were discharged, died or suffered from re-do (revision) surgery were censored from the analysis of cumulative incidence of postoperative reduced oxygenation index (<300 mm Hg) since the day of the event. Censored data are indicated in the figure by vertical ticks. An oxygenation index of <300 mm Hg on postoperative day 0 (arrival on ICU) was not judged as postoperative event. #Indicates differences in the cumulative incidence of postoperative respiratory impairment (oxygenation index <300 mm HG) between both groups at postoperative day 10 (p < 0.001).
Discussion
Respiratory complications are one major clinical problem in thoracic surgery, leading to unacceptably high rates of morbidity, disability and mortality3–9,11. Several studies have shown extraordinary high rates of pulmonary complications, especially of up to 40% pneumonia and up to 25% ARDS after ILE7,12,18,19. Perioperative atelectasis due to single-lung ventilation, postoperative pain after thoracotomy impairing respiratory physiology, manipulation and injury of the thoracic cavity and the lung during surgery, as well as potential laryngeal nerve injury caused by extended lymph node dissection or cervical esophageal preparation with an increased postoperative risk for aspiration were suggested to contribute to high pulmonary complication rates9,13,14. Except the obviously higher rate of aspiration due to delayed gastric emptying and a loss of the functional esophago-gastric junction in ILE-patients (10.8%, p = 0.006), these factors apply not only to ILE, but also to MPR with conventional thoracotomy. However, the previously reported pulmonary complication rates, especially for pneumonia (up to 6%20–22) and ARDS (approximately 4% in the early phase after anatomic lung resection20,23) are considerably lower after MPR compared with ILE, although – as shown in the present study – the rate of preexisting chronic pulmonary diseases is obviously higher in MPR patients.
In the present study, the complication rates after ILE correspond to those of previously reported patient cohorts7. Especially the rate of postoperative respiratory insufficiency leading to re-intubation of ILE patients is similar or even lower than those reported in the recent literature7,24. Nevertheless, pneumonia was assessed retrospectively through the “Uniform Pneumonia Score”17. The revised scoring system by Weijs et al.17 was herein applied in a slightly modified version with regard to the current “International Guidelines for the Management of Severe Sepsis and Septic Shock 2012”25 by using a body temperature ≥38.0 °C or ≤36.0 °C. This resulted in a high sensitivity of the scoring system for retrospective assessment of pneumonia in our patient cohort and a slightly higher rate of postoperative pneumonia of ILE and MPR patients (39.8% and 20.4%, respectively) than those reported in the recent literature7,15,16,24.
In addition to the clinical diagnoses of pneumonia and ARDS, the Horovitz OI (PaO2/FiO2) below 300 mm Hg was used as a sensitive indicator of respiratory impairment, irrespective of the underlying pulmonary disease26. Of note, the clinical diagnosis of ARDS (acute onset, bilateral pulmonary infiltrates, reduced OI26) was made in only a minority of our patients with an OI < 300 mm Hg. To the best of our knowledge, we are the first to describe an extraordinarily high cumulative incidence of respiratory impairment during POD 1–10 after ILE that is significantly higher than that of MPR patients (p < 0.001). In the light of a higher preoperative prevalence of preexisting chronic lung disease in MLR patients that is reflected by the first intraoperative OI value, the poor lung function of ILE patients on POD 1 and thereafter is even more striking. However, in both surgical approaches (ILE or MPR) the access to the thoracic cavity was the same standardized right-sided anterolateral thoracotomy and all patients underwent single-lung ventilation as well as most patients underwent disease-specific mediastinal lymph node dissection.
Development of postoperative respiratory complications after ILE and MPR is certainly a multifactorial process9: surgical tissue damage leads to an extensive inflammatory response, which may increase pulmonary endothelial permeability. Vascular leakage was previously suspected to contribute to acute lung injury and respiratory impairment after esophagectomy27–30. This would explain the early postoperative onset of ARDS after esophagectomy reported by Howells et al.12. However, pulmonary vascular leakage was not directly investigated in our study.
Among the various factors that can lead to respiratory impairment, transfusion-related acute lung injury is not expected to be responsible for the differences between ILE and MPR, because transfusion rates did not differ. The duration of single-lung ventilation was reported as a significant contributing factor for postoperative ARDS in the study by Tandon et al.21, while it did not seem to contribute to postoperative ARDS in the study by Morita et al.31. In our study, the total surgical procedure of ILE lasted longer than that of MPR. However, in contrast to the results of Tandon et al.21, the duration of the thoracic part of ILE was significantly shorter compared to MPR in our study (p < 0.0001). Hence, we can also exclude that differences in the duration of single-lung ventilation are responsible for the differences in respiratory impairment between ILE and MPR.
Also, the extent of tissue damage and the resulting release of pro-inflammatory damage-associated molecular patterns have to be considered. ILE is expected to generally cause more tissue damage compared to MPR, because, in addition to the surgical manipulations in the thorax, extensive surgery is performed in the abdominal cavity. However, MPR patients had higher leukocyte counts until POD 2, which might be explained by preoperative inflammation in this patient group. Further, CRP levels were similar on POD 1 and POD 2 and started to be higher in ILE patients as late as POD 3. Hence, the increased CRP levels in ILE patients are probably not directly related to differences in the extent or localization of surgery-associated trauma. However, we cannot fully exclude differences in trauma-associated inflammation, because unfortunately no pro-calcitonin or cytokine data were available in this retrospective study. Inflammatory markers should be carefully investigated in future prospective studies. In addition to trauma-associated inflammation caused by a more extensive surgery, the second operation site (laparotomy or laparoscopy) in ILE procedures could also contribute to pulmonary complications by increasing postoperative pain. For trans-thoracic procedures it is well known that minimally-invasive approaches result in reduced postoperative pain and lower pulmonary complication rates compared to conventional open thoracic surgery16,31–33. This has not only been shown for totally but also for hybrid minimally-invasive ILE procedures34–37. Briez et al. found that a hybrid minimally-invasive approach with laparoscopy for ILE procedures as well as pain management with epidural anesthesia are independent factors predicting against major postoperative pulmonary complications35. Also in a recently published well-controlled multicenter trial, Mariette et al. discussed reduced pain in response to their laparoscopic, hybrid minimally-invasive approach to ILE as the reason for a decreased rate of major pulmonary complications36. Further studies are needed to clarify this potential protective effect, especially with regard to perioperative oxygenation indexes.
The rate of neoadjuvant treatment clearly differed between both groups. The role of multimodal therapeutic approaches and especially induction therapies prior to ILE as a cause for an increased rate of postoperative pulmonary complications is still disputed. Reynolds et al. reported a higher rate of postoperative septic and pulmonary complications in patients, who underwent esophagectomy after induction therapy38, whereas Zingg et al. did not identify neoadjuvant treatment as a risk factor for postoperative pulmonary complications in their large patient cohort3. If alterations of the lung parenchyma including radiation pneumonitis and lung fibrosis, which can be observed in patients after irradiation of thoracic carcinomas39, play a role in the pathogenesis of perioperative respiratory impairment and postoperative pulmonary complications remains to be elucidated. However, an increased inflammatory status in ILE patients following radio-chemo induction should be reflected in an early elevation of leukocyte counts and CRP levels, which was obviously not the case.
Furthermore, the role of anastomotic complications in the development of respiratory impairment remains unclear. In this patient cohort only two patients of the ILE-group presented respiratory insufficiency (OI < 300 mm Hg) synchronously with the clinical diagnosis of anastomotic leakage requiring treatment. The other ILE patients either developed no respiratory impairment postoperatively (n = 1), presented a reduced OI (<300 mm Hg) metachronously (≥three days before anastomotic leakage, n = 4 patients) or were excluded from the Kaplan Meier analysis for a cumulative incidence of reduced postoperative OI due to re-do surgery. If the OI is sufficient to predict postoperative (pulmonary) complications in abdomino-thoracic surgery had – to the best of our knowledge – never been investigated before and should be evaluated in lager patient cohorts.
The surgical access route by thoracotomy is, however, notoriously a cause for postoperative pain, impairing postoperative breathing and respiratory physiology9. Previous reports have shown not only a reduction in pulmonary complications and pneumonia rates in patients undergoing (hybrid) minimally-invasive approaches for ILE or MPR as discussed above but also for abdominal-only transhiatal approaches for distal esophagectomy3,13,14,32,33,40–43. Especially after transhiatal esophagectomy, the rate of pulmonary complications is lower compared with the transthoracic approach12–14,40. However, the extent of mediastinal lymph node dissection and the location regarding the thoracic height of intrathoracic resection margins of the esophagus itself is technically limited by transhiatal esophageal surgery.
Nevertheless, the localization of esophageal resection margins with regard to the mediastinal level after transthoracic or transhiatal esophagectomy could explain the reported high rate of respiratory impairment in ILE patients as well as the observed major differences between both groups: some kind of neurogenic pulmonary damage has to be considered and discussed as a possible cause for the high rate of respiratory impairment after ILE. A dysbalance in the autonomic nervous system with a sympathetic hyperreaction is well-known in patients after brain damage, including the medulla and hypothalamic injury leading to neurogenic derived pulmonary edema formation by pulmonary vasculature constriction and pressure overload44–46. Vice versa, it had been shown in animal experiments that formation of pulmonary edema was either caused by bilateral (cervical) vagotomy44 or prevented by sympathetic denervation46. During ILE, surgeons usually transect the vagus nerve at the level, where the azygos vein joins the vena cava, which results in extensive or even complete vagal denervation of the lung47. Vagal branches carrying all parasympathetic and the vast majority of sensory fibers to the lung emerge from the vagal main trunk distally from this transection site down to the inferior margin of the main bronchus, so that this pathway is interrupted by cutting the vagus nerve more cranially47. Contrastingly, the sympathetic fibers innervating pulmonary structures, together with a few spinal sensory fibers, derive from the paravertebrally located sympathetic trunk, take a route along the bronchial artery47,48 and are obviously not injured to the same extent as parasympathetic and vagal sensory fibers during surgery. A vagus-sparing esophagectomy technique previously showed reductions in the rate of postoperative pneumonia, ARDS and delayed gastric emptying. This technique was, however, not combined with mediastinal lymph node assessment and is not suitable for oncologic surgical purposes with regard to oncological radicality47,49. A frequent and well-known postoperative clinical manifestation of bilateral truncal vagotomy during ILE is delayed gastric emptying and gastric outlet obstruction through pyloric denervation and consecutive spasm50. Even the high rate of postoperative atrial fibrillation after ILE might be partially caused by similar imbalances in the autonomic nervous system through higher mediastinal denervation41,48,51. We hypothesize, that a classical ILE with high thoracic esophageal resection margins and mediastinal lymph node dissection even leads to parasympathetic and sensory denervation of trachea, bronchi and pulmonary vasculature (especially pulmonary arteries) by vagotomy, the consecutive dysbalance of the autonomic nervous system innervating pulmonary structures would result in a higher sympathetic drive and thus may impair respiratory function and gas exchange widely analogous to the situation of neurogenic pulmonary edema.
Retrospectively conducted patient analyses like this study are only suited to generate hypotheses, which should be further tested in larger prospectively conducted trials with a high standardization of surgical procedures. Based on our findings, respiratory impairment beyond pulmonary complications after ILE need to be further evaluated, because these will have a major negative impact on the outcome of affected patients and prevention of postoperative pulmonary impairment is mandatory. This study shows, that beyond the surgical access route and the necessity of single-lung ventilation during surgery or inflammatory marker profile, other causes of lung function impairment after ILE must be considered. The impact of a dysbalanced autonomic nervous system on the perioperative pulmonary and cardiac function caused by ILE due to the concomitant vagotomy should therefore be examined in further prospectively trials, that should include e.g. a perioperative monitoring of heart rate variability, pulmonary arterial wedge pressure, pulmonary edema formation and immunological parameters.
Materials and Methods
Patients
The study was performed in accordance to the latest version of the Declaration of Helsinki and was approved by the local ethics committee of University of Giessen (AZ214/15). As this study was conducted retrospectively using routine patient data and as patient data were evaluated fully anonymized, written informed consent of the retrospectively included patients was waived by the local ethics committee in accordance to local legislation. 181 consecutive patients who underwent right-sided anterolateral thoracotomy for either ILE (n = 83) or MPR (lobectomy and bi-lobectomy, n = 98) were retrospectively identified during a seven-year period and included into this single-center analysis. Patients who underwent ILE together with any kind of pulmonary resection were included in the ILE-group. Patients who underwent left-sided thoracic surgery, right-sided anterolateral thoracotomy with minor lung resection (less than lobectomy: segmentectomy or (multiple) wedge resection) or pneumonectomy as well as patients who underwent transhiatal esophagectomy without thoracotomy were excluded from the analysis. All patients were treated according to the institutional standard-of-care. Patient data were analyzed retrospectively from the prospectively maintained institutional database. Patient data were analyzed during the first ten postoperative days (POD 0–10) regarding the Horovitz oxygenation index (OI), a key parameter to evaluate perioperative pulmonary function. OI-values were available for patients staying on ICU. Patients on a normal ward were excluded from analysis of OI-values and indicated in the tables as “missing values”. Consequently, discharge from the ICU was interpreted as absence of respiratory impairment or failure and an OI ≥ 300 mm Hg was assumed.
As described previously26, the OI was calculated as the ratio of the arterial pressure of oxygen (PaO2) and the fraction of inspired oxygen (FiO2) (PaO2/FiO2) at the beginning of mechanical ventilation (in most cases under double-lung ventilation), at the end of surgery, upon arrival at the ICU (POD 0), and on POD 1–10. If the PaO2 and FiO2 were assessed more than once a day, the first measurement of the day was used. According to the Berlin classification, an OI ≤ 300 mm Hg is an important criterion for the clinical definition of ARDS (mild: 201–300 mm Hg, moderate: 101–200 mm Hg and severe: ≤100 mm Hg)26. For mechanically ventilated patients (either invasively or non-invasively) the FiO2 was available. Patients who were not mechanically ventilated (invasively or non-invasively), a FiO2 of 30% was anticipated. An OI < 300 mm Hg was considered to indicate respiratory impairment or failure.
Surgery
Right-sided anterolateral thoracotomy in the 4th or 5th intercostal space was the standard procedural access to the thoracic cavity for ILE as well as right-sided MPR. Regarding the local clinical standard ILE was performed as a synchronous two-stage procedure with laparotomy or laparoscopy for gastric tube building and gastric pull-up followed by an anterolateral thoracotomy on the right side to complete the subtotal esophagectomy and reconstruct the esophago-gastric continuity. Two-field lymph node dissection was performed as the standard procedure during oncologic esophagectomy, except in cases of cervical anastomosis, lymph node dissection was completed as a three field procedure following international recommendations1. The decision for laparoscopy or laparotomy was made based on the surgeon’s preferences and tumor stages. In cases of colon reconstruction, patients underwent primary laparotomy. Gastric tube was stapled from the small gastric curvature and anastomosis was usually made by using circular stapler devices. The time of the thoracic incision or of the intraoperative rearrangement of the patients into a lateral decubitus position for thoracotomy, respectively, was used to estimate retrospectively the time spent for of the transthoracic part of ILE, indicating the duration of single-lung ventilation during two-stage ILE.
For right-sided anatomic MPR a standard anterolateral thoracotomy in the 4th or 5th intercostal space was performed. Hilar structures were dissected and individually ligated, sutured or stapled. Systematic lymph node dissection in patients undergoing MPR for primary lung cancer was performed according to international recommendations52,53. Principles of the institutional standard in systematic lymph node dissection during lung cancer surgery were described previously in more detail in a patient cohort undergoing minimally-invasive oncologic pulmonary surgery by video-assisted thoracoscopy54.
In the postoperative phase, patients were treated by principles of an “enhanced recovery after surgery” protocol with extubation as soon as possible, early enteral nutrition and mobilization at the earliest convenience55. Usually, patients were monitored at the ICU after ILE or MPR for at least for one day (POD 1) and – if cardiorespiratorily stable – discharged from the ICU. One important clinical criterion for discharge from the ICU to a normal ward after major thoracic surgery is an adequate respiratory function. Patients on normal ward are classified as “normal oxygenation” with an OI ≥ 300 mm Hg (“no respiratory impairment” according to the ARDS definition26) to make them comparable to patients on ICU with regard to the rate of postoperative respiratory impairment or failure (OI < 300 mm Hg). Pneumonia was assessed and defined retrospectively in this study during the hospital stay using the “Uniform Pneumonia Score” introduced initially by van der Sluis et al. and revised by Weijs et al. as a standardized methodology to define pneumonia after esophagectomy17,56. However, apart from the revised “Uniform Pneumonia Score”, but according to current “International Guidelines for the Management of Severe Sepsis and Septic Shock 2012” we decided to use a body temperature ≥38.0 °C or ≤36.0 °C, respectively, as the threshold for pneumonia scoring in our study17,25.
Statistical analyses
Statistical analyses were performed using GraphPad Prism (Version 5.00 for Windows, GraphPad Software, San Diego California USA, www.graphpad.com) for two group comparisons and SPSS (IBM SPSS Statistics for Windows, Version 24.0).
For descriptive statistics, data of both groups were analyzed using Fisher’s exact or Pearson’s X2 test for categorical data in cross-tabulation. Two group comparisons were performed by Mann-Whitney-U test. Patients who died were censored from analysis upon the day of death (indicated in the tables).
To determine statistical dependence, the total duration of surgery was correlated with the duration of cumulative (perioperative) mechanical ventilation as well as the intraoperative and postoperative (POD 1–3) OI by Spearman’s Rho rank correlation coefficient.
Cumulative incidences of postoperative pneumonia during POD 0–30 and postoperative respiratory impairment or failure (defined by an OI < 300 mm Hg) during POD 1–10 of patients after ILE or MPR were analyzed by Kaplan-Meier estimator. Patients who were discharged or died were censored from the analysis of cumulative incidences of postoperative pneumonia since the day the event. Patients who were discharged, died or underwent re-do surgery were censored from the analysis of cumulative incidences of postoperative reduced OI (<300 mm Hg) since the day of the event. Censored data are indicated in the figures by vertical ticks. An OI < 300 mm Hg on POD 0 (arrival on ICU) was not judged as postoperative event.
Data are given in tables as median and ranges; p-values ≤ 0.05 were considered to indicate statistical significance.
Compliance with ethical standards
The data are collected, the manuscript is written and submitted in accordance to the COPE guidelines.
The acquisition of data and the study was formally approved by the local ethics committee of University of Giessen (AZ214/15). As this is a retrospective, fully anonymized patient data analysis, for this type of study formal written consent from the patients is waived by the local ethics committee in accordance to local legislation. All patients were treated by the current local standard of care. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Acknowledgements
We thank Professor Wolfgang Kummer from the Institute of Anatomy and Cell Biology, Justus-Liebig-University Giessen, Germany, for helpful discussions.
Author Contributions
M.R., F.U., C.K., M.W. and A.H. made substantial contributions of the conception and design of the work. M.R. and M.S. obtained the data. M.R. and F.U. analyzed the data. M.R., J.B., M.H., R.H., V.G., W.P., M.W. and A.H. interpreted and discussed the data substantially. M.R. and F.U. drafted the work. C.K., J.B., M.H., R.H., V.G., W.P., M.W. and A.H. revised the work substantively. All authors read and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 2.McCulloch P, Ward J, Tekkis PP. Mortality and morbidity in gastro-oesophageal cancer surgery: initial results of ASCOT multicentre prospective cohort study. BMJ. 2003;327:1192–1197. doi: 10.1136/bmj.327.7425.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zingg U, et al. Factors associated with postoperative pulmonary morbidity after esophagectomy for cancer. Ann. Surg. Oncol. 2011;18:1460–1468. doi: 10.1245/s10434-010-1474-5. [DOI] [PubMed] [Google Scholar]
- 4.Avendano CE, Flume PA, Silvestri GA, King LB, Reed CE. Pulmonary complications after esophagectomy. Ann. Thorac. Surg. 2002;73:922–926. doi: 10.1016/S0003-4975(01)03584-6. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson MK, Durkin AE. Preoperative prediction of the risk of pulmonary complications after esophagectomy for cancer. J. Thorac. Cardiovasc. Surg. 2002;123:661–669. doi: 10.1067/mtc.2002.120350. [DOI] [PubMed] [Google Scholar]
- 6.Law S, Wong KH, Kwok KF, Chu KM, Wong J. Predictive factors for postoperative pulmonary complications and mortality after esophagectomy for cancer. Ann. Surg. 2004;240:791–800. doi: 10.1097/01.sla.0000143123.24556.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blencowe NS, et al. Reporting of short-term clinical outcomes after esophagectomy: A systematic review. Ann. Surg. 2012;255:658–666. doi: 10.1097/SLA.0b013e3182480a6a. [DOI] [PubMed] [Google Scholar]
- 8.Seesing MFJ, et al. Defining pneumonia after esophagectomy for cancer: Validation of the Uniform Pneumonia Score in a high volume center in North America. Dis. Esophagus. 2018;31:1–8. doi: 10.1093/dote/doy002. [DOI] [PubMed] [Google Scholar]
- 9.Molena D, Mungo B, Stem M, Lidor AO. Incidence and Risk Factors for Respiratory Complications in Patients Undergoing Esophagectomy for Malignancy: A NSQIP Analysis. Semin. Thorac. Cardiovasc. Surg. 2014;26:287–294. doi: 10.1053/j.semtcvs.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Kinugasa S, et al. Postoperative pulmonary complications are associated with worse short- and long-term outcomes after extended esophagectomy. J. Surg. Oncol. 2004;88:71–77. doi: 10.1002/jso.20137. [DOI] [PubMed] [Google Scholar]
- 11.Park KU, Rubinfeld I, Hodari A, Hammoud Z. Prolonged Length of Stay after Esophageal Resection: Identifying Drivers of Increased Length of Stay Using the NSQIP Database. J. Am. Coll. Surg. 2016;223:286–290. doi: 10.1016/j.jamcollsurg.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 12.Howells P, et al. The impact of the acute respiratory distress syndrome on outcome after oesophagectomy. Br. J. Anaesth. 2016;117:375–381. doi: 10.1093/bja/aew178. [DOI] [PubMed] [Google Scholar]
- 13.Boshier PR, Anderson O, Hanna GB. Transthoracic versus transhiatal esophagectomy for the treatment of esophagogastric cancer: A meta-analysis. Ann. Surg. 2011;254:894–906. doi: 10.1097/SLA.0b013e3182263781. [DOI] [PubMed] [Google Scholar]
- 14.Bhayani NH, et al. Esophagectomies with thoracic incisions carry increased pulmonary morbidity. JAMA Surg. 2013;148:733–738. doi: 10.1001/jamasurg.2013.2356. [DOI] [PubMed] [Google Scholar]
- 15.Sawada S, Suehisa H, Ueno T, Yamashita M. Changes in post-operative complication and mortality rates after lung cancer resection in the 20-year period 1995–2014. Acta Med. Okayama. 2016;70:183–188. doi: 10.18926/AMO/54417. [DOI] [PubMed] [Google Scholar]
- 16.Laursen LØ, et al. Video-assisted thoracoscopic surgery lobectomy for lung cancer is associated with a lower 30-day morbidity compared with lobectomy by thoracotomy. Eur. J. Cardio-thoracic Surg. 2016;49:870–875. doi: 10.1093/ejcts/ezv205. [DOI] [PubMed] [Google Scholar]
- 17.Weijs Teus J., Seesing Maarten F. J., van Rossum Peter S. N., Koëter Marijn, van der Sluis Pieter C., Luyer Misha D. P., Ruurda Jelle P., Nieuwenhuijzen Grard A. P., van Hillegersberg Richard. Internal and External Validation of a multivariable Model to Define Hospital-Acquired Pneumonia After Esophagectomy. Journal of Gastrointestinal Surgery. 2016;20(4):680–687. doi: 10.1007/s11605-016-3083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tandon S, et al. Peri-operative risk factors for acute lung injury after elective oesophagectomy. Br. J. Anaesth. 2001;86:633–638. doi: 10.1093/bja/86.5.633. [DOI] [PubMed] [Google Scholar]
- 19.Howells PA, et al. ARDS following oesophagectomy: a comparison of two trials. BMJ open Respir. Res. 2017;4:e000207. doi: 10.1136/bmjresp-2017-000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Licker M, et al. Impact of intraoperative lung-protective interventions in patients undergoing lung cancer surgery. Crit. Care. 2009;13:1–10. doi: 10.1186/cc7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitson BA, Groth SS, Duval SJ, Swanson SJ, Maddaus MA. Surgery for Early-Stage Non-Small Cell Lung Cancer: A Systematic Review of the Video-Assisted Thoracoscopic Surgery Versus Thoracotomy Approaches to Lobectomy. Ann. Thorac. Surg. 2008;86:2008–2018. doi: 10.1016/j.athoracsur.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Yan TD, Black D, Bannon PG, McCaughan BC. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J. Clin. Oncol. 2009;27:2553–2562. doi: 10.1200/JCO.2008.18.2733. [DOI] [PubMed] [Google Scholar]
- 23.Amar D, et al. Protective lung ventilation and morbidity after pulmonary resection: A propensity score-matched analysis. Anesth. Analg. 2017;125:190–199. doi: 10.1213/ANE.0000000000002151. [DOI] [PubMed] [Google Scholar]
- 24.Michelet P, et al. Non-invasive ventilation for treatment of postoperative respiratory failure after oesophagectomy. Br. J. Surg. 2009;96:54–60. doi: 10.1002/bjs.6307. [DOI] [PubMed] [Google Scholar]
- 25.Hecker A., Reichert M., Reuß C. J., Schmoch T., Riedel J. G., Schneck E., Padberg W., Weigand M. A., Hecker M. Intra-abdominal sepsis: new definitions and current clinical standards. Langenbeck's Archives of Surgery. 2019;404(3):257–271. doi: 10.1007/s00423-019-01752-7. [DOI] [PubMed] [Google Scholar]
- 26.Ranieri VM, et al. Acute respiratory distress syndrome: The Berlin definition. JAMA - J. Am. Med. Assoc. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 27.Reid PT, et al. Pulmonary endothelial permeability and circulating neutrophil-endothelial markers in patients undergoing esophagogastrectomy. Crit. Care Med. 2000;28:3161–3165. doi: 10.1097/00003246-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Shiozaki A, et al. Risk factors for postoperative respiratory complications following esophageal cancer resection. Oncol. Lett. 2012;3:907–912. doi: 10.3892/ol.2012.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boshier PR, Marczin N, Hanna GB. Pathophysiology of acute lung injury following esophagectomy. Dis. Esophagus. 2015;28:797–804. doi: 10.1111/dote.12295. [DOI] [PubMed] [Google Scholar]
- 30.Morita M, et al. Acute lung injury following an esophagectomy for esophageal cancer, with special reference to the clinical factors and cytokine levels of peripheral blood and pleural drainage fluid. Dis. Esophagus. 2008;21:30–36. doi: 10.1111/j.1442-2050.2007.00725.x. [DOI] [PubMed] [Google Scholar]
- 31.Kwon ST, et al. Evaluation of acute and chronic pain outcomes after robotic, video-assisted thoracoscopic surgery, or open anatomic pulmonary resection. J. Thorac. Cardiovasc. Surg. 2017;154:652–659.e1. doi: 10.1016/j.jtcvs.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Sihag S, et al. Comparison of perioperative outcomes following open versus minimally invasive Ivor Lewis oesophagectomy at a single, high-volume centre. Eur. J. Cardio-thoracic Surg. 2012;42:430–437. doi: 10.1093/ejcts/ezs031. [DOI] [PubMed] [Google Scholar]
- 33.Nozaki I, et al. Impact of laparoscopy on the prevention of pulmonary complications after thoracoscopic esophagectomy using data from JCOG0502: a prospective multicenter study. Surg. Endosc. 2018;32:651–659. doi: 10.1007/s00464-017-5716-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berlth F, et al. Total minimally invasive esophagectomy for esophageal adenocarcinoma reduces postoperative pain and pneumonia compared to hybrid esophagectomy. Surg. Endosc. 2018;32:4957–4965. doi: 10.1007/s00464-018-6257-2. [DOI] [PubMed] [Google Scholar]
- 35.Briez N, et al. Effects of hybrid minimally invasive oesophagectomy on major postoperative pulmonary complications. Br. J. Surg. 2012;99:1547–1553. doi: 10.1002/bjs.8931. [DOI] [PubMed] [Google Scholar]
- 36.Mariette C, et al. Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer. N. Engl. J. Med. 2019;380:152–162. doi: 10.1056/NEJMoa1805101. [DOI] [PubMed] [Google Scholar]
- 37.van der Sluis PC, et al. Robot-assisted Minimally Invasive Thoracolaparoscopic Esophagectomy Versus Open Transthoracic Esophagectomy for Resectable Esophageal Cancer: A Randomized Controlled Trial. Ann. Surg. 2019;269:621–630. doi: 10.1097/SLA.0000000000003031. [DOI] [PubMed] [Google Scholar]
- 38.Reynolds JV, et al. Neoadjuvant chemoradiation may increase the risk of respiratory complications and sepsis after transthoracic esophagectomy. J. Thorac. Cardiovasc. Surg. 2006;132:549–555. doi: 10.1016/j.jtcvs.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 39.Schroder C, Engenhart-Cabillic R, Kirschner S, Blank E, Buchali A. Changes of lung parenchyma density following high dose radiation therapy for thoracic carcinomas - an automated analysis of follow up CT scans. Radiat. Oncol. 2019;14:72. doi: 10.1186/s13014-019-1276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schlottmann F, Strassle PD, Patti MG. Transhiatal vs. Transthoracic Esophagectomy: A NSQIP Analysis of Postoperative Outcomes and Risk Factors for Morbidity. J. Gastrointest. Surg. 2017;21:1757–1763. doi: 10.1007/s11605-017-3572-1. [DOI] [PubMed] [Google Scholar]
- 41.McCormack O, et al. New-onset atrial fibrillation post-surgery for esophageal and junctional cancer: Incidence, management, and impact on short- And long-term outcomes. Ann. Surg. 2014;260:772–778. doi: 10.1097/SLA.0000000000000960. [DOI] [PubMed] [Google Scholar]
- 42.Wei MT, et al. Transthoracic vs transhiatal surgery for cancer of the esophagogastric junction: A meta-analysis. World J. Gastroenterol. 2014;20:10183–10192. doi: 10.3748/wjg.v20.i29.10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hulscher JBF, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N. Engl. J. Med. 2002;347:1662–1669. doi: 10.1056/NEJMoa022343. [DOI] [PubMed] [Google Scholar]
- 44.Sedy J, Zicha J, Kunes J, Jendelova P, Sykova E. Mechanisms of neurogenic pulmonary edema development. Physiol. Res. 2008;57:499–506. doi: 10.33549/physiolres.931432. [DOI] [PubMed] [Google Scholar]
- 45.Busl KM, Bleck TP. Neurogenic Pulmonary Edema. Crit. Care Med. 2015;43:1710–1715. doi: 10.1097/CCM.0000000000001101. [DOI] [PubMed] [Google Scholar]
- 46.Davison DL, Terek M, Chawla LS. Neurogenic pulmonary edema. Crit. Care. 2012;16:212. doi: 10.1186/cc11226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weijs TJ, et al. Topography and extent of pulmonary vagus nerve supply with respect to transthoracic oesophagectomy. J. Anat. 2015;227:431–439. doi: 10.1111/joa.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen MJ, Zipes DP. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ. Res. 2014;114:1004–1021. doi: 10.1161/CIRCRESAHA.113.302549. [DOI] [PubMed] [Google Scholar]
- 49.Weijs TJ, et al. Preserving the pulmonary vagus nerve branches during thoracoscopic esophagectomy. Surg. Endosc. 2016;30:3816–3822. doi: 10.1007/s00464-015-4683-y. [DOI] [PubMed] [Google Scholar]
- 50.Maus MKH, et al. Gastric Outlet Obstruction After Esophagectomy: Retrospective Analysis of the Effectiveness and Safety of Postoperative Endoscopic Pyloric Dilatation. World J. Surg. 2016;40:2405–2411. doi: 10.1007/s00268-016-3575-1. [DOI] [PubMed] [Google Scholar]
- 51.Park H-W, et al. Neural mechanisms of atrial fibrillation. Curr. Opin. Cardiol. 2012;27:24–28. doi: 10.1097/HCO.0b013e32834dc4e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest. 1997;111:1718–1723. doi: 10.1378/chest.111.6.1718. [DOI] [PubMed] [Google Scholar]
- 53.Lardinois D, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur. J. Cardio-thoracic Surg. 2006;30:787–792. doi: 10.1016/j.ejcts.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 54.Reichert M, et al. A standardized technique of systematic mediastinal lymph node dissection by video-assisted thoracoscopic surgery (VATS) leads to a high rate of nodal upstaging in early-stage non-small cell lung cancer. Surg. Endosc. 2016;30:1119–1125. doi: 10.1007/s00464-015-4312-9. [DOI] [PubMed] [Google Scholar]
- 55.Low Donald E., Allum William, De Manzoni Giovanni, Ferri Lorenzo, Immanuel Arul, Kuppusamy MadhanKumar, Law Simon, Lindblad Mats, Maynard Nick, Neal Joseph, Pramesh C. S., Scott Mike, Mark Smithers B., Addor Valérie, Ljungqvist Olle. Guidelines for Perioperative Care in Esophagectomy: Enhanced Recovery After Surgery (ERAS®) Society Recommendations. World Journal of Surgery. 2018;43(2):299–330. doi: 10.1007/s00268-018-4786-4. [DOI] [PubMed] [Google Scholar]
- 56.van der Sluis Pieter C., Verhage Roy J.J., van der Horst Sylvia, van der Wal Willem M., Ruurda Jelle P., van Hillegersberg Richard. A New Clinical Scoring System to Define Pneumonia following Esophagectomy for Cancer. Digestive Surgery. 2014;31(2):108–116. doi: 10.1159/000357350. [DOI] [PubMed] [Google Scholar]


