Abstract
Populations see their range fluctuate in relation to environmental variations, including climate change, and their survival is linked to the maintenance of large enough populations and broad enough distributions during these variations. Most amphibian populations are threatened by numerous ecological and anthropogenic variables acting in synergy with climate change. Accumulating basic ecological data such as range enables the development of population and range dynamics, themselves resulting on adequate conservation plans. Karsenia koreana is the only known Asian plethodontic salamander, occurring in a very restricted area only. Based on presence data, we created an ecological model using six bioclimatic factors with low multicollinearity to define the adequate habitat of the species, and we modelled the predicted suitability of the Korean landscape following four Representative Concentration Pathways (RCPs) predicting climate change scenarios based on CO2 concentrations in 2050 and 2070. The maximum entropy model for the current distribution produced a landscape suitability considerably wider than the current known distribution. The projected ranges for each RCP indicated marked increases, decreases and shifts in areas with suitable landscapes due to climate change. The lowest RCP prediction resulted in an increase in suitable area, although potentially without connectivity with current populations, while the highest RCP predictions resulted in a decrease. Our results highlight the potential negative impact of climate change, thus requiring updates in conservation plans for K. koreana. The methods used here can be replicated with any land-dwelling species, and our results reflect expected range shifts for most amphibians of the northern hemisphere.
Subject terms: Climate-change ecology, Projection and prediction
Introduction
Range variations due to climate change have been described in several species and tend to result in the shift of climate envelopes towards the poles and higher elevations1. For instance, a three degree Celsius increase matches with a 350 km shift in latitude, or a 500 m shift in altitude2. Patterns of range shifts resulting from climate change have been well documented in migratory species despite large yearly variations1,3,4, and a wide range of taxa displays such a range displacement (reviewed by Hughes, 20005). Several studies demonstrated a range shift for 22 species of non-migratory European butterflies6, Edith’s checkerspot butterfly7, mosquito species in equatorial areas8, birds, anoline lizards9, 59 British bird species10, 14 North American bird species11, 19 North American mammals12, the bacterium Pseudomonas syringae in China13 and benthic species in the Mediterranean sea14. This is also the case for amphibians, with species such as red-eyed stream frogs (Hyla uranochroa) predicted to shift distribution as a result of climate change9.
Species distribution models (SDMs) are spatial representations of species presence probability or abundance. They are created using responses to environmental predictor variables, spatial relationships such as convex hulls or interpolation, or a combination of the two. SDMs are easily projected across landscapes, and can therefore be helpful in circumstances where acquiring occurrence data on the distribution of species is not achievable, or when the ecological variables related to the distribution of the species have changed (reviewed by Guisan and Thuiller 200515). The change may result from long-term shifts in environmental variables, as illustrated by the range shift between the paleo and current distributions of the Japanese Treefrog, Dryophytes japonicus, in North East Asia16. SDMs can also be used to predict variations in the distribution of species due to future anomalies resulting from climate change9,13,14,17–19. Ecological models thus allow determining the range of species in conditions where it would not be otherwise doable or when the range boundaries are not known20–22. For instance, eight new localities of Vipera ursinii graeca were found through landscape and climate modelling, doubling the known range of the species23. Spatial modelling for some clades without clear geographic boundaries, such as fish or birds24 is inherently more complex. However, species like amphibians are perfect model species as they do not disperse over long distances25, nor do they migrate over numerous climatic zones, and thus have generally continuous distribution patterns26.
Amphibians are comparatively more endangered and less studied than their counterparts, be them flying or apex predator27, and the range of most species is not accurately defined, with many species classified as Data Deficient under the IUCN criteria28. This is especially true for geographic areas where ecological research is less widespread. For instance, the Suweon Treefrog (Dryophytes suweonensis) in the Republic of Korea saw its known range doubled between 201229 and 201730,31.
Here we focus on the only plethodontid salamander in Asia, the Korean Crevice Salamander, Karsenia koreana. It is considered one of the most elusive Korean amphibian species, and although it was first collected in 197132, it was only described in 200533. Accordingly, very little is known about its morphology34–37 and genetics38–41. In additions, K. koreana is not stringently bound to the aquatic environment as a result of the reacquisition of its larval stage42 and unusual breeding behaviour43,44. Little or no information is available about the potential range shift for K. koreana due to climate change45, but the latter is expected to have a negative impact on habitat suitability for plethodontids in general46–48. Climate change has likely caused body size reductions in other plethodontid salamander species49, which affects fecundity (reviewed by Wells, 200750) and rates of water loss51,52. In turn this may impact surface activity time53, and consequently affect foraging, dispersal, reproduction, and might change future species’ distributions54. As the climate on the Korean peninsula is getting warmer55,56 and dryer57, K. koreana is also going to be affected by climatic variations and related ecological changes. The aims of this study were (1) to define the potential current distribution of K. koreana through the development of a habitat suitability model and a set of bioclimatic and vegetation variables; (2) to investigate the potential impact of climate change on the distribution of K. koreana in the Korean peninsula using four Representative Concentration Pathways (RCPs), which are climate change scenarios based on greenhouse gas concentration trajectories.
Material and Methods
Species and habitat
Very little is known about Karsenia koreana. Its breeding behaviour was observed for the first time in 2016, but it still lacks any formal description in the wild44. The species is not as stringently bound to the aquatic environment as other amphibians due to the absence of a larval stage42, and to unusual breeding behaviour43,44. Like most plethodontids, K. koreana breeds and lays eggs on land and where they develop directly into adults without a larval stage34. Similarly, very little is known about the morphology34,35,37 or the genetics of the species38–41.
Until recently, K. koreana had only been found at a few locations under rocks on moist hills of montane woodlands with limestone soils33, while other plethodontid salamanders are described in a range of different habitats e.g., aquatic, fossorial, arboreal, stream, terrestrial or cave58. The behaviour of K. koreana in relation to habitat use is not known, although Buckley, et al.34 suggested the use of shelters in tight spaces between rocks, based on the species’ skull morphology.
Field survey
A total of 143 opportunistic field surveys were conducted between the first week of April 2007 and the last week of August 2017. Initial surveys were focused around Daejeon (Republic of Korea; Fig. 1), the area where the species was first found (2007–2008), and later expanded to similar types of habitat (2009–2016). Each observation was recorded with date, time and GPS coordinates (latitude and longitude).
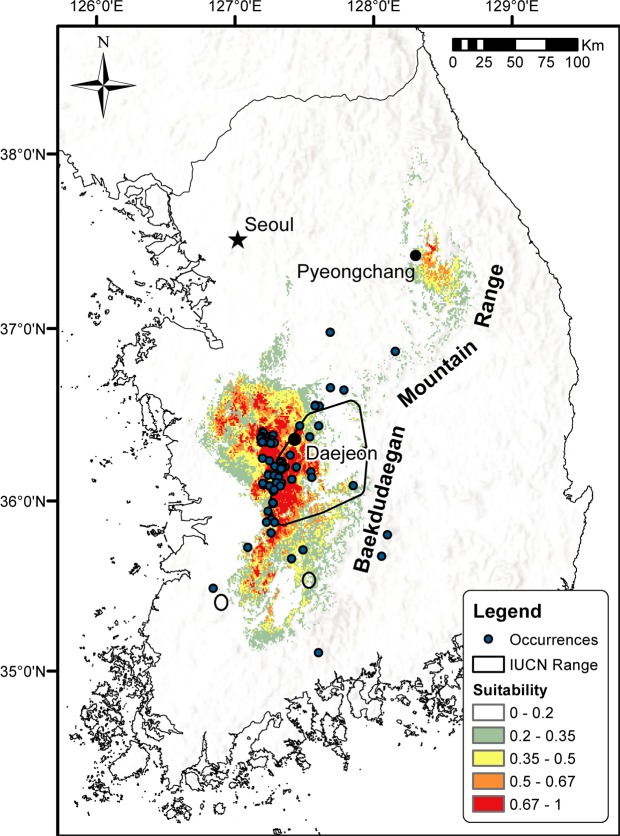
Figure 1.
Map of the Korean peninsula showing habitat suitability for Karsenia koreana based on 19 bioclimatic variables. Red areas indicate higher suitability. Observation points and IUCN range are also shown. Map generated in ArcMap 10.6.
Spatial model, current suitability and field validation
We used Maxent version 3.4.059, the maximum entropy approach (http://biodiversityinformatics.amnh.org/open_source/maxent/, accessed April 2018) to assess the potential distribution of K. koreana using species’ occurrences and environmental variables to develop habitat suitability maps. The reliability of the prediction depends on the variables selected and their resolutions. The 19 bioclimatic variables traditionally used for SDM60–63 were tested for multicollinearity using a Pearson’s correlation test, and the variables retained in model testing (Table 1) included Bio2 (mean diurnal range), Bio3 (isothermality), Bio4 (temperature seasonality), Bio6 (minimum temperature of coldest month), Bio14 (precipitation of driest month), and Bio16 (precipitation of wettest quarter). All variables were represented by rasters with resolutions of 30 arc seconds (~1 km) and had Pearson’s correlation coefficients less than 0.8. These variables were selected to represent climatic limitations, such as minimum and maximum temperature and precipitation, as such variables have been shown to have physiological implications for individual survival64,65.
Table 1.
Bioclimatic variables (Hijmans et al. 2005) used as Maxent environmental factors and in RCP projections to model the current and future habitat suitability for Karsenia koreana.
| Bioclimatic Variable | Description |
|---|---|
| BIO2 | Mean Diurnal Range (Mean of monthly (max temp-min temp) |
| BIO3 | Isothermality ((BIO2/BIO7)*100) |
| BIO4 | Temperature Seasonality (standard deviation*100) |
| BIO6 | Min Temperature of Coldest Month |
| BIO14 | Precipitation of Driest Month |
| BIO16 | Precipitation of Wettest Quarter |
To determine the current suitability range for K. koreana, Maxent was run with 82 occurrences, reduced from 139 by removing duplicate presence records within 1 km2 to reduced spatial autocorrelation caused by sample bias21,66. Ten bootstrap runs with a 30 percent random test percentage were output as cloglog, which is considered appropriate for estimating presence probability59, with a threshold at 10-percentile training presence. The averages of all runs were used as final models, and jackknife analysis was used to determine the factors contributing the greatest amount to habitat suitability. The final model was evaluated by area under the curve (AUC), true skill statistic (TSS67) and percent of occurrences within the 10-percentile training presence area. Additionally, we verified the accuracy of our prediction by comparing predicted suitable areas with the species’ reported distribution68,69.
Projected suitability
We predicted the potential future distribution of K. koreana using four climate change scenarios or Representative Concentration Pathways (RCPs) based on atmospheric CO2 trajectories. These four RCPs correspond to increases of 2.6, 4.5, 6.0, and 8.5 watts/m2 by 2100, with wattage increasing with projected CO2 concentrations (Table 2)70–73. SDMs were developed for two time-steps: 2050 (average for 2041–2060) and 2070 (average for 2061–2080) for all four RCPs. Among the global circulation models (GCMs) we used for all projections the Community Climate System Model 4.0 (CCSM4) from the Coupled Model Intercomparison Project Phase 5 (CMIP5) developed by the Intergovernmental Panel on Climate Change (IPCC) at 30 arc seconds, or approximately 1 km2 resolution, a choice confirmed by the jackknife analysis74–76.
Table 2.
Climate scenarios RCP 2.6, 4.5, 6.0, and 8.5 used to predict the range of Karsenia koreana in 2050 and 2070.
| Scenario | Radiative forcing increase (watts/m2 increase in 2100) | Year of peak greenhouse gas emissions | Decline during 21st century? |
|---|---|---|---|
| 2.6 | 2.6 | 2010–2020 | Yes |
| 4.5 | 4.5 | 2040 | Yes |
| 6.0 | 6.0 | 2080 | Yes |
| 8.5 | 8.5 | n/a | No |
Results
Current suitable range
The results of our SDMs (Fig. 1) showed a marked increase of potential suitable range for Karsenia koreana in respect to that described by previous studies45. The IUCN range did not include the areas with high suitability in the northwestern and southern regions of the country. The potential distribution resulting from our models covered a large part of western Korea, situated between ca. 100 and 700 meters above sea level west of the Baekdudaegan Mountain Range (Fig. 1). There is also a disjunct area of high suitability located to the northeast in the Pyeongchang area, which matches with reports of the species in that region68.
The Maxent modelling of current climate conditions (Fig. 1) produced a range of suitable habitat (cloglog output above the 10-percentile training presence threshold of 0.1964) was 10,261 km2. This model had an averaged AUC of 0.984 ± 0.007 and TSS of 0.869 ± 0.017 and could therefore be accepted as a model for the species’ habitat suitability. Suitable area thresholded at the 10-percentile training presence covered 93.6% of total presence points including 14 of the 15 counties, cities, and districts reported in The Encyclopedia of Korean Amphibians68 and at most locations reported in the Arrow guide of amphibians and reptiles69.
Climate scenario projections and jackknife analysis
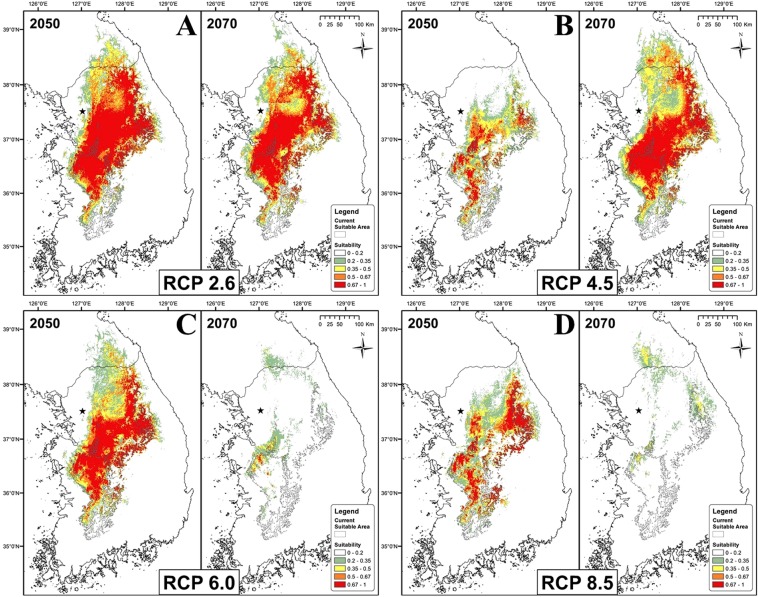
The climate projections showed a shift of the current suitability range for the species towards the northern and eastern regions, characterized by higher altitude (Fig. 2, Table 3). Following the RCP 2.6 and 4.5 predictions, the area of suitability will increase to the north and to higher elevations through 2070, while RCPs 6.0 and 8.5 show increases by 2050 but marked decreases by 2070 (Fig. 3). The two latter RCPs are clearly negative for the habitat suitability of K. koreana, highlighting a significant loss of suitable habitat by 2070.
Figure 2.
Projected suitable range for Karsenia koreana in RCP 2.6 (A), RCP 4.5 (B), RCP 6.0 (C), RCP 8.5 (D) for 2050 and 2070. Map generated in ArcMap 10.6.
Table 3.
Suitable area in km2 and percentage of current occurrences within suitable area for Karsenia koreana in the Republic of Korea under four climate change scenarios in 2050 and 2070.
| Scenario | 2050 | 2070 | ||
|---|---|---|---|---|
| Area (km2) | Percentage | Area (km2) | Percentage | |
| RCP 2.6 | 36.033 | 96.4 | 35.172 | 96.4 |
| RCP 4.5 | 19.417 | 95.7 | 38.506 | 96.4 |
| RCP 6.0 | 33.744 | 97.1 | 6.156 | 25.9 |
| RCP 8.5 | 23.947 | 95.0 | 6.219 | 18.0 |
Figure 3.
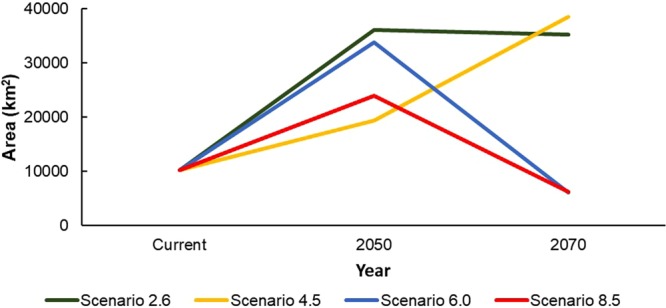
Comparison for the area of suitability (>0.1964) for Karsenia koreana based on four RCP scenarios. Suitable area in km2.
In all RCPs, suitable area is expected to increase by 2050. However, for RCPs 6.0 and 8.5, suitable area will decrease significantly by 2070. The most favorable scenarios for suitable habitat (RCPs 2.6 and 4.5) resulted in increases of 243% and 275%, respectively, in suitable habitat area by 2070, while the less favorable scenarios (RCPs 6.0 and 8.5) resulted in decreases of 40% and 39%, respectively, in suitable area by 2070 (Table 3, Fig. 3). Similarly, occurrence points located within suitable areas will be maintained through 2070 for RCPs 2.6 and 4.5 but will fall to 25.9% and 18.0% in RCPs 6.0 and 8.5, respectively (Table 3, Fig. 4). Here, higher percentages indicate a lower range shift for the species, while lower percentages indicate a higher range shift.
Figure 4.
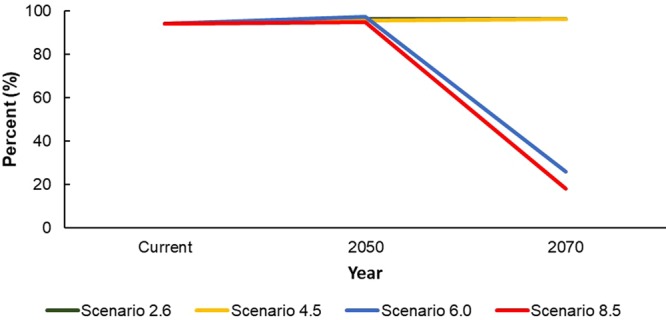
Percentage of current observation locations present in suitable areas (>0.1964) for Karsenia koreana under the four RCPs.
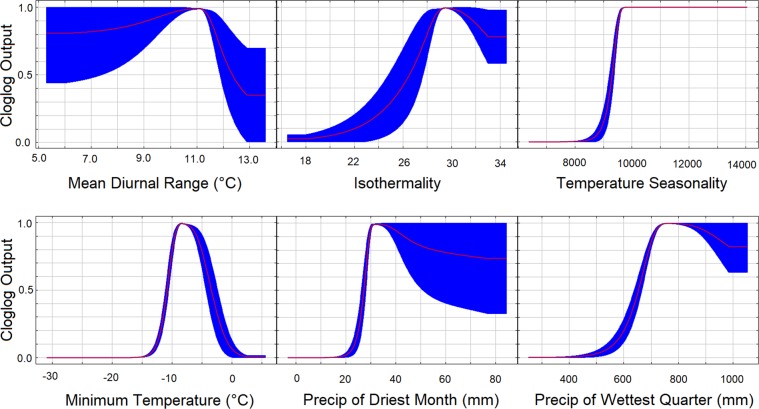
In jackknife analysis (Fig. 5), the three highest-contributing bioclimatic variables in order were Bio14 (precipitation of driest month), Bio4 (temperature seasonality), and Bio3 (isothermality). Similarly, Bio14 had the highest contribution and second highest permutation importance, the response to which was an increase in presence probability with precipitation in mm during the driest month. The variables with the lowest contributions were Bio2 (mean diurnal range), Bio6 (minimum temperature of coldest month), and Bio16 (precipitation of wettest quarter). However, Bio6 had the highest average permutation importance.
Figure 5.
Response curves of Karsenia koreana to bioclimatic variables used in Maxent modelling.
Discussion
Our results highlight a significantly larger habitat suitable for Karsenia koreana than is the current known distribution of the species45,68. The fact that the model matches with the known presence in counties68 and five other data points77–81. highlights the accuracy of our prediction. Discrepancies are not impossible as precise spatial modelling from a low number of samples can result in large variations82. The range of the species may be restricted by additional factors that could not be included in the model, such as competition. As an example, within plethodontid salamander, spatial distributions can be influenced by interference competition to complete competitive exclusion83–85. Second, salamanders may have been extirpated from some parts of the range, for instance during the Korean War when logging was intensive. However, Plethodontids are lungless ectotherms, and it appears that their activity and geographic distribution is predominantly controlled by climate86–88. Finally, salamanders may exist in areas where they have not been discovered yet, which introduces a bias in the ecological preferences of the species, and on the SDM as a result. Additional field surveys are required to clearly record the occurrence of the species, and further observations would improve the precision of habitat suitability models.
Despite this potentially larger new range, the threat status of K. koreana is still unresolved, as species with a limited range have often larger projected habitat loss and are more prone to extinction48,89,90, and a present point of concern in the Republic of Korea91. The results of Maxent modelling identify areas that are potentially important habitats for K. koreana and provide researchers with new potential survey locations. These results can inform future conservation efforts for the species in terms of protecting important habitat areas; the ranges calculated for habitat suitability fall within different threat levels of the IUCN red list of endangered species92, and K. koreana would thus join the list of endangered species from the Korean Peninsula93. Additionally, acquiring the exact area of occupancy of the species would be important for ecological monitoring as K. koreana is likely to be sensitive to the newly emerged Chytrid fungus Batrachochytrium salamandrivorans94. This fungus may soon be present in the Republic of Korea because of the pet trade, the same way it found its way to Europe95, and the same way the anuran Chytrid fungus entered Korea96.
Studies evaluating the effect of climate change on amphibian populations are based on both breeding phenology97,98 and range shifts9. Furthermore, the impact of agriculture on amphibian assemblages is well-studied and has been found to have two main types of impacts: landscape fragmentation (reviewed by Cushman 200699) and chemical use (reviewed by Mann et al.100). The predicted range of suitability resulting from Maxent modelling is an increase of 80% from the current IUCN range of 5,687 km2 (or 1.8 times the IUCN range). This area contains 93.8% of the observed points, so it can be considered an improvement of the IUCN range, which only contains 51.4% of the observed points. Because some of the predicted areas are fragmented or too small, or because the species may not manage to disperse there, it is likely that the actual range will be smaller than the predicted 10,261 km2. Additionally, there are areas with medium to high suitability where the species has yet to be recorded.
The jackknife analysis indicates that precipitation is likely a limiting environmental factor in the range of K. koreana, and that the species requires sufficient precipitation in the dry winter months to persist. Projections for all RCPs indicate that future habitats for K. koreana will be fragmented. In all cases, the suitable range for the species will shift, in which case the species may require translocation to persist. With continued development of natural areas by humans, translocation to more remote areas that already have protected status may be necessary. For instance, Baekdudaegan Mountains Reserve is currently outside the suitable range for K. koreana, but projections indicate the species may be able to inhabit areas in the reserve in the future. Since the goal of this modelling was to determine effects of climate change on K. koreana, this model leaves out other potentially important environmental factors, such as greenness and wetness. These factors will likely change along with the climate, and therefore, current maps of these factors could not be used in projections for this study.
Acknowledgements
This project was funded by the grant 2017R1A2B2003579 from the National Research Foundation of Korea and by the grant PJ012285 from the Rural Development Administration to J.Y.
Author Contributions
A.B., H.K. and Y.B. collected data; A.B., D.A., J.G. conceived and designed the experiments and analysed the data; A.B., D.A., J.G., H.K., Y.B. and Y.J. wrote the manuscript. All authors provided editorial advice.
Data Availability
All data used for the analyses will uploaded to an online data repository upon acceptation of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Amaël Borzée, Desiree Andersen and Jordy Groffen contributed equally.
References
- 1.Walther G-R, et al. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- 2.Houghton, J. T. Climate change 1995: The science of climate change: contribution of working group I to the second assessment report of the Intergovernmental Panel on Climate Change. Vol. 2 (Cambridge University Press, 1996).
- 3.Easterling DR, et al. Climate extremes: observations, modeling, and impacts. Science. 2000;289:2068–2074. doi: 10.1126/science.289.5487.2068. [DOI] [PubMed] [Google Scholar]
- 4.McCarty JP. Ecological consequences of recent climate change. Conserv Biol. 2001;15:320–331. doi: 10.1046/j.1523-1739.2001.015002320.x. [DOI] [Google Scholar]
- 5.Hughes L. Biological consequences of global warming: is the signal already apparent? Trends Ecol Evol. 2000;15:56–61. doi: 10.1016/S0169-5347(99)01764-4. [DOI] [PubMed] [Google Scholar]
- 6.Parmesan C, et al. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature. 1999;399:579–583. doi: 10.1038/21181. [DOI] [Google Scholar]
- 7.Parmesan C. Climate and species’ range. Nature. 1996;382:765–766. doi: 10.1038/382765a0. [DOI] [Google Scholar]
- 8.Epstein PR, et al. Biological and physical signs of climate change: focus on mosquito-borne diseases. Bull Amer Meteor. 1998;79:409–417. doi: 10.1175/1520-0477(1998)079<0409:BAPSOC>2.0.CO;2. [DOI] [Google Scholar]
- 9.Pounds JA, Fogden MP, Campbell JH. Biological response to climate change on a tropical mountain. Nature. 1999;398:611–615. doi: 10.1038/19297. [DOI] [Google Scholar]
- 10.Thomas CD, Lennon JJ. Birds extend their ranges northwards. Nature. 1999;399:213. doi: 10.1038/20335. [DOI] [Google Scholar]
- 11.Johnson NK. Pioneering and natural expansion of breeding distributions in western North American birds. Studies in Avian Biology. 1994;15:27–44. [Google Scholar]
- 12.Davis R, Callahan J. Post-Pleistocene dispersal in the Mexican vole (Microtus mexicanus): an example of an apparent trend in the distribution of southwestern mammals. Great Basin nat. 1992;52:262–268. [Google Scholar]
- 13.Wang R, et al. Modeling and mapping the current and future distribution of Pseudomonas syringae pv. actinidiae under climate change in China. PloS one. 2018;13:e0192153. doi: 10.1371/journal.pone.0192153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moraitis ML, Valavanis VD, Karakassis I. Modelling the effects of climate change on the distribution of benthic indicator species in the Eastern Mediterranean Sea. Science of The Total Environment. 2019;667:16–24. doi: 10.1016/j.scitotenv.2019.02.338. [DOI] [PubMed] [Google Scholar]
- 15.Guisan A, Thuiller W. Predicting species distribution: offering more than simple habitat models. Ecol Lett. 2005;8:993–1009. doi: 10.1111/j.1461-0248.2005.00792.x. [DOI] [PubMed] [Google Scholar]
- 16.Dufresnes C, et al. Phylogeography reveals an ancient cryptic radiation in East-Asian tree frogs (Hyla japonica group) and complex relationships between continental and island lineages. BMC Evol Biol. 2016;16:253. doi: 10.1186/s12862-016-0814-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearson RG, Dawson TP. Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Global ecol biogeogr. 2003;12:361–371. doi: 10.1046/j.1466-822X.2003.00042.x. [DOI] [Google Scholar]
- 18.Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 19.Mohammadi S, Ebrahimi E, Moghadam MS, Bosso L. Modelling current and future potential distributions of two desert jerboas under climate change in Iran. Ecol Inform. 2019;52:7–13. doi: 10.1016/j.ecoinf.2019.04.003. [DOI] [Google Scholar]
- 20.Franco JN, et al. The ‘golden kelp’ Laminaria ochroleuca under global change: Integrating multiple eco‐physiological responses with species distribution models. Journal of Ecology. 2018;106:47–58. doi: 10.1111/1365-2745.12810. [DOI] [Google Scholar]
- 21.Smeraldo S, et al. Ignoring seasonal changes in the ecological niche of non-migratory species may lead to biases in potential distribution models: lessons from bats. Biodiver Conserv. 2018;27:2425–2441. doi: 10.1007/s10531-018-1545-7. [DOI] [Google Scholar]
- 22.Williams PH, Lobo JM, Meseguer AS. Bumblebees take the high road: climatically integrative biogeography shows that escape from Tibet, not Tibetan uplift, is associated with divergences of present-day Mendacibombus. Ecography. 2018;41:461–477. doi: 10.1111/ecog.03074. [DOI] [Google Scholar]
- 23.Mizsei E, et al. Species distribution modelling leads to the discovery of new populations of one of the least known European snakes, Vipera ursinii graeca, in Albania. Amphibia Reptilia. 2016;37:55–68. doi: 10.1163/15685381-00003031. [DOI] [Google Scholar]
- 24.Planque B, Loots C, Petitgas P, LindstrøM U, Vaz S. Understanding what controls the spatial distribution of fish populations using a multi-model approach. Fisheries Oceanography. 2011;20:1–17. doi: 10.1111/j.1365-2419.2010.00546.x. [DOI] [Google Scholar]
- 25.Smith MA, Green DM. Dispersal and the metapopulation paradigm in amphibian ecology and conservation: are all amphibian populations metapopulations. Ecography. 2005;28:110–128. doi: 10.1111/j.0906-7590.2005.04042.x. [DOI] [Google Scholar]
- 26.Duellman, W. E. Patterns of distribution of amphibians: a global perspective. (Johns Hopkins University Press, 1999).
- 27.Pimm SL, et al. The biodiversity of species and their rates of extinction, distribution, and protection. Science. 2014;344:987–997. doi: 10.1126/science.1246752. [DOI] [PubMed] [Google Scholar]
- 28.Howard SD, Bickford DP. Amphibians over the edge: silent extinction risk of Data Deficient species. Divers Distrib. 2014;20:837–846. doi: 10.1111/ddi.12218. [DOI] [Google Scholar]
- 29.Kim I-H, Son S-H, Kang S-W, Kim J-B. Distribution and habitat characteristics of the endangered Suweon-Tree Frog (Hyla suweonensis) Kor J Herpetol. 2012;4:15–22. [Google Scholar]
- 30.Borzée A, Yu SH, Jang Y. Dryophytes suweonensis (Suweon Treefrog) Herpetol Rev. 2016;47:418. [Google Scholar]
- 31.Borzée A, Kim K, Heo K, Jablonski PG, Jang Y. Impact of land reclamation and agricultural water regime on the distribution and conservation status of the endangered Dryophytes suweonensis. PeerJ. 2017;5:e3872. doi: 10.7717/peerj.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishikawa K. The first specimen of Karsenia koreana (Caudata: Plethodontidae) collected 34 years before its description. Curr Herpetol. 2009;28:27–28. doi: 10.3105/018.028.0104. [DOI] [Google Scholar]
- 33.Min MS, et al. Discovery of the first Asian plethodontid salamander. Nature. 2005;435:87–90. doi: 10.1038/nature03474. [DOI] [PubMed] [Google Scholar]
- 34.Buckley D, Wake MH, Wake DB. Comparative skull osteology of Karsenia koreana (Amphibia, Caudata, Plethodontidae) Journal of morphology. 2010;271:533–558. doi: 10.1002/jmor.10816. [DOI] [PubMed] [Google Scholar]
- 35.Wake DB. Taxonomy of salamanders of the family Plethodontidae (Amphibia: Caudata) Zootaxa. 2012;3484:75–82. doi: 10.11646/zootaxa.3484.1.5. [DOI] [Google Scholar]
- 36.Wake DB. The enigmatic history of the European, Asian and American plethodontid salamanders. Amphibia Reptilia. 2013;34:323–336. doi: 10.1163/15685381-00002893. [DOI] [Google Scholar]
- 37.Sever DM, Pinsoneault AD, Mackenzie BW, Siegel DS, Staub NL. A description of the skin glands and cloacal morphology of the plethodontid salamander Karsenia koreana. Copeia. 2016;104:816–823. doi: 10.1643/CG-16-468. [DOI] [Google Scholar]
- 38.Vieites DR, Min M-S, Wake DB. Rapid diversification and dispersal during periods of global warming by plethodontid salamanders. Proc Natl Acad Sci. 2007;104:19903–19907. doi: 10.1073/pnas.0705056104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sessions SK, et al. Cytogenetic analysis of the asian plethodontid salamander, Karsenia koreana: Evidence for karyotypic conservation, chromosome repatterning, and genome size evolution. Chromosome Research. 2008;16:563–574. doi: 10.1007/s10577-008-1197-7. [DOI] [PubMed] [Google Scholar]
- 40.Vieites DR, Román SN, Wake MH, Wake DB. A multigenic perspective on phylogenetic relationships in the largest family of salamanders, the Plethodontidae. Mol Phylogenet Evol. 2011;59:623–635. doi: 10.1016/j.ympev.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Jeong TJ, et al. DNA barcode reference data for the Korean herpetofauna and their applications. Mol Ecol Resour. 2013;13:1019–1032. doi: 10.1111/1755-0998.12055. [DOI] [PubMed] [Google Scholar]
- 42.Bonett RM, Mueller RL, Wake DB. Why should reaquisition of larval stages by Desmognathine salamanders surprise us? Herpetol Rev. 2005;36:112. [Google Scholar]
- 43.Lee J. Use of breeding habitats by different amphibian species in the Woraksan National Park. Journal of Environmental Research. 2005;2:23–35. [Google Scholar]
- 44.Moon KY, Park D. Report of Karsenia koreana eggs oviposited within a semi-natural terrarium constructed at natural habitat. Kor J Herpetol. 2016;7:1–5. [Google Scholar]
- 45.Vieites, D., Karsen, S., Mi-Sook, M., Yang, S. Y. & Wake, D. Karsenia koreana. Vol. e.T61903A12569677 (IUCN, 2006).
- 46.Struecker B, Milanovich J. Predicted suitable habitat declines for midwestern United States amphibians under future climate and land-use change scenarios. Herpetological Conservation and Biology. 2017;12:635–654. [Google Scholar]
- 47.Connette GM, Crawford JA, Peterman WE. Climate change and shrinking salamanders: alternative mechanisms for changes in plethodontid salamander body size. Global Change Biol. 2015;21:2834–2843. doi: 10.1111/gcb.12883. [DOI] [PubMed] [Google Scholar]
- 48.Milanovich JR, Peterman WE, Nibbelink NP, Maerz JC. Projected loss of a salamander diversity hotspot as a consequence of projected global climate change. PloS one. 2010;5:e12189. doi: 10.1371/journal.pone.0012189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caruso NM, Sears MW, Adams DC, Lips KR. Widespread rapid reductions in body size of adult salamanders in response to climate change. Global Change Biol. 2014;20:1751–1759. doi: 10.1111/gcb.12550. [DOI] [PubMed] [Google Scholar]
- 50.Wells, K. Complex life cycles and the ecology of amphibian metamorphosis. (University of Chicago Press, 2007).
- 51.Tracy CR, Christian KA, Tracy CR. Not just small, wet, and cold: effects of body size and skin resistance on thermoregulation and arboreality of frogs. Ecology. 2010;91:1477–1484. doi: 10.1890/09-0839.1. [DOI] [PubMed] [Google Scholar]
- 52.Peterman W, Locke J, Semlitsch R. Spatial and temporal patterns of water loss in heterogeneous landscapes: using plaster models as amphibian analogues. Can J Zoolog. 2013;91:135–140. doi: 10.1139/cjz-2012-0229. [DOI] [Google Scholar]
- 53.Peterman W, Semlitsch RD. Spatial variation in water loss predicts terrestrial salamander distribution and population dynamics. Oecologia. 2014;176:357–369. doi: 10.1007/s00442-014-3041-4. [DOI] [PubMed] [Google Scholar]
- 54.Gifford ME, Kozak KH. Islands in the sky or squeezed at the top? Ecological causes of elevational range limits in montane salamanders. Ecography. 2012;35:193–203. doi: 10.1111/j.1600-0587.2011.06866.x. [DOI] [Google Scholar]
- 55.Chung YS, Yoon MB, Kim HS. On climate variations and changes observed in South Korea. Clim Change. 2004;66:151–161. doi: 10.1023/B:CLIM.0000043141.54763.f8. [DOI] [Google Scholar]
- 56.Choi W, et al. Season-dependent warming characteristics observed at 12 stations in South Korea over the recent 100 years. Int J Climatol. 2018;38:4092–4101. doi: 10.1002/joc.5554. [DOI] [Google Scholar]
- 57.Azam M, Park HK, Maeng SJ, Kim HS. Regionalization of drought across South Korea using multivariate methods. Water. 2017;10:24. doi: 10.3390/w10010024. [DOI] [Google Scholar]
- 58.Wake, D. B. Comparative osteology and evolution of the lungless salamanders, family Plethodontidae. Vol. 4 (Southern California Academy of Sciences, 1966).
- 59.Phillips, S. J., Dudík, M. & Schapire, R. E. Maxent software for modeling species niches and distributions (Version 3.4.1) (2017).
- 60.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25:1965–1978. doi: 10.1002/joc.1276. [DOI] [Google Scholar]
- 61.Ray D, Behera MD, Jacob J. Predicting the distribution of rubber trees (Hevea brasiliensis) through ecological niche modelling with climate, soil, topography and socioeconomic factors. Ecol Res. 2016;31:75–91. doi: 10.1007/s11284-015-1318-7. [DOI] [Google Scholar]
- 62.Kabir M, et al. Habitat suitability and movement corridors of Grey wolf (Canis lupus) in Northern Pakistan. PloS one. 2017;12:e0187027. doi: 10.1371/journal.pone.0187027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boria RA, Blois JL. The effect of large sample sizes on ecological niche models: analysis using a North American rodent, Peromyscus maniculatus. Ecol Model. 2018;386:83–88. doi: 10.1016/j.ecolmodel.2018.08.013. [DOI] [Google Scholar]
- 64.Layne JR, Jr, Claussen DL. Seasonal variation in the thermal acclimation of critical thermal maxima (CTMax) and minima (CTMin) in the salamander Eurycea bislineata. J Therm Biol. 1982;7:29–33. doi: 10.1016/0306-4565(82)90016-X. [DOI] [Google Scholar]
- 65.Spotila JR. Role of temperature and water in the ecology of lungless salamanders. Ecological Monographs. 1972;42:95–125. doi: 10.2307/1942232. [DOI] [Google Scholar]
- 66.Kwon H, Kim B, Jang G. Modelling the spatial distribution of wildlife animals using presence and absence data. Contemporary Problems of Ecology. 2016;9:515–518. doi: 10.1134/S1995425516050085. [DOI] [Google Scholar]
- 67.Allouche O, Tsoar A, Kadmon R. Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS) J Appl Ecol. 2006;43:1223–1232. doi: 10.1111/j.1365-2664.2006.01214.x. [DOI] [Google Scholar]
- 68.Lee, J.-H. & Park, D. The encyclopedia of Korean amphibians., Vol. 17 248 (Checklist of Organisms in Korea 17, 2016).
- 69.Kim, H.-T., Kim, H., Jeon, G. & Kim, D. Arrow guide of amphibians and reptiles. (Econature, 2019).
- 70.Moss, R. et al. Towards new scenarios for the analysis of emissions: climate change, impacts and response strategies. (Intergovernmental Panel on Climate Change Secretariat (IPCC), 2008).
- 71.Weyant, J. et al. In Towards New Scenarios for Analysis of Emissions, Climate Change, Impacts, and Response Strategies. (ed Intergovernmental Panel on Climate Change, 2009).
- 72.Meinshausen M, et al. The RCP greenhouse gas concentrations and their extensions from 1765 to 2300. Clim Change. 2011;109:213. doi: 10.1007/s10584-011-0156-z. [DOI] [Google Scholar]
- 73.Stocker BD, Feissli F, Strassmann KM, Spahni R, Joos F. Past and future carbon fluxes from land use change, shifting cultivation and wood harvest. Tellus B: Chemical and Physical Meteorology. 2014;66:23188. doi: 10.3402/tellusb.v66.23188. [DOI] [Google Scholar]
- 74.Yuan HS, Wei YL, Wang XG. Maxent modeling for predicting the potential distribution of Sanghuang, an important group of medicinal fungi in China. Fungal. Ecology. 2015;17:140–145. [Google Scholar]
- 75.Gholamrezaei M, Mohebali M, Hanafi-Bojd AA, Sedaghat MM, Shirzadi MR. Ecological niche modeling of main reservoir hosts of zoonotic cutaneous leishmaniasis in Iran. Acta Tropica. 2016;160:44–52. doi: 10.1016/j.actatropica.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 76.Bosso L, De Conno C, Russo D. Modelling the risk posed by the zebra mussel Dreissena polymorpha: Italy as a case study. Environmental Management. 2017;60:304–313. doi: 10.1007/s00267-017-0882-8. [DOI] [PubMed] [Google Scholar]
- 77.Song J-Y, et al. Roadkill of amphibians in the Korean National Parks. Korean J Environ Ecol. 2009;23:187–193. [Google Scholar]
- 78.Song J-Y. Herpetofauna of the Korean National Parks in Baekdudaegan. Journal of National Park Research. 2010;1:216–219. [Google Scholar]
- 79.Kim, J., Min, M., Park, D. & Song, J. Red data book of endangered amphibians and reptiles in Korea. (National Institute of Biological Resources, 2011).
- 80.Ra, N., Ham, C., Lee, Y., Han, H. & Sung, H. In The Korean Society of Herpetologists, Vol. 2015 (ed The Korean Society of Herpetologists) (The Korean Society of Herpetologists, Wonju, 2015).
- 81.Kwon S-R, et al. Herpetofauna in the Jangjeon Valley of Mt. Gariwang, South Korea. Kor J Herpetol. 2016;7:23–29. [Google Scholar]
- 82.Pearson RG, et al. Model-based uncertainty in species range prediction. J Biogeogr. 2006;33:1704–1711. doi: 10.1111/j.1365-2699.2006.01460.x. [DOI] [Google Scholar]
- 83.Hairston, N. A. & Hairston, N. G. Community ecology and salamander guilds. (Cambridge University Press, 1988).
- 84.Marvin GA. Interspecific aggression and spatial relationships in the salamanders Plethodon kentucki and Plethodon glutinosus: evidence of interspecific interference competition. Can J Zoolog. 1998;76:94–103. doi: 10.1139/z97-158. [DOI] [Google Scholar]
- 85.Maerz, J. C. & Madison, D. M. In The biology of plethodontid salamanders 395–406 (Springer, 2000).
- 86.Feder ME. Integrating the ecology and physiology of plethodontid salamanders. Herpetologica. 1983;39:291–310. [Google Scholar]
- 87.Marshall JL, Camp CD. Environmental correlates of species and genetic richness in lungless salamanders (family Plethodontidae) Acta Oecol. 2006;29:33–44. doi: 10.1016/j.actao.2005.07.008. [DOI] [Google Scholar]
- 88.Bernardo J, Spotila JR. Physiological constraints on organismal response to global warming: mechanistic insights from clinally varying populations and implications for assessing endangerment. Biol Lett. 2006;2:135–139. doi: 10.1098/rsbl.2005.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lynch M, Conery J, Burger R. Mutation accumulation and the extinction of small populations. Am Nat. 1995;164:489–518. doi: 10.1086/285812. [DOI] [Google Scholar]
- 90.Jacquemyn H, Vandepitte K, Brys R, Honnay O, Roldán-Ruiz I. Fitness variation and genetic diversity in small, remnant populations of the food deceptive orchid Orchis purpurea. Biol Cons. 2007;139:203–210. doi: 10.1016/j.biocon.2007.06.015. [DOI] [Google Scholar]
- 91.Borzée A, Struecker M-Y, Yi Y, Kim D, Kim H. Time for Korean wildlife conservation. Science. 2019;363:1161–1162. doi: 10.1126/science.aaw9023. [DOI] [PubMed] [Google Scholar]
- 92.IUCN. The IUCN Red List of threatened species. Vol, http://www.iucnredlist.org, (IUCN, 2017).
- 93.Lee S-D, Miller-Rushing AJ. Degradation, urbanization, and restoration: a review of the challenges and future of conservation on the Korean Peninsula. Biol Cons. 2014;176:262–276. doi: 10.1016/j.biocon.2014.05.010. [DOI] [Google Scholar]
- 94.Martel A, et al. Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proc Natl Acad Sci. 2013;110:15325–15329. doi: 10.1073/pnas.1307356110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nguyen TT, Van Nguyen T, Ziegler T, Pasmans F, Martel A. Trade in wild anurans vectors the urodelan pathogen Batrachochytrium salamandrivorans into. Europe. Amphibia Reptilia. 2017;38:554–556. doi: 10.1163/15685381-00003125. [DOI] [Google Scholar]
- 96.Borzée A, Kosch TA, Kim M, Jang Y. Introduced bullfrogs are associated with increased Batrachochytrium dendrobatidis prevalence and reduced occurrence of Korean treefrogs. PloS one. 2017;12:e0177860. doi: 10.1371/journal.pone.0177860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Beebee TJC. Amphibian breeding and climate change. Nature. 1995;374:219–220. doi: 10.1038/374219a0. [DOI] [Google Scholar]
- 98.Forchhammer MC, Post E, Stenseth NC. Breeding phenology and climate…. Nature. 1998;391:29–30. doi: 10.1038/34070. [DOI] [Google Scholar]
- 99.Cushman SA. Effects of habitat loss and fragmentation on amphibians: a review and prospectus. Biol Cons. 2006;128:231–240. doi: 10.1016/j.biocon.2005.09.031. [DOI] [Google Scholar]
- 100.Mann RM, Hyne RV, Choung CB, Wilson SP. Amphibians and agricultural chemicals: review of the risks in a complex environment. Environ Pollut. 2009;157:2903–2927. doi: 10.1016/j.envpol.2009.05.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used for the analyses will uploaded to an online data repository upon acceptation of the manuscript.