Abstract
Human longevity is heritable, but genome-wide association (GWA) studies have had limited success. Here, we perform two meta-analyses of GWA studies of a rigorous longevity phenotype definition including 11,262/3484 cases surviving at or beyond the age corresponding to the 90th/99th survival percentile, respectively, and 25,483 controls whose age at death or at last contact was at or below the age corresponding to the 60th survival percentile. Consistent with previous reports, rs429358 (apolipoprotein E (ApoE) ε4) is associated with lower odds of surviving to the 90th and 99th percentile age, while rs7412 (ApoE ε2) shows the opposite. Moreover, rs7676745, located near GPR78, associates with lower odds of surviving to the 90th percentile age. Gene-level association analysis reveals a role for tissue-specific expression of multiple genes in longevity. Finally, genetic correlation of the longevity GWA results with that of several disease-related phenotypes points to a shared genetic architecture between health and longevity.
Subject terms: Genome-wide association studies, Diseases, Ageing, Risk factors
Genome-wide association studies have only revealed a handful of genetic loci for longevity. Here, in a case–control design based on phenotype definitions of individuals surviving at or beyond the age corresponding to the 90th and 99th survival percentile, the authors report two additional loci located in the APOE locus and near GPR78.
Introduction
The average human life expectancy has been increasing for centuries1. Based on twin studies, the heritability of human lifespan has been estimated to be ~25%, although this estimate differs among studies2. On the other hand, the heritability of lifespan based on the correlation of the mid-parent (i.e., the average of the father and mother) and offspring difference between age at death and expected lifespan was estimated to be 12%3. A recent study has indicated that the different heritability estimates may be inflated due to assortative mating, leaving a true heritability that is below 10%4. The heritability of lifespan, estimated using the sibling relative risk, increases with age5 and is assumed to be enriched in long-lived families, particularly when belonging to the 10% longest-lived of their generation6. To identify genetic associations with human lifespan, several genome-wide association (GWA) studies have been performed7–20. These studies have used a discrete (i.e., older cases versus younger controls) or a continuous phenotype (such as age at death of individuals or their parents). The selection of cases for the studies using a discrete longevity phenotype has been based on the survival to ages above 90 or 100 years or belonging to the top 10% or 1% of survivors in a population. Studies defining cases using a discrete longevity phenotype often need to rely on controls from more contemporary birth cohorts, because all others from the case birth cohorts have died before sample collection. Previous GWA studies have identified several genetic variants, but the only locus that has shown genome-wide significance (P ≤ 5 × 10−8) in multiple independent meta-analyses of GWA studies is apolipoprotein E (APOE)21, where the ApoE ε4 variant is associated with lower odds of being a long-lived case.
The lack of replication for many reported associations with longevity could be due, at least partly, to the use of different definitions for cases and controls between studies. Furthermore, even within a study, the use of a single age cut-off phenotype for men and women and for individuals belonging to different birth cohorts will give rise to heterogeneity, as survival probabilities differ by sex and birth cohort22, and genetic effects are known to be age- and birth cohort-specific5,23. In an attempt to mitigate the effects of heterogeneous case and control groups, we use country-, sex- and birth cohort-specific life tables to identify ages that correspond to different survival percentiles to define cases and controls in our meta-analyses of GWA studies of longevity. Furthermore, most studies in our meta-analyses use controls from the same study population as the cases, which limits the impact of sampling biases that could confound associations. The current meta-analyses include individuals from 20 cohorts from populations of European, East Asian, or African American descent. Two sets of cases are examined: individuals surviving at or beyond the age corresponding to the 90th survival percentile (90th percentile cases) or the 99th survival percentile (99th percentile cases) based on life tables specific to the country where each cohort was based, sex, and birth cohort (i.e., birth year). The same country-, sex-, and birth cohort-specific life tables are used to define the age threshold for controls, corresponding to the 60th percentile of survival. We identify two genome-wide significant loci, of which one is replicated in two independent European cohorts that use de novo genotyping. We also perform a gene-level association analysis based on tissue-specific gene expression and identify additional longevity genes. In addition, using linkage disequilibrium (LD) score regression24, we show that longevity is genetically correlated with multiple diseases and traits.
Results
Genome-wide association meta-analyses
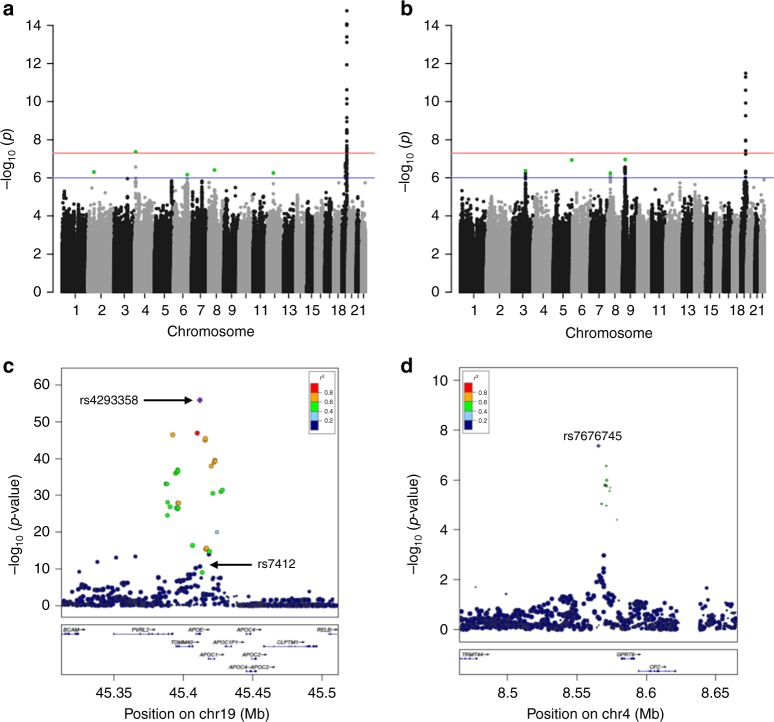
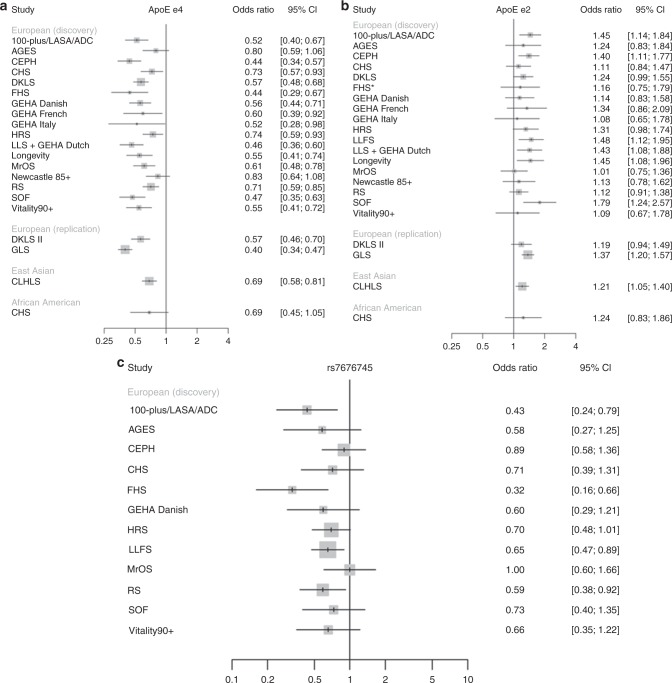
We performed two meta-analyses in individuals of European ancestry combining cohort-specific genome-wide association data generated using 1000 Genomes imputation: (1) 90th percentile cases versus all controls and (2) 99th percentile cases versus all controls. The numbers of cases and controls in each study are shown in Table 1. For both case definitions, multiple genetic variants at the well-replicated APOE locus reached genome-wide significance (P ≤ 5 × 10−8) (Table 2, Fig. 1 and Supplementary Fig. 1). Consistent with previous reports, rs429358 (ApoE ε4) was associated with lower odds of surviving to the 90th or 99th percentile age at the genome-wide significance level. In addition, we report a genome-wide significant association of rs7412 (ApoE ε2) with higher odds of surviving to the 90th and the 99th percentile age. Conditional analysis in two of the cohorts with individuals of European ancestry, CEPH and LLS (combined with GEHA Dutch) (representing 18% of the 90th percentile cases and 6% of all controls), indicated that the signal at the APOE locus was explained by these two independent variants, i.e., rs429358 (ApoE ε4) and rs7412 (ApoE ε2). There was no evidence of heterogeneity of effect across cohorts for ApoE ε2 (P-value for heterogeneity (Phet) = 0.619, Table 2). For ApoE ε4, on the other hand, there was evidence of heterogeneity (Phet = 0.004, Table 2), although the direction of effect of this variant was consistent across cohorts (Fig. 2). Besides ApoE ε4 and ε2, one additional variant, rs7676745, located on chromosome 4 near GPR78, showed a genome-wide significant association in the 90th percentile cases versus all controls analysis (P = 4.3 × 10−8, Table 2). The rare allele of this variant (A) was associated with lower odds of surviving to the 90th percentile age and there was no evidence of heterogeneity of effect across cohorts (Phet = 0.462, Table 2). The regional association and forest plots for this locus are depicted in Figs. 1 and 2.
Table 1.
Samples included in the different genome-wide association meta-analyses or the replication and validation
| Study | Ancestry | 90th percentile cases | 99th percentile cases | All controls | Dead controls |
|---|---|---|---|---|---|
| Discovery | |||||
| 100-plus/LASA/ADC | European | 373 | 301 | 2271 | 245 |
| AGES | European | 300 | 1001 | 466 | |
| CEPHa | European | 1234 | 1112 | 831 | |
| CHS | European | 905 | 68 | 558 | 539 |
| DKLSa | European | 960 | 610 | 1917 | |
| FHS | European | 332 | 1444 | 539 | |
| GEHA Danisha | European | 451 | 127 | 900 | |
| GEHA French | European | 271 | 81 | 358 | |
| GEHA Italy | European | 182 | 184 | ||
| HRS | European | 361 | 3312 | 657 | |
| LLFS | European | 1110 | 339 | 552 | 82 |
| LLS + GEHA Dutch | European | 1037 | 377 | 712 | |
| Longevity | European | 548 | 271 | 584 | |
| MrOS | European | 1171 | 82 | 386 | 320 |
| Newcastle 85 + a | European | 215 | 5159 | ||
| RS | European | 774 | 79 | 2965 | 1731 |
| SOF | European | 812 | 37 | 354 | 300 |
| Vitality 90 + a | European | 226 | 1995 | ||
| Total | 11,262 | 3484 | 25,483 | 4879 | |
| Replication | |||||
| DKLSIIa | European | 944 | 298 | 772 | |
| GLS | European | 1613 | 1613 | 4215 | |
| Total | 2557 | 1911 | 4987 | ||
| Validation | |||||
| UK Biobank | European | 19,742 | 928 | 19,698 | |
| Trans-ethnic | |||||
| CLHLS | East Asian | 2178 | 2178 | 2299 | |
| CHS | African American | 177 | 211 | ||
| Total | 13,617 | 5662 | 27,993 | ||
100-plus 100-plus Study, LASA Longitudinal aging study of Amsterdam, ADC Amsterdam dementia cohort, AGES Age/Gene Environment Susceptibility Study, CEPH CEPH centenarian cohort, CHS Cardiovascular Health Study, DKLS Danish longevity study, FHS Framingham Heart Study, GEHA Genetics of Healthy Aging Study, HRS Health and Retirement Study, LLFS Long Life Family Study, LLS Leiden Longevity Study, Longevity Longevity Gene Project, MrOS Osteoporotic Fractures in Men Study, Newcastle 85 + Newcastle 85 + Study, RS Rotterdam study, SOF Study of Osteoporotic Fracture, Vitality 90 + Vitality 90 + project, GLS German longevity study, CLHLS Chinese Longitudinal Healthy Longevity Survey
aFor these studies, controls were provided by a separate cohort. Further details of the cohorts are provided in Supplementary Data 4
Table 2.
Results of the European genome-wide association meta-analyses and replication in the de novo genotyped cohorts
| rsID | Chr:Position | Candidate/ closest gene | Alleles (EA/OA) | EAF | OR | 95% CI | P | I2 (%) | P het |
|---|---|---|---|---|---|---|---|---|---|
| 90th percentile cases versus all controls (Discovery) | |||||||||
| rs116362179 | 2:53,380,757 | − | T/C | 0.05 | 1.34 | 1.20–1.50 | 4.9 × 10−7 | 0 | 0.457 |
| rs7676745a | 4:8,565,547 | GPR78 | A/G | 0.04 | 0.67 | 0.57–0.77 | 4.3 × 10−8 | 0 | 0.462 |
| rs7754015 | 6:127,206,068 | − | G/T | 0.43 | 0.90 | 0.86–0.94 | 6.8 × 10−7 | 0 | 0.670 |
| rs35262860 | 8:55,478,909 | RP1 | GCT/G | 0.39 | 1.11 | 1.07–1.15 | 3.9 × 10−7 | 0 | 0.941 |
| rs3138136 | 12:56,117,570 | RDH5 | T/C | 0.10 | 0.83 | 0.77–0.89 | 5.4 × 10−7 | 14.5 | 0.284 |
| rs429358 | 19:45,411,941 | APOE | C/T | 0.13 | 0.60 | 0.56–0.64 | 1.3 × 10−56 | 54.3 | 0.004 |
| rs7412 | 19:45,412,079 | APOE | T/C | 0.09 | 1.28 | 1.19–1.37 | 2.4 × 10−11 | 0 | 0.619 |
| 90th percentile cases versus all controls (Replication) | |||||||||
| rs429358 | 19:45,411,941 | APOE | C/T | 0.45 | 0.40–0.51 | 5.2 × 10−36 | 85.4 | 0.009 | |
| rs7412 | 19:45,412,079 | APOE | T/C | 1.32 | 1.18–1.48 | 2.4 × 10−6 | 16.6 | 0.274 | |
| 99th percentile cases versus all controls (Discovery) | |||||||||
| rs3830412 | 3:124,397,321 | KALRN | A/AT | 0.22 | 1.21 | 1.12–1.30 | 4.3 × 10−7 | 0 | 0.767 |
| rs138762279 | 5:173,710,197 | − | AT/A | 0.16 | 0.79 | 0.72–0.86 | 1.2 × 10−7 | 0 | 0.769 |
| rs62502826 | 8:28,982,295 | KIF13B | A/G | 0.15 | 1.23 | 1.13–1.33 | 5.6 × 10−7 | 14.9 | 0.298 |
| rs7039467 | 9:22,056,213 | CDKN2A/B | A/G | 0.48 | 1.20 | 1.12–1.28 | 1.1 × 10−7 | 0 | 0.843 |
| rs429358 | 19:45,411,941 | APOE | C/T | 0.13 | 0.52 | 0.47–0.58 | 3.9 × 10−34 | 0 | 0.833 |
| rs7412 | 19:45,412,079 | APOE | T/C | 0.09 | 1.47 | 1.32–1.64 | 3.2 × 10−12 | 0 | 0.639 |
| 99th percentile cases versus all controls (Replication) | |||||||||
| rs429358 | 19:45,411,941 | APOE | C/T | 0.44 | 0.38–0.50 | 4.0 × 10−32 | 84.0 | 0.012 | |
| rs7412 | 19:45,412,079 | APOE | T/C | 1.35 | 1.19–1.53 | 2.0 × 10−6 | 0 | 0.534 | |
EA effect allele, OA other allele, EAF effect allele frequency, OR odds ratio (i.e., odds to become long-lived when carrying the effect allele); 95% CI 95% confidence interval, I2 heterogeneity statistic, Phet P-value for heterogeneity
aWe were not able to replicate the effect of this genetic variant, since there was no Taqman SNP Genotyping Assay available. We only report the most significant genetic variant for the loci with at least one variant with a P-value ≤ 1 × 10–6. The rsID is based on dbSNP build 150. The Chr:Position is based on Genome Reference Consortium Human Build 37 (GRCh37)
Fig. 1.
Results of the European genome-wide association meta-analyses. Manhattan plot presenting the –log10 P-values from the European genome-wide association meta-analysis of the 90th percentile cases versus all controls (a) and 99th percentile cases versus all controls (b). The red line indicates the threshold for genome-wide significance (P ≤ 5 × 10−8), while the blue line indicates the threshold for genetic variants that showed a suggestive significant association (P ≤ 1 × 10−6). The variants that are reported in Table 2 are highlighted in green. For representation purposes, the maximum of the y-axis was set to 14. Regional association plot for the APOE (c) and GPR78 (d) loci based on the results from the 90th percentile cases versus all controls meta-analysis. The colour of the variants is based on the linkage disequilibrium with rs429358 (ApoE ε4) (c) or rs7676745 (d)
Fig. 2.
Study-specific results for the genetic variants in APOE and GPR78. Forest plots for the ApoE ε4 (a) and ε2 (b) variants and rs7676745 (c) based on the results from the 90th percentile versus all controls analysis. The size of the boxes represents the sample size of the cohort. We had no data available for ApoE ε4 in LLFS and for rs7676745 in DKLS, GEHA Italy, GEHA Danish, LLS (combined with GEHA Dutch), Longevity, and Newcastle 85 + . The data for ApoE ε2 in FHS was based on imputation using the Haplotype Reference Consortium reference panel due to the low-imputation quality of this variant when using the 1000 Genomes reference panel
Most of the variants reported in Table 2 show stronger effects in the 99th percentile as compared to the 90th percentile analysis (Supplementary Fig. 2), indicating that the use of a more extreme phenotype results in stronger effects.
Replication
The effects of ApoE ε4 and ε2 were replicated in the two cohorts (i.e., DKLSII and GLS) in which de novo genotyping, using predesigned Taqman SNP Genotyping Assays, was applied (Table 2). However, we were not able to replicate the effect of rs7676745 in these cohorts, since there was no Taqman SNP Genotyping Assay available for this variant.
Validation in parental age-based data sets
Given that all available studies with genome-wide genetic data that met our inclusion criteria were included in our genome-wide association meta-analyses, we additionally set out to validate our findings in two UK Biobank parental longevity data sets (Table 1) and the parental lifespan data set recently created by Timmers and colleagues20. Since the genotyped individuals in the UK Biobank were recruited at relatively young ages (40–69 years), these data sets were based on the age reached by the parents of the study participants. Hence, the phenotypes used for validation were different from those used in our meta-analyses, resulting in smaller effect sizes. Moreover, the reference panels used to impute the genetic variants (a merged panel of UK10K, 1000G Phase 3, and Haplotype Reference Consortium (HRC) for parental longevity and HRC alone for parental lifespan)20 were different from the one used in our meta-analyses (1000G Phase 1), which could have influenced the outcome of the analyses. Of the variants that showed a P-value ≤ 1 × 10−6 in our meta-analyses (Table 2), only ApoE ε4 and ε2 were significantly associated with both parental longevity and lifespan (P < 0.05) in these data sets (Table 3). Moreover, the rare allele (A) of the second most significant variant at the CDKN2A/B locus, rs2184061, was associated with increased parental lifespan (P = 8.4 × 10−6), but not with parental longevity (P = 0.329). However, we had adequate power to validate all of our identified variants, even when the effect sizes were halved in the parental longevity data sets.
Table 3.
Results of the validation in the UK Biobank parental age-based data sets
| rsID | Chr:Position | Candidate/ closest gene | Alleles (EA/OA) | EAF | OR | 95% CI | P |
|---|---|---|---|---|---|---|---|
| 90th percentile cases versus all controls (Parental longevity) | |||||||
| rs116362179 | 2:53,380,757 | − | T/C | 0.04 | 1.01 | 0.94–1.08 | 0.775 |
| rs7676745 | 4:8,565,547 | GPR78 | A/G | 0.04 | 0.98 | 0.92–1.06 | 0.667 |
| rs7754015 | 6:127,206,068 | − | G/T | 0.43 | 1.00 | 0.97–1.03 | 0.832 |
| rs35262860 | 8:55,478,909 | RP1 | GCT/G | 0.39 | 0.97 | 0.94–0.99 | 0.021 |
| rs3138136 | 12:56,117,570 | RDH5 | T/C | 0.11 | 1.00 | 0.95–1.04 | 0.863 |
| rs429358 | 19:45,411,941 | APOE | C/T | 0.16 | 0.85 | 0.81–0.88 | 1.1 × 10−16 |
| rs7412 | 19:45,412,079 | APOE | T/C | 0.08 | 1.12 | 1.06–1.18 | 2.2 × 10−5 |
| 90th percentile cases versus all controls (Parental lifespan) | |||||||
| rs116362179 | 2:53,380,757 | − | T/C | 0.04 | 1.00 | 0.98–1.02 | 0.697 |
| rs7676745 | 4:8,565,547 | GPR78 | A/G | 0.05 | 1.01 | 0.99–1.03 | 0.247 |
| rs3138136 | 12:56,117,570 | RDH5 | T/C | 0.11 | 0.99 | 0.98–1.00 | 0.135 |
| rs429358 | 19:45,411,941 | APOE | C/T | 0.15 | 0.90 | 0.89–0.91 | 3.1 × 10−83 |
| rs7412 | 19:45,412,079 | APOE | T/C | 0.08 | 1.06 | 1.05–1.08 | 7.6 × 10−17 |
| 99th percentile cases versus all controls (Parental longevity) | |||||||
| rs3830412 | 3:124,397,321 | KALRN | A/AT | 0.20 | 1.11 | 0.99–1.24 | 0.081 |
| rs138762279 | 5:173,710,197 | − | AT/A | 0.34 | 1.05 | 0.95–1.17 | 0.299 |
| rs62502826 | 8:28,982,295 | KIF13B | A/G | 0.14 | 1.04 | 0.90–1.19 | 0.614 |
| rs7039467 | 9:22,056,213 | CDKN2A/B | A/G | 0.69 | 0.93 | 0.83–1.05 | 0.245 |
| rs2184061 | 9:22,061,562 | CDKN2A/B | A/C | 0.40 | 0.95 | 0.87–1.05 | 0.329 |
| rs429358 | 19:45,411,941 | APOE | C/T | 0.16 | 0.76 | 0.66–0.87 | 9.6 × 10−5 |
| rs7412 | 19:45,412,079 | APOE | T/C | 0.08 | 1.23 | 1.05–1.45 | 0.011 |
| 99th percentile cases versus all controls (Parental lifespan) | |||||||
| rs62502826 | 8:28,982,295 | KIF13B | A/G | 0.14 | 1.00 | 0.99–1.02 | 0.376 |
| rs2184061 | 9:22,061,562 | CDKN2A/B | A/C | 0.40 | 1.02 | 1.01–1.03 | 8.4 × 10−6 |
| rs429358 | 19:45,411,941 | APOE | C/T | 0.15 | 0.90 | 0.89–0.91 | 3.1 × 10−84 |
| rs7412 | 19:45,412,079 | APOE | T/C | 0.08 | 1.06 | 1.05–1.08 | 7.6 × 10−17 |
For the CDKN2A/B locus we have also reported the second most significant variant in this locus (rs2184061), since the allele frequency of the most significant variant (rs7039467) is not comparable between the meta-analyses and UK Biobank data sets due to difference in the reference panel used for imputation. The rsID is based on dbSNP build 150. The Chr:Position is based on Genome Reference Consortium Human Build 37 (GRCh37)
EA effect allele, OA other allele, EAF effect allele frequency, OR odds ratio (i.e., odds of parent(s) to become long-lived when carrying the effect allele), 95% CI 95% confidence interval
Trans-ethnic meta-analyses
We subsequently performed two trans-ethnic meta-analyses (90th and 99th percentile cases versus all controls) to see if the increase in sample size would lead to identification of additional longevity loci. In this analysis we included individuals of European (all previously used data sets), East Asian (CLHLS), and African American (CHS) ancestry. However, with the exception of APOE and rs2069837, located in IL6, which has previously been associated with longevity in CLHLS9, this analysis did not identify additional genome-wide significant loci (Table 4, Fig. 3 and Supplementary Fig. 3). The observed association of the genetic variant in IL6 in the trans-ethnic meta-analyses was mainly driven by the association in the East Asian population. The other variant previously associated with longevity in CLHLS9, rs2440012, located in ANKRD20A9P, did not pass quality control in the large majority of the included cohorts from populations of European descent and was thus not analysed in the trans-ethnic meta-analyses.
Table 4.
Results of the trans-ethnic genome-wide association meta-analyses
| rsID | Chr:Position | Candidate/ closest gene | Alleles (EA/OA) | EAF | OR | 95% CI | P | I2 (%) | P het |
|---|---|---|---|---|---|---|---|---|---|
| 90th percentile cases versus all controls | |||||||||
| rs12143832 | 1:21,705,436 | ECE1 | C/T | 0.46 | 0.90 | 0.87–0.94 | 2.0 × 10−7 | 0 | 0.722 |
| rs7676745 | 4:8,565,547 | GPR78 | A/G | 0.04 | 0.67 | 0.58–0.78 | 1.7 × 10−7 | 1.8 | 0.428 |
| rs1262476 | 6:126,986,996 | − | A/G | 0.24 | 1.12 | 1.07–1.17 | 9.8 × 10−7 | 0 | 0.574 |
| rs2069837 | 7:22,768,027 | IL6 | G/A | 0.08 | 0.90 | 0.82–0.99 | 5.2 × 10−8 | 50.7 | 0.005 |
| rs35262860 | 8:55,478,909 | RP1 | GCT/G | 0.39 | 1.11 | 1.07–1.15 | 5.6 × 10−7 | 0 | 0.955 |
| rs62127362 | 19:33,458,479 | CEP89 | C/G | 0.13 | 0.87 | 0.82–0.93 | 4.3 × 10−7 | 21.4 | 0.190 |
| rs429358 | 19:45,411,941 | APOE | C/T | 0.13 | 0.60 | 0.55–0.66 | 1.0 × 10−61 | 52.1 | 0.004 |
| rs7412 | 19:45,412,079 | APOE | T/C | 0.09 | 1.26 | 1.19–1.35 | 1.7 × 10−12 | 0 | 0.718 |
| 99th percentile cases versus all controls | |||||||||
| rs2758603 | 1:156,198,994 | PMF1 | C/T | 0.34 | 1.12 | 1.02–1.22 | 9.8 × 10−7 | 57.2 | 0.005 |
| rs3830412 | 3:124,397,321 | KALRN | A/AT | 0.22 | 1.21 | 1.12–1.30 | 8.2 × 10−7 | 0 | 0.767 |
| rs138762279 | 5:173,710,197 | − | AT/A | 0.16 | 0.79 | 0.72–0.86 | 2.2 × 10−7 | 0 | 0.769 |
| rs2069837 | 7:22,768,027 | IL6 | G/A | 0.09 | 0.90 | 0.76–1.08 | 1.4 × 10−8 | 67.7 | 3.5 × 10−4 |
| rs7039467 | 9:22,056,213 | CDKN2A/B | A/G | 0.48 | 1.20 | 1.12–1.28 | 2.1 × 10−7 | 0 | 0.843 |
| rs429358 | 19:45,411,941 | APOE | C/T | 0.13 | 0.55 | 0.50–0.61 | 1.3 × 10−36 | 20.0 | 0.247 |
| rs7412 | 19:45,412,079 | APOE | T/C | 0.09 | 1.39 | 1.26–1.53 | 1.7 × 10−12 | 10.0 | 0.347 |
We only report the most significant genetic variant for the loci with at least one variant with a P-value ≤ 1 × 10−6. The reported P is the P-value from the Han-Eskin random-effects (RE2) model from METASOFT. The rsID is based on dbSNP build 150. The Chr:Position is based on Genome Reference Consortium Human Build 37 (GRCh37)
EA effect allele, OA other allele, EAF effect allele frequency (based on individuals of European ancestry only), OR odds ratio (i.e., odds to become long-lived when carrying the effect allele), 95% CI 95% confidence interval, I2 heterogeneity statistic, Phet P-value for heterogeneity
Fig. 3.
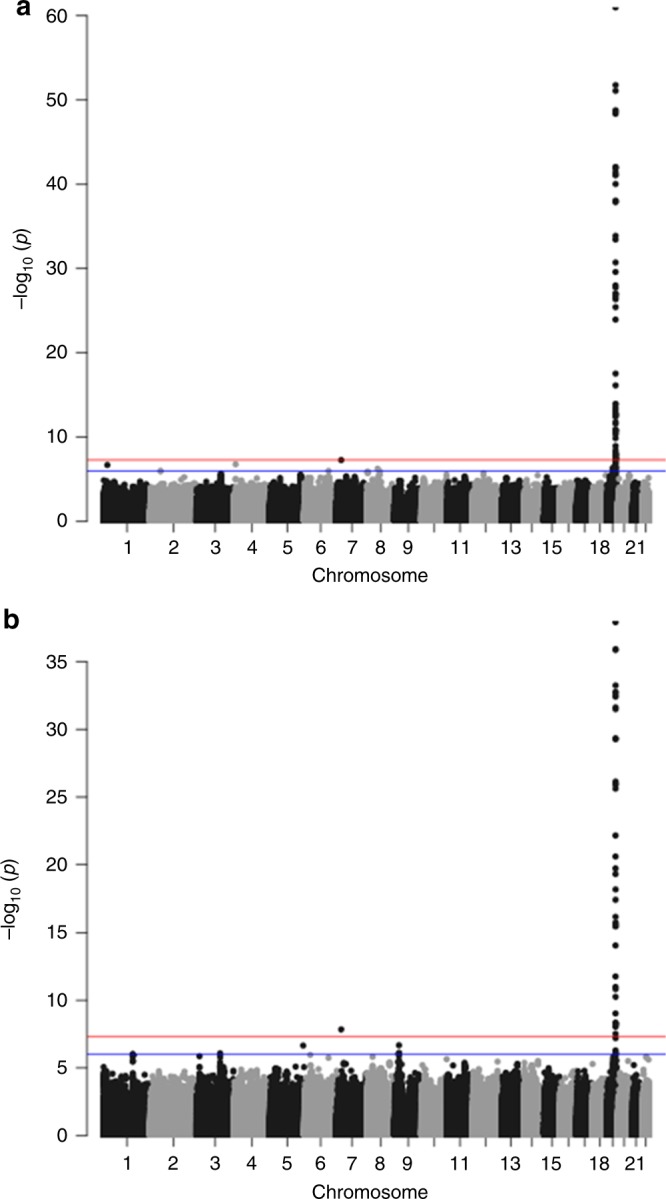
Results of the trans-ethnic genome-wide association meta-analyses. Manhattan plot presenting the –log10 P-values from the trans-ethnic genome-wide association meta-analysis of the 90th percentile cases versus all controls (a) and 99th percentile cases versus all controls (b). The red line indicates the threshold for genome-wide significance (P ≤ 5 × 10−8), while the blue line indicates the threshold for genetic variants that showed a suggestive significant association (P ≤ 1 × 10−6)
Comparison of control definitions
To examine the impact of the definition of controls, we performed a sensitivity analysis in which we compared the results of the meta-analysis using the same case definition (90th percentile) with (1) all controls and (2) dead controls only. For this analysis, only cohorts that contributed results using both control definitions were considered (i.e., 100-plus/LASA/ADC, AGES, CHS, FHS, HRS, LLFS, MrOS, RS, and SOF). The results of the two meta-analyses with different control groups were very similar (Supplementary Fig. 4). Among the three loci with at least one genetic variant with a P-value ≤ 1 × 10−6 in either meta-analysis (and analysed in the same cohorts in both meta-analyses), the most significant variants had odds ratios (ORs) that differed by <1% (Supplementary Table 1).
Replication of previously identified loci for human lifespan
To determine the association of previously identified loci for human lifespan and longevity, we performed a look-up of the reported genetic variants within these loci in our meta-analyses data sets. The only previously identified loci that contained variants that showed a significant (P < 7.8 × 10−4, i.e., Bonferroni adjusted for the number of tested loci (n = 64)) and directionally consistent associations in our study were FOXO3 and CDKN2A/B (Supplementary Data 1). As depicted in Supplementary Fig. 5, the effects of the most frequently reported variants within these loci (i.e., rs2802292 and rs1556516) fluctuate between cohorts and there seems to be no correlation with the genetic background of the included populations. However, for the reported variants within both loci, the odds of surviving to the 99th percentile age is higher than the odds of surviving to the 90th percentile age, indicating they likely affect both early and late-life mortality.
Several of the loci that have been associated with increased parental lifespan in the most recent and largest meta-analysis of GWA studies for this phenotype (i.e., KCNK3, HTT, LPA, ATXN2/BRAP, and LDLR)20 contain genetic variants that show a nominal significant association (P < 0.05) with higher odds of surviving to the 90th and/or 99th percentile age. Since the phenotypes used in our study (i.e., cases surviving at or beyond the age corresponding to the 90th/99th survival percentile) were different from the one used in the previous study (i.e., parental lifespan), we performed an additional look-up of these variants in one of the UK Biobank data sets we created for validation of our findings (i.e., the 90th percentile cases versus all controls data set). With the exception of the variant in HTT, all variants showed a nominal significant association in this data set (Supplementary Table 2), indicating that the lack of significant replication of these loci in our discovery phase data set is not likely to be due to a difference in the used phenotype.
Gene-level association analysis
In addition to genetic variant associations, GWA studies can also be used to identify gene-level associations by integrating results from expression quantitative trait locus (eQTL) studies that relate variants to gene expression. In order to identify gene-level associations, we used MetaXcan, an analytic approach that uses tissue-specific eQTL results from the GTEx project to estimate gene-level associations with the trait examined from summary-level GWA study results25. Tissue-specific genetically predicted expression of 14 genes (ANKRD31, BLOC1S1, KANSL1, CRHR1, ARL17A, LRRC37A2, ERCC1, RELB, DMPK, CD3EAP, PVRL2, GEMIN7, BLOC1S3, and APOC2) was significantly associated with survival to the 90th and/or 99th percentile age after adjustment for multiple testing (Table 5). Eight of these genes (ERCC1, RELB, DMPK, CD3EAP, PVRL2, GEMIN7, BLOC1S3, and APOC2) are located near the APOE gene, raising the likely possibility that these associations reflected the influence of variants in this well-established longevity-associated locus. The remaining genes are located on chromosome 5, 12, and 17. As depicted in Supplementary Data 2, distinct sets of genetic variants were used by MetaXcan for all significant tissue-specific gene expression associations with survival to the 90th and/or 99th percentile age.
Table 5.
Results of the gene-level association analyses
| Genes | Ensembl ID | Chromosome band | Tissue | OR90 | P 90 | OR99 | P 99 |
|---|---|---|---|---|---|---|---|
| ANKRD31 | ENSG00000145700 | 5q13.3 | Stomach | 0.63 | 1.1 × 10−6 | 0.61 | 9.0 × 10−4 |
| BLOC1S1 | ENSG00000135441 | 12q13.2 | Adipose subcutaneous | 0.49 | 4.5 × 10−7 | 0.56 | 0.009 |
| KANSL1 | ENSG00000120071 | 17q21.31 | Skin sun exposed lower leg | 1.22 | 1.5 × 10−6 | 1.26 | 1.9 × 10−4 |
| CRHR1 | ENSG00000120088 | 17q21.31 | Nerve tibial | 1.54 | 3.4 × 10−7 | 1.81 | 6.2 × 10−6 |
| ARL17A | ENSG00000185829 | 17q21.31 | Artery aorta | 1.24 | 8.1 × 10−7 | 1.31 | 5.9 × 10−5 |
| ARL17A | ENSG00000185829 | 17q21.31 | Breast mammary tissue | 1.18 | 1.8 × 10−6 | 1.22 | 3.2 × 10−4 |
| ARL17A | ENSG00000185829 | 17q21.31 | Colon sigmoid | 1.21 | 2.2 × 10−6 | 1.21 | 0.002 |
| LRRC37A2 | ENSG00000238083 | 17q21.31 | Minor salivary gland | 1.17 | 2.2 × 10−6 | 1.20 | 4.4 × 10−4 |
| ERCC1 | ENSG00000012061 | 19q13.32 | Ovary | 1.19 | 2.8 × 10−7 | 1.24 | 1.8 × 10−4 |
| RELB | ENSG00000104856 | 19q13.32 | Lung | 0.57 | 2.0 × 10−7 | 0.44 | 2.9 × 10−6 |
| DMPK | ENSG00000104936 | 19q13.32 | Stomach | 1.64 | 1.7 × 10−6 | 2.31 | 1.8 × 10−6 |
| CD3EAP | ENSG00000117877 | 19q13.32 | Brain substantia nigra | 0.51 | 8.0 × 10−17 | 0.36 | 3.8 × 10−15 |
| PVRL2 | ENSG00000130202 | 19q13.32 | Artery coronary | 1.36 | 5.0 × 10−7 | 1.59 | 1.6 × 10−6 |
| PVRL2 | ENSG00000130202 | 19q13.32 | Oesophagus muscularis | 1.62 | 6.6 × 10−7 | 2.31 | 4.4 × 10−8 |
| GEMIN7 | ENSG00000142252 | 19q13.32 | Brain nucleus accumbens basal ganglia | 0.85 | 1.5 × 10−4 | 0.70 | 1.4 × 10−7 |
| BLOC1S3 | ENSG00000189114 | 19q13.32 | Oesophagus muscularis | 2.80 | 6.4 × 10−16 | 4.47 | 1.3 × 10−13 |
| APOC2 | ENSG00000234906 | 19q13.32 | Skin not sun exposed suprapubic | 0.75 | 4.2 × 10−7 | 0.74 | 9.3 × 10−4 |
OR odds ratio (i.e., odds to become long-lived when having an increased tissue-specific gene expression). P-values highlighted in bold are significant after adjustment for multiple testing of 247,999 longevity associations with gene-tissue pairs (Storey q-value < 0.05). OR90 and P90 are based on the analysis of the 90th percentile cases versus all controls meta-analysis data set, while OR99 and P99 are based on the analysis of the 99th percentile cases versus all controls meta-analysis data set
Genetic correlation analyses
LD score regression was performed to determine the genetic correlation between the different case definitions used for our meta-analyses (based on the results from the European cohorts only), and between longevity and other traits and diseases24. The genetic correlation (rg) between the 90th and 99th percentile analysis, using all controls for both groups, was 1.01 (SE = 0.06, P = 3.9 × 10−66). Using LD Hub26, which performs automated LD score regression, we subsequently estimated the genetic correlation of our phenotypes with 246 diseases and traits available in their database. We found a significant genetic correlation of our phenotypes with the father’s age at death phenotype from the UK Biobank. The most significant (negative) genetic correlation of both our phenotypes was with coronary artery disease (CAD) (rg (SE) = −0.40 (0.07) and rg (SE) = −0.29 (0.07), respectively) and several traits involved in type 2 diabetes (T2D) also showed a significant association with one or both phenotypes after Bonferroni adjustment for multiple testing (Table 6 and Supplementary Data 3).
Table 6.
Results of the genetic correlation analyses of the 90th and 99th percentile phenotypes with other diseases and traits
| Disease/trait | rg90 | SE90 | P 90 | rg99 | SE99 | P 99 |
|---|---|---|---|---|---|---|
| Coronary artery disease | −0.40 | 0.07 | 1.7 × 10−8 | −0.29 | 0.07 | 1.2 × 10−5 |
| Fathers age at death | 0.74 | 0.13 | 2.5 × 10−8 | 0.54 | 0.13 | 2.7 × 10−5 |
| HDL cholesterol | 0.36 | 0.07 | 1.0 × 10−7 | 0.22 | 0.07 | 0.002 |
| Age of first birth | 0.33 | 0.07 | 3.8 × 10−7 | 0.16 | 0.07 | 0.019 |
| Years of schooling 2016 | 0.26 | 0.05 | 9.6 × 10−7 | 0.12 | 0.05 | 0.017 |
| Waist circumference | −0.26 | 0.05 | 2.4 × 10−6 | −0.19 | 0.06 | 0.001 |
| Type 2 diabetes | −0.44 | 0.10 | 4.4 × 10−6 | −0.42 | 0.10 | 2.0 × 10−5 |
| Overweight | −0.28 | 0.06 | 1.2 × 10−5 | −0.23 | 0.07 | 9.0 × 10−4 |
| Fasting insulin main effect | −0.45 | 0.11 | 3.0 × 10−5 | −0.33 | 0.11 | 0.002 |
| Urate | −0.26 | 0.07 | 5.0 × 10−5 | −0.15 | 0.06 | 0.013 |
| Body mass index | −0.21 | 0.05 | 9.2 × 10−5 | −0.19 | 0.07 | 0.004 |
| Cigarettes smoked per day | −0.49 | 0.13 | 1.0 × 10−4 | −0.31 | 0.13 | 0.016 |
| Mothers age at death | 0.51 | 0.14 | 2.0 × 10−4 | 0.14 | 0.13 | 0.289 |
| Waist-to-hip ratio | −0.24 | 0.07 | 2.0 × 10−4 | −0.15 | 0.07 | 0.028 |
P-values highlighted in bold are significant after Bonferroni adjustment for multiple testing (P < 0.05/246). rg90, SE90, and P90 are based on the analysis of the 90th percentile cases versus all controls meta-analysis data set, while rg99, SE99, and P99 are based on the analysis of the 99th percentile cases versus all controls meta-analysis data set
rg genetic correlation, SE standard error of the rg estimate, HDL high-density lipoprotein
Discussion
We brought together studies from all over the world to perform GWA study meta-analyses in over 13,000 long-lived individuals of diverse ethnic background, including European, East Asian and African American ancestry, to characterise the genetic architecture of human longevity. We used the 1000 Genomes reference panel for imputation to expand the coverage of the genome in comparison to previous GWA studies of longevity. Consistent with previous reports, rs429358, defining ApoE ε4, was associated with decreased odds of becoming long-lived. Moreover, we report a genome-wide significant association of rs7412, defining ApoE ε2, with increased odds of becoming long-lived. We additionally found a genome-wide significant association of a locus near GPR78. Gene-level association analysis revealed association of increased KANSL1, CRHR1, ARL17A, and LRRC37A2 expression and decreased ANKRD31 and BLOC1S1 expression with increased odds of becoming long-lived. Genetic correlation analysis showed that our longevity phenotypes are genetically correlated with father’s age at death, CAD and T2D-related phenotypes.
Genetic variation in APOE is well known to be associated with longevity and lifespan, with the first report more than two decades ago in a small candidate gene study27. Since then, there have been numerous candidate gene studies, including individuals of diverse ancestry, which have identified associations of ApoE with longevity28–32. However, thus far, rs7412, the ApoE ε2-defining, genetic variant has not been reported to show a genome-wide significant association in GWA studies of longevity and lifespan. This could be due to the fact that we performed imputation using the 1000 Genomes reference panel, while earlier GWA studies used the HapMap reference panel, which has limited coverage of this variant. ApoE mediates cholesterol metabolism in peripheral tissues and is the principal cholesterol carrier in the brain. The ApoE ε2 and ε4 variants have previously been associated with a decreased (ε2) or increased (ε4) risk for several age-related diseases, such as cardiovascular disease and Alzheimer’s disease33, which could explain their effect on longevity. The fact that the two variants in ApoE show opposite effects may be attributable to differences in structural and biophysical properties of the protein, since ApoE ε2 shows high stability and ApoE ε4 low stability upon folding34.
We also found a genome-wide significant association of rs7676745, located on chromosome 4 near GPR78. We have to note that this locus would benefit from replication in independent cohorts in the future, given that we were not able to replicate this variant in the cohorts in which de novo genotyping was applied. There is no report of association of this locus with other traits according to Phenoscanner (http://www.phenoscanner.medschl.cam.ac.uk/)35, although other genetic variants in this gene have been associated with several diseases and traits in the UK Biobank, including death due to a variety of disorders. The GPR78 protein, belongs to the family of G-protein-coupled receptors, whose main function is to mediate physiological responses to various extracellular signals, including hormones and neurotransmitters36. However, the specific function of GPR78 is still largely unknown, although it has been shown to play a role in lung cancer metastasis37.
To maximise power for discovery, we meta-analysed results from all of the studies that contained long-lived individuals that met our 90th and/or 99th percentile case definitions, had genome-wide genetic data, and were able to participate. Hence, we were not able to replicate our findings in an independent cohort with genome-wide genotype data and participants reaching the age of our case definitions. Therefore, we tried to validate our findings using two related phenotypes, parental longevity and lifespan, in the UK Biobank. We applied our case and control definitions to the parental lifespan of genotyped middle-aged UK Biobank participants rather than the participants themselves, as none of the latter fulfilled the age criteria for cases in our study. Although this resulted in relatively large data sets for both the 90th and 99th percentile analysis, the power to replicate our findings using the parental longevity traits was lower in comparison to replication using the traits based on the genotyped individuals themselves, since these individuals share only half of their parental genomes. In addition, many of the genotyped individuals, who were 40–69 years at recruitment, will never reach the age belonging to the 90th, let alone the 99th, percentile of their birth cohort. This may explain why we were unable to validate any of our suggestive associations (P ≤ 1 × 10−6), with the exception of the genetic variants at the APOE locus in these data sets. On the other hand, we were able to validate one additional locus, CDKN2A/B, in the parental lifespan data set. This is not surprising, since this locus had already been reported to associate with parental lifespan20. However, it is unclear why our reported variants at this locus, rs7039467 and rs2184061, are not associated with parental longevity, given that the most significant parental lifespan-associated variant at this locus, rs1556516, also shows a nominal significant effect on parental longevity (see Supplementary Table 2). We hypothesise that this may be due to a difference in the LD structure of the reference panels used for imputation.
We were able to detect significant genetic associations at two previously identified longevity/lifespan-related loci, FOXO3 and CDKN2A/B. For the other loci, we did not find evidence for replication (P > 7.8 × 10−4), despite having adequate power (≥ 0.8) for replication of all but one of the examined genetic variants (rs28926173) associated with the discrete longevity phenotypes. We were not able to calculate our power to replicate the variants associated with the continuous lifespan-related phenotypes, although we should have had adequate power to replicate variants with a minor allele frequency (MAF) > 12% and an OR > 1.1 (based on the 90th percentile versus all controls analysis). However, several of the variants associated with parental lifespan show a directionally consistent and nominal significant association with our phenotypes, indicating they may also be relevant for longevity. The failure to replicate previously reported loci could be due to the use of a different longevity phenotype then what was used in previous studies, the small effect size of some of the variants associated with parental lifespan, and the modest power of our study. The fact that we detect significant associations of variants in the FOXO3 locus is not surprising, since this locus was previously reported in the longevity GWA study from the CHARGE consortium7, from which many cohorts are included in these meta-analyses. So far, three functional longevity-associated variants have been identified at the FOXO3 locus (rs2802292, rs12206094, and rs4946935). For all of them, an allele-specific response to cellular stress was observed. Consistently, the longevity-associated alleles of all three variants were shown to induce FOXO3 expression38,39. The CDKN2A/B locus has previously been associated with parental lifespan and parents’ attained age in the UK Biobank as well as a diversity of age-related diseases13,20,40. The longevity-associated allele of the most significant variant at this locus (rs1556516) has also been associated with lower odds of developing CAD41. Although the molecular mechanism behind this association is still unclear, it is known that genes encoded at the CDKN2A/B locus are involved in cellular senescence42, a known hallmark of ageing in animal models43.
The gene-level association analysis identified several associations between increased (KANSL1, CRHR1, ARL17A, and LRRC37A2) or decreased (ANKRD31 and BLOC1S1) genetically driven tissue-specific gene expression with survival to the 90th percentile age. The increased expression of KANSL1, CRHR1, ARL17A, and LRRC37A2 on chromosome 17q21.31 is regulated by different genetic variants, indicating that these associations may be independent. More functional work is needed to determine the exact relationship between the altered genetically driven tissue-specific expression of these genes and longevity in humans.
A limitation of MetaXcan is that the underlying GTEx models might not have been adequately adjusted for age, which could be problematic for an age-related phenotype like longevity. However, MetaXcan has successfully been used to identify gene-level associations with age-related diseases and traits, such as Alzheimer’s disease and age-related macular degeneration25.
The genetic correlation analyses showed that survival to ages corresponding to the 90th and 99th percentile shared genetic associations with father’s age at death, CAD and T2D-related phenotypes, suggesting that survival to old ages may at least partially be explained by protective influences on the mechanisms underlying these traits. The genetic correlation with CAD and T2D-related phenotypes is expected, since it has previously been reported that individuals from long-lived families show a decreased prevalence of cardiovascular disease and T2D44,45. The higher genetic correlation of our longevity phenotypes with father’s in comparison to mother’s age at death may be explained by the difference in the prevalence of cardiovascular diseases and T2D between men and women in the last century46,47, which may be, at least partially, attributable to a difference in smoking prevalence48. Hence, the correlation of our longevity phenotypes with the parental age at death phenotypes from UK Biobank is likely due to the absence of death from specific diseases (i.e., those with a higher prevalence in men). For longevity-specific loci, on the other hand, one would expect that they will have beneficial effects on multiple diseases simultaneously, since long-lived individuals show a delay in overall morbidity49.
Our study design imposed an age gap between cases and controls to reduce outcome misclassification, which we expected could potentially increase power by increasing the genetic effect size. It has been correctly noted that longevity study designs that include an age gap between cases and controls result in an effect estimate that is based on an OR and a relative risk (RR) term, which could lead to the identification of genetic variant associations related to early mortality (OR), rather than survival past the case age threshold (RR) (for more details see Sebastiani et al.)50. However, we have presented evidence that imposing a case–control age gap did not greatly influence our results or prevent our replication of variant associations previously discovered using study designs without a case–control age gap. First, our sensitivity analysis indicated that reducing the age gap between cases and controls had a minimal effect on our results. Our sensitivity analysis compared results using dead controls, where all individuals had died before they reached the 60th percentile age, and all controls, which included dead controls and individuals whose age at last contact was below the 60th percentile age but whose age of death was unknown. There is likely to be some outcome misclassification of the living controls, since a small percentage may survive beyond the age corresponding to the 90th or 99th survival percentile. On the other hand, the age gap between cases and controls was narrower for all controls compared to dead controls. However, despite the narrower age gap, the suggestively significant results in all controls and dead controls comparisons with 90th percentile cases were essentially unchanged, and there was a very high genetic correlation between the results of these two meta-analyses, indicating that the age gap had little or no impact on our results. Second, if we had discovered a large number of genome-wide significant variant associations in our study, it could be argued that the OR, reflecting early mortality, contributed to some or all of them. However, the only genome-wide significant variant associations we detected were in the APOE locus, which have been identified using multiple study designs, including designs with no pre-specified age gap between cases and controls14, and the GPR78 locus. Third, it is unlikely that our study design prevented the replication of findings from previous GWA studies of survival to extreme ages (i.e., 99th percentile cases) that did not include a case–control age gap, since such studies would only identify variants associated with survival past the minimum case age and not with early mortality. For variants with no early mortality association, it would be expected that the association estimate in our study would have an OR equal to one and a RR greater than one. Nothing prevents our study design from also detecting this type of variant association, as our estimated association parameter reflects both the OR and RR.
The majority of the previously performed GWA studies of longevity used the survival of individuals to a pre-defined age threshold (i.e., 85, 90, or 100 years) as selection criterion to define long-lived cases. Although these studies used a consistent phenotype for each cohort included in the GWA study, this type of selection may gave rise to heterogeneity, given that survival probabilities differ between sexes and birth cohorts22. Moreover, it was recently shown that the heritable component of longevity is strongest in individuals belonging to the top 10% survivors of their birth cohort6. Hence, instead of using a pre-defined age threshold, we decided to select cases based on country-, sex- and birth cohort-specific life tables. For the definition of controls we used the 60th percentile age, since we wanted to include as many controls as possible (preferably from the same cohort as the cases), while leaving a large enough age gap between our cases and controls. Using the 1920 birth cohort as an example, the difference between the 60th and 90th percentile age is 14 years (men) or 11 years (women), which is quite substantial. The difference between the 70th and 90th percentile age, on the other hand, is considerably smaller (9 years (men) or 7 years (women)) and the living controls are more likely to reach the 90th percentile age, which increases the risk of outcome misclassification. Moreover, even when selecting the 60th percentile controls from much later birth cohorts (i.e., 1940) than the cases (i.e., 1900) the ages will not overlap.
Our study has several limitations. First, we did not analyse the sex and mitochondrial chromosomes, since we were unable to gather enough cohorts that could contribute to the analysis of these chromosomes. However, these chromosomes may harbour loci associated with longevity that we thus have missed. Second, although we included as many cohorts as possible, the sample size of our study is still relatively small (especially for the 99th percentile analysis) in comparison to GWA studies of age-related diseases, such as T2D and cardiovascular disease, and parental age at death11,51,52. Hence, this limited our power to detect loci with a low MAF (<1%) that contribute to longevity. Third, we did not perform sex-stratified analyses and may thus have missed sex-specific longevity-related genetic variants. The reason for this is that (1) we only identified a limited number of suggestive significant associations in our unstratified 90th and 99th percentile analyses, (2) our sample size is modest (especially when stratified by sex), and (3) thus far, there has been no report of any genome-wide significant sex-specific longevity locus.
Given that we have included nearly all cohorts with long-lived individuals with genome-wide genetic data in our study, it will be challenging to increase the sample size in future GWA studies using the same extreme phenotypes. Future genetic studies of longevity may therefore benefit from the use of alternative phenotypes or more rigorous phenotype definitions. Alternative phenotypes that could be used are the parental lifespan or healthspan-related phenotypes that were analysed in the UK Biobank or biomarkers of healthy aging20,53,54. One way to strengthen the longevity phenotype is by selecting cases from families with multiple individuals belonging to the top 10% survivors of their birth cohort6. Moreover, given the limited number of longevity-associated genetic variants identified through GWA studies and the availability of affordable exome and whole-genome sequencing, future genetic studies of longevity may also benefit from the analysis of rare genetic variants. Ideally, such studies should also try to include participants from genetically diverse populations. Most cohorts that are currently included in genetic longevity studies originate from populations of European descent, while some longevity loci may be specific for non-European populations, as exemplified by the previously reported genome-wide associations of genetic variants in IL6 and ANKRD20A9P in Han Chinese9. Moreover, a recent genetic study of multiple complex traits has shown the benefit of analysis of diverse populations55.
In conclusion, we performed a genome-wide association study of longevity-related phenotypes in individuals of European, East Asian and African American ancestry and identified the APOE and GPR78 loci to be associated with these phenotypes in our study. Moreover, our gene-level association analyses highlight a role for tissue-specific expression of genes at chromosome 5q13.3, 12q13.2, 17q21.31, and 19q13.32 in longevity. Genetic correlation analyses show that our longevity-related phenotypes are genetically correlated with several disease-related phenotypes, which in turn could help to identify phenotypes that could be used as potential biomarkers for longevity in future (genetic) studies.
Methods
Study populations
In this collaborative effort, we included cohorts that participated in one or more of the previously published GWA studies on longevity7–9. The sample sizes and descriptive characteristics of the cohorts used in this study are provided in Table 1, Supplementary Data 4, and the Supplementary Methods.
We have complied with all relevant ethical regulations for work with human subjects. All participants provided written informed consent and the studies were approved by the relevant institutional review boards.
Case and control definitions
Cases were individuals who lived to an age above the 90th or 99th percentile based on cohort life tables from census data from the appropriate country, sex, and birth cohort. Controls were individuals who died at or before the age at the 60th percentile or whose age at the last follow-up visit was at or before the 60th percentile age. Hence, the number of selected cases and controls is defined by the ages of their birth cohort corresponding to the 60th or 90th/99th percentile age and is independent of the study population used (i.e., the number of controls and cases within a study population is not based on the percentiles of that specific population, but instead on that of their birth cohorts). As part of their recruitment protocol, many of the studies enroled participants that were already relatively old at the time of recruitment (i.e., close to (or even over) the 60th percentile age). The majority of these individuals subsequently survived past the 60th percentile age threshold of their respective birth cohorts, resulting in a small number of controls in comparison to the number of cases for some of these studies.
The cohort life tables were available through the Human Mortality Database (www.mortality.org)56, the United States Social Security Administration (https://www.ssa.gov/oact/NOTES/as120/LifeTables_Tbl_7.html)22 or National registries; https://opendata.cbs.nl/statline/portal.html?_la = nl&_catalog = CBS&tableId = 80333ned&_theme = 90; http://webarchive.nationalarchives.gov.uk/20160129121820/http://www.ons.gov.uk/ons/rel/npp/national-population-projections/2012-based-extra-variants/index.html). For example, the 60th, 90th, and 99th percentile correspond to ages of 75, 89, and 98 years for men and 83, 94, and 102 years for women for the 1920 birth cohort from the US. For cohort life tables providing birth cohort by decade, linear model predictions were used to estimate the ages corresponding to survival percentiles at yearly birth cohorts.
For the parental longevity analyses in the UK Biobank, cases were individuals with at least one parent achieving an age above the 90th or 99th percentile and who had not themselves died, while controls were individuals for whom both parents died at or before the age at the 60th percentile.
Genome-wide association analysis of individual cohorts
Details on the genotyping (platform and quality control criteria), imputation and genome-wide association analyses for each cohort are provided in Supplementary Data 5. In all cohorts, genetic variants were imputed using the 1000G Phase 1 version 3 reference panel. The logistic regression analyses were adjusted for clinical site, known family relationships, and/or the first four principal components (if applicable). All cohorts used a Hardy–Weinberg equilibrium (HWE) P-value that was between 1 × 10−4 and 1 × 10−6 to exclude variants not in HWE, which is considered standard in GWA studies. However, this may have resulted in removal of variants that were out of HWE in the cases due to mortality selection57.
Quality control of individual cohorts
Quality control of the summary statistics from each cohort was performed using the EasyQC software and the standard script (fileqc_1000G.ecf) available on their website (http://www.uni-regensburg.de/medizin/epidemiologie-praeventivmedizin/genetische-epidemiologie/software/)58. The only difference was that we used the expected minor allele count (eMAC) instead of the MAC. To this end, we first calculated the ‘Effective N’ (2/(1/Ncases + 1/Ncontrols)) for each cohort. The use of the ‘Effective N’ instead of the ‘Total N’ leads to a more stringent filtering of genetic variants and decreases the chance of false positive findings due to an imbalance between the number of cases and controls58. The ‘Effective N’ was subsequently used to calculate the eMAC (2 × minor allele frequency × ‘Effective N’ × imputation quality) for each variant. Variants were excluded when eMAC < 10, with the exception of the Newcastle 85 + (90th percentile cases versus all controls) and the RS (99th percentile cases versus all controls) data sets in which we excluded variants when eMAC < 25 due to the large imbalance between the number of cases and controls in these data sets (1:24 and 1:38, respectively) in comparison to the other ones (all < 1:10). For the CLHLS and LLFS data sets, we flipped the strands of several variants based on the discordance of allele frequencies with the reference panel. We only flipped palindromic variants with a MAF < 0.4 and an allele frequency that differed from the reference panel by <10% after switching.
Meta-analyses
The fixed-effect meta-analyses based on the data sets with individuals of European ancestry were performed on the cleaned files using METAL59, with the ‘Effective N’ as weight and adjustment for genomic control (lambda (λ)) for each cohort. Cohorts with an ‘Effective N’ < 50 were excluded from the meta-analyses. We did not apply genomic control on the meta-analyses results, since there was limited inflation (all λ < 1.04, Supplementary Fig. 1).
The trans-ethnic meta-analyses were performed using the random-effects model of Han and Eskin, implemented in METASOFT60. This model separates hypothesis testing from the estimation of the effect size, which allows the test to better model the between-study heterogeneity that is typically encountered in a trans-ethnic meta-analysis. Prior to using METASOFT, study-specific results were filtered as described above, which included removing genetic variants with eMAC < 10, and applying genomic control by multiplying each variant’s standard error by the inverse of the square root of the lambda for cohorts with λ > 1.
Genetic variants for which the total ‘Effective N’ was less than half of the maximum ‘Effective N’ were removed from the meta-analyses results.
Conditional analyses
Conditional analyses were performed using the ‘-condition_on’ option implemented in SNPTEST to determine the number of independent signals at the APOE locus. We performed this analysis in the cohorts that were analysed using SNPTEST and for which both the ApoE ε4 and ApoE ε2 variant showed a significant association in the unadjusted analysis (i.e., CEPH and LLS (combined with GEHA Dutch)). In both cohorts, the association of ApoE ε2 remained significant (P < 0.05) after adjustment for ApoE ε4, indicating an independent effect.
Gene-level association analysis
MetaXcan was used to identify genetically predicted tissue-specific expression associations with longevity using the results from the 90th and 99th percentile cases versus all controls meta-analyses25. GTEx version 7 tissue models of genetically predicted expression were used. To maximize the number of genetic variants that MetaXcan could match with tissue models, the MetaXcan SNP annotation file (gtex_v7_hapmapceu_dbsnp150_snp_annot.txt) was used to map variants from the GWA study results file to rsIDs by chromosome, position, and alleles. To control for the false discovery rate when testing multiple genes across multiple tissues, the Storey q-value was applied and a q-value < 0.05 was considered significant61.
Colocalization of the tissue-specific eQTL results from the GTEx project and our longevity meta-analyses results was performed using the ‘coloc.abf’ function implemented in the R-package coloc62.
Genetic correlation analysis
To estimate the genetic correlation between the different phenotypes used in this study, we used LD score regression24. The genetic correlation between the results from the 90th and 99th percentile cases versus all controls meta-analyses and 246 diseases and traits were estimated using the LD Hub web portal (http://ldsc.broadinstitute.org/ldhub/)26. Since LD score regression is currently only possible with data from individuals of European ancestry, we used our meta-analyses results based on the cohorts from populations of European descent only.
Power calculation
The power calculations for the validation in the UK Biobank and for the replication of previously identified loci associated with human lifespan were performed using the Genetic Association Study Power Calculator (http://csg.sph.umich.edu/abecasis/cats/gas_power_calculator/index.html) using an additive disease model and a disease prevalence of 0.1 (90th percentile) or 0.01 (99th percentile).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
A full list of acknowledgements, including support for each of the participating cohorts, is provided in Supplementary Note 1.
Author contributions
J.D., D.S.E., P.E.S., and J.M.M. designed and supervised the study. J.D. and D.S.E. performed the meta-analyses and follow-up experiments. J.D., D.S.E., D.P.K., K.L., P.E.S., and J.M.M. interpreted the data. J.D., D.S.E., D.E.A., N.T., M.N., X.L., M.K.W., M.L.B., A.vdS., G.A., E.B.W., C.S., A.V.S., I.S., H.J.C., J.Do., N.A., A.M.A., K.L.A., E.J.B., B.D.,C.J., A.K., L.-P.L., J.M., P.S., S.J.vdL., K.Y., and W.Z. performed association analyses for individual cohorts. G.A., M.B., J.D.F., E.S.O., J.Do., H.B., J-F.D., P.G., C.M.-R., C.N., J.I.R., J.S., K.D.T., A.G.U., J.C.C., S.C., S.R.C., K.D., V.G., D.K., W.L., A.B.N., M.J.T.R., J.-M.R., D.vH., and J.W.V. were involved in data acquisition and/or genotyping of the individual cohorts. N.B., H.B., K.C., L.C., S.R.C., J.-F.D., R.D., C.F., P.G., V.G., T.B.H., M.H., M.A.H., C.J., I.J., M.J., M.K., S.L.R.K., T.B.L.K., L.J.L., T.L., A.N., E.A.N., E.S.O., T.T.P., M.A.P., B.M.P., O.T.R., T.I.A.S., W.vdF., C.M.vD., D.W., Y.Z., H.H., D.P.K., K.L., P.E.S., and J.M.M. were involved in supervision of individual cohorts. J.D., D.S.E., P.E.S., and J.M.M. wrote the manuscript. All authors read and approved the final version of the manuscript.
Data availability
The full meta-analyses summary statistics are available for download at www.longevitygenomics.org/downloads and through GRASP (https://grasp.nhlbi.nih.gov/FullResults.aspx) and the NHGRI-EBI GWAS Catalog (https://www.ebi.ac.uk/gwas/downloads/summary-statistics). All other data that supports the findings of this study are available from the corresponding authors upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information: Nature Communications thanks Anatoliy Yashin, Yousin Suh and the other anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Joris Deelen, Daniel S. Evans, P. Eline Slagboom, Joanne M. Murabito.
Contributor Information
Joris Deelen, Email: Joris.Deelen@age.mpg.de.
Daniel S. Evans, Email: DEvans@sfcc-cpmc.net
P. Eline Slagboom, Email: P.Slagboom@lumc.nl.
Joanne M. Murabito, Email: murabito@bu.edu
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-11558-2.
References
- 1.Oeppen J, Vaupel JW. Demography. Broken limits life Expect. Sci. 2002;296:1029–1031. doi: 10.1126/science.1069675. [DOI] [PubMed] [Google Scholar]
- 2.van den Berg N, Beekman M, Smith KR, Janssens A, Slagboom PE. Historical demography and longevity genetics: back to the future. Ageing Res. Rev. 2017;38:28–39. doi: 10.1016/j.arr.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Kaplanis J, et al. Quantitative analysis of population-scale family trees with millions of relatives. Science. 2018;360:171–175. doi: 10.1126/science.aam9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruby JG, et al. Estimates of the heritability of human longevity are substantially inflated due to assortative mating. Genetics. 2018;210:1109–1124. doi: 10.1534/genetics.118.301613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sebastiani P, Nussbaum L, Andersen SL, Black MJ, Perls TT. Increasing sibling relative risk of survival to older and older ages and the importance of precise definitions of “aging,” “life span,” and “longevity”. J. Gerontol. A Biol. Sci. Med. Sci. 2016;71:340–346. doi: 10.1093/gerona/glv020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Berg N, et al. Longevity defined as top 10% survivors and beyond is transmitted as a quantitative genetic trait. Nat. Commun. 2019;10:35. doi: 10.1038/s41467-018-07925-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broer L, et al. GWAS of longevity in CHARGE consortium confirms APOE and FOXO3 candidacy. J. Gerontol. A Biol. Sci. Med. Sci. 2015;70:110–118. doi: 10.1093/gerona/glu166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deelen J, et al. Genome-wide association meta-analysis of human longevity identifies a novel locus conferring survival beyond 90 years of age. Hum. Mol. Genet. 2014;23:4420–4432. doi: 10.1093/hmg/ddu139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng Y, et al. Novel loci and pathways significantly associated with longevity. Sci. Rep. 2016;6:21243. doi: 10.1038/srep21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flachsbart F, et al. Immunochip analysis identifies association of the RAD50/IL13 region with human longevity. Aging Cell. 2016;15:585–588. doi: 10.1111/acel.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joshi PK, et al. Genome-wide meta-analysis associates HLA-DQA1/DRB1 and LPA and lifestyle factors with human longevity. Nat. Commun. 2017;8:910. doi: 10.1038/s41467-017-00934-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDaid AF, et al. Bayesian association scan reveals loci associated with human lifespan and linked biomarkers. Nat. Commun. 2017;8:15842. doi: 10.1038/ncomms15842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pilling LC, et al. Human longevity: 25 genetic loci associated in 389,166 UK biobank participants. Aging (Albany NY) 2017;9:2504–2520. doi: 10.18632/aging.101334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sebastiani P, et al. Four genome-wide association studies identify new extreme longevity variants. J. Gerontol. A Biol. Sci. Med. Sci. 2017;72:1453–1464. doi: 10.1093/gerona/glx027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newman AB, et al. A meta-analysis of four genome-wide association studies of survival to age 90 years or older: the Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65:478–487. doi: 10.1093/gerona/glq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nebel A, et al. A genome-wide association study confirms APOE as the major gene influencing survival in long-lived individuals. Mech. Ageing Dev. 2011;132:324–330. doi: 10.1016/j.mad.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Deelen J, et al. Genome-wide association study identifies a single major locus contributing to survival into old age; the APOE locus revisited. Aging Cell. 2011;10:686–698. doi: 10.1111/j.1474-9726.2011.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng, Y. et al. Sex differences in genetic associations with longevity. JAMA Netw. Open1, pii: e181670 (2018). [DOI] [PMC free article] [PubMed]
- 19.Yashin AI, et al. Genetics of human longevity from incomplete data: new findings from the long life family study. J. Gerontol. A Biol. Sci. Med. Sci. 2018;73:1472–1481. doi: 10.1093/gerona/gly057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Timmers, P. R. et al. Genomics of 1 million parent lifespans implicates novel pathways and common diseases and distinguishes survival chances. Elife8, pii: e39856 (2019). [DOI] [PMC free article] [PubMed]
- 21.Partridge L, Deelen J, Slagboom PE. Facing up to the global challenges of ageing. Nature. 2018;561:45–56. doi: 10.1038/s41586-018-0457-8. [DOI] [PubMed] [Google Scholar]
- 22.Bell, F. C. & Miller, M. L. Life Tables for the United States Social Security Area 1900–2100. Social Security Administration, Office of the Chief Actuary, SSA Pub. No. 11-11536 (2005).
- 23.Nygaard M, et al. Birth cohort differences in the prevalence of longevity-associated variants in APOE and FOXO3A in Danish long-lived individuals. Exp. Gerontol. 2014;57:41–46. doi: 10.1016/j.exger.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bulik-Sullivan BK, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 2015;47:291–295. doi: 10.1038/ng.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbeira AN, et al. Exploring the phenotypic consequences of tissue specific gene expression variation inferred from GWAS summary statistics. Nat. Commun. 2018;9:1825. doi: 10.1038/s41467-018-03621-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng J, et al. LD Hub: a centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics. 2017;33:272–279. doi: 10.1093/bioinformatics/btw613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schachter F, et al. Genetic associations with human longevity at the APOE and ACE loci. Nat. Genet. 1994;6:29–32. doi: 10.1038/ng0194-29. [DOI] [PubMed] [Google Scholar]
- 28.Sebastiani P, et al. APOE alleles and extreme human longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2019;74:44–51. doi: 10.1093/gerona/gly174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryu S, Atzmon G, Barzilai N, Raghavachari N, Suh Y. Genetic landscape of APOE in human longevity revealed by high-throughput sequencing. Mech. Ageing Dev. 2016;155:7–9. doi: 10.1016/j.mad.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shadyab AH, et al. Replication of genome-wide association study findings of longevity in White, African American, and Hispanic Women: the women’s health initiative. J. Gerontol. A Biol. Sci. Med. Sci. 2017;72:1401–1406. doi: 10.1093/gerona/glx037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garatachea N, et al. ApoE gene and exceptional longevity: insights from three independent cohorts. Exp. Gerontol. 2014;53:16–23. doi: 10.1016/j.exger.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Liu G, et al. APOE gene polymorphism in long-lived individuals from a central China population. Sci. Rep. 2017;7:3292. doi: 10.1038/s41598-017-03227-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahley RW, Rall SC., Jr. Apolipoprotein E far more than a lipid transport protein. Annu. Rev. Genom. Hum. Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- 34.Huang Y, Weisgraber KH, Mucke L, Mahley RW. Apolipoprotein E: diversity of cellular origins, structural and biophysical properties, and effects in Alzheimer’s disease. J. Mol. Neurosci. 2004;23:189–204. doi: 10.1385/JMN:23:3:189. [DOI] [PubMed] [Google Scholar]
- 35.Staley JR, et al. PhenoScanner: a database of human genotype-phenotype associations. Bioinformatics. 2016;32:3207–3209. doi: 10.1093/bioinformatics/btw373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong DD, Zhou H, Li G. GPR78 promotes lung cancer cell migration and metastasis by activation of Galphaq-Rho GTPase pathway. BMB Rep. 2016;49:623–628. doi: 10.5483/BMBRep.2016.49.11.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grossi V, et al. The longevity SNP rs2802292 uncovered: HSF1 activates stress-dependent expression of FOXO3 through an intronic enhancer. Nucl. Acids Res. 2018;46:5587–5600. doi: 10.1093/nar/gky331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flachsbart F, et al. Identification and characterization of two functional variants in the human longevity gene FOXO3. Nat. Commun. 2017;8:2063. doi: 10.1038/s41467-017-02183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeck WR, Siebold AP, Sharpless NE. Review: a meta-analysis of GWAS and age-associated diseases. Aging Cell. 2012;11:727–731. doi: 10.1111/j.1474-9726.2012.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schunkert H, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat. Genet. 2011;43:333–338. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baker DJ, Jin F, van Deursen JM. The yin and yang of the Cdkn2a locus in senescence and aging. Cell Cycle. 2008;7:2795–2802. doi: 10.4161/cc.7.18.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newman AB, et al. Health and function of participants in the Long Life Family Study: a comparison with other cohorts. Aging (Albany NY) 2011;3:63–76. doi: 10.18632/aging.100242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Westendorp RG, et al. Nonagenarian siblings and their offspring display lower risk of mortality and morbidity than sporadic nonagenarians: The Leiden Longevity Study. J. Am. Geriatr. Soc. 2009;57:1634–1637. doi: 10.1111/j.1532-5415.2009.02381.x. [DOI] [PubMed] [Google Scholar]
- 46.Mosca L, Barrett-Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124:2145–2154. doi: 10.1161/CIRCULATIONAHA.110.968792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gale EA, Gillespie KM. Diabetes and gender. Diabetologia. 2001;44:3–15. doi: 10.1007/s001250051573. [DOI] [PubMed] [Google Scholar]
- 48.Ng M, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA. 2014;311:183–192. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- 49.Andersen SL, Sebastiani P, Dworkis DA, Feldman L, Perls TT. Health span approximates life span among many supercentenarians: compression of morbidity at the approximate limit of life span. J. Gerontol. A Biol. Sci. Med. Sci. 2012;67:395–405. doi: 10.1093/gerona/glr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sebastiani P, et al. Limitations and risks of meta-analyses of longevity studies. Mech. Ageing Dev. 2017;165:139–146. doi: 10.1016/j.mad.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.CARDIoGRAMplusC4D Consortium. et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat. Genet. 2013;45:25–33. doi: 10.1038/ng.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DIAbetes Genetics Replication Meta-analysis Consortium. et al. Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat. Genet. 2014;46:234–244. doi: 10.1038/ng.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zenin A, et al. Identification of 12 genetic loci associated with human healthspan. Commun. Biol. 2019;2:41. doi: 10.1038/s42003-019-0290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lara J, et al. A proposed panel of biomarkers of healthy ageing. BMC Med. 2015;13:222. doi: 10.1186/s12916-015-0470-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wojcik GL, et al. Genetic analyses of diverse populations improves discovery for complex traits. Nature. 2019;570:514–518. doi: 10.1038/s41586-019-1310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.University of California, Berkeley (USA) & Max Planck Institute for Demographic Research (Germany). Human Mortality Database.
- 57.Kulminski A, Culminskaya I, Yashin AI. Letter to the editor: standardization of genetic association studies, pros and cons, reaffirmed. Age (Dordr.) 2014;36:945–947. doi: 10.1007/s11357-013-9602-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winkler TW, et al. Quality control and conduct of genome-wide association meta-analyses. Nat. Protoc. 2014;9:1192–1212. doi: 10.1038/nprot.2014.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han B, Eskin E. Random-effects model aimed at discovering associations in meta-analysis of genome-wide association studies. Am. J. Hum. Genet. 2011;88:586–598. doi: 10.1016/j.ajhg.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc. Natl Acad. Sci. USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Giambartolomei C, et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 2014;10:e1004383. doi: 10.1371/journal.pgen.1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The full meta-analyses summary statistics are available for download at www.longevitygenomics.org/downloads and through GRASP (https://grasp.nhlbi.nih.gov/FullResults.aspx) and the NHGRI-EBI GWAS Catalog (https://www.ebi.ac.uk/gwas/downloads/summary-statistics). All other data that supports the findings of this study are available from the corresponding authors upon request.