Abstract
Background
Depression is a well-known co-morbidity of coronary heart disease (CHD) and these two diseases share common risk mechanisms. Here, the aim of this study was to investigate the possible link between energy homeostasis regulation and CHD patients comorbid with depression.
Methods
Two hundred and nine CHD patients and 101 matched healthy individuals were included. Demographic, clinical data were collected, serum irisin, adropin, preptin and brain-derived neurotrophic factor (BDNF) levels were determined by a double-antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA), and the depression was scored by Patient Health Questionnaire-9 (PHQ-9). Correlation analysis as well as multiple linear regression was used to assess the relationship between the three peptides, BDNF serum levels and PHQ-9 scores.
Results
Irisin serum level was significantly lower in CHD patients without depression as compared with healthy controls (P=0.002), as well as adropin (P=0.000), preptin (P=0.000) and BDNF (P=0.000). Furthermore, similar trends were observed in CHD patients with depression in terms of irisin, adropin and BDNF as compared with CHD patients without depression (P=0.006; P=0.003; P=0.002; respectively). Multiple logistic regression results confirmed the contribution of irisin and BDNF to the occurrence of depression in CHD. Interestingly, correlations analysis revealed significant negative correlations between PHQ-9 scores and irisin, adropin, BDNF level (r=−0.43, P<0.01; r=−0.29, P<0.05; r=−0.45, P<0.001 respectively), and irisin serum level was positively correlated with BDNF (r=0.38, P<0.01).
Conclusions
Our study firstly identified the role of energy homeostasis in the susceptibility to depression in CHD patients, and the interaction between irisin and BDNF could trigger the imbalance of energy homeostasis that occurs in depression of CHD patients.
Keywords: Irisin, adropin, preptin, coronary heart disease (CHD), depression
Introduction
As one of the leading causes of mortality worldwide, coronary heart disease (CHD) confers significant individual health and societal consequences including decreased quality of life, high medical costs and heavy socioeconomic burdens. Due to the low adherence to secondary prevention treatments, poor health-related quality of life, and higher rates of adverse events, depression and CHD are highly comorbid (1), with depression being at least 3 times more common in CHD patients than in the general population (2-4). Conversely, emergence of adverse cardiovascular outcomes such as CHD or heart failure was reported in relation to even transient provocation of depressed or sad mood (5). This two-way relationship suggests the possibility for a causal relationship, and warrants the clarification of plausible shared neurobiological pathways underlying this link.
As far as we know, vascular endothelial dysfunction, vascular inflammation and lipid metabolism disorder are the main potential contributors to the increasing incidence of CHD (6). More recently, three newly discovered peptides irisin, adropin and preptin, contributing to the maintenance of energy homeostasis, have drawn a lot of attention. Overwhelming evidence suggests that irisin is beneficial for the energy consumption due to its property of converting white adipose tissue to brown adipose tissue, plays a role in the prevention of obesity (7), directly contributes to structural stabilization and functional improvement in vascular endothelium (8-10), and is involved in the development of cardiovascular disease. Besides irisin, adropin is purported to strongly up-regulate vascular endothelial growth factor receptor 2 (VEGFR2), activate its two downstream signaling pathways (phosphatidylinositol-3 kinase/serine-threonine kinase, PI3K/Akt; extracellular signal-regulated kinases 1/2, ERK 1/2), enhance endothelial form of nitric oxide synthase (eNOS), modulate nitric oxide (NO) bioavailability, and thus exert its endothelial protection (11). Additionally, identification of adropin linking energy homeostasis and lipid metabolism indicated its potential role in disorders associated with dyslipidosis, such as CHD (12). In contrast, although not exhaustive studies demonstrated that preptin was related to the etiopathology of a wide array of diseases for instance obesity (13), polycystic ovary syndrome (PCOS) (14), diabetes (15) and osteoporosis (16), yet a study revealed that it might be involved in the progression of atherosclerosis or the vascular complications of hypertension (17).
In addition to previous studies focusing on the significant associations between energy homeostasis related to CHD, recent studies have investigated the association between serum irisin, adropin, preptin concentrations and the development of various central nervous system (CNS) disorders. A recent survey revealed that irisin can be involved in depression by inducing the expression of BDNF through the peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1a)/fibronectin type III domain containing 5 (FNDC5) pathway (18). Likewise, adropin can activate ERK 1/2 signaling pathway and its downstream cascades of substances BDNF via VEGFR2. Considering the critical role in neurogenesis, synapse formation, plasticity, learning, and memory (19), BDNF elicits neuroprotective functions in depression, which makes adropin an interesting candidate in the occurrence of depression. Furthermore, recent studies demonstrated that BDNF expresses in the peripheral vasculature, promotes the survival of endothelial cells and maintains vascular integrity, and thus is associated with cardiovascular system (20). Given the complex relationship among energy homeostasis, BDNF, CHD and depression, it is feasible to speculate that irisin, adropin, preptin and BDNF serum levels may be related to the susceptibility to depression in CHD patients. To verify the hypothesis, we determined circulating irisin, adropin, preptin and BDNF serum levels in 209 CHD patients with varying scores of PHQ-9.
Methods
Subjects
To evaluate the role of energy homeostasis in CHD patients with depression, 209 CHD patients and 101 matched healthy individuals were selected at the outpatient clinic of Jining First People’s Hospital in Shandong Province. This study was approved by the medical ethics committee of the Jining First People’s Hospital, and all participants provided written informed consent prior to the study.
All CHD patients were angiographically confirmed by at least two experienced cardiologists. Individuals with at least one of the three major coronary artery or major branches significant coronary artery stenosis ≥50% were defined as cases. Patients were excluded on the basis of having severe autoimmunity disease, valvular heart disease, cancer or severe liver and/or kidney disease. Additionally, the 101 matched healthy controls were non-CHD adults after being assessed by a series of examination, such as clinical physical examination, radiographic chest examination, electrocardiogram (ECG) and medical history.
All CHD patients suffering from depression or not were judged according to the 5th edition of Diagnosis and Statistical Manual of Mental Disorders (DSM-5). Then the depression was scored by Patient Health Questionnaire-9 (PHQ-9), a 9-item questionnaire which is commonly used to screen for symptoms of depression in outpatients. The sensitivity and specificity had been validated (21). Significantly, to minimize the environmental factors, all patients were asked to complete the questionnaire alone in a separate room unless they asked for help to write or read. The cutoff used for depression analysis of PHQ-9 scale was 5 points or greater (22-24).
Blood chemistry analysis
After an overnight fasting, venous blood samples were collected. Prior to analyses, serum samples were isolated by centrifugation at 3,000 rpm for 10 minutes and maintained at −80 °C. Irisin, adropin preptin and BDNF serum levels in all participants were measured by a double-antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA) (GUXI, China). Firstly, standards and samples were added to appropriate wells, which were pre-coated with anti-human monoclonal antibody. Then the plates were incubated for 30 min at 37 °C after sealed by a membrane. Second antibody labeled by horseradish peroxidase (HRP) was added to all wells to form into antibody-antigen-HRP labelled antibody complex. After incubated and washed again, substrate solution A and substrate solution B were added and the color reaction was developed for 10 min. Finally, stop solution was added and the color became yellow. All of the serum samples were routinely analyzed by ELISA in duplicate, and the results were averaged. Quality characteristics of each ELISA method including limit of detection, intra- and inter-run coefficient of variation are described as Table S1.
Table S1. Quality characteristics of each ELISA method.
| Variables | Detection limit | Intra-run coefficient of variation (%) | Inter-run coefficient of variation (%) |
|---|---|---|---|
| Irisin (pg/mL) | 30–1,400 | 6.5 | 8.9 |
| Adropin (pg/mL) | 100–2,500 | 7.2 | 9.3 |
| Preptin (pg/mL) | 75–2,400 | 8.8 | 10.6 |
| BDNF (ìg/L) | 1.5–50 | 6.4 | 8.2 |
BDNF, brain-derived neurotrophic factor; ELISA, enzyme-linked immunosorbent assay.
Statistical analysis
All statistical analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, USA). Continuous variables were described as mean with standard deviation (SD) and the differences were compared by student’s t-test for normal distribution or non-parametric Mann-Whitney U tests for skewed variables. Categorical variables were compared by the χ2 test. Correlations between three peptides and BDNF serum levels with each other or with PHQ-9 score were analyzed by Spearman correlation analysis. A multiple logistic regression analysis was performed to assess the presence of depression in CHD patients. Significance was defined as P value <0.05 in all analyses.
Results
Basic characteristics of subjects
Overall, 209 patients with CHD and 101 healthy individuals were included in these analyses. The demographic and clinical characteristics of these subjects are summarized in Table 1. As shown, CHD patients did not differ with regard to age, gender, BMI, smoking and drinking status from those healthy individuals (P>0.05). Likewise, when considering comorbid with depression or not, no statistical difference in terms of demographic and clinical characteristics above was still observed (P>0.05).
Table 1. Demographic and clinical characteristics of the study participants.
| Variables | CHD [209] | Controls [101] | P value | CHD-D [156] | P value | CHD+D [53] | P value |
|---|---|---|---|---|---|---|---|
| Age (years) | 60.14±11.42 | 58.44±8.144 | 0.133a | 60.33±10.74 | 0.111b | 59.58±13.32 | 0.684c |
| Gender (M/F, n) | 116/93 | 64/37 | 0.188a | 86/70 | 0.191b | 30/23 | 0.852c |
| Smoking (n, %) | 86 (41.1) | 33 (32.7) | 0.150a | 57 (36.5) | 0.526b | 19 (35.8) | 0.928c |
| Drinking (n, %) | 81 (38.7) | 39 (38.6) | 0.981a | 63 (40.4) | 0.777b | 18 (34.0) | 0.407c |
| BMI (kg/m2) | 24.15±3.573 | 23.46±3.655 | 0.113a | 23.81±3.631 | 0.446b | 24.22±3.602 | 0.479c |
Data are expressed as means ± SEM. a, CHD versus controls; b, CHD-D versus controls; c, CHD-D versus CHD+D. CHD, coronary heart disease; CHD+D, CHD with depression; CHD-D, CHD without depression.
Serum levels of irisin, adropin, preptin and BDNF in CHD patients with or without depression
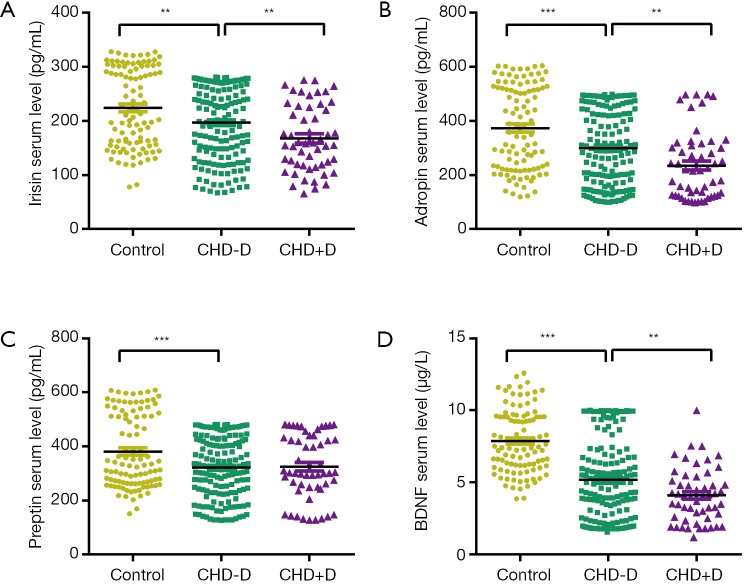
As demonstrated in Figure 1, irisin concentrations were significantly lower in CHD patients without depression as compared with healthy controls (P=0.002), so did adropin (P=0.000), preptin (P=0.000) and BDNF (P=0.000). Furthermore, CHD patients with depression appeared as significantly lower serum levels in terms of irisin, adropin and BDNF as compared with CHD patients without depression (P=0.006; P=0.003; P=0.002; respectively), indicating lower serum levels of these factors may be related to susceptibility to depression in CHD patients. The median concentrations and ranges of irisin, adropin, preptin and BDNF are described in Table S2.
Figure 1.
Comparisons of irisin (A), adropin (B), preptin (C) and BDNF (D) serum levels among CHD patients with, without depression and healthy individuals. **, P<0.01, ***, P<0.001 between groups. CHD, coronary heart disease; CHD+D, CHD with depression; CHD-D, CHD without depression; BDNF, brain-derived neurotrophic factor.
Table S2. The median concentrations and ranges of three peptides and BDNF serum levels between CHD patients and healthy controls.
| Variables | Controls [101] | CHD-D [156] | CHD+D [53] |
|---|---|---|---|
| Irisin (pg/mL) | 213.1 (77.76–327.9) | 203.7 (66.79–280.9) | 158.1 (65.35–275.5) |
| Adropin (pg/mL) | 357.3 (119.0–603.7) | 289.3 (100.2–498.6) | 216.9 (98.29–497.9) |
| Preptin (pg/mL) | 333.7 (150.7–607.8) | 318.8 (127.0–480.5) | 302.5 (127.7–480.8) |
| BDNF (ìg/L) | 7.471 (3.859–12.61) | 4.933 (1.541–9.993) | 4.065 (1.190–9.989) |
CHD+D, CHD with depression; CHD-D, CHD without depression; CHD, coronary heart disease; BDNF, brain-derived neurotrophic factor.
Multiple logistic regression for the presence of depression in CHD patients
Multiple logistic regression was used to identify three peptides and BDNF risk factors of the occurrence of depression in CHD patients. The multiple logistic regression model revealed that irisin (OR: 0.994; 95% CI: 0.989–1.000; P=0.039) and BDNF (OR: 0.860; 95% CI: 0.746–0.990; P=0.036) serum levels were significant in contributing to the occurrence of depression in CHD. The detailed results are shown in Table 2.
Table 2. Logistic regression analysis for the presence of depression in CHD patients.
| Variables | OR (95% CI) | P value |
|---|---|---|
| Irisin (pg/mL) | 0.994 (0.989–1.000) | 0.039 |
| Adropin (pg/mL) | 0.997 (0.995–1.000) | 0.055 |
| Preptin (pg/mL) | 1.002 (0.999–1.005) | 0.274 |
| BDNF (μg/L) | 0.860 (0.746–0.990) | 0.036 |
OR, odds ratio; CI, confidence intervals; CHD, coronary heart disease.
Correlations among PHQ-9 score, three peptides, and BDNF levels
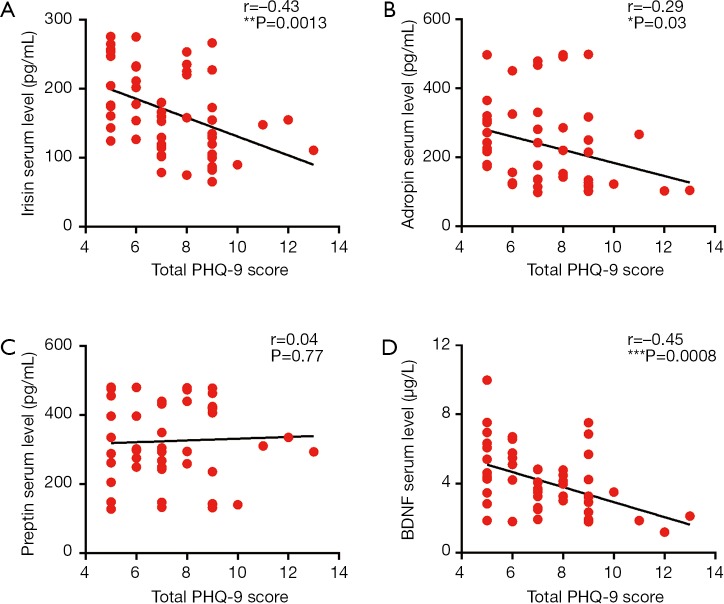
Upon assessing the correlation between PHQ-9 score and irisin, adropin, preptin, BDNF serum levels, we found a significant negative correlation between PHQ-9 score and irisin (r=−0.43, P<0.01), between PHQ-9 score and adropin (r=−0.29, P<0.05), between PHQ-9 score and BDNF (r=−0.45, P<0.001) respectively (shown as Figure 2). Further correlation analysis between three peptides and BDNF showed that irisin serum level was positively correlated with BDNF serum level (r=0.38, P<0.01; shown as Figure 3).
Figure 2.
The correlations between serum levels of irisin (A), adropin (B), preptin (C), BDNF (D) and PHQ-9 scores in CHD patients comorbid with depression. *, P<0.05, **, P<0.01, ***, P<0.001 between groups. CHD, coronary heart disease; BDNF, brain-derived neurotrophic factor.
Figure 3.
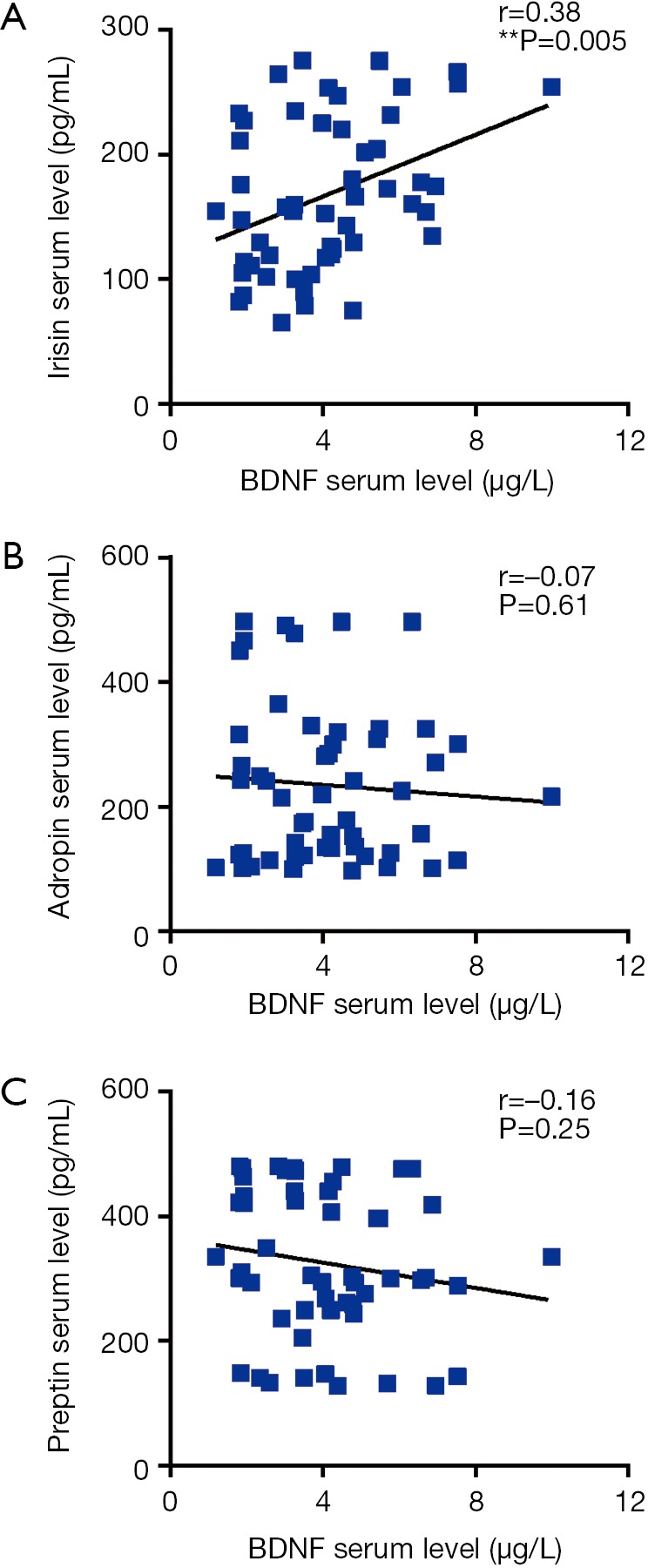
The correlations between serum levels of irisin (A), adropin (B), preptin (C) and BDNF in CHD patients comorbid with depression. **, P<0.01 between groups. CHD, coronary heart disease; BDNF, brain-derived neurotrophic factor.
Discussion
The present study introduces what is to our knowledge a novel shared aetiology energy homeostasis involved in the susceptibility to depression in CHD patients. Among 209 CHD patients and 101 healthy individuals studied, three peptides irisin, adropin, preptin which are responsible for the maintenance of energy homeostasis and BDNF serum levels were determined. Interestingly, we found a significantly lower irisin level in CHD patients as compared with healthy controls. Consistent with our results, Deng (25) reported that circulating irisin level was significantly lower in CHD patients and irisin can be as an independent risk factor for CHD. Besides, serum irisin level is purported to predict the severity of coronary artery disease in patients with stable angina (26). Additionally, accumulating evidences showed that the involvement of irisin in numerous mechanisms such as inhibition of nuclear factor-kappaB (NF-κB)/inducible nitric oxide synthase (iNOS) signaling pathway (27) and activation of AMP-activated protein kinase (AMPK)-PI3K-Akt-eNOS signaling pathway (9) can reduce oxidative/nitrative stresses and consequently exert its endothelial protective function, further strengthening our conclusion. Similarly, adropin can also modulate expression of eNOS and increase the endothelial cells proliferation, migration (11), which may act as a potential protective regulator of atherogenesis and cardiovascular diseases (28-30). In accordance with the finding that diminished serum adropin level in patients with cardiac syndrome X and stable coronary artery disease (29,31), CHD patients showed a significant reduction in adropin level compared with healthy controls in our study. In contrast, few researches indicated that low plasma preptin level might participate in the progression of carotid atherosclerosis by regulating bone anabolism combined with specific receptors (17), a significant decrease of preptin level in CHD patients was also observed in our study, firstly identifying its crucial role in CHD.
To gain further insights into the association of energy homeostasis with susceptibility to the comorbidity of CHD and depression, 209 CHD patients were scored by PHQ-9, an indicator of the presence and severity of depression. Comparisons of serum levels between CHD patients with or without depression showed that there was a remarkable decrease in irisin serum level, indicating that lower irisin serum level may be related to susceptibility to depression in CHD patients. Additionally, animal study demonstrated the crucial role of irisin in inducing antidepressant-like effects in chronic unpredictable stress (CUS) rats, which may be attributed to its regulation to energy metabolism in the prefrontal cortex (32). Likewise, adropin can activate Akt by stimulating Ser-473 phosphorylation and trigger intracellular ligands such as mammalian target of rapamycin (mTOR)—which plays an important role in angiogenesis, neuronal regeneration, synaptic plasticity, inflammatory responses, and apoptosis (33,34). As expected, significant reduction of adropin serum level was observed in CHD patients with depression as well. Although multiple logistic regression analysis confirmed the contribution of only irisin to the occurrence of depression in CHD, the correlation analysis identified the negative correlation between irisin, adropin and PHQ-9 scores of depression in CHD patients, further highlighting their clinical significance of biomarkers to predict the severity of depression in CHD patients.
Notably, BNDF has attracted considerable attention by virtue of its role not only in cardiovascular disease, but also in CNS disorders. There was a remarkable decrease in BDNF serum level in CHD patients compared with healthy individuals in our study, which is consistent with previous study (35). Interestingly, the similar trend still existed in CHD patients with depression as compared with CHD patients without depression, and multiple logistic regression analysis as well as the negative correlation between BDNF and PHQ-9 scores further emphasized its key role in the development of depression in CHD patients. More recently, a meta-analysis showed that the activation of PGC1α-FNDC5/irisin pathway by excise contributed to the up-regulation of BDNF and induced a neuroprotective gene program (36). Correspondingly, the current study found irisin serum level was positively correlated with BDNF serum level, suggesting the interaction between the irisin and BDNF might trigger the imbalance of energy homeostasis that occurs in depression of CHD patients. Crucially, although adropin is considered to activate ERK 1/2 and its downstream cascades of substances such as BDNF via VEGFR2, correlation between adropin and PHQ-9 score was failed to identify, which may due to a variety of external instability. In addition, the negative result of preptin and the depression in CHD patients can be interpreted by many reasons. Firstly, little is currently known whether preptin can cross the blood brain barrier to exert its neuroprotective function. Second, the unassociated relationship between preptin and the occurrence of depression in CHD may due to its own instability or easily concealed by other factors. Third, the negative result may attribute to the relative small sample size and regional bias, thus larger-scale correlation analysis and functional studies investigating the association between the novel shared aetiology energy homeostasis and CHD comorbid with depression are necessary to be carried out from multiple regions to gain further insights into its pathogenesis.
In summary, our present study for the first time demonstrated lower irisin, adropin serum levels may be relevant to the CHD patients comorbid with depression, indicating the crucial role of energy homeostasis in the increased susceptibility to depression in CHD patients of Chinese population. Furthermore, the interaction between the irisin and BDNF may trigger the imbalance of energy homeostasis that occurs in depression of CHD patients. Future mechanism studies targeting the irisin—BDNF interaction are needed to further support this notion.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (81602846 to P Jiang, 81571334 to G Li) and Shandong Natural Science Foundation (ZR2016HQ21 to P Jiang).
Ethical Statement: The study was approved by the medical ethics committee of the Jining First People’s Hospital (No. 20150021), and all participants provided written informed consent prior to the study.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Rocca WA, Boyd CM, Grossardt BR, et al. Prevalence of multimorbidity in a geographically defined American population: patterns by age, sex, and race/ethnicity. Mayo Clin Proc 2014;89:1336-49. 10.1016/j.mayocp.2014.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruo B, Rumsfeld JS, Hlatky MA, et al. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA 2003;290:215-21. 10.1001/jama.290.2.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rutledge T, Reis VA, Linke SE, et al. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol 2006;48:1527-37. 10.1016/j.jacc.2006.06.055 [DOI] [PubMed] [Google Scholar]
- 4.Cohen BE, Edmondson D, Kronish IM. State of the Art Review: Depression, Stress, Anxiety, and Cardiovascular Disease. Am J Hypertens 2015;28:1295-302. 10.1093/ajh/hpv047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gan Y, Gong Y, Tong X, et al. Depression and the risk of coronary heart disease: a meta-analysis of prospective cohort studies. BMC Psychiatry 2014;14:371. 10.1186/s12888-014-0371-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu XH, Qian K, Jiang N, et al. ABCG5/ABCG8 in cholesterol excretion and atherosclerosis. Clin Chim Acta 2014;428:82-8. 10.1016/j.cca.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 7.Boström P, Wu J, Jedrychowski MP, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012;481:463-8. 10.1038/nature10777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han F, Zhang S, Hou N, et al. Irisin improves endothelial function in obese mice through the AMPK-eNOS pathway. Am J Physiol Heart Circ Physiol 2015;309:H1501-8. 10.1152/ajpheart.00443.2015 [DOI] [PubMed] [Google Scholar]
- 9.Lu J, Xiang G, Liu M, et al. Irisin protects against endothelial injury and ameliorates atherosclerosis in apolipoprotein E-Null diabetic mice. Atherosclerosis 2015;243:438-48. 10.1016/j.atherosclerosis.2015.10.020 [DOI] [PubMed] [Google Scholar]
- 10.Wu F, Song H, Zhang Y, et al. Irisin Induces Angiogenesis in Human Umbilical Vein Endothelial Cells In Vitro and in Zebrafish Embryos In Vivo via Activation of the ERK Signaling Pathway. PLoS One 2015;10:e0134662. 10.1371/journal.pone.0134662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovren F, Pan Y, Quan A, et al. Adropin is a novel regulator of endothelial function. Circulation 2010;122:S185-92. 10.1161/CIRCULATIONAHA.109.931782 [DOI] [PubMed] [Google Scholar]
- 12.Kumar KG, Trevaskis JL, Lam DD, et al. Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab 2008;8:468-81. 10.1016/j.cmet.2008.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozkan Y, Timurkan ES, Aydin S, et al. Acylated and desacylated ghrelin, preptin, leptin, and nesfatin-1 Peptide changes related to the body mass index. Int J Endocrinol 2013;2013:236085. 10.1155/2013/236085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Celik O, Celik N, Hascalik S, et al. An appraisal of serum preptin levels in PCOS. Fertil Steril 2011;95:314-6. 10.1016/j.fertnstert.2010.08.058 [DOI] [PubMed] [Google Scholar]
- 15.Aslan M, Celik O, Karsavuran N, et al. Maternal serum and cord blood preptin levels in gestational diabetes mellitus. J Perinatol 2011;31:350-5. 10.1038/jp.2010.125 [DOI] [PubMed] [Google Scholar]
- 16.Li N, Zheng YB, Han J, et al. Lower circulating preptin levels in male patients with osteoporosis are correlated with bone mineral density and bone formation. BMC Musculoskelet Disord 2013;14:49. 10.1186/1471-2474-14-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai H, Liu Q, Dong X, et al. Plasma preptin levels are decreased in patients with essential hypertension. Pharmazie 2018;73:274-8. [DOI] [PubMed] [Google Scholar]
- 18.Wrann CD, White JP, Salogiannnis J, et al. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab 2013;18:649-59. 10.1016/j.cmet.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab 2014;25:89-98. 10.1016/j.tem.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin H, Chen Y, Wang B, et al. Association between brain-derived neurotrophic factor and von Willebrand factor levels in patients with stable coronary artery disease. BMC Cardiovasc Disord 2018;18:23. 10.1186/s12872-018-0762-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gelaye B, Williams MA, Lemma S, et al. Validity of the Patient Health Questionnaire-9 for depression screening and diagnosis in East Africa. Psychiatry Res 2013;210:653-61. 10.1016/j.psychres.2013.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duko B, Geja E, Zewude M, et al. Prevalence and associated factors of depression among patients with HIV/AIDS in Hawassa, Ethiopia, cross-sectional study. Ann Gen Psychiatry 2018;17:45. 10.1186/s12991-018-0215-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gezie LD, Yalew AW, Gete YK, et al. Socio-economic, trafficking exposures and mental health symptoms of human trafficking returnees in Ethiopia: using a generalized structural equation modelling. Int J Ment Health Syst 2018;12:62. 10.1186/s13033-018-0241-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng W. Association of Serum Irisin Concentrations with Presence and Severity of Coronary Artery Disease. Med Sci Monit 2016;22:4193-7. 10.12659/MSM.897376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Efe TH, Acar B, Ertem AG, et al. Serum Irisin Level Can Predict the Severity of Coronary Artery Disease in Patients with Stable Angina. Korean Circ J 2017;47:44-9. 10.4070/kcj.2016.0079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu D, Wang H, Zhang J, et al. Irisin improves endothelial function in type 2 diabetes through reducing oxidative/nitrative stresses. J Mol Cell Cardiol 2015;87:138-47. 10.1016/j.yjmcc.2015.07.015 [DOI] [PubMed] [Google Scholar]
- 28.Wu L, Fang J, Chen L, et al. Low serum adropin is associated with coronary atherosclerosis in type 2 diabetic and non-diabetic patients. Clin Chem Lab Med 2014;52:751-8. 10.1515/cclm-2013-0844 [DOI] [PubMed] [Google Scholar]
- 29.Zhao LP, Xu WT, Wang L, et al. Serum adropin level in patients with stable coronary artery disease. Heart Lung Circ 2015;24:975-9. 10.1016/j.hlc.2015.03.008 [DOI] [PubMed] [Google Scholar]
- 30.Li L, Xie W, Zheng XL, et al. A novel peptide adropin in cardiovascular diseases. Clin Chim Acta 2016;453:107-13. 10.1016/j.cca.2015.12.010 [DOI] [PubMed] [Google Scholar]
- 31.Celik A, Balin M, Kobat MA, et al. Deficiency of a new protein associated with cardiac syndrome X; called adropin. Cardiovasc Ther 2013;31:174-8. 10.1111/1755-5922.12025 [DOI] [PubMed] [Google Scholar]
- 32.Wang S, Pan J. Irisin ameliorates depressive-like behaviors in rats by regulating energy metabolism. Biochem Biophys Res Commun 2016;474:22-8. 10.1016/j.bbrc.2016.04.047 [DOI] [PubMed] [Google Scholar]
- 33.Chen H, Qu Y, Tang B, et al. Role of mammalian target of rapamycin in hypoxic or ischemic brain injury: potential neuroprotection and limitations. Rev Neurosci 2012;23:279-87. 10.1515/revneuro-2012-0001 [DOI] [PubMed] [Google Scholar]
- 34.Li W, Yang Y, Hu Z, et al. Neuroprotective effects of DAHP and Triptolide in focal cerebral ischemia via apoptosis inhibition and PI3K/Akt/mTOR pathway activation. Front Neuroanat 2015;9:48. 10.3389/fnana.2015.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang H, Liu Y, Zhang Y, et al. Association of plasma brain-derived neurotrophic factor and cardiovascular risk factors and prognosis in angina pectoris. Biochem Biophys Res Commun 2011;415:99-103. 10.1016/j.bbrc.2011.10.020 [DOI] [PubMed] [Google Scholar]
- 36.Wrann CD. FNDC5/irisin - their role in the nervous system and as a mediator for beneficial effects of exercise on the brain. Brain Plast 2015;1:55-61. 10.3233/BPL-150019 [DOI] [PMC free article] [PubMed] [Google Scholar]