Abstract
Purpose
To determine the utility of 18F-sodium fluoride positron emission tomography-computed tomography (18F-NaF PET/CT) in the imaging assessment of therapy response in men with osseous-only metastatic prostate cancer.
Methods
In this Institutional Review Board–approved single institution retrospective investigation, we evaluated 21 18F-NaF PET/CT scans performed in 14 patients with osseous metastatic disease from prostate cancer and no evidence of locally recurrent or soft-tissue metastatic disease who received chemohormonal therapy. Imaging-based qualitative and semi-quantitative parameters were defined and compared with changes in serum PSA level.
Results
Qualitative and semi-quantitative image-based assessments demonstrated > 80% concordance with good correlation (SUVmax κ = 0.71, SUVavg κ = 0.62, SUVsum κ = 0.62). Moderate correlation (κ = 0.43) was found between SUVmax and PSA-based treatment response assessments. There was no statistically significant correlation between PSA-based disease progression and semi-quantitative parameters. Qualitative imaging assessment was moderately correlated (κ = 0.52) with PSA in distinguishing responders and non-responders.
Conclusion
18F-NaF PET/CT is complementary to biochemical monitoring in patients with bone-only metastases from prostate cancer which can be helpful in subsequent treatment management decisions.
Keywords: 18F-NaF, PET/CT, Prostate, Cancer, Metastasis, Bone
Introduction
Prostate cancer is the most common cancer in men and the second leading cause of cancer death, with a high incidence of osseous metastases [1]. Traditionally, conventional computed tomography (CT) and bone scintigraphy with 99mTc-based radiotracers, such as 99mTc methylene diphosphonate (MDP), have been used for the detection and monitoring of bony metastases [2]. However, while 99mTc-based radiotracers are sensitive, radiotracers are taken up in a variety of other disease processes, including infection, trauma, non-infectious inflammation, and metabolic bone diseases limiting its specificity for malignancy and treatment monitoring [3, 4]. Due to these limitations, there has been an increased interest in the role of positron emission tomography (PET) for the evaluation and monitoring of metastatic prostate cancer [5].
18F-sodium fluoride (18F-NaF) is a positron-emitting radiopharmaceutical analog of the hydroxyl group found in hydroxyapatite bone crystals, first used for skeletal scintigraphy in the 1970s [6]. With fast bone uptake and rapid blood clearance, 18F-NaF provides high target to background uptake, leading to high-quality images in less than an hour after intravenous administration [7]. However, due to its shorter half-life, technical limitations, and cost at the time, it was replaced by 99mTc-phosphonates. Yet, with the expansion of availability and access to PET/CT, there has been a resurgence of interest in the use of 18F-NaF for bone metastasis imaging [8]. In a cohort of patients with high-risk prostate cancer, Evan-Sapir et al. showed 18F-NaF PET/CT to have a sensitivity and specificity of 100% for the detection of osseous metastases, compared with 70% and 57% for 99mTc-MDP [9], with several subsequent studies supporting the utility of 18F-NaF PET/CT [10–12].
Currently, monitoring the treatment response of bony metastases involves a combination of clinical assessment, biochemical markers with prostate-specific antigen (PSA) and serum alkaline phosphatase (ALP), and imaging with CT and 99mTc-MDP [13]. However, these methods have been inadequate and often non-specific compared with those used for soft tissue disease [14]. The purpose of this study was to investigate the utility of 18F-NaF PET bone scans in evaluating response of osseous metastatic prostate cancer to treatment.
Methods
Institutional review board approval was obtained for this retrospective study. Patient selection criteria for this study were as follows: (1) men above the age of 21 years with a prior histological diagnosis of prostate adenocarcinoma; (2) PSA relapse, defined as post-radical prostatectomy PSA level exceeding 0.2 ng/mL [15] or post-radiation therapy PSA rise of 2 ng/mL or more above the nadir after external beam radiation therapy [16]; (3) patients with bone-only metastases who underwent a baseline and follow-up 18F-NaF PET/CT scan after undergoing medical treatment. Patients undergoing treatment for both metastatic castrate-sensitive prostate cancer (mCSPC) and metastatic castrate-resistant prostate cancer (mCRPC) were included in this study. Exclusion criteria included history of cancer other than prostate cancer, active infection, active inflammatory conditions, recent or complicated nonhealing fracture, and hip or knee arthroplasty. Medical therapy was chosen at the discretion of the treating physicians, who were made aware of the results of the scans.
Records of all men who underwent baseline and follow-up 18F-NaF PET/CT scans from 2010 to 2012 were obtained and reviewed for specific eligibility criteria as defined above. All 18F-NaF PET/CT scans (Biograph Duo LSO; Siemens) were performed 60 min after the intravenous administration of 10 mCi of 18F-NaF. The fused 18F-NaF PET/CT studies were interpreted by a board-certified fellowship-trained nuclear radiologist with more than 20 years of experience of interpreting PET/CT studies. Suspicious skeletal lesions were identified based on previously published guidelines [17]. The maximum standardized uptake values (SUVs) of suspicious lesions, defined as foci of nonphysiological uptake above regional background bone activity, were obtained using 3D region of interest (ROI) software (Siemens). PET variables included maximum SUV value of the most active lesion (SUVmax), average maximum SUV value of all lesions (SUVavg), and the sum of the maximum SUV values of all lesions (SUVsum). PSA labs were available within 50 days of each scan.
Semi-quantitative imaging treatment response and PSA-based treatment response criteria were defined as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) (Table 1). Qualitative imaging assessment of the 18F-NaF PET/CT was the general impression that was reported by the interpreting nuclear radiologist in the final report combining the overall PET and CT findings. Patients were further categorized if they demonstrated progressive disease, progressors (P=PD) vs. non-progressors (NP=SD+PR+CR), and if they demonstrated complete or partial response, responders (R=CR+PR) vs non-responders (NR=SD+PD).
Table 1.
Definition of treatment response criteria
| Semi-quantitative imaging treatment response criteria | PSA-based treatment response criteria | |
|---|---|---|
| CR | Disappearance of all target lesions | PSA decline to undetectable (< 0.2 ng/mL) |
| PR | ≥ 25% decrease in SUVsum, SUVavg, or SUVmax | ≥ 25% PSA decline and ≥ 2 ng/mL decline |
| SD | < 25% decrease/increase in SUVsum, SUVavg, or SUVmax | < 25% PSA decline/rise or < 2 ng/mL decline/rise |
| PD | Any new lesions or a ≥ 25% increase in SUVsum, SUVavg, or SUVmax | ≥ 25% PSA increase and ≥ 2 ng/mL increase |
CR complete response, PR partial response, SD stable disease, PD progressive disease
For patients who underwent multiple 18F-NaF PET/CT scans, each interval scan was analyzed separately, with the most recent 18F-NaF PET/CT scan serving as the new baseline. Descriptive statistics were used to summarize the data, and Bowker’s test was performed to assess the concordance of PSA-based treatment response, semi-quantitative treatment response, and qualitative imaging treatment response. Statistical analysis was performed in (STATA, College Station, TX), reporting unequal variance two-sided p values at preset significant level of 0.05.
Results
On review of all patients who underwent 18F-NaF PET/CT scans, there were 14 patients who met our eligibility criteria, 7 of which underwent multiple scans, allowing for 21 interval comparisons. All patients’ disease was confined to the bones, with no evidence of soft tissue disease by CT. At the time of the baseline 18F-NaF PET/CT scan, the average PSA was 29.3 ng/mL (range 0.3–130.6 ng/mL). The average time between baseline and follow-up scans was 218 days (range 85–489 days). The baseline 18F-NaF PET/CT scans with avid lesions demonstrated average values for SUVsum of 200.8 (range 19.7–549.5), SUVavg of 39.8 (range 6.6–95.0), and SUVmax of 53.3 (range 8.8–172). One patient with biochemically recurrent disease had no detectable lesions on baseline scan.
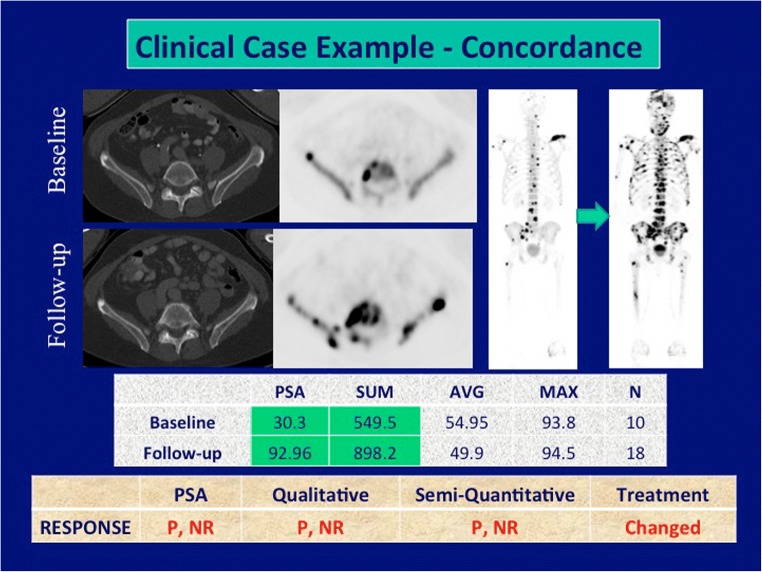
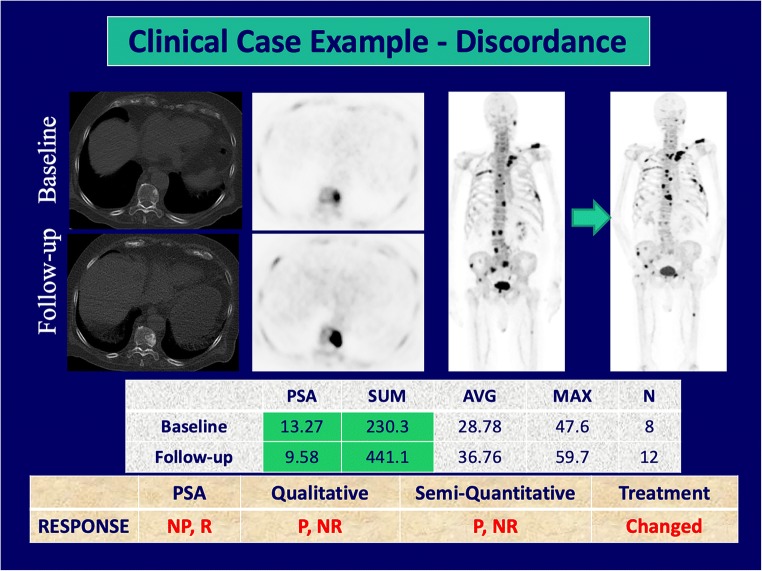
Tumor response assessment by PSA-based treatment response, semi-quantitative imaging treatment response, and qualitative imaging treatment response can be found in Table 2. Qualitative imaging demonstrated a moderate correlation with PSA in assessing responders and non-responders, (κ = 0.52, 95%CI 0.16–0.89), and was concordant in 76% of cases (Fig. 1). However, no correlation was noted between qualitative reports and PSA in the assessment of progressors vs. non-progressors (κ = 0.05, 95%CI − 0.38–0.47, concordant in 52% of cases). Of the 10 patients with no disease progression by PSA-based treatment response criteria, four demonstrated progressive disease on qualitative assessment with 18F-NaF PET/CT, two of which showed new metabolically active lesions (Fig. 2).
Table 2.
Tumor response assessment results
| PSA-based treatment response | Semi-quantitative imaging treatment response | Qualitative imaging treatment response | |||
|---|---|---|---|---|---|
| SUVsum | SUVavg | SUVmax | |||
| PD | 11 | 9 | 9 | 9 | 9 |
| SD | 3 | 6 | 6 | 7 | 8 |
| PR | 6 | 6 | 6 | 5 | 4 |
| CR | 1 | 0 | 0 | 0 | 0 |
Entries are number of scans compared with a prior scan
CR complete response, PR partial response, SD stable disease, PD progressive disease
Fig. 1.
Concordance among treatment response criteria, treatment changed (P progressor, NP non-progressor, R responder, NR non-responder, N number of lesions)
Fig. 2.
Discordance among response criteria, treatment changed (P progressor, NP non-progressor, R responder, NR non-responder, N number of lesions)
Semi-quantitative imaging treatment response demonstrated a moderate correlation between SUVmax and PSA-based treatment response (κ = 0.43, 95%CI 0.04–0.82) and was concordant in 71% of cases. PSA-based treatment response and the semi-quantitative features SUVsum and SUVavg were both concordant in 67% of cases; however, no statistically significant correlation was demonstrated. PSA-based disease progression and semi-quantitative parameters demonstrated no significant correlation, with 43% concordance between PSA-based disease progression and all measurements. The qualitative reports and semi-quantitative analysis demonstrated good correlation in assessing treatment response: SUVmax (κ = 0.71, 95%CI 0.41–1.0, concordant in 86% of cases), SUVavg (κ = 0.62, 95%CI 0.28–0.95, concordant in 81% of cases), SUVsum (κ = 0.62, 95%CI 0.28–0.95, concordant in 81% of cases), and very good correlation in assessing disease progression (κ = 0.81, 95%CI 0.56–1.0, concordant in 91% of cases for all semi-quantitative measurements).
Discussion
There is a wide array of therapies available for patients with metastatic prostate cancer. However, regardless of initial treatment choice, a significant number of patients will develop resistance sometime during therapy [18]. Thus, accurately monitoring tumor response during therapy is crucial to ensure patients are receiving the most optimal treatment. Yet, current methods of biochemical monitoring and imaging of osseous metastases with CT and 99mTc-MDP are not sufficiently sensitive and specific [13]. Advances in the understanding of the complex biology of prostate cancer have paved the way for molecular imaging with PET. Several PET-based radiotracers are in use or under active investigation in prostate cancer, including 18F- or 11C-choline, radiotracers based on prostate-specific membrane antigen (PSMA), 16β-18F-fluoro-5α-dihydrotestosterone targeted to the androgen receptor, and the synthetic L-leucine analog 18F-fluciclovine [12]. 18F-FDG PET/CT has shown both prognostic value in patients with metastatic prostate cancer and correlation with successful treatment response [19–21]. Assessing therapy response with 18F-choline PET/CT [22, 23] and 68Ga-PSMA PET/CT [24] has also shown promising results, although larger studies are needed to confirm their utility.
The recent resurgence of 18F-NaF PET imaging has offered a potentially better method for the evaluation of osseous metastases. Even-Sapir et al. showed 18F-NaF PET to have a sensitivity of 100% and specificity of 62% for the detection of bony metastases compared with 70% sensitivity and 57% specificity with 99mTc-MDP, with a sensitivity and specificity of 100% for combined 18F-NaF PET/CT scans [10]. Furthermore, 18F-NaF PET/CT has been shown to be a useful diagnostic tool in otherwise radiologic occult metastases [25]. Shen et al. conducted a meta-analysis on the diagnostic performance of 18F-NaF PET/CT for the detection of bone metastases and found a sensitivity and specificity of 96% and 91% respectively, with better diagnostic accuracy compared with 99mTc-MDP bone scan (sensitivity 88%, specificity 80%) and FDG PET/CT (sensitivity 73%, specificity 98%) [26].
Initial studies evaluating the utility of 18F-NaF PET in the monitoring treatment response have shown encouraging results [27]. Cooke et al., in a pilot study of five patients with metastatic castrate-resistant prostate cancer treated with 223RaCl2, showed concordance between the mean SUVmax of patients bone metastases and PSA-treatment response [28]. The functional burden of metastatic castrate-resistant prostate cancer has also been shown to have a strong correlation with response to chemotherapy and androgen receptor pathway inhibitors, and can be predictive of progression-free survival [29]. In an imaging companion trial of a multicenter metastatic castration-resistant prostate cancer tissue biomarker-guided therapeutic trial from the American College of Radiology Imaging Network (ACRIN 6687), changes of 18F-NaF PET average SUVmax correlated with bone alkaline phosphatase levels, although there was no correlation with PSA or progression-free survival [30, 31]. Results from the National Oncology PET Registry (NOPR) have demonstrated that not only 18F-NaF PET is useful in assessing treatment response, but can change treatment plans in up to 40% of patients [32]. Additional NOPR data have also shown that 18F-NaF PET results are highly associated with patient survival and subsequent hospice claims, aiding patients and their physicians in the decisions on whether to continue treatment or pursue palliative care [33].
Our study demonstrated a strong concordance between PSA-based treatment response and qualitative and semi-quantitative imaging, especially when further stratifying patients into responders vs. non-responders. These findings suggest that 18F-NaF PET/CT may serve as a highly accurate method for identifying patients who do not respond to treatment, which can potentially lead to changes in treatment management. Of note, the one patient with complete response by PSA-based criteria had residual stable disease by 18F-NaF PET/CT and subsequently had a rise of PSA on follow-up studies. This is suggestive of 18F-NaF PET/CT detecting residual smoldering disease. Additionally, in patients with no progressive disease by PSA-based criteria, two of the four patients with discordant 18F-NaF PET/CT findings had new metabolically active lesions. Therapy-induced flare phenomenon has been reported for 18F-NaF PET [34]; however, this process is thought to be limited to sites of treated metastases with increased osteoblastic activity as demonstrated on CT. While few cases of discordance may reflect treated small marrow only lesions that were previously radiologically occult, it is more likely that 18F-NaF PET/CT provided a better evaluation of overall disease burden and disease progression. This suggests 18F-NaF PET/CT may offer advantages over biochemical monitoring in bone-dominant metastases and at the least should be used in conjunction with PSA-monitoring for determining treatment management.
Several limitations must be considered when interpreting the results of our study. The study had a relatively small sample size and was conducted at a single institution. Differences in clinical referrals, equipment, and imaging protocols may have an influence on the outcomes in an alternative setting. Patients with both mCSPC and mCRPC were analyzed as a group despite the differing tumor biology and treatment regimen. Additional larger studies are needed to assess potential differences in the utility of 18F-NaF PET/CT between mCSPC and mCRPC. Furthermore, conventional 99mTc-MDP imaging was not performed for a majority of the patients; thus, the comparison between 99mTc-MDP treatment response and 18F-NaF PET/CT response was out of the scope of this study. Additionally, there was a wide range of time intervals between the 18F-NaF PET/CT studies, which may limit the efficacy of treatment assessment. Lastly, the length of time between PSA collection and 18F-NaF PET/CT scans may have influenced results; however, all patients had PSA levels drawn within 50 days from the 18F-NaF PET/CT scans and most prostate cancers are relatively slow growing within this time frame [35].
Conclusion
18F-NaF PET/CT is an accurate imaging modality in the assessment of treatment response in patients with bone-only metastases from prostate cancer. This modality is complementary to biochemical monitoring and potentially can serve as a useful tool for determining further treatment management.
Abbreviations
- CT
computed tomography
- PET
positron emission tomography
- PSA
prostate-specific antigen
- MDP
methylene diphosphonate
- NaF
sodium fluoride
- P
progressor
- NP
non-progressor
- R
responder
- NR
non-responder
Funding Information
We acknowledge National Institutes of Health grants R01-CA111613 (PI: H. Jadvar) and P30-CA014089 (USC Norris Comprehensive Cancer Center).
Compliance with Ethical Standards
Conflict of Interest
Erik M. Velez, Bhushan Desai, and Hossein Jadvar declare no conflicts of interest. There was no direct funding for this study.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.O’Sullivan GJ, Carty FL, Cronin CG. Imaging of bone metastasis: an update. World J Radiol. 2015;7:202–211. doi: 10.4329/wjr.v7.i8.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jadvar H, Desai B, Conti PS. Sodium 18F-fluoride PET/CT of bone, joint and other disorders. Semin Nucl Med. 2015;45:58–65. doi: 10.1053/j.semnuclmed.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langsteger W, Rezaee A, Pirich C, Beheshti M. 18F-NaF-PET/CT and 99mTc-MDP bone scintigraphy in the detection of bone metastases in prostate cancer. Semin Nucl Med. 2016;46:491–501. doi: 10.1053/j.semnuclmed.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Iagaru AH, Mittra E, Colletti PM, Jadvar H. Bone-targeted imaging and radionuclide therapy in prostate cancer. J Nucl Med. 2016;57(Suppl 3):19S–24S. doi: 10.2967/jnumed.115.170746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czernin J, Satyamurthy N, Schiepers C. Molecular mechanisms of bone 18F-NaF deposition. J Nucl Med. 2010;51:1826–1829. doi: 10.2967/jnumed.110.077933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bridges RL, Wiley CR, Christian JC, Strohm AP. An introduction to Na(18)F bone scintigraphy:basic principles, advanced imaging concepts, and case examples. J Nucl Med Technol. 2007;35:64–76. doi: 10.2967/jnmt.106.032870. [DOI] [PubMed] [Google Scholar]
- 8.Kulshrestha RK, Vinjamuri S, England A, Nightingale J, Hogg P. The role of 18F-sodium fluoride PET/CT bone scans in the diagnosis of metastatic bone disease from breast and prostate Cancer. J Nucl Med Technol. 2016;44:217–222. doi: 10.2967/jnmt.116.176859. [DOI] [PubMed] [Google Scholar]
- 9.Even-Sapir E. Imaging of malignant bone involvement by morphologic, scintigraphic, and hybrid modalities. J Nucl Med. 2005;46:1356–1367. [PubMed] [Google Scholar]
- 10.Even-Sapir E, Metser U, Mishani E, Lievshitz G, Lerman H, Leibovitch I. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med. 2006;47:287–297. [PubMed] [Google Scholar]
- 11.Beheshti M, Rezaee A, Geinitz H, Loidl W, Pirich C, Langsteger W. Evaluation of prostate cancer bone metastases with 18F-NaF and 18F-fluorocholine PET/CT. J Nucl Med. 2016;57(Suppl 3):55S–60S. doi: 10.2967/jnumed.115.169730. [DOI] [PubMed] [Google Scholar]
- 12.Jadvar H. Molecular imaging of prostate cancer: PET radiotracers. AJR Am J Roentgenol. 2012;199:278–291. doi: 10.2214/AJR.12.8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clamp A, danson S, Nguyen H, Cole D, Clemons M. Assessment of therapeutic response in patients with metastatic bone disease. Lancet Oncol. 2004;5:607–616. doi: 10.1016/S1470-2045(04)01596-7. [DOI] [PubMed] [Google Scholar]
- 14.Woolf DK, Padhani AR, Makris A. Assessing response to treatment of bone metastases from breast cancer: what should be the standard of care? Ann Oncol. 2015;26:1048–1057. doi: 10.1093/annonc/mdu558. [DOI] [PubMed] [Google Scholar]
- 15.Cookson MS, Aus G, Burnett AL, Canby-Hagino ED, D’Amico AV, Dmochowski RR, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for localized prostate cancer update panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007;177:540–545. doi: 10.1016/j.juro.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 16.Roach M, 3rd, Hanks G, Thames H, Jr, Schellhammer P, Shipley WU, Sokol GH, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix consensus conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 17.Segall G, Delbeke D, Stabin MG, Even-Sapir E, Fair J, Sajdak R, et al. SNM practice guideline for sodium 18F-fluoride PET/CT bone scans 1.0. J Nucl Med. 2010;51:1813–1820. doi: 10.2967/jnumed.110.082263. [DOI] [PubMed] [Google Scholar]
- 18.Ceci F, Castellucci P, Nanni C, Fanti S. PET/CT imaging for evaluating response to therapy in castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:2103–2104. doi: 10.1007/s00259-016-3493-3. [DOI] [PubMed] [Google Scholar]
- 19.Simoncic U, Perlman S, Liu G, Staab MJ, Sraus JE, Jeraj R. Comparison of NaF and FDG PET/CT for assessment of treatment response in castration-resistant prostate cancers with osseous metastases. Clin Genitourin Cancer. 2015;13:e7–e17. doi: 10.1016/j.clgc.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jadvar H, Desai B, Ji L, Conti PS, Dorff TB, Groshen SG, et al. Baseline 18F-FDG PET/CT parameters as imaging biomarkers of overall survival in castrate-resistant metastatic prostate cancer. J Nucl Med. 2013;54:1195–1201. doi: 10.2967/jnumed.112.114116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oyama N, Akino H, Suzuki Y, Kanamaru H, Ishida H, Tanase K, et al. FDG PET for evaluating the change of glucose metabolism in prostate cancer after androgen ablation. Nucl Med Commun. 2001;22:963–969. doi: 10.1097/00006231-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 22.De Giorgi U, Caroli P, Burgio SL, Menna C, Coteduca V, Bianchi E, et al. Early outcome prediction on 18F-fluorocholine PET/CT in metastatic castration-resistant prostate cancer patients treated with abiraterone. Oncotarget. 2014;5:12448–12458. doi: 10.18632/oncotarget.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Giorgi U, Caroli P, Scarpi E, Conteduca V, Burgio SL, Menna C, et al. 18F-Fluorocholine PET/CT for early response assessment in patients with metastatic castration-resistant prostate cancer treated with enzalutamide. Eur J Nucl Med Mol Imaging. 2015;42:1276–1283. doi: 10.1007/s00259-015-3042-5. [DOI] [PubMed] [Google Scholar]
- 24.Seitz AK, Rauscher I, Hallr B, Kronke M, Luther S, Heck MM, et al. Preliminary results on response assessment using 68Ga-HBED-CC-PSMA PET/CT in patients with metastatic prostate Cancer undergoing docetaxel chemotherapy. Eur J Nucl Med Mol Imaging. 2018;45:602–612. doi: 10.1007/s00259-017-3887-x. [DOI] [PubMed] [Google Scholar]
- 25.Jadvar H, Desai B, Ji L, Conti PS, Dorff TB, Grshen SG, et al. Prospective evaluation of 18F-NaF and 18F-FDG PET/CT in detection of occult metastatic disease in biochemical recurrence of prostate cancer. Clin Nucl Med. 2012;37:637–643. doi: 10.1097/RLU.0b013e318252d829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen CT, Qiu ZL, Han TT, Luo QY. Performance of 18F-fluoride PET or PET/CT for the detection of bone metastases: a meta-analysis. Clin Nucl Med. 2015;40:103–110. doi: 10.1097/RLU.0000000000000592. [DOI] [PubMed] [Google Scholar]
- 27.Jadvar H, Colletti PM. 18F-NaF/223RaCl2 theranostics in metastatic prostate cancer: treatment response assessment and prediction of outcome. Br J Radiol. 2018;91(1091):20170948. [DOI] [PMC free article] [PubMed]
- 28.Cook G, Jr, Parker C, Chua S, Johnson B, Aksnes AK, Lweington VJ. 18F-fluoride PET: changes in uptake as a method to assess response in bone metastases from castrate-resistant prostate cancer patients treated with 223Ra-chloride (Alpharadin) EJNMMI Res. 2011;1:4. doi: 10.1186/2191-219X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harmon SA, Perk T, Lin C, Eickhoff J, Choyke PL, Dahut WL, et al. Quantitative assessment of early [18F]sodium fluoride positron emission tomography/computed tomography response to treatment in men with metastatic prostate cancer to bone. J Clin Oncol. 2017;35:2829–2837. doi: 10.1200/JCO.2017.72.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muzi M, O’Sullivan F, Muzi J, Mankofff D, Yu E. Whole body 18F-fluoride PET SUV imaging to monitor response to dasatinib therapy in castration-resistant prostate cancer bone metastases: secondary results from ACRIN 6687. J Nucl Med. 2018;59(suppl. 1):1465. doi: 10.3390/tomography7020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu EY, Duan F, Muzi M, Deng X, Chin BB, Alumkal JJ, et al. Castration-resistant prostate cancer bone metastasis response measured by 18F-fluoride PET after treatment with dasatinib and correlation with progression-free survival: results from American College of Radiology Imaging Network 6687. J Nucl Med. 2015;56:354–360. doi: 10.2967/jnumed.114.146936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hillner BE, Siegel BA, Hanna L, Duan F, Quinn B, Shields AF. 18F-fluoride PET used for treatment monitoring of systemic cancer therapy: results from the National Oncologic PET Registry. J Nucl Med. 2015;56:222–228. doi: 10.2967/jnumed.114.150391. [DOI] [PubMed] [Google Scholar]
- 33.Gareen IF, Hillner BE, Hann L, Makineni R, Duan F, Shields AF, et al. Hospice admission and survival after 18F-fluoride PET performed for evaluation of osseous metastatic disease in the National Oncologic PET Registry. J Nucl Med. 2018;59:427–433. doi: 10.2967/jnumed.117.205120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wade AA, Scott JA, Kuter I, Fischman AJ. Flare response in 18F-fluoride ion PET bone scanning. AJR Am J Roentgenol. 2006;186:1783–1786. doi: 10.2214/AJR.05.0225. [DOI] [PubMed] [Google Scholar]
- 35.Penney KL, Stampfer MJ, Jahn JL, Sinnott JA, Flavin R, Rider JR, et al. Gleason grade progression is uncommon. Cancer Res. 2013;73:5163–5168. doi: 10.1158/0008-5472.CAN-13-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]