Abstract
Gap prepulse inhibition of acoustic startle (GPIAS) method has been used effectively for the objective assessment of tinnitus in animals. Among two types of enclosures for the GPIAS, the unconstrained type carries less risk of animal death due to the absence of binding stress in the enclosure, and lack of need for alteration to animal size variation as it grows. However, animals’ voluntary movements, which have no relation to the startles evoked by acoustic stimuli, are problematic, as they cannot be excluded in the case of the unconstrained enclosure based GPIAS measurement system. In order to discount voluntary movements which are not associated with external acoustic stimuli, we propose the conditional random inter-stimulus interval (CR ISI) method for unconstrained enclosure based GPIAS measurement. With the proposed ISI method, the unconstrained enclosure based acoustic startle response measurement system has been implemented in this paper. As a result, the effectiveness of the proposed CR ISI method has been verified and compared with those of conventional ISI methods through animal experiments using SD-rats. The experimental results showed that abnormal startle responses and invalid GPIAS values caused by motion were prevented when our proposed CR ISI method was applied to our implemented system. It was also verified that our proposed CR ISI method is advantageous in reducing the total experimental time for acquiring normal startle responses and valid GPIAS values, compared to conventional ISI methods, since our proposed CR ISI can begin the acoustic stimulation only when the animal gets stable and motionless.
Keywords: GPIAS, Startle response, Tinnitus, Conditional random ISI, Acceleration sensor
Introduction
Lots of people feel uncomfortable in their lives due to the tinnitus evoked by the increased environmental acoustic noise and mental stress nowadays [1]. The conventional test for detecting tinnitus is not completely accurate since it only depends on patients’ subjective expression. Therefore, much research has been undertaken to find an objective tinnitus detection method. Turner et al. [2] reported the gap pre-pulse inhibition of acoustic startle (GPIAS) method to determine objectively whether the animal has tinnitus. The GPIAS method is now widely used in researching animal tinnitus because the correct use of the method does not require long periods of training to make the animal show a startle response against intentionally presented stimulations [2–5]. The present GPIAS measurement system for animals can be divided into two types: the constrained type and the unconstrained type, according to the kind of animal enclosure where the motion sensor is attached. Like SR-Lab’s startle response system (San Diego Instrument, US), the constrained type GPIAS measurement system makes use of the animal enclosure with a fixed size so that the animal can be tightly fitted to the enclosure. In that kind of GPIAS system, irrelevant movements which do not occur due to the sound stimulus can be restrained, but animal death might occur due to excessive binding stress or choking. Especially, the packing problem can occur as animals grow up during a period of the experiment time. In contrast, unconstrained enclosure based GPIAS measurement systems can solve the problems of the constrained type one by providing a marginal space for the animal, but the motion artifacts of animals’ voluntary movements which have no relation to the startles evoked by acoustic stimuli cannot be excluded. So, the artifacts can directly affect the sensor output signals and GPIAS values, and lead to incorrect experimental results.
In this paper, we have proposed the conditional random inter-stimulus interval (CR ISI) method for the unconstrained enclosures based GPIAS measurement system. Conventionally, the time intervals of consecutive acoustic stimuli have been fixed or unconditionally randomized without considering the animal’s moving status. Such kinds of ISI methods inevitably involve the uncorrelated movement with the presented stimulus because sound stimulation and sensor signal acquisition begin regardless of the current animal moving condition. Our proposed CR ISI method can exclude such motion artifacts since a CR ISI based measurement system emits sound stimuli to the animal and obtains the startle response simultaneously only after a stable condition of the animal has been determined by the motion sensor output signal. With the proposed ISI method, we have implemented the unconstrained enclosure based acoustic startle response measurement system for measuring GPIAS values, as well as noise burst pre-pulse inhibition of acoustic startle (NBPIAS) values. In order to prove the effectiveness of the proposed CR ISI method, our method has been verified and compared with that of conventional ISI methods, such as fixed ISI and unconditional random ISI (UR ISI), through animal experiments using five sprague–dawley (SD) rats and our implemented system. Through the experimental results, we found that our proposed CR ISI method could prevent abnormal startle responses and invalid GPIAS values caused by motion artifacts unlike the conventional ISI ones, and our method had the possibility to reduce the total experiment time for acquiring necessary number of normal startle responses and valid GPIAS values.
Materials and methods
Implementation of unconstrained enclosure based GPIAS measurement system
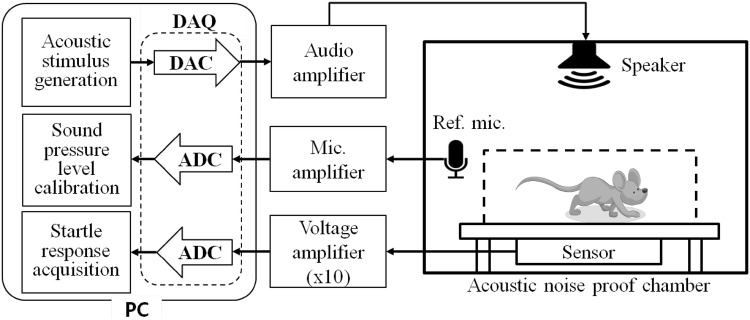
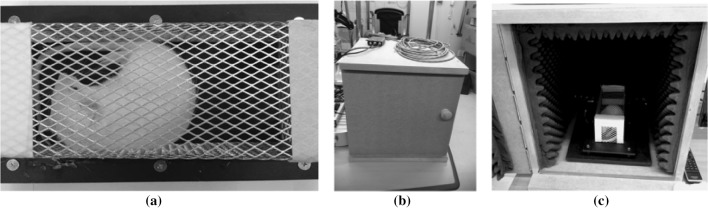
As shown in Fig. 1, the implemented GPIAS system consists of an unconstrained enclosure, an anti-noise chamber, an audio amplifier/speaker for sound stimulation, a reference microphone for sound level calibration, a motion sensor and sensor signal amplifier for sensing animal startling, and a PC based DAQ system for capturing the startle response. The unconstrained enclosure has been designed to have the size of 110(W) × 110(H) × 225(D) mm, so that a SD-rat with the weight of 250–310 g can move freely within in the enclosure. For better sound spreading within the enclosure, its roof and walls were covered by aluminum mesh layers. The bottom was constructed with a compressed polyvinyl chloride (PVC) form plate of 2.5 mm thickness. A 3-axis accelerometer IC (MMA7260Q3, Freescale, US) was attached beneath the center of the enclosure bottom plate in order to sense the animal’s movement. In addition, an elastic rubber shoe was placed over each supporting column for holding up the bottom plate of the enclosure, to improve the sensitivity for animal startle responses. Figure 2a shows our implemented unconstrained enclosure. Like Fig. 2b, c, the acoustic noise proof chamber for the enclosure has been designed to have a hexahedral structure with a size of 50 cm (H) × 50 cm (W) × 50 cm (D) made from medium density fiberboard (MDF) plates, and its internal walls are covered by sound barrier sheets and sound absorption sponge blocks for blocking the external sound inflow and reducing internal sound reverberation.
Fig. 1.
Block diagram of our implemented unconstrained enclosure based GPIAS measurement system
Fig. 2.
a The implemented unconstrained type enclosure, b the acoustic noise proof chamber, c its internal view
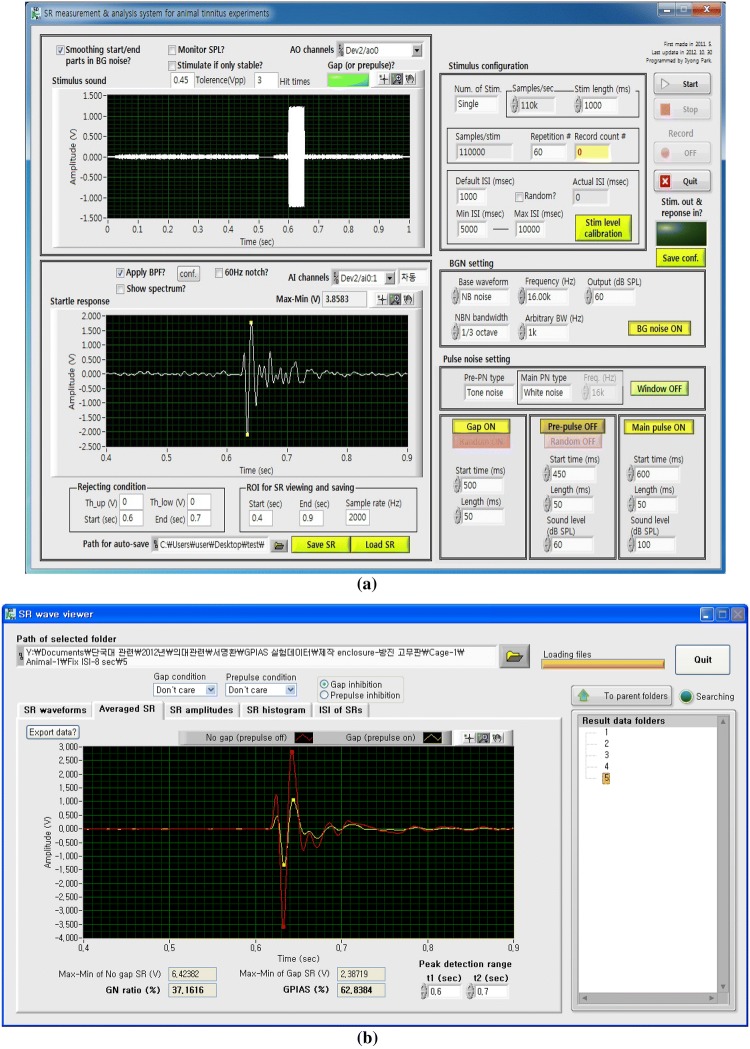
For the presentation of acoustic stimuli, an audio amplifier (PM-5004, Marantz, US) and a full-range speaker unit (TC0FSD13, Peerless, Denmark) have been used for our GPIAS measurement system. A reference microphone (40PH, GRAS, Denmark) was adopted for the calibration of stimulant volume. The sensor signal amplifying part was set to have the gain of 10 considering the amplitudes of acceleration sensor output signals evoked by the startle responses. In order to acquire and store the amplified sensor output signals in a personal computer, a data acquisition (DAQ) device (PCIe-6321, National Instrument, US) was used, along with startle response measurement software, which has the functions of a stimulation generation, a startle response capture, an obtained signal conditioning, and a result analysis has been implemented by the use of NI LabVIEW. Figure 3a shows the graphic user interface (GUI) of the implemented startle response measurement software which can control data acquisition conditions and various sound stimulus parameters, such as frequencies, amplitudes, time durations, start times of a gap, a background sound, and a pulse for generating stimuli. The startle response viewer was implemented as depicted in Fig. 3b, and can promptly extract the information of analysis, such as GPIAS values, amplitudes of startle responses, inter-stimulus intervals, etc. from the obtained data files, and the analyzed results can be directly exported to Microsoft Excel for compatibility with other programs.
Fig. 3.
GUIs of a the implemented startle response measurement software, b the startle response viewer
Proposal of an ISI method for unconstrained enclosure based GPIAS measurement systems
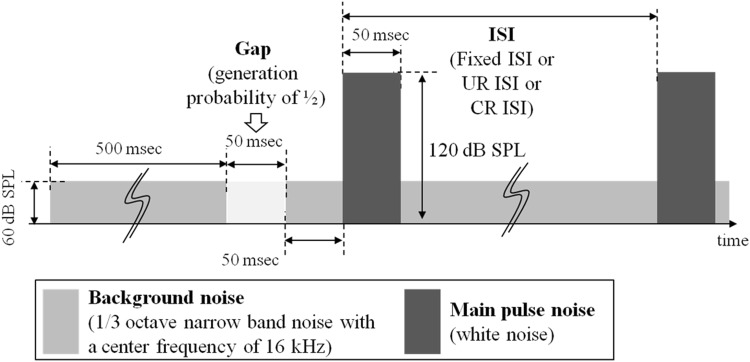
Inter-stimulus interval (ISI) indicates a time interval between repeated acoustic stimuli presented to the animals, as indicated in Fig. 4a. Conventionally, the unconditional random ISI (UR ISI) was randomly determined by the computer software without considering the animal’s moving status in the enclosure [5]. Since that kind of ISI method can cause the problems of the motion artifact in acquiring animal startle responses when the unconstrained type enclosure is used, a simple and effective ISI method to solve the problem has been proposed. In the proposed conditional random ISI (CR ISI) method, as shown in Fig. 4b, the animal movement is monitored by the use of an accelerometer for sensing animal startle responses before applying the intended sound stimulus. Thus, if the sensor output is continuously maintained lower than the voltage tolerance level for a predefined time, the acoustic stimulation and the signal acquisition are started simultaneously by our implemented GPIAS measurement system. As the CR ISI shows irregular time intervals like the UR ISI, due to voluntary and free movements of the animal in the unconstrained enclosure before beginning the sound stimulation, the proposed method ensures that the effect of the movement is remarkably reduced. The proposed CR ISI method has been adopted for our implemented GPIAS measurement hardware and software, reducing the required time for determination of movement responses.
Fig. 4.
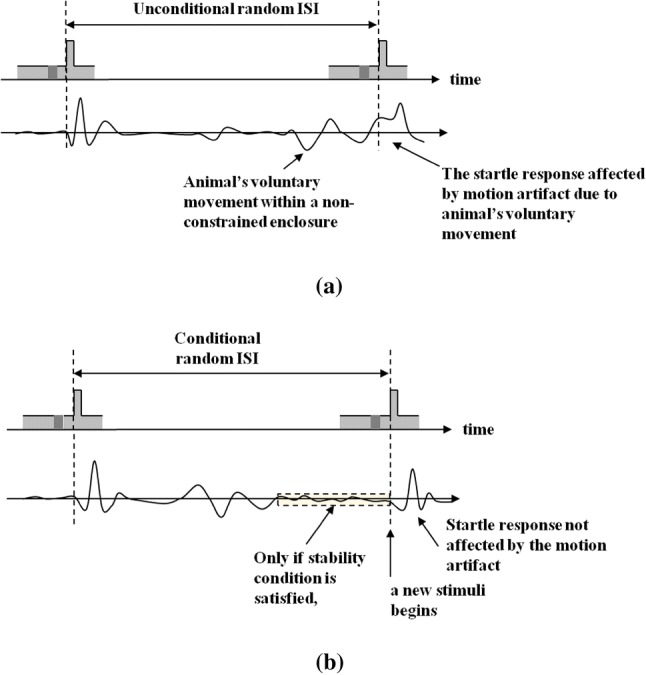
Sound stimulation sequences of a the conventional UR ISI method, b our proposed CR ISI
Experimental methods
In order to evaluate the external sound attenuation performance of the implemented acoustic noise proof chamber, the internal sound pressure level in the 3rd octave band within the chamber has been measured by the use of a reference microphone when the white noise sound was presented by an external speaker and its total power was maintained at 95 dB SPL at the outside of the chamber.
The dropping impact experiment using a metal mass and an electromagnet has been performed to verify the difference of the sensor output according to the different impact positions on the bottom plate of the implemented enclosure. At 9 positions on the bottom plate, with a fixed distance between each position, the metal mass of 2 g attached to an electromagnet was allowed to drop from a height of 20 mm from the bottom plate 5 times. The sensor output values were then obtained. The significance test among the sensor output data for each position was performed using statistical software, PASW. An electromagnet, JE-1A (NSmagnet, Korea) was used in order to ensure uniform conditions in each trial. The GPIAS values have been measured using the implemented system and normal SD rats. The results were then compared one another. The GPIAS values indicate the quantitative amount of startle response inhibition due to the gap pre-pulse, based on the following equation
| 1 |
where SRno gap and SRgap are the peak to peak values of the waveforms averaged for 30 each of startle responses by the stimuli both with and without the gap pre-pulse. For 5 SD-rats (290–310 g), one measurement session consisting of 60 presentations of acoustic stimuli for obtaining startle responses, as shown in Fig. 5, was repeated 3 times per animal, using our implemented GPIAS measurement system which adopted 3 ISI methods, such as a fixed ISI, a UR ISI, and the proposed CR ISI. Between two sessions, a rest time of 2 min was allowed.
Fig. 5.
Stimulus pattern for the animal startle response experiment
The gap pre-pulse has been inserted into the stimulus pattern, as depicted in Fig. 5, with the probability of 50% for the whole acoustic stimuli. And the gap duration is 50 ms, beginning 100 ms before applying the main pulse to startle the animal. For our proposed CR ISI method, we assumed that the minimum condition to determine the lack of motion in an animal is the maintenance of a sensor output lower than the threshold voltage level of 0.3 Vpeak-to-peak for 3 s. If the system regards the minimum condition as being satisfied, the acoustic stimulation can be presented to the animal. In the results of the startle response experiments adopting the CR ISI method with the minimum condition, the mean ISI for 300 acoustic stimuli was about 4 s. Based on the mean ISI value, other ISIs have been set (Table 1) to make their means meet about 4 s. After acquiring all the startle responses (SR), the abnormal SR data have been excluded by rejection criterion. In other words, the startle responses which have less than 0.3 Vpeak to peak amplitudes within a latency of 50 ms have been regarded as abnormal SR data and have been rejected. The rejection criterion was determined by considering that the sensor output signal level showed a maximum peak to peak amplitude of 0.25 V when the enclosure was empty. The startle response latency time of 50 ms was based on that of a published result [6, 7], and the rejection rate for 3 kinds of ISI methods have been calculated for the SR data following Eq. (2) and compared with one another
| 2 |
Table 1.
Stimulus interval configurations according to three different ISI methods
| Fixed ISI | UR ISI | CR ISI | |
|---|---|---|---|
| ISI (sec) | 4 | Randomly selected from 2 to 6 | More than 3 and determined by the amount of animal voluntary movement |
| ISI setting condition | Unconditionally fixed | Unconditionally random | Conditionally random |
| Measured ISI (n = 5) | 4 | 4.16 ± 1.25 | 4.92 ± 1.75 |
The GPIAS values and the probability of abnormal GPIAS value occurrences for 3 different ISI methods have been compared by using the SR data to which the rejection criterion was applied. All animal experiments were performed under protocols approved by the Animal Care and Use Committee of Dankook University (DKU-12-057).
Results and discussion
Performance of the implemented acoustic soundproof chamber
When the white noise sound of 95 dB SPL is externally applied to the outside of the acoustic soundproof chamber, the measured total sound pressure power at the distance of 80 mm away from the internal wall of the chamber was 66 dB SPL. Hence, we found that our implemented soundproof chamber had the attenuation of about 29 dB against the external sound, and the attenuation at the 16 kHz background noise band, which is similar to the tinnitus sound, was about 60 dB. This means that it is quiet enough for the background noise sound to be applied to the animal without being affected by the environmental acoustic noise.
Evaluation of sensor output deviation of the unconstrained enclosure
In order to identify the differences of sensor output according to the different locations of the enclosure bottom, the sensor output was measured at 9 different locations where mass weights dropped repeatedly, as shown in Fig. 6a. The Fig. 6b shows that the sensor outputs from different positions of the enclosure are not statistically significant (p > 0.05). The used statistical methods were Tukey HSD test and Scheffe test. Through Tukey HSD test, the significance probability p values for each sensor output at every position were from 0.141 to 1.000. And Scheffe test resulted in p values of 0.450–1.000 for every position.
Fig. 6.
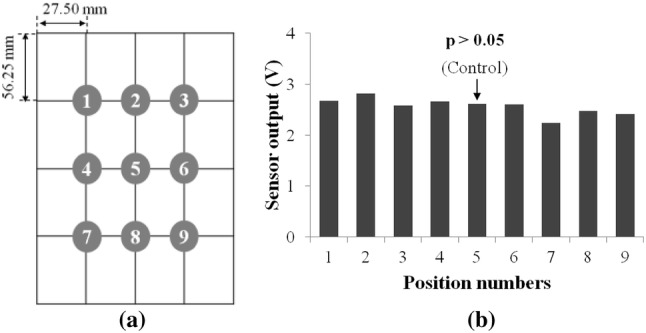
a 9 Positions (gray filled circles) for dropping a weight, b the measured sensor output voltage levels according to the different weight impact positions (those are not statistically significant, p > 0.05)
Comparison of measured GPIAS values according the different ISI methods
Through all the measured results, no voluntary movements unrelated with acoustic stimuli were observed in the measured startle responses when the CR ISI method was used. However, motion artifacts occurred in other ISI methods, and these artifacts affected the GPIAS values. Figure 7 shows the differences between no gap startling response peak-to-peak amplitudes and gap ones according to different ISI methods. As shown in Fig. 7, the largest amplitude was obtained in case of the CR ISI method. Also, the SR rejection rate is shown in Fig. 8. The figure indicates that the CR ISI method can produce the minimum value among the three ISI methods. Unlike the significant differences between Fixed ISI and CR ISI, there were no significant differences in the startle response (SR) amplitudes and the SR rejection rate between CR ISI and UR ISI (p > 0.05). It might be caused by the limited number of experimental animals and large standard deviation characteristics of startle reflex amplitudes. But UR ISI results didn’t show the significant difference against Fixed ISI in the SR amplitude comparison like Fig. 7. That means that the effect of CR ISI randomization may be greater than that of UR ISI one against fixed ISI method. Also, CR ISI method didn’t show an inferior effect on SR amplitude increase against UR ISI. The calculated GPIAS values, after applying the condition of the SR data rejection and excluding negative GPIAS values, are shown in Fig. 9. These results in cases of all ISI methods of the Fig. 9 are similar one another. However, the probability of having invalid negative GPIAS values for normal SD-rats was lowest in case of the CR ISI method, as can be seen in Fig. 10. The probabilities of having valid GPIAS values during 15 GPIAS sessions in each method of Fixed ISI, UR ISI, and CR ISI were 66.7% (10 valid GPIAS values), 80% (12 ones), and 100% (15 ones). This means that the CR ISI method can reduce the probability of leading invalid GPIAS values because of the animals’ voluntary movement unrelated with acoustic stimuli. Like other reported GPIAS results using the acoustic stimulation conditions which are similar to our experimental ones, the startle responses are normally inhibited by gap pre-pulses and the GPIAS values are mainly positive [3–6]. Negative GPIAS value which means a gap pre-pulse facilitation can be occurred in some specific cases such as long interval condition (> 500 ms) between a gap pre-pulse and an acoustic startle stimulus, a different animal state to external stimuli, and so on [5]. But the exact reason of a facilitation has not turned out yet. So, we regarded the negative GPIAS value as invalid ones under our experimental condition. As shown in Fig. 10, CR ISI shows no invalid GPIAS value for our all GPIAS sessions. That means CR ISI has higher probability to get a valid GPIAS in a single session per animal than other two ISI methods. In that point, adopting the CR ISI method has the possibility of decreasing the measurement time for acquiring the necessary valid GPIAS values, and hence, animal stress can be reduced.
Fig. 7.
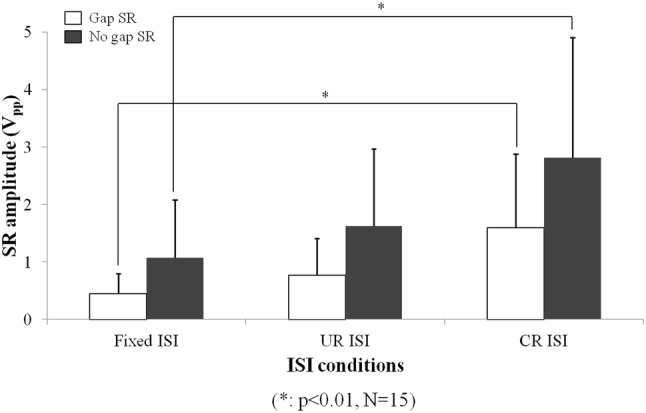
Comparison of measured peak-to-peak amplitudes of no gap startle responses and gap ones according to the different ISI methods
Fig. 8.
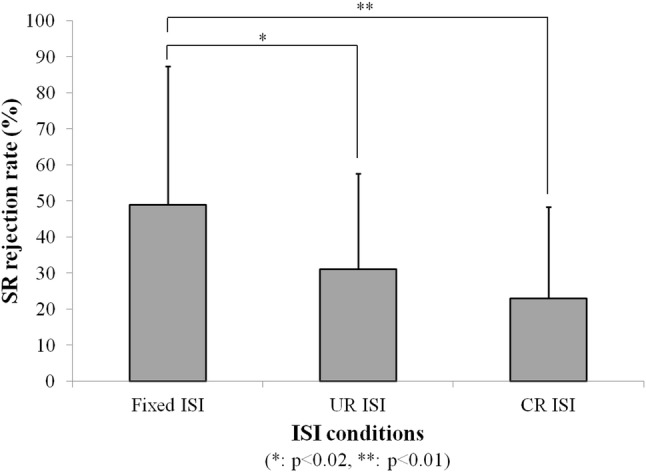
Comparison of the SR data rejection rate according to different ISI methods
Fig. 9.
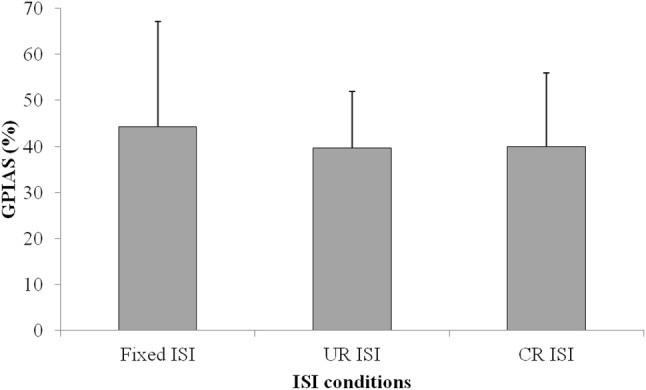
Comparison of GPIAS values according to the ISI methods (negative GPIAS values are excluded)
Fig. 10.
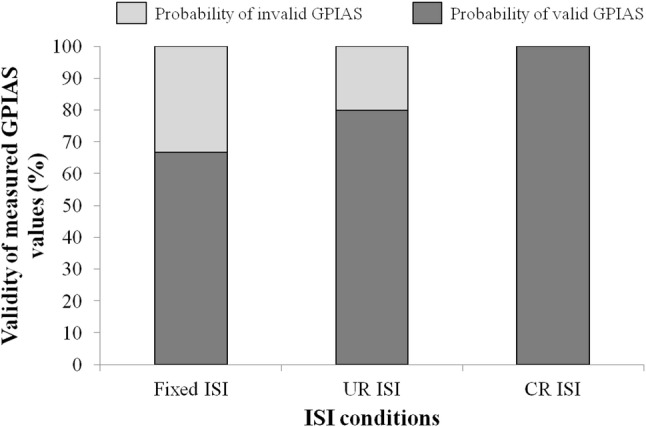
Comparison of the validity of measured GPIAS values according to different ISI methods
Conclusion
In this paper, we have designed and implemented the GPIAS measurement system based on an unconstrained enclosure, and have proposed the CR ISI (conditional random inter-stimulus interval) method to remove the effect of animals’ voluntary movement in the unconstrained type enclosure. The implemented GPIAS measurement system is composed of PC based signal instrumentation software developed by LabVIEW for measuring animal startle responses, a DAQ device, a sound-proof chamber, a microphone, a sound speaker, and an audio amplifier. In order to prove the effectiveness of the proposed CR ISI method, our method has been verified and compared with ISI methods, such as a fixed ISI and an unconditional random ISI (UR ISI) through animal experiments using five SD-rats. Through the experimental results, we found that our proposed CR ISI method prevented abnormal startle responses and invalid GPIAS values, unlike conventional ISI methods and had the possibility of reducing the total experiment time for acquiring the necessary valid GPIAS values.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All animal experiments were performed under protocols approved by the Animal Care and Use Committee of Dankook University (DKU-12-057).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jeong SH, Kang SH, Lee ZN, Kang BJ, Rim HD. Review of psychiatric approaches to tinnitus. J Korean Soc Biol Ther Psychiatry. 1997;3(2):250–258. [Google Scholar]
- 2.Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, Caspary DM. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci. 2006;120(1):188–195. doi: 10.1037/0735-7044.120.1.188. [DOI] [PubMed] [Google Scholar]
- 3.Baeuer CA. Animal model of tinnitus. Otolaryngol Clin North Am. 2003;36(2):267–285. doi: 10.1016/S0030-6665(02)00171-8. [DOI] [PubMed] [Google Scholar]
- 4.Turner JG, Kinder JM. Tinnitus testing device and method. US patent application publication 2009; Pub. No. US 2009/0163828 A1.
- 5.Galazyuk A, Sylvie H. Gap-prepulse inhibition of the acoustic startle reflex (GPIAS) for tinnitus assessment: current status and future directions. Front Neurol. 2015;6(88):1–12. doi: 10.3389/fneur.2015.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seaman RL, Chen J. Sensing platform for acoustic startle responses from rat forelimbs and hindlimbs. IEEE Trans Biomed Eng. 1996;43(2):221–225. doi: 10.1109/10.481992. [DOI] [PubMed] [Google Scholar]
- 7.Ralli M, Lobarinas E, Fetoni AR, Stolzberg D, Paludetti G, Salvi R. Comparison of salicylate and quinine induced tinnitus in rats; development, time course and evaluation of audiological correlates. Otol Neurotol. 2010;31(5):823–831. doi: 10.1097/MAO.0b013e3181de4662. [DOI] [PMC free article] [PubMed] [Google Scholar]