Abstract
Purpose
To investigate the correlation between the tenosynovitis pattern on two-phase bone scintigraphy (2P-BS) and clinical manifestation in patients with suspected rheumatoid arthritis (RA).
Method
2P-BS including technetium–99m-methylene diphosphonate blood pool and bone phase imaging in 402 consecutive patients with clinically suspected RA were retrospectively reviewed. According to 2010 RA Classification Criteria, patients were grouped as RA and non-RA. Visual assessment of all fingers, toes, wrists, and ankles on 2P-BS was performed. Clinical suspected tenosynovitis was evaluated on physical examination. Rheumatoid factor, anti-cyclic citrullinated protein antibody, C-reactive protein, and estimated sedimentation rate were obtained. Radiographic findings were also used to define early and established arthritis.
Results
Tenosynovitis pattern was detected in 12.7% (51/402 patients) on 2P-BS. A total of 94.1% (48/51) were diagnosed as RA vs. 5.9% (3/51) as non-RA. Of the 48 RA patients with positive 2P-BS finding, 85.4% (41/48) had early arthritis and 14.6% (7/48) had established arthritis. On physical examination, tenosynovitis was suspected in 21.9% (88/402). A total of 56.8% (50/88) belonged to the RA group and 43.2% (38/88) to the non-RA group. The tenosynovitis pattern of 2P-BS and physical examination showed statistical difference and moderate agreement. The positive tenosynovitis pattern on 2P-BS represented up to 26.408 of odds ratio which was highest among the RA-associated factors.
Conclusion
Tenosynovitis pattern on 2P-BS was more commonly detected in the RA group and was more frequently associated with early arthritis pattern. Therefore, 2P-BS could give additional information for the detection of subclinical tenosynovitis in early or preclinical RA patients.
Keywords: Tenosynovitis, Bone scintigraphy, MDP, Rheumatoid arthritis
Introduction
Bone scintigraphy with technetium–99m-methyl diphosphonate (99mTc-MDP) has been used for the evaluation of various musculoskeletal diseases including arthritis, soft tissue inflammation, trauma, and metastasis. Two-phase bone scintigraphy (2P-BS) consists of the delayed bone phase imaging and additional blood pool phase imaging which can give supplementary information of the inflammation activity and extent that the bone phase imaging alone can hardly do [1, 2].
Rheumatoid arthritis (RA) is a systemic inflammatory disease that involves peripheral small joints, tendons, ligaments, and the destruction of bone and cartilage. Tenosynovitis as its definition is inflammation of a tendon and its synovial sheath. In RA, tenosynovitis can occur as isolated or predominant. There were reports that about 50% of RA patients had tenosynovitis on hands and up to 80% of preclinical RA patients with positive anti-cyclic citrullinated protein antibody had tenosynovitis which was confirmed by magnetic resonance imaging (MRI) [3–5]. In addition, the early detection of RA becomes more essential for the strategy of RA management as the early treatment of RA with disease-modifying anti-rheumatic drugs achieves promising results [6–12]. For such an effort of the early RA recognition, there are clinical trials that investigate the relationship between tenosynovitis and early RA by using ultrasonography and MRI [13–20].
To evaluate tenosynovitis, plain radiography has been initially used in daily practice with its easy access and convenience. The use of ultrasonography and MRI is increasing as promising imaging modalities for the detection of tenosynovitis. However, those imaging modalities have limitations that plain radiography is difficult to delineate soft tissue lesions including tenosynovitis. Ultrasonography is operator-dependent and time-consuming for the evaluation of whole interesting areas, and MRI is expensive and limited for the examination of whole extremities. Instead, 2P-BS can identify tenosynovitis and takes advantage of the one-step procedure for the whole body evaluation [1, 2, 21–23].
Even so, no study has been reported for the correlation between tenosynovitis pattern on 2P-BS and clinical manifestation which may be useful for the early detection of RA. Therefore, this study aims to show the tenosynovitis patterns on 2P-BS and its relevant clinical manifestation in patients with suspected RA.
Methods
Population
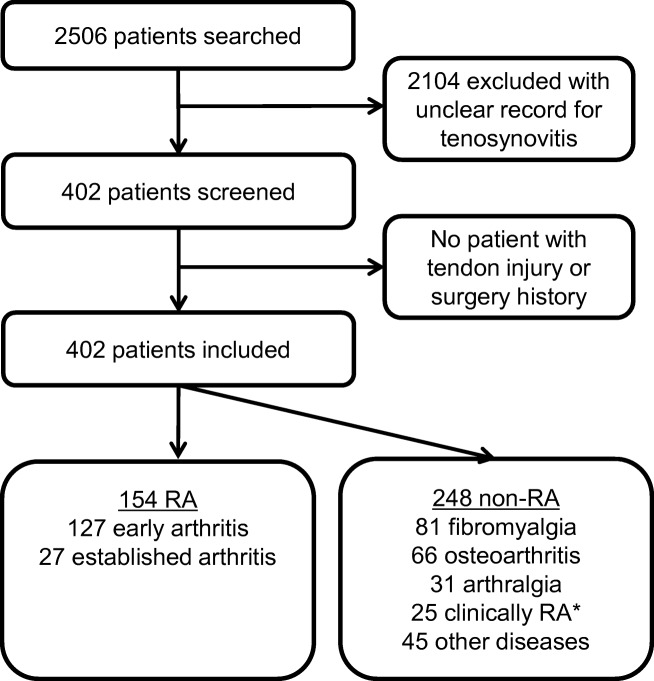
Consecutive patients who were referred from rheumatology and underwent 2P-BS with the blood pool phase imaging and regional delayed bone phase imaging for the evaluation of multiple joint pain and clinically suspected RA between January 2015 and May 2018 were reviewed. Patients with available records of history, physical examination, plain radiographs, and laboratory data including rheumatoid factor (RF), anti-cyclic citrullinated protein antibody (ACPA), C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR) were included. Any patients who had had history of tendon injury or surgery were excluded. Consequently, a total of 402 patients were enrolled (Fig. 1).
Fig. 1.
Overview of patient enrollment. RA rheumatoid arthritis. *Clinically RA without satisfying 2010 RA classification criteria
Imaging Protocol
For 2P-BS imaging, the blood pool phase imaging was acquired within 5 min and the delayed bone imaging in 4 h after injection of 740 MBq of 99mTc-MDP using a dual-head gamma camera (ECAM, Siemens Medical Solutions USA Inc., Malvern, Pennsylvania, USA) equipped with a low-energy high-resolution collimator in matrix of 128 × 128. Additionally, the regional views of hands, wrists, ankles, and feet were obtained during the blood pool and delayed bone phases. Two nuclear medicine physicians with consensus performed visual assessment of all fingers, toes, radial and ulnar sides of both wrists, and medial and lateral sides of both ankles on 2P-BS. Linearly increased activity of each region on the blood pool image with/without focal bone uptake was interpreted as tenosynovitis. The positive arthritis pattern on 2P-BS was defined as increased radiotracer fusiform uptake around joints on the blood pool phase with or without increased periarticular bone uptake on the bone phase in typical areas of RA joint involvement (proximal interphalangeal, metacarpophalangeal, thumb interphalangeal, wrist, elbow, shoulder, knee, ankle, and metatarsophalangeal joints) described on the 2010 Rheumatoid Arthritis classification criteria [24].
Follow-Up and Outcomes
With at least 6 months of follow-up, all patients were diagnosed as RA and non-RA by the same rheumatologist with 2010 Rheumatoid Arthritis Classification Criteria [24]. Joint involvement, serologic markers (RF and ACPA), acute-phase reactants (CRP and ESR), and duration of symptoms were used categories for the classification criteria. The normal ranges of those serologic markers in our hospital were ≤ 15 IU/mL, < 25 U/mL, ≤ 0.3 mg/dL, and ≤ 20 mm/h accordingly. A summed score of higher than 6 was defined as definite RA.
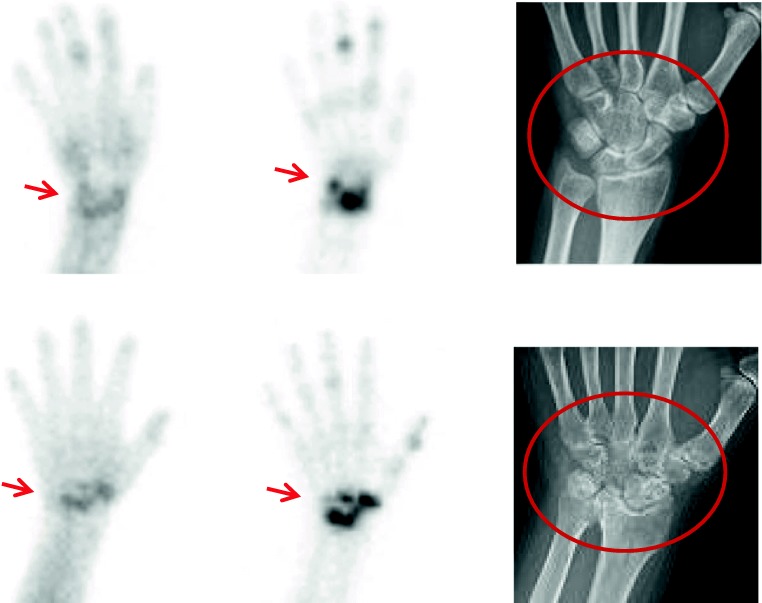
The reported results of relevant radiographic imaging were reviewed and used to define early and established arthritis. The early arthritis was defined with the positive 2P-BS and negative plain X-ray finding. When discrete bone change suggestive of RA involvement was shown on the plain X-ray, it was regarded as the established arthritis (Fig. 2).
Fig. 2.
Examples of early and established arthritis in RA patients. (Top row) Early arthritis of wrist was defined when the plain radiograph showed normal (right, circle) in addition to the positive two-phase bone scintigraphic images (left and middle, arrows). (Bottom row) For established arthritis, the blood pool and bone phase imaging was positive (left and middle, arrows), and bone involvement was shown on the plain radiography (right, circle) compared with that of early arthritis. RA rheumatoid arthritis
Statistical Analysis
For the comparison of parametric and non-parametric values between RA and non-RA group, the Student t test and Mann–Whitney test were used accordingly. The agreement and difference of both 2P-BS and physical examination for the detection of tenosynovitis was evaluated by kappa values and McNemar’s test, respectively. When prevalence was too low to estimate the agreement, a prevalence-adjusted and bias-adjusted kappa (PABAK) was used [25–27]. The odds ratio, sensitivity, specificity, accuracy, positive predictive value, and negative predictive value were described for the association and diagnostic performance of the tenosynovitis pattern on 2P-BS and physical examination for RA. To analyze the influence of sex, age, duration of pain, serologic markers, acute-phase reactants on the association of RA with the tenosynovitis pattern on 2P-BS, and clinically suspected tenosynovitis, univariable and multivariable logistic regression analyses with the enter method were performed. For the logistic regression analyses, age was grouped into intervals of 10 years. RF and ACPA were divided into negative, low-positive, and high-positive, and CRP and ESR were into normal and abnormal as defined in the 2010 Rheumatoid Arthritis Classification Criteria. Statistical significance was defined as lower than 0.05 of the p value. All statistical analyses except PABAK were performed with IBM SPSS Statistics 22.0 (IBM Corp, Armonk, New York, USA) software.
Ethical Consideration
The institutional internal review board ethics (IRB) approved a waiver of consent for this retrospective study (IRB No. 2018-11-001). There was no potential conflict of interest relevant to this study.
Results
Clinical and Demographic Features of the Patients
Four hundred two patients with multiple-joint pain (72 males and 330 females; 48.3 ± 12.6 years) were enrolled. The mean duration of pain was 26.7 ± 21 months, and there was no patient who had had history of tendon injury or surgery. The mean of RF, ACPA, CRP, and ESR were 166 ± 485 IU/mL, 246 ± 512 U/mL, 0.5 ± 1.3 mg/dL, and 27 ± 26 mm/h, respectively. One hundred fifty-four of the 402 patients (38.3%) were finally diagnosed as RA and 248 (61.7%) as non-RA. In the RA group, the duration of pain (31.8 ± 45.2 months), RF (321 ± 574 IU/mL), ACPA (585 ± 670 U/mL), CRP (1.0 ± 1.7 mg/dL), and ESR (42 ± 30 mm/h) were statistically different with those of the non-RA group (p < 0.018). The sex and age were not statistically different between the two groups. Detailed characteristics of patients are summarized in Table 1.
Table 1.
Baseline characteristics of enrolled patients
| N = 402 | RA (n = 154) | Non-RA (n = 248) |
|---|---|---|
| Sex (male/female) | 28:126 | 44:204 |
| Age, years (range) | 48 (19–81) | 49 (19–78) |
| Duration of pain, months (range) | 31.8 (0.5–240) | 38.7* (0.25–276) |
| RF, IU/mL (normal range 0–15) | 321 ± 574* | 68 ± 389 |
| ACPA, U/mL (normal range < 25) | 585 ± 670* | 31 ± 158 |
| CRP, mg/dL (normal range 0–0.3) | 1.0 ± 1.7* | 0.2 ± 0.9 |
| ESR, mm/h (normal range 0–20) | 42 ± 30* | 18 ± 18 |
RA rheumatoid arthritis, RF rheumatoid factor, ACPA anti-cyclic citrullinated protein antibody, CRP C-reactive protein, ESR erythrocyte sedimentation rate, 2P-BS two-phase bone scintigraphy
*Statistical difference was significant with less than 0.05 of the p value
Tenosynovitis Pattern on 2P-BS and Physical Examination
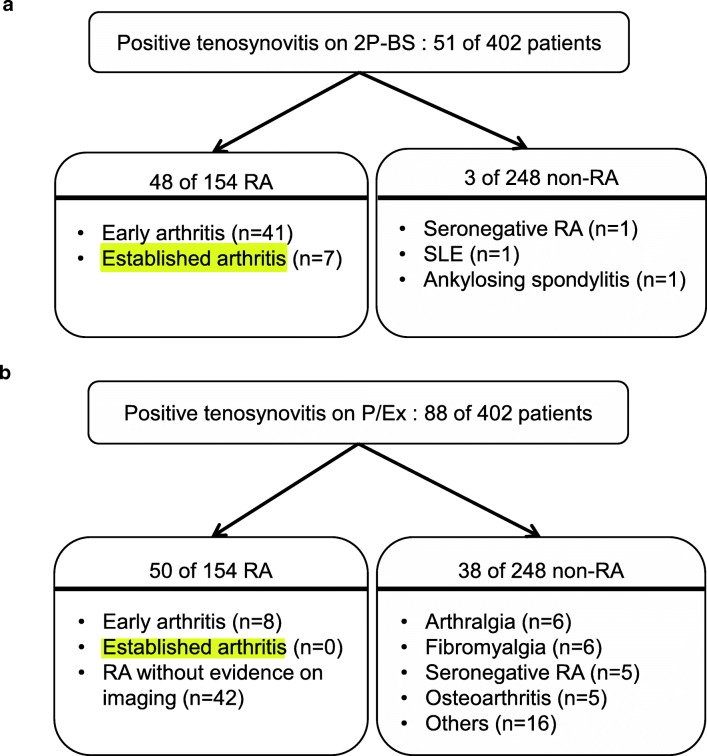
Tenosynovitis pattern was detected in 51 on 2P-BS of 402 patients (12.7%). Of the 51 patients, 48 patients (94.1%) were diagnosed as RA vs. three patients (5.9%) as non-RA including 1 of clinically seronegative RA but not satisfying the 2010 RA criteria score, 1 of SLE, and 1 of ankylosing spondylitis. Of the 48 RA patients with positive 2P-BS finding, 41 patients (85.4%) had early arthritis and 7 patients (14.6%) had established arthritis (Fig. 3a). On physical examination, tenosynovitis was suspected in 88 of 402 patients (21.9%). A total of 56.8% belonged to the RA group (50/88) and 43.2% to the non-RA group (38/88) including 6 of unspecified arthralgia, 6 of fibromyalgia, 5 of clinically seronegative RA but not satisfying 2010 RA criteria score, 5 of osteoarthritis, and others (Fig. 3b). Twenty-nine patients had tenosynovitis on both 2P-BS and physical examination. Patients with positive 2P-BS and negative physical examination for tenosynovitis were 22. Fifty-nine patients showed tenosynovitis on physical examination but not on 2P-BS. There were 292 patients whose 2P-BS and physical examination for tenosynovitis was negative (Table 2).
Fig. 3.
Clinical outcomes of enrolled 402 patients. a On 2P-BS, 51 patients showed tenosynovitis. Among them, 41 patients were in early arthritis and 7 in established arthritis. b Physical examination suggested tenosynovitis on 88 patients. Fifty of them were in the RA group with 8 early arthritis and no established arthritis. 2P-BS two-phase bone scintigraphy, RA rheumatoid arthritis, SLE systemic lupus erythematosus, P/Ex physical examination
Table 2.
The agreement between 2P-BS and physical examination in the detection of tenosynovitis
| Physical examination | 2P-BS | Agreement | ||||
|---|---|---|---|---|---|---|
| Total patients | Positive | Negative | Total | Kappa | PABAK | |
| Positive | 29 | 59 | 88 | 0.306* | 0.597 | |
| Negative | 22 | 292 | 314 | |||
| Total | 51 | 351 | 402 | |||
| RA | Positive | 27 | 23 | 50 | 0.342* | 0.429 |
| Negative | 21 | 83 | 104 | |||
| Total | 48 | 106 | 154 | |||
| Non-RA | Positive | 2 | 36 | 38 | 0.077* | 0.702 |
| Negative | 1 | 209 | 210 | |||
| Total | 3 | 245 | 248 | |||
2P-BS two-phase bone scintigraphy, PABAK prevalence-adjusted and bias-adjusted kappa, RA rheumatoid arthritis
*The kappa coefficient was significantly different (p < 0.001)
Agreement of Tenosynovitis Between 2P-BS and Physical Examination
In a total of 402 patients, the detection results of 2P-BS and physical examination showed to be statistically different by McNemar’s test (p < 0.001) and fair agreement by 0.306 of the kappa coefficient (p < 0.001) while PABAK was 0.597 (Table 2). In 154 RA patients, the kappa coefficient and PABAK between 2P-BS and physical examination for the detection of tenosynovitis were 0.342 (p < 0.001) and 0.429 while 0.077 and 0.702 in 248 non-RA patients (p = 0.013), respectively.
Tenosynovitis on 2P-BS and Physical Examination in the Prediction of RA
The univariable logistic analysis showed that the tenosynovitis pattern on 2P-BS, ACPA, RF, CRP, ESR, and clinically suspected tenosynovitis were associated with RA accordingly. In consequential multivariable logistic analysis, the positive tenosynovitis pattern on 2P-BS, CRP, ACPA, RF, ESR, and duration of pain were statistically associated with RA in descending order. The positive tenosynovitis pattern on 2P-BS represented 26.408 of odds ratio which was highest among those of the RA-associated factors. Sex, age, clinically suspected tenosynovitis, and the body distribution of tenosynovitis were not associated with RA. The throughout results of univariable and multivariable logistic analyses are shown in Tables 3 and 4.
Table 3.
The association of RA and contributing factors on univariable logistic regression (enter method)
| B | OR (95% C.I.) | p | |
|---|---|---|---|
| Sex* | 0.030 | 1.030 (0.611–1.739) | 0.911 |
| Age# | 0.035 | 1.035 (0.886–1.210) | 0.661 |
| Duration of pain (years) | 0.052 | 1.054 (0.995–1.116) | 0.074 |
| Tenosynovitis | |||
| 2P-BS | 3.610 | 36.981 (11.268–121.373) | < 0.001 |
| P/Ex | 0.977 | 2.657 (1.639–4.306) | < 0.001 |
| RF† | 1.694 | 5.439 (4.023–7.353) | < 0.001 |
| ACPA† | 2.436 | 11.423 (7.770–16.795) | < 0.001 |
| CRP‡ | 1.832 | 6.244 (3.648–10.689) | < 0.001 |
| ESR‡ | 1.802 | 6.064 (3.854–9.541) | < 0.001 |
RA rheumatoid arthritis, OR Odds ratio, C.I. confidence interval, 2P-BS two-phase bone scintigraphy, P/Ex physical examination, RF rheumatoid factor, ACPA anti-cyclic citrullinated protein antibody, CRP C-reactive protein, ESR estimated sedimentation rate
*Sex was divided into male and female
#Age was grouped into intervals of 10 years
†RF and ACPA were divided into negative with their normal references (≤ 15 IU/mL and < 25 U/mL, respectively), low-positive (higher than the upper normal reference but ≤ 3 times), and high-positive (> 3 times the upper normal reference)
‡CRP and ESR were into normal when their values were within their normal ranges (≤ 0.8 mg/dL and 20 mm/h respectively). Higher values than the normal ranges were defined as abnormal
Table 4.
Comparison of 2P-BS and physical examination for the association of RA and contributing factors on multivariable logistic regression (enter method)
| B | OR (95% C.I.) | p | B | OR (95% C.I.) | p | |
|---|---|---|---|---|---|---|
| 2P-BS | P/Ex | |||||
| Sex* | − 0.651 | 0.521 (0.150–1.809) | 0.305 | − 0.136 | 0.873 (0.291–2.616) | 0.808 |
| Age# | − 0.201 | 0.818 (0.586–1.141) | 0.237 | − 0.170 | 0.843 (0.622–1.144) | 0.273 |
| Duration of pain (years) | 0.151 | 1.163 (1.034–1.309) | 0.012 | 0.159 | 1.173 (1.045–1.316) | 0.007 |
| Tenosynovitis | 3.274 | 26.408 (5.057–137.901) | < 0.001 | 0.757 | 2.132 (0.763–5.955) | 0.149 |
| RF† | 1.217 | 3.378 (1.921–5.940) | < 0.001 | 1.156 | 3.177 (1.878–5.374) | < 0.001 |
| ACPA† | 2.241 | 9.402 (5.411–16.335) | < 0.001 | 2.326 | 10.237 (6.039–17.355) | < 0.001 |
| CRP‡ | 2.897 | 18.118 (4.753–69.066) | < 0.001 | 2.651 | 14.175 (4.015–50.039) | < 0.001 |
| ESR‡ | 1.115 | 3.173 (1.158–8.697) | < 0.001 | 1.280 | 3.598 (1.381–9.371) | 0.009 |
2P-BS two-phase bone scintigraphy, RA rheumatoid arthritis, OR Odds ratio, C.I. confidence interval, P/Ex physical examination, RF rheumatoid factor, ACPA anti-cyclic citrullinated protein antibody, CRP C-reactive protein, ESR estimated sedimentation rate
*Sex was divided into male and female
#Age was grouped into intervals of 10 years
†RF and ACPA were divided into negative with their normal references (≤ 15 IU/mL and < 25 U/mL respectively), low-positive (higher than the upper normal reference but ≤ 3 times), and high-positive (> 3 times the upper normal reference)
‡CRP and ESR were into normal when their values were within their normal ranges (≤ 0.8 mg/dL and 20 mm/h respectively). Higher values than the normal ranges were defined as abnormal
Discussion
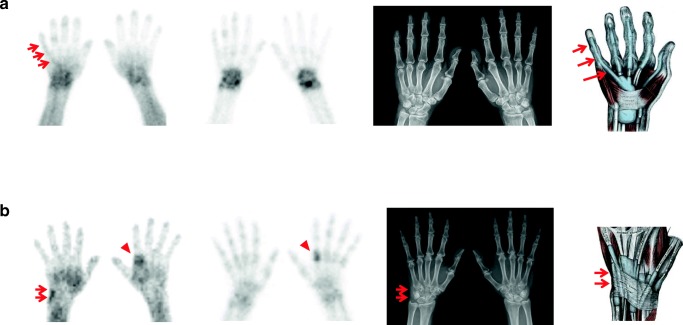
2P-BS is a bone scintigraphy technique that consists of the blood pool phase within 5 min and delayed bone phase imaging in 4 h after the injection of 99mTc-MDP. Because the blood pool phase of 2P-BS can represent the activity of inflammation, 2P-BS is useful for the evaluation of tenosynovitis which is inflammation of a tendon and its synovial sheath. Indeed, this study revealed positive blood pool imaging for tenosynovitis (Figs. 4 and 5) though there was no report of RA patients with depicted tenosynovitis on the blood pool imaging, but several studies represented the ability of blood pool phase imaging in case of de Quervain [22, 29].
Fig. 4.
Tenosynovitis of the flexor digitorum profundus tendon on the right hand and extensor carpi ulnaris tendon on the right wrist of a rheumatoid arthritis patient. a The blood pool image showed the curvilinear activity (left, arrows) which was not clearly visualized on the bone phase image (middle left) and the plain radiograph image (middle right). The curvilinear activity was matched with the flexor digitorum profundus tendon (right, arrows) on the illustration of the hand [28]. b The linearly increased blood pool phase activity (left, arrows) with negative bone phase scan (middle left), and the ill-defined soft tissue swelling on the plain radiography (middle right, arrows) was shown on the ulnar side of the right wrist, which was matched with the extensor carpi ulnaris tendon (right, arrows) on the illustration of the wrist [28]. The second metacarpophalangeal joint of the left hand had arthritis on both of the blood pool (left, arrowhead) and bone phase image (middle left, arrowhead)
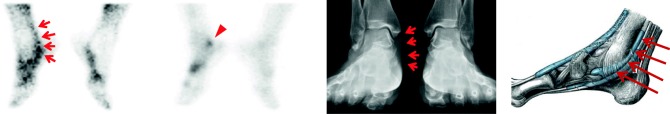
Fig. 5.
Tenosynovitis of posterior tibialis tendon on the right ankle of a rheumatoid arthritis patient. There was a curvilinear blood pool phase activity (left, arrows), adjacent bone had an increased uptake on the bone scan (middle left, arrowhead), and there was an asymmetric soft tissue swelling on the plain radiograph (middle right, arrows) along the matched posterior tibialis tendon of the right ankle medial side with the illustration of the right ankle (right, arrows) [28]
In RA, tenosynovitis is an isolated or predominant clinical manifestation with about 50% of prevalence [30]. There was a report that the flexor tenosynovitis of hands had played an important role in the prediction of the early RA [31]. Another study represented that up to 80% of preclinical RA with positive ACPA antibody and MRI-confirmed tenosynovitis had developed overt RA [3–5]. Möttönen reported that the flexor tenosynovitis of hands was highly correlated with the number of joint erosion in RA [32]. Though the pathophysiology is still poorly understood, the pathologic findings of tenosynovitis in RA may be similar to that of synovitis with inflammatory granulation change called pannus [33]. Therefore, the clinical diagnosis of tenosynovitis may be easier when the typical clinical manifestations of RA coexisted. On the other hand, no evidence of overt arthritis especially in the preclinical or early stage of RA may bring harder-to-diagnose tenosynovitis. Noticeably, 41 of 51 patients who had tenosynovitis on 2P-BS were in early RA compared with seven established RA patients in this study. Though this study is not a cohort study, the results may be similar to those prior studies. In addition, Lindegaard et al. reported that tenosynovitis was present in 60% of early RA patients at baseline and decreased to 28% at 6 months [34]. The report may be a clue why only seven established RA patients had tenosynovitis in this study. Taken together, tenosynovitis may be more common in early RA than in established RA and may be a reasonable predictor of RA progression in preclinical settings against the retrospective nature of this study.
Interestingly, there was statistical difference for evaluation of tenosynovitis between 2P-BS and physical examination. In this study, the agreement of 2P-BS and physical examination was similar in the total population and the RA group (kappa coefficient, 0.306 and 0.342, respectively) but lower in the non-RA group (kappa coefficient, 0.077). PABAK was 0.597, 0.429, and 0.702 respectively, which turned the less than fair agreement to moderate or even good. It was thought that the deviated prevalence between those groups might affect the agreement. Collected laboratory data were less helpful to explain the difference and moderate agreement. The lack of gold standard for tenosynovitis in this study may mainly contribute the difference. Nonetheless, the discordance and higher prevalence (85.4%) of early RA on patients with RA and tenosynovitis detected by 2P-BS may postulate that 2P-BS could depict preclinical RA-related subclinical tenosynovitis in addition to clinically detected tenosynovitis, as physical examination had a low negative predictive value in detection of tenosynovitis against its high sensitivity, specificity, and positive predictive values [17].
The association of the tenosynovitis pattern on 2P-BS with RA was strongest among all contributing factors in this study. CRP and ACPA revealed the second and third strongest association with RA. Duration of pain had the weakest association with RA though it was included as an important factor for RA classification criteria [35]. As those results represented, the existence of tenosynovitis on 2P-BS was positively associated with a higher odds ratio for RA than other factors that were already included in RA Classification criteria. Therefore, 2P-BS could give additional usefulness to predict RA in conjunction with current categorical factors of RA Classification criteria.
This study had limitations. The retrospective design took into account selection bias, and the lack of gold standard for tenosynovitis affected the limited evaluation of the discordance between 2P-BS and physical examination. Comparison with other imaging modalities including MRI and ultrasonography was not performed as the retrospective nature. Those limitations might have arisen whether 2P-BS brought a false-positive result for differentiating arthritis from soft tissue inflammation. To minimize such possibilities, the target population in this study was limited to patients with suspected RA complaining multiple-joint pain. The record of physical examination was carefully reviewed in order to identify soft tissue inflammation that might have affected the false positivity of 2P-BS for arthritis. The positivity of arthritis on 2P-BS was made only when fusiform blood pool activity and periarticular uptake near frequently involved joints in RA were clearly shown while soft tissue inflammation might not have been done. Although such limitations still remain, this study shows that 2P-BS may give additional diagnostic values into the physical examination for the detection of tenosynovitis especially in case of early or preclinical RA. Further investigation such as a prospective case–control study and comparison with MRI and ultrasonography should be assigned for the ability of 2P-BS in such a clinical scenario.
Conclusion
2P-BS can detect tenosynovitis earlier than radiographs by only adding blood pool phase imaging to the delayed bone phase imaging. Also, it is easy to access compared to MRI and highly reproducible compared to ultrasonography. Additionally, it can depict arthritis earlier than radiographs in polyarthralgia. In this study, either 2P-BS or physical examination detected tenosynovitis more commonly in the RA group than in the non-RA group. Tenosynovitis of 2P-BS had a more frequent association with early arthritis than with established arthritis. Though there was discordance on the evaluation of tenosynovitis between 2P-BS and physical examination in this retrospective study, it in turn postulated that 2P-BS might be helpful for additional detection of subclinical tenosynovitis in early or preclinical RA patients.
Compliance with Ethical Standards
Conflict of Interest
Hyung Jin Choi, Soo Jin Lee, Ji Young Kim, Yoon-Kyoung Sung, and Yun Young Choi declare no conflict of interest.
Ethical Statement
All procedures followed were performed in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2013.
Informed Consent
The study design of the retrospective analysis and exemption of informed consent were approved by the Institutional Review Board of Hanyang University Medical Center (HYUH 2018-11-001).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sandrock D, Backhaus M, Burmester G, Munz DL. Imaging techniques in rheumatology: scintigraphy in rheumatoid arthritis. Z Rheumatol. 2003;62:476–480. doi: 10.1007/s00393-003-0515-x. [DOI] [PubMed] [Google Scholar]
- 2.Kim JY, Choi YY, Kim CW, Sung YK, Yoo DH. Bone scintigraphy in the diagnosis of rheumatoid arthritis: is there additional value of bone scintigraphy with blood pool phase over conventional bone scintigraphy? J Korean Med Sci. 2016;31:502–509. doi: 10.3346/jkms.2016.31.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray RG, Gottlieb NL. Hand flexor tenosynovitis in rheumatoid arthritis. Prevalence, distribution, and associated rheumatic features. Arthritis Rheum. 1977;20:1003–1008. doi: 10.1002/art.1780200414. [DOI] [PubMed] [Google Scholar]
- 4.Rowbotham EL, Freeston JE, Emery P, Grainger AJ. The prevalence of tenosynovitis of the interosseous tendons of the hand in patients with rheumatoid arthritis. Eur Radiol. 2016;26:444–450. doi: 10.1007/s00330-015-3859-0. [DOI] [PubMed] [Google Scholar]
- 5.Kleyer A, Krieter M, Oliveira I, Faustini F, Simon D, Kaemmerer N, et al. High prevalence of tenosynovial inflammation before onset of rheumatoid arthritis and its link to progression to RA-A combined MRI/CT study. Semin Arthritis Rheum. 2016;46:143–150. doi: 10.1016/j.semarthrit.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Bukhari MA, Wiles NJ, Lunt M, Harrison BJ, Scott DG, Symmons DP, et al. Influence of disease-modifying therapy on radiographic outcome in inflammatory polyarthritis at five years: results from a large observational inception study. Arthritis Rheum. 2003;48:46–53. doi: 10.1002/art.10727. [DOI] [PubMed] [Google Scholar]
- 7.Davies A, Cifaldi MA, Segurado OG, Weisman MH. Cost-effectiveness of sequential therapy with tumor necrosis factor antagonists in early rheumatoid arthritis. J Rheumatol. 2009;36:16–26. doi: 10.3899/jrheum.080257. [DOI] [PubMed] [Google Scholar]
- 8.Emery P, Seto Y. Role of biologics in early arthritis. Clin Exp Rheumatol. 2003;21:S191–S194. [PubMed] [Google Scholar]
- 9.Hammer HB, Kvien TK, Terslev L. Tenosynovitis in rheumatoid arthritis patients on biologic treatment: involvement and sensitivity to change compared to joint inflammation. Clin Exp Rheumatol. 2017;35:959–965. [PubMed] [Google Scholar]
- 10.Pincus T, O'Dell JR, Kremer JM. Combination therapy with multiple disease-modifying antirheumatic drugs in rheumatoid arthritis: a preventive strategy. Ann Intern Med. 1999;131:768–774. doi: 10.7326/0003-4819-131-10-199911160-00009. [DOI] [PubMed] [Google Scholar]
- 11.van der Heide A, Jacobs JW, Bijlsma JW, Heurkens AH, van Booma-Frankfort C, van der Veen MJ, et al. The effectiveness of early treatment with “second-line” antirheumatic drugs. A randomized, controlled trial. Ann Intern Med. 1996;124:699–707. doi: 10.7326/0003-4819-124-8-199604150-00001. [DOI] [PubMed] [Google Scholar]
- 12.van Dongen H, van Aken J, Lard LR, Visser K, Ronday HK, Hulsmans HM, et al. Efficacy of methotrexate treatment in patients with probable rheumatoid arthritis: a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2007;56:1424–1432. doi: 10.1002/art.22525. [DOI] [PubMed] [Google Scholar]
- 13.Ammitzboll-Danielsen M, Ostergaard M, Naredo E, Terslev L. Validity and sensitivity to change of the semi-quantitative OMERACT ultrasound scoring system for tenosynovitis in patients with rheumatoid arthritis. Rheumatology (Oxford) 2016;55:2156–2166. doi: 10.1093/rheumatology/kew317. [DOI] [PubMed] [Google Scholar]
- 14.Boutry N, Larde A, Lapegue F, Solau-Gervais E, Flipo RM, Cotten A. Magnetic resonance imaging appearance of the hands and feet in patients with early rheumatoid arthritis. J Rheumatol. 2003;30:671–679. [PubMed] [Google Scholar]
- 15.Danielsen MA. Ultrasonography for diagnosis, monitoring and treatment of tenosynovitis in patients with rheumatoid arthritis. Dan Med J. 2018;65. [PubMed]
- 16.Haavardsholm EA, Ostergaard M, Ejbjerg BJ, Kvan NP, Kvien TK. Introduction of a novel magnetic resonance imaging tenosynovitis score for rheumatoid arthritis: reliability in a multireader longitudinal study. Ann Rheum Dis. 2007;66:1216–1220. doi: 10.1136/ard.2006.068361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hmamouchi I, Bahiri R, Srifi N, Aktaou S, Abouqal R, Hajjaj-Hassouni N. A comparison of ultrasound and clinical examination in the detection of flexor tenosynovitis in early arthritis. BMC Musculoskelet Disord. 2011;12:91. doi: 10.1186/1471-2474-12-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navalho M, Resende C, Rodrigues AM, Ramos F, Gaspar A, Pereira da Silva JA, et al. Bilateral MR imaging of the hand and wrist in early and very early inflammatory arthritis: tenosynovitis is associated with progression to rheumatoid arthritis. Radiology. 2012;264:823–833. doi: 10.1148/radiol.12112513. [DOI] [PubMed] [Google Scholar]
- 19.Szkudlarek M, Court-Payen M, Jacobsen S, Klarlund M, Thomsen HS, Ostergaard M. Interobserver agreement in ultrasonography of the finger and toe joints in rheumatoid arthritis. Arthritis Rheum. 2003;48:955–962. doi: 10.1002/art.10877. [DOI] [PubMed] [Google Scholar]
- 20.Zabotti A, Salvin S, Quartuccio L, De Vita S. Differentiation between early rheumatoid and early psoriatic arthritis by the ultrasonographic study of the synovio-entheseal complex of the small joints of the hands. Clin Exp Rheumatol. 2016;34:459–465. [PubMed] [Google Scholar]
- 21.Stein F, Miale A, Jr, Stein A. Enhanced diagnosis of hand and wrist disorders by triple phase radionuclide bone imaging. Bull Hosp Jt Dis Orthop Inst. 1984;44:477–484. [PubMed] [Google Scholar]
- 22.Sopov W, Rozenbaum M, Rosner I, Groshar D. Scintigraphy of de Quervain's tenosynovitis. Nucl Med Commun. 1999;20:175–177. doi: 10.1097/00006231-199902000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Kaya M, Tuna H, Tuncbilek N, Cermik TF, Sardogan K. Scintigraphic findings in plant thorn tenosynovitis of finger. Clin Nucl Med. 2008;33:131–132. doi: 10.1097/RLU.0b013e31815ef7fd. [DOI] [PubMed] [Google Scholar]
- 24.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–1588. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- 25.Byrt T, Bishop J, Carlin JB. Bias, prevalence and kappa. J Clin Epidemiol. 1993;46:423–429. doi: 10.1016/0895-4356(93)90018-V. [DOI] [PubMed] [Google Scholar]
- 26.Tooth LR, Ottenbacher KJ. The kappa statistic in rehabilitation research: an examination. Arch Phys Med Rehabil. 2004;85:1371–1376. doi: 10.1016/j.apmr.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Spitznagel EL, Helzer JE. A proposed solution to the base rate problem in the kappa statistic. Arch Gen Psychiatry. 1985;42:725–728. doi: 10.1001/archpsyc.1985.01790300093012. [DOI] [PubMed] [Google Scholar]
- 28.Wikimedia Commons contributors. Gray’s Anatomy plates, Wikimedia Commons, the free media repository. https://commons.wikimedia.org/wiki/Gray%27s_Anatomy_plates. Accessed 6 Dec 2018.
- 29.Leslie WD. The scintigraphic appearance of de Quervain tenosynovitis. Clin Nucl Med. 2006;31:602–604. doi: 10.1097/01.rlu.0000238297.43948.b5. [DOI] [PubMed] [Google Scholar]
- 30.Ansell BM, Bywaters EG. Finger contractures due to tendon lesions as a mode of presentation of rheumatoid arthritis. Ann Rheum Dis. 1953;12:283–289. doi: 10.1136/ard.12.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eshed I, Feist E, Althoff CE, Hamm B, Konen E, Burmester GR, et al. Tenosynovitis of the flexor tendons of the hand detected by MRI: an early indicator of rheumatoid arthritis. Rheumatology (Oxford) 2009;48:887–891. doi: 10.1093/rheumatology/kep136. [DOI] [PubMed] [Google Scholar]
- 32.Mottonen TT. Prediction of erosiveness and rate of development of new erosions in early rheumatoid arthritis. Ann Rheum Dis. 1988;47:648–653. doi: 10.1136/ard.47.8.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jain A, Nanchahal J, Troeberg L, Green P, Brennan F. Production of cytokines, vascular endothelial growth factor, matrix metalloproteinases, and tissue inhibitor of metalloproteinases 1 by tenosynovium demonstrates its potential for tendon destruction in rheumatoid arthritis. Arthritis Rheum. 2001;44:1754–1760. doi: 10.1002/1529-0131(200108)44:8<1754::AID-ART310>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 34.Lindegaard HM, Vallo J, Horslev-Petersen K, Junker P, Ostergaard M. Low-cost, low-field dedicated extremity magnetic resonance imaging in early rheumatoid arthritis: a 1-year follow-up study. Ann Rheum Dis. 2006;65:1208–1212. doi: 10.1136/ard.2005.049213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rantapaa-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]