Abstract
Disease-modifying anti-rheumatic drugs (DMARDs) can reduce inflammation and slow progression of rheumatoid arthritis (RA). It remains unknown what impact DMARDs may have on dementia, where inflammation also plays a critical role in pathogenesis. Patients without a prior history of dementia who were newly diagnosed with RA between 2000 and 2005 were identified from Taiwan’s National Health Insurance Research Database. The participants were stratified by age and gender. The outcome of interest was all-cause dementia, and Cox regression models were used to estimate the hazard ratio (HR) of dementia. The cumulative DMARD dosage was stratified by quartiles of defined daily doses. A total of 20,707 patients were diagnosed with RA, and 62,121 non-RA individuals aged 20 years or older were included. Cox proportional hazard regression analysis revealed that the RA cohort was 37% less likely to develop dementia compared with the non-RA cohort [adjusted HR 0.63, 95% confidence interval (CI) 0.55–0.72]. Further decreased risk was noted in DMARD users (adjusted HR 0.48, 95% CI 0.39–0.58) with dose-dependent effect. Subgroup analysis identified DMARD use as having a protective effect against developing dementia in female and younger patients. Also, in male and older patients, DMARD use further reduced the risk. These observations suggest that DMARDs may reduce the risk of developing dementia, and its effect is further increased at high cumulative dosages. These findings warrant further examination in randomized control trials.
Key Words: Disease-modifying anti-rheumatic drugs, dementia, inflammation, rheumatoid arthritis
Introduction
Over the past few decades as the elderly population has rapidly increased, the prevalence of dementia has also continuously increased in developed countries, and cognitive decline has had a strong impact on society and the economy [1]. Although pharmacological interventions are recommended for the treatment of cognitive impairment in Alzheimer’s disease (AD), they do not modify the disease course and are not recommended in other types of dementia, such as vascular dementia [2, 3]. Inflammation plays a critical role in the pathogenesis of AD [4, 5], and persistent inflammation may also contribute to the development of atherosclerosis [6] and vascular cognitive impairment [7]. Medications that suppress inflammation, such as steroid or non-steroidal anti-inflammatory drugs (NSAIDs), are believed to protect against dementia [8–10]. Rheumatoid arthritis (RA) is an autoimmune disease, which is characterized by persistent synovitis and systemic inflammation. Uncontrolled active disease causes joint damage, disability, and decreased quality of life as well as cardiovascular and other comorbidities [11]. Many studies have been conducted to demonstrate the association between autoimmune diseases (e.g., RA) and dementia [12–15]. However, the results of the studies remain controversial.
Disease-modifying anti-rheumatic drugs (DMARDs) are the principal therapeutic options for RA; they can reduce synovitis and systemic inflammation and slow disease progression and structure damage [11, 16, 17]. In a systemic review, DMARD use was associated with a reduced risk of cardiovascular disease events in patients with RA where inflammatory insult was caused [18]. However, there have only been a few studies regarding the association between DMARD use and dementia risk in patients with RA [19, 20] and the results of these studies were conflicting. Chou et al. [20] reported that DMARD use was associated with an increased risk of dementia in patients with RA. However, another study conducted by Judge et al. [19] suggested that DMARD users were at reduced risk of dementia compared with non-DMARD users. Whether an association exists between different ages or genders is still inconclusive.
The present study aimed to clarify the association between RA and dementia and to explore whether the use of DMARDs could reduce the risk of developing dementia in patients with RA through a population-based case–control study.
Methods
Data Source
The present study was conducted using claims data from the Taiwan’s National Health Insurance Research Database (NHIRD), which is managed by the National Health Research Institute of Taiwan. The National Health Insurance (NHI) program reimburses healthcare costs of 99% of Taiwan’s population (approximately 23 million people). The NHIRD contains comprehensive healthcare information for all of Taiwan’s insured individuals, including demographic data, dates of clinical visits, diagnostic codes, and prescription details. For the present study, a subset of the NHIRD, known as the Longitudinal Health Insurance Database (LHID) 2000, was used. The subset contains historical ambulatory and inpatient care data for 1 million randomly sampled beneficiaries enrolled into the NHI system in the year 2000. The LHID 2000 allows researchers to follow up the medical service utilization of these 1 million subjects. According to the National Health Research Institute, there are no statistically significant differences in age, sex, or healthcare costs between the LHID 2000 and the NHIRD. Patient diagnoses were coded according to the International Classification of Diseases-Ninth Revision-Clinical Modification (ICD-9-CM). The Registry for Catastrophic Illness Patient Database (RCIPD) was also used to confirm the diagnoses of patients with RA. In Taiwan, rheumatologists can apply for a catastrophic illness card for patients with RA who meet 4 or more diagnostic criteria based on the 1987 American College of Rheumatology criteria [21].
Study Patients
The present case–control study was conducted using the NHIRD and RCIPD. Patients aged ≥ 20 years who were newly diagnosed with RA (ICD-9-CM:714) between January 2000 and December 2005 were included in the RA cohort. This RA cohort was propensity matched with a comparison cohort, which consisted of subjects who had never been diagnosed with RA and who were randomly sampled from the remainder of the LHID 2000 data set; they were matched (1:3) on the basis of their propensity score, including age, sex, index year, and comorbidities. The date of RA diagnosis for each patient was defined as the index date. Subjects were excluded if they were < 20 years of age, their demographic data was incomplete, or they had been previously diagnosed with dementia before the index date. The demographic data were collected, including age and sex.
Cumulative Exposure of DMARDs
Drug usage information was obtained from the outpatient pharmacy prescription database; it included the prescribed drug dosage, date of prescription, number of days supplied, and total number of pills dispensed. The DMARDs selected for the present study were methotrexate, azathioprine, leflunomide, cyclophosphamide, hydroxychloroquine, sulfasalazine, ciclosporin, etanercept, adalimumab, golimumab, tocilizumab, ustekinumab, abatacept, and rituximab. As patients may discontinue or restart drug therapy, the analyses assumed that a patient’s exposure to DMARDs contributed both cumulatively and continuously to their long-term risk of dementia. Defined daily dose (DDD) is the assumed average maintenance dose per day for a drug used for its primary usage in adults [22]. To investigate the effect of dose, the cumulative use of DMARDs was calculated as the total prescribed DDD (i.e., the same as the total dispensed DDD). The cumulative DMARD dosage was calculated at the time a dementia event occurred and represents the total DDD from drug initiation to the day before the dementia event. For those who were still at risk (event free and uncensored) in the cohort, the cumulative doses were recorded and ranked at each event. Participants were then classified into mutually exclusive dosage categories based on the quartiles of cumulative dosage distribution. As time passed and the accumulated DMARD dosages changed during the follow-up period, a participant could be reassigned to either a higher or a lower quartile.
Diagnosis of Dementia
A diagnosis of dementia was based on at least 2 outpatient visits or at least 1 inpatient visit with a diagnosis of dementia listed on the submitted claims filing. The outcome of interest was a diagnosis of either ICD-9-CM:290.0 (senile dementia, uncomplicated), ICD-9-CM:290.4x (arteriosclerotic dementia), ICD-9-CM:294.1 (dementia in conditions classified elsewhere), or ICD-9-CM:331.0 (AD). Patients were followed from the index date until either the occurrence of dementia, death, disenrollment from the NHI program, or the end of the study date (31 December 2009), whichever came first. Patient identifiers were scrambled before their data was used for research purposes to protect confidentiality; therefore, the requirement for written or verbal consent from patients for data linkage was waived.
Covariates
Inpatient and outpatient claim filings from the year prior to the index date were used to obtain information on comorbidities, including diabetes mellitus (ICD-9-CM:250.xx), hypertension (ICD-9-CM:401x), hyperlipidemia (ICD-9-CM:272x), cardiovascular disease (ICD-9-CM:433–438), depression (ICD-9-CM:296.x-,300.4, 311), and cerebral vascular disease (ICD-9-CM:433–438). Comorbidities were considered in a patient if they were diagnosed for any of the aforementioned diseases on at least 2 outpatient claims or 1 inpatient claim during the examined period.
Statistical Analysis
Pearson’s chi-square test was performed to evaluate differences in categorical data between the DMARD cohort and the comparison cohort, including demographic data and comorbidities. Cox proportional hazards regression analysis was performed to examine the risk of dementia in the DMARD cohort versus the comparison cohort during the follow-up period. The DDDs recommended by the World Health Organization were used to quantify the use of DMARDs. Cumulative DDD was estimated as the sum of the dispensed DDDs of DMARDs from 1 January 2000 to the date of dementia diagnosis or until the end of the study. The patients on DMARDs were categorized into low-dose (< 616 DDDs), moderate-dose (616–1744 DDDs), and high-dose (> 1744 DDDs) users. Several covariables, including age, sex, and comorbidities, were adopted in the statistical analysis model. Hazard ratios (HRs) and 95% confidence intervals (CIs), using the comparison cohort as the reference, were calculated to show the risk of dementia in patients on DMARDs and in the dose–response analysis. Subgroup analysis was performed according to gender or age (< 65 years or ≥ 65 years). A Kaplan–Meier curve was used to estimate the probability of all-cause dementia, and a log-rank test was used to evaluate the differences between the DMARD cohort and the comparison cohort. All statistical analyses were performed using the SAS 9.3 statistical package (SAS Institute, Cary, NC, USA). All p values were 2-sided, and a p value < 0.05 was considered to indicate a statistically significant difference.
Results
A total of 20,707 patients who were diagnosed with RA between the 1st of January 2000 and the 31st of December 2005 were included in the present study. After propensity score matching, 20,707 RA patients were matched with 62,121 non-RA subjects. A summary of the RA and non-RA cohort characteristics is shown in Table 1, and all covariates were comparable after matching. A total of 245 (1.2%) RA patients developed all-cause dementia, while 1258 (2.0%) of the non-RA group developed all-cause dementia. Among the RA cohorts, 139 (2.3%) of the non-DMARD users developed dementia, and 106 (0.7%) of the DMARD users developed dementia. Cox proportional hazards regression analysis revealed that the RA cohort was 38% less likely to develop dementia compared with the matched non-RA cohort (crude HR 0.62, 95% CI 0.54–0.71; p < 0.001). The decreased risk of dementia remained significant after adjusting for potential confounders (adjusted HR 0.63, 95% CI 0.55–0.72; p < 0.001). A further decreased risk was observed when the DMARD users (crude HR 0.36, 95% CI 0.30–0.44; p < 0.001; adjusted HR 0.48, 95% CI 0.39–0.58; p < 0.001) were examined in isolation (Table 2). Figure 1 illustrates the level of all-cause dementia between the non-RA and RA cohorts, by the use of DMARDs.
Table 1.
Demographic data between the non-RA and RA groups (n = 82,828)
| Characteristics | Non-RA group (N = 62,121) | RA group (N = 20,707) | p value |
|---|---|---|---|
| Age (years) | 52.6 ± 14.7 | 53.0 ± 14.5 | 0.743 |
| < 50 | 26,563 (42.8) | 8865 (42.8) | 0.246 |
| 50–64 | 21,372 (34.4) | 6951 (33.6) | |
| ≥ 65 | 14,186 (22.8) | 4891 (23.6) | |
| Gender | |||
| Female | 44,895 (72.3) | 14,946 (72.2) | 0.798 |
| Male | 17,226 (27.7) | 5761 (27.8) | |
| Comorbidities | |||
| Diabetes | 3789 (6.1) | 1266 (6.1) | 0.940 |
| Hypertension | 9604 (15.5) | 3164 (15.3) | 0.534 |
| Cerebral vascular disease | 1212 (2.0) | 410 (2.0) | 0.794 |
| Depression | 168 (0.3) | 59 (0.3) | 0.730 |
| Hyperlipidemia | 3059 (4.9) | 1009 (4.9) | 0.766 |
| Cardiovascular disease | 598 (1.0) | 205 (1.0) | 0.728 |
p values compare the non-RA and RA groups using Student’s t test or a chi-square test. Data are shown as the mean ± standard deviation for quantitative variables and n (%) for qualitative variables
RA = rheumatoid arthritis
Table 2.
Risk of all-cause of dementia between the non-RA and RA cohort (n = 82,828)
| Cohort group | Case events | cHR (95% CI) | p value | aHR (95% CI) | p value |
|---|---|---|---|---|---|
| Non-RA cohort (n = 62,121) | 1258 (2.0) | Reference | Reference | ||
| RA cohort | 245 (1.2) | 0.62 (0.54–0.71) | < 0.001 | 0.63 (0.55–0.72) | < 0.001 |
| Non-DMARD user (n = 6118) | 139 (2.3) | 1.33 (1.22–1.59) | 0.001 | 0.82 (0.69–0.98) | 0.031 |
| DMARD user (n = 14,589) | 106 (0.7) | 0.36 (0.30–0.44) | < 0.001 | 0.48 (0.39–0.58) | < 0.001 |
Data are shown as n (%) for qualitative variables. The results were adjusted for age, gender, and all comorbidities
RA = rheumatoid arthritis; DMARD = disease-modifying anti-rheumatic drug; cHR = crude hazard ratio; aHR = adjusted hazard ratio; CI = confidence interval
Fig. 1.
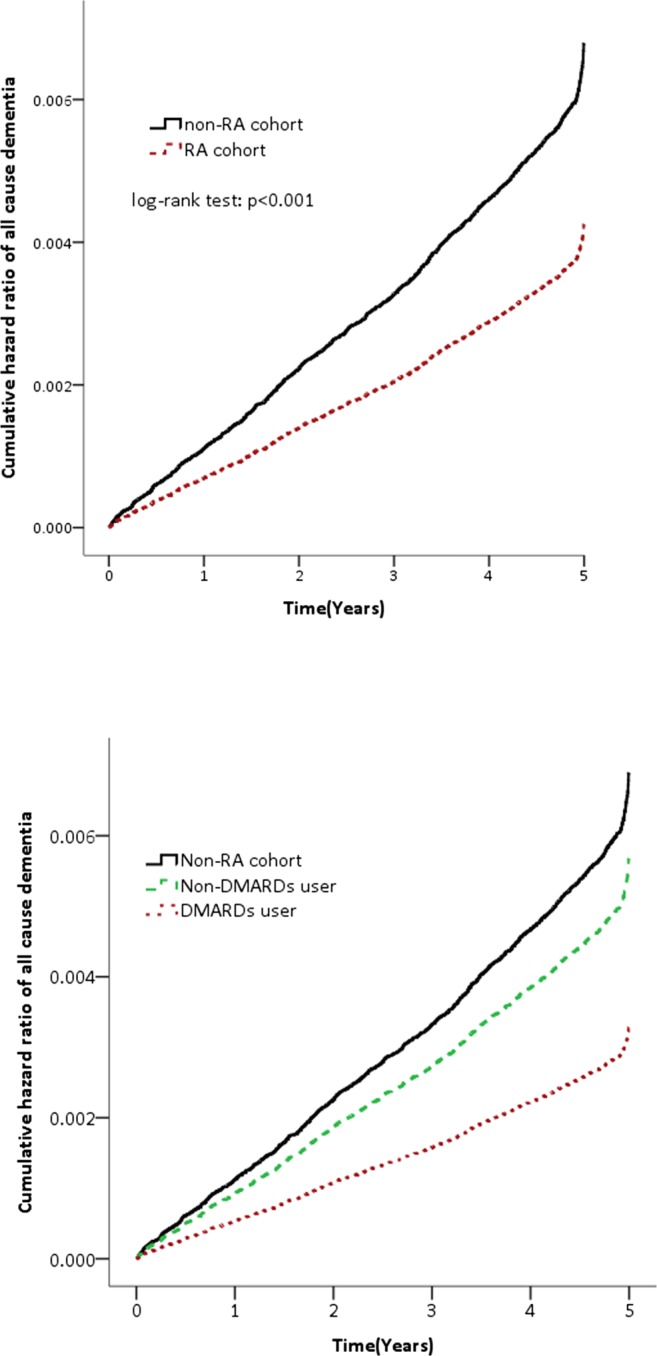
All-cause of dementia between the non-RA and RA cohorts and by use of disease-modifying anti-rheumatic drugs. RA =rheumatoid arthritis.
DMARDs were further evaluated as a dose-dependent factor for dementia by dividing the patients into 3 subgroups based on their cumulative DDD of DMARDs (low dose, < 616 DDD; moderate dose, 616–1744 DDD; high dose, > 1744 DDDs). The incidence of dementia was 1.1%, 0.9%, and 0.2% in the low-, moderate-, and high-dose groups, respectively. After adjusting for potential confounders, their HRs for developing dementia were 0.68 (95% CI 0.49–0.94; p = 0.021), 0.63 (95% CI 0.44–0.90; p = 0.012), and 0.20 (95% CI 0.11–0.38; p < 0.001) (Table 3). These results indicate that DMARDs significantly and dose dependently decrease the risk of dementia.
Table 3.
Risk of all-cause of dementia associated with the dose–response of DMARD usage (n = 82,828)
| Cohort | Case events | Model I | Model II | ||
|---|---|---|---|---|---|
| aHR (95% CI) | p value | aHR (95% CI) | p value | ||
| Non-RA cohort | 1258 (2.0) | Reference | |||
| RA without DMARD use (n = 6118) | 139 (2.3) | 0.82 (0.69–0.98) | 0.032 | Reference | |
| DMARD cumulative DDDs (n = 14,589) | |||||
| < 616 DDDs | 52 (1.1) | 0.59 (0.45–0.78) | 0.032 | 0.68 (0.49–0.94) | 0.021 |
| 616–1744 DDDs | 43 (0.9) | 0.57 (0.42–0.77) | < 0.001 | 0.63 (0.44–0.90) | 0.012 |
| > 1744 DDDs | 11 (0.2) | 0.18 (0.10–0.33) | < 0.001 | 0.20 (0.11–0.38) | < 0.001 |
Data are shown as n (%) for qualitative variables. The results were adjusted for age, gender, and all comorbidities
RA = rheumatoid arthritis; DMARD = disease-modifying anti-rheumatic drug; DDD = defined daily dose; aHR = adjusted hazard ratio; CI = confidence interval
The results were then stratified by gender and age. The incidence of dementia decreased in the female DMARD patients (adjusted HR 0.54, 95% CI 0.43–0.69; p < 0.001) but not in the female non-DMARD patients (adjusted HR 0.82, 95% CI 0.65–1.03; p = 0.081). In patients < 65 years of age, the incidence of dementia decreased in DMARD users (adjusted HR 0.53, 95% CI 0.35–0.80; p = 0.002) but not in non-DMARD users (adjusted HR 1.27, 95% CI 0.87–1.85; p = 0.222). These results suggest that compared with other medications used in RA patients, DMARD use is protective against the development of dementia in female and younger patients. However, in male or older patients, DMARDs use was associated with further lowering the risk of developing dementia compared with non-users (Table 4).
Table 4.
The risk of all-cause of dementia between the non-RA and RA cohorts stratified by age and gender
| Characteristic | Case events | cHR (95% CI) | p value | aHR (95% CI) | p value |
|---|---|---|---|---|---|
| Gender | |||||
| Female | |||||
| Non-RA cohort | 771 (1.7) | Reference | Reference | ||
| Non-DMARD user | 82 (2.2) | 1.51 (1.21–1.90) | < 0.001 | 0.82 (0.65–1.03) | 0.081 |
| DMARD user | 74 (0.7) | 0.38 (0.30–0.49) | < 0.001 | 0.54 (0.43–0.69) | < 0.001 |
| Male | |||||
| Non-RA cohort | 487 (2.8) | Reference | Reference | ||
| Non-DMARD user | 57 (2.3) | 1.00 (0.76–1.32) | 0.999 | 0.71 (0.54–0.95) | 0.016 |
| DMARD user | 32 (1.0) | 0.35 (0.24–0.49) | < 0.001 | 0.47 (0.33–0.68) | < 0.001 |
| Age (years) | |||||
| < 65 | |||||
| Non-RA cohort | 232 (0.5) | Reference | Reference | ||
| Non-DMARD user | 31 (0.8) | 1.90 (1.31–2.77) | 0.001 | 1.27 (0.87–1.85) | 0.222 |
| DMARD user | 26 (0.2) | 0.46 (0.31–0.69) | < 0.001 | 0.53 (0.35–0.80) | 0.002 |
| ≥ 65 | |||||
| Non-RA cohort | 1026 (7.2) | Reference | Reference | ||
| Non-DMARD user | 108 (4.8) | 0.82 (0.67–1.00) | 0.052 | 0.69 (0.57–0.85) | < 0.001 |
| DMARD user | 80 (3.0) | 0.41 (0.33–0.52) | < 0.001 | 0.51 (0.41–0.64) | < 0.001 |
Data are shown as n (%) for qualitative variables. The results were adjusted for age, gender, and all comorbidities
RA = rheumatoid arthritis; DMARD = disease-modifying anti-rheumatic drug; cHR = crude hazard ratio; aHR = adjusted hazard ratio; CI = confidence interval
Discussion
The present study found that individuals with RA were less likely to develop all-cause dementia, and that DMARD use is a protective factor with a dose–response relationship among patients without a prior history of dementia. In addition, the effects were different according to age group and gender. In young females, a reduced risk of developing dementia was observed if they used DAMRDs. However, the risk of developing dementia was further reduced in male DAMRD users compared with non-users. To the best of our knowledge, this is the first population-based, propensity-matched cohort study to explore the link between DMARD use and the risk of dementia in different age groups and genders.
RA and dementia may share similar inflammatory mechanisms, and the risk of dementia is thought to increase in patients with RA. However, in the present study, RA was negatively associated with dementia, which is consistent with the results of previous studies [8, 12, 23]. While it has been commonly assumed that NSAID use prohibits the onset of dementia, NSAIDs were not proven to be protective in an AD clinical trial [24]. The intrinsic factors within RA pathogenesis may underlie the protective effect of RA. Granulocyte–macrophage colony-stimulating factors, which are upregulated in RA, reverse cognitive impairment and amyloidosis in Alzheimer’s disease mice [25]. This effect may also be related to DMARD use in RA.
In the current study, DMARDs were observed to reduce the risk of developing dementia. These results add to the evidence that inflammation may exacerbate cognitive impairment, and that anti-inflammatory agents may be disease modifying. The result of the present study is consistent with previous studies [19]. In addition, the effect was dose dependent, which may be explained by higher cumulative dosages suppressing inflammation more intensely. However, these results do contradict the results of a previous study. A higher risk of dementia in patients with RA using DMARDs was reported in a case–control study by Chou et al. [20]. This controversial finding may have been due to the category of DMARDs observed. According to Chou et al., of the conventional synthetic DMARDs, methotrexate, but not azathioprine and cyclosporine, is associated with dementia development, while biological DMARDs were shown to non-significantly reduce the risk of dementia. The DMARDs selected in the present study included conventional synthetic DMARDs and biological DMARDs. Also, our patients are younger than Chou et al.’s (the mean age, 53 years vs 74 years). The central nervous system toxicity of methotrexate is more common in older patients [26], which might explain the discrepancy. The results of the present study also showed a sex and age difference. DMARD use reduces the dementia risk in young and female RA patients compared with non-DMARD users. This result may be related to gender differences observed in autoimmune diseases, such as elevated immune responses and the higher incidence of autoimmune diseases in females compared with males [27, 28]. Most autoimmune diseases affect younger and middle-aged people, as opposed to the elderly [29]. Therefore, the effect of DMARDs is more significant in these patients.
It should be noted that the study had several limitations. Firstly, the database did not contain lifestyle information, education level, inflammatory biomarkers, family history, and genetic factors, which might confound the association. Secondly, medication adherence was not available. Therefore, it was not possible to ensure DMARD adherence in the patients. Another limitation was that it was not possible to differentiate the etiologies of dementia into AD, vascular dementia, or mixed type. However, the strength of the study is that the NHI covers almost all of Taiwan’s population and provides a detailed medication and disease history of patients throughout the follow-up period.
In conclusion, the present case–control cohort study found that DMARDs, which reduce systemic inflammation, may also reduce the risk of dementia, a disease for which no treatment currently exists. This finding required verification in large observation studies and may provide an important basis for pharmacological treatment of dementia in a future randomized control trial.
Acknowledgments
This study was supported by grants from Kaohsiung Municipal Ta-Tung Hospital (kmtth-105-021) and Kaohsiung Medical University Hospital (KMUH102-2T-05).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brookmeyer R, Johnson E, Ziegler-Graham K, et al. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 2.Baskys A, Hou AC. Vascular dementia: pharmacological treatment approaches and perspectives. Clin Interv Aging. 2007;2:327–335. [PMC free article] [PubMed] [Google Scholar]
- 3.Galimberti D, Scarpini E. Disease-modifying treatments for Alzheimer’s disease. Ther Adv Neurol Disord. 2011;4:203–216. doi: 10.1177/1756285611404470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Latta CH, Brothers HM, Wilcock DM. Neuroinflammation in Alzheimer’s disease; A source of heterogeneity and target for personalized therapy. Neuroscience. 2015;302:103–111. doi: 10.1016/j.neuroscience.2014.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 7.Thiel A, Cechetto DF, Heiss WD, et al. Amyloid burden, neuroinflammation, and links to cognitive decline after ischemic stroke. Stroke. 2014;45:2825–2829. doi: 10.1161/STROKEAHA.114.004285. [DOI] [PubMed] [Google Scholar]
- 8.McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: a review of 17 epidemiologic studies. Neurology. 1996;47:425–432. doi: 10.1212/WNL.47.2.425. [DOI] [PubMed] [Google Scholar]
- 9.Chang KH, Hsu YC, Hsu CC, et al. Prolong Exposure of NSAID in Patients With RA Will Decrease the Risk of Dementia: A Nationwide Population-Based Cohort Study. Medicine (Baltimore) 2016;95:e3056. doi: 10.1097/MD.0000000000003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alisky JM. Intrathecal corticosteroids might slow Alzheimer’s disease progression. Neuropsychiatr Dis Treat. 2008;4:831–833. doi: 10.2147/NDT.S3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 12.Kao LT, Kang JH, Lin HC, et al. Rheumatoid Arthritis Was Negatively Associated with Alzheimer’s Disease: A Population-Based Case-Control Study. PLoS One. 2016;11:e0168106. doi: 10.1371/journal.pone.0168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallin K, Solomon A, Kareholt I, et al. Midlife rheumatoid arthritis increases the risk of cognitive impairment two decades later: a population-based study. J Alzheimers Dis. 2012;31:669–676. doi: 10.3233/JAD-2012-111736. [DOI] [PubMed] [Google Scholar]
- 14.Wotton CJ, Goldacre MJ. Associations between specific autoimmune diseases and subsequent dementia: retrospective record-linkage cohort study, UK. J Epidemiol Community Health. 2017;71:576–583. doi: 10.1136/jech-2016-207809. [DOI] [PubMed] [Google Scholar]
- 15.Ungprasert P, Wijarnpreecha K, Thongprayoon C. Rheumatoid arthritis and the risk of dementia: A systematic review and meta-analysis. Neurol India. 2016;64:56–61. doi: 10.4103/0028-3886.173623. [DOI] [PubMed] [Google Scholar]
- 16.Negrei C, Bojinca V, Balanescu A, et al. Management of rheumatoid arthritis: Impact and risks of various therapeutic approaches. Exp Ther Med. 2016;11:1177–1183. doi: 10.3892/etm.2016.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yazici Y. Treatment of rheumatoid arthritis: we are getting there. Lancet. 2009;374:178–180. doi: 10.1016/S0140-6736(09)60792-3. [DOI] [PubMed] [Google Scholar]
- 18.Westlake SL, Colebatch AN, Baird J, et al. The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology (Oxford) 2010;49:295–307. doi: 10.1093/rheumatology/kep366. [DOI] [PubMed] [Google Scholar]
- 19.Judge A, Garriga C, Arden NK, et al. Protective effect of antirheumatic drugs on dementia in rheumatoid arthritis patients. Alzheimers Dement (N Y) 2017;3:612–621. doi: 10.1016/j.trci.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou MH, Wang JY, Lin CL, et al. DMARD use is associated with a higher risk of dementia in patients with rheumatoid arthritis: A propensity score-matched case-control study. Toxicol Appl Pharmacol. 2017;334:217–222. doi: 10.1016/j.taap.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 21.Silman AJ. The 1987 revised American Rheumatism Association criteria for rheumatoid arthritis. Br J Rheumatol. 1988;27:341–343. doi: 10.1093/rheumatology/27.5.341. [DOI] [PubMed] [Google Scholar]
- 22.Sinnott SJ, Polinski JM, Byrne S, et al. Measuring drug exposure: concordance between defined daily dose and days’ supply depended on drug class. J Clin Epidemiol. 2016;69:107–113. doi: 10.1016/j.jclinepi.2015.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenkinson ML, Bliss MR, Brain AT, et al. Rheumatoid arthritis and senile dementia of the Alzheimer’s type. Br J Rheumatol. 1989;28:86–88. doi: 10.1093/rheumatology/28.1.86-b. [DOI] [PubMed] [Google Scholar]
- 24.Group AR. Martin BK, Szekely C, et al. Cognitive function over time in the Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch Neurol. 2008;65:896–905. doi: 10.1001/archneur.2008.65.7.nct70006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyd TD, Bennett SP, Mori T, et al. GM-CSF upregulated in rheumatoid arthritis reverses cognitive impairment and amyloidosis in Alzheimer mice. J Alzheimers Dis. 2010;21:507–518. doi: 10.3233/JAD-2010-091471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wernick R, Smith DL. Central nervous system toxicity associated with weekly low-dose methotrexate treatment. Arthritis Rheum. 1989;32:770–775. doi: 10.1002/anr.1780320616. [DOI] [PubMed] [Google Scholar]
- 27.Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol. 2014;35:347–369. doi: 10.1016/j.yfrne.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Schuurs AH, Verheul HA. Effects of gender and sex steroids on the immune response. J Steroid Biochem. 1990;35:157–172. doi: 10.1016/0022-4731(90)90270-3. [DOI] [PubMed] [Google Scholar]
- 29.Walsh SJ, Rau LM. Autoimmune diseases: a leading cause of death among young and middle-aged women in the United States. Am J Public Health. 2000;90:1463–1466. doi: 10.2105/AJPH.90.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]


