This cohort study examines whether daytime and nighttime blood pressure levels measured outside the clinic vs inside the clinic are associated with cardiovascular disease and all-cause mortality.
Key Points
Question
Among African American individuals, are daytime and nighttime blood pressure levels measured outside the clinic associated with cardiovascular disease independent of blood pressure levels measured inside the clinic?
Findings
In this cohort study of 1034 African American individuals, higher daytime and nighttime systolic blood pressures were associated with an increased risk for cardiovascular disease events independent of blood pressure levels measured in the clinic.
Meaning
Measurement of daytime and nighttime blood pressure using ambulatory monitoring during a 24-hour period may help identify African American individuals who have an increased cardiovascular disease risk.
Abstract
Importance
Little is known regarding health outcomes associated with higher blood pressure (BP) levels measured outside the clinic among African American individuals.
Objective
To examine whether daytime and nighttime BP levels measured outside the clinic among African American individuals are associated with cardiovascular disease (CVD) and all-cause mortality independent of BP levels measured inside the clinic.
Design, Setting, and Participants
This prospective cohort study analyzed data from 1034 African American participants in the Jackson Heart Study who completed ambulatory BP monitoring at baseline (September 26, 2000, to March 31, 2004). Mean daytime and nighttime BPs were calculated based on measurements taken while participants were awake and asleep, respectively. Data were analyzed from July 1, 2017, to April 30, 2019.
Main Outcomes and Measures
Cardiovascular disease events, including coronary heart disease and stroke, experienced through December 31, 2014, and all-cause mortality experienced through December 31, 2016, were adjudicated. The associations of daytime BP and nighttime BP, separately, with CVD events and all-cause mortality were determined using Cox proportional hazards regression models.
Results
A total of 1034 participants (mean [SD] age, 58.9 [10.9] years; 337 [32.6%] male; and 583 [56.4%] taking antihypertensive medication) were included in the study. The mean daytime systolic BP (SBP)/diastolic BP (DBP) was 129.4/77.6 mm Hg, and the mean nighttime SBP/DBP was 121.3/68.4 mm Hg. During follow-up (median [interquartile range], 12.5 [11.1-13.6] years for CVD and 14.8 [13.7-15.6] years for all-cause mortality), 113 CVD events and 194 deaths occurred. After multivariable adjustment, including in-clinic SBP and DBP, the hazard ratios (HRs) for CVD events for each SD higher level were 1.53 (95% CI, 1.24-1.88) for daytime SBP (per 13.5 mm Hg), 1.48 (95% CI, 1.22-1.80) for nighttime SBP (per 15.5 mm Hg), 1.25 (95% CI, 1.02-1.51) for daytime DBP (per 9.3 mm Hg), and 1.30 (95% CI, 1.06-1.59) for nighttime DBP (per 9.5 mm Hg). Nighttime SBP was associated with all-cause mortality (HR per 1-SD higher level, 1.24; 95% CI, 1.06-1.45), but no association was present for daytime SBP (HR, 1.13; 95% CI, 0.97-1.33) and daytime (HR, 0.95; 95% CI, 0.81-1.10) and nighttime (HR, 1.06; 95% CI, 0.90-1.24) DBP.
Conclusions and Relevance
Among African American individuals, higher daytime and nighttime SBPs were associated with an increased risk for CVD events and all-cause mortality independent of BP levels measured in the clinic. Measurement of daytime and nighttime BP using ambulatory monitoring during a 24-hour period may help identify African American individuals who have an increased cardiovascular disease risk.
Introduction
Blood pressure (BP) has been primarily measured in the clinic setting for the diagnosis and treatment of hypertension.1,2 However, measurements taken in the clinic may not accurately reflect BP levels that a person experiences at home, at work, and while asleep.3,4,5 Ambulatory BP monitoring (ABPM) measures BP outside the clinic setting, usually during a 24-hour period, including during sleep, and is the reference standard method for assessing out-of-clinic BP.5 Community-based studies in Europe and Asia suggest that higher mean BP measured outside the clinic setting with the use of ABPM is associated with an increased risk for cardiovascular disease (CVD) events independent of BP measured in the clinic setting.6,7,8,9,10
African American individuals are disproportionally affected by hypertension-related CVD compared with other racial/ethnic groups in the United States11 and have higher daytime and nighttime BP levels than white and Asian individuals.12,13,14 A previous study found15 that the prevalence of high nighttime BP (mean nighttime systolic BP [SBP]/diastolic BP [DBP] ≥120/70 mm Hg using ABPM) was higher than the prevalence of high daytime BP (mean daytime SBP/DBP≥135/85 mm Hg on ABPM) among African American individuals. Furthermore, in prior studies,7,15 nondipping status (nighttime to daytime SBP ratio >0.90) has been present in more than 50% of African American individuals and 30% of white and Asian individuals. At present, little is known about whether, among African American individuals, (1) higher daytime and nighttime BP levels are associated with an increased risk of CVD events, (2) nondipping BP is associated with CVD events, and (3) these associations are independent of clinic BP levels.
Using data from the Jackson Heart Study (JHS), a community-based cohort of African American adults,16,17 we examined the associations of daytime BP levels, nighttime BP levels, and nondipping vs dipping BP with CVD events and whether the associations are independent of clinic-measured BP. We also examined whether discrimination of participants with and without CVD events was improved beyond clinic-measured BP by assessing daytime and nighttime BP levels. All-cause mortality was examined as a secondary outcome.
Methods
Study Population
The JHS is a community-based, prospective cohort study designed to identify CVD risk factors among African American individuals.16,17 From September 26, 2000, to March 31, 2004, a total of 5306 noninstitutionalized African American adults 21 years or older from the Jackson, Mississippi, metropolitan area enrolled in the JHS. The current analysis was restricted to 1146 JHS participants who completed ABPM as part of their baseline study visit (visit 1 in 2000-2004). Participants who did not meet the International Database18 on ABPM in Relation to Cardiovascular Outcomes (IDACO) criteria for a complete ABPM recording (n = 83) or who were missing information on clinic BP (n = 5) were excluded from the analyses. All participants provided written informed consent, and all data were deidentified. We also excluded 24 participants who did not consent to be followed up for outcome events. After these exclusions were applied, the analyses included 1034 participants. The institutional review boards of the University of Mississippi Medical Center, Jackson State University, and Tougaloo College approved the JHS protocol. The statistical analysis of JHS data was approved by the University of Alabama at Birmingham Institutional Review Board.
Data Collection and BP Measurement
A detailed description of data and specimen collection and specimen processing at baseline is included in the eMethods in the Supplement.16,17 At the baseline examination, trained research staff measured brachial artery BP 2 times in the right arm separated by 1 minute after the participant had been sitting in a quiet room for 5 minutes, using a Hawksley random-zero sphygmomanometer (Hawksley and Sons Ltd). The random-zero BP measurements were calibrated to an oscillometric device using robust regression as previously described.19 The mean of the 2 measurements was used to define clinic BP (eMethods in the Supplement).
After the visit 1 examination, participants underwent ABPM for 24 hours using the SpaceLabs model 90207 device (SpaceLabs Healthcare) fitted on their nondominant arm.20,21 Blood pressure readings were obtained every 20 minutes during a 24-hour period. We defined daytime and nighttime periods during ABPM based on self-reported awake and asleep times from a sleep diary. The BP readings during the awake period were used to calculate mean daytime BP, and the readings during the asleep period were used to calculate mean nighttime BP. Participants were considered to have a complete ABPM recording if they met the IDACO criteria, defined as having 10 or more daytime and 5 or more nighttime valid SBP and DBP measurements.18 This approach has been used in previous outcome studies using ABPM.7,8 A previous study22 found that the prevalence of daytime and nighttime hypertension defined using the IDACO criteria was similar to that defined using the 2013 European Society of Hypertension criteria. In the current study, daytime BPs were defined with measurements obtained from the mean (SD) of 33.2 (14.4) readings, and nighttime BPs were defined with measurements obtained from the 20.3 (8.9) readings. Additional details of the ABPM BP measurement procedure are described in the eMethods in the Supplement.
High daytime BP was defined as a mean daytime SBP of 135 mm Hg or higher or a DBP of 85 mm Hg or higher.1,23 High nighttime BP was defined as a mean nighttime SBP of 120 mm Hg or higher or DBP of 70 mm Hg or higher.1,23
With use of mean daytime and nighttime SBP, the percentage nighttime dipping in SBP was calculated as the nighttime to daytime ratio. In primary analyses, nighttime SBP dipping was categorized into 2 groups: nondipping (nighttime to daytime ratio >0.90) and dipping (nighttime to daytime ratio ≤0.90). In secondary analyses, nighttime SBP dipping was categorized into 3 groups: dipping (nighttime to daytime ratio ≤0.90), nondipping BP (nighttime to daytime ratio >0.90 to ≤1.00), or reverse-dipping BP (nighttime to daytime ratio >1.00).3,4
CVD Events and All-Cause Mortality
Adjudication procedures for CVD and all-cause mortality during follow-up have been described previously17 and in the eMethods in the Supplement. The CVD events included the occurrence of myocardial infarction, acute coronary heart disease death, or stroke. Adjudicated CVD events were available through December 31, 2014, and all-cause mortality was available through December 31, 2016.
Statistical Analysis
Data were analyzed from July 1, 2017, to April 30, 2019. Summary statistics for characteristics of JHS participants who were included and not included in the current analysis were calculated. The statistical significance of differences between these 2 groups was determined using 2-sample t tests for continuous variables and χ2 tests for categorical variables. Using Cox proportional hazards regression models, we calculated the hazard ratios (HRs) and 95% CIs for CVD events and all-cause mortality, separately, associated with daytime and nighttime SBP, modeled as continuous variables. The analyses were repeated for daytime and nighttime DBP. Next, we calculated the HRs and 95% CIs for CVD events and all-cause mortality, separately, associated with daytime and nighttime SBP, modeled as tertiles. The analyses were repeated for daytime and nighttime DBP. The HRs and 95% CIs were also calculated for CVD events and all-cause mortality, separately, associated with high daytime BP and high nighttime BP. In secondary analyses, these latter analyses were performed again, defining high daytime BP and high nighttime BP using the 2017 American College of Cardiology (ACC)/American Heart Association (AHA) BP guideline thresholds: high daytime BP (mean daytime SBP ≥130 mm Hg or DBP ≥80 mm Hg) and high nighttime BP (mean nighttime SBP ≥110 mm Hg or DBP ≥65 mm Hg).24 Nighttime SBP dipping was modeled first as 2 categories and then as 3 categories. The proportionality assumption for the Cox proportional hazards regression analyses was confirmed graphically and with the inclusion of a time × BP component interaction terms in the regression models.
The HRs were calculated in an unadjusted model (model 1), after adjustment for age and sex (model 2), and after adjustment for educational level, body mass index (calculated as weight in kilograms divided by height in meters squared), current smoking, diabetes, myocardial infarction, stroke, total cholesterol level, high-density lipoprotein cholesterol level, C-reactive protein level, estimated glomerular filtration rate25 less than 60 mL/min/1.73 m2, urinary albumin to creatinine ratio of 30 mg/g or higher, and statin and antihypertensive medication use (model 3). We imputed missing data for covariates (eTable 1 in the Supplement) using chained equations and 10 data sets.26 For model 3, we adjusted for a composite risk score rather than individual covariates because of concerns of overfitting the model because of a limited number of outcomes among JHS participants who underwent ABPM.27 For CVD events, the risk score was created in the overall JHS population (5306 participants and 534 CVD events) by determining the 10-year probability of a CVD event for each participant using a Cox proportional hazards regression model with CVD as the outcome and the covariates used in model 3 as independent variables. For all-cause mortality, the risk score was created in the overall JHS population (5306 participants and 992 deaths) using a Cox proportional hazards regression model with all-cause mortality as the outcome and covariates used in model 3 as independent variables. To assess whether daytime or nighttime BP is associated with outcomes independent of clinic BP, we included the variables in model 3 and clinic BP as covariates into a fourth model (model 4). Clinic SBP and daytime or nighttime SBP were modestly correlated (Pearson r = 0.5), and the variable inflation factor for clinic SBP and daytime or nighttime SBP, when both were included in the same model, was 1.91. Conversely, daytime SBP and nighttime SBP were highly correlated (Pearson r = 0.80), and the variable inflation factor for daytime SBP and nighttime SBP, when both were included in the same model, was greater than 3.0.28 In the primary analysis, we did not include daytime and nighttime BP in the same multivariable model because of the possible high degree of collinearity. We included daytime BP and nighttime BP in the same multivariable model in a secondary analysis.
We tested for heterogeneity in the association between each SBP measure and outcomes by antihypertensive medication use with the inclusion of multiplicative interaction terms. Stratified analyses were performed when a statistically significant interaction was present (P < .05).
We computed the change in the Harrell concordance (C) statistic by comparing models that included the composite risk score, clinic SBP, clinic DBP, and daytime or nighttime SBP vs models that consisted of the composite risk score, clinic SBP, and clinic DBP. We applied a 1000-bootstrap resampling to calculate the 95% CIs for the differences in C statistics and applied multiple imputation in each bootstrap replicate to account for uncertainty in missing values.
We conducted a sensitivity analysis, excluding participants with a history of myocardial infarction and stroke at baseline. Statistical analyses were performed using SAS statistical software, version 9.3 (SAS Institute Inc), Stata, version 13.1 (StataCorp), and R, version 3.4.2 (R Foundation for Statistical Computing). Statistical significance was defined by a 2-sided P < .05.
Results
A total of 1034 participants (mean [SD] age, 58.9 [10.9] years; 337 [32.6%] male; and 583 [56.4%] taking antihypertensive medication) were included in the study. Compared with the 4272 JHS participants not included in the analysis, the sample of 1034 included participants were older (mean [SD] age, 58.9 [10.9] vs 53.9 [13.1] years), had higher mean (SD) high-density lipoprotein cholesterol levels (54.0 [15.0] vs 51.2 [14.5] mg/dL [to convert to millimoles per liter, multiply by 0.0259]), and had lower mean (SD) body mass indexes (31.2 [6.4] vs 31.9 [7.4]) (eTable 2 in the Supplement). Included participants were also less likely to be men (337 of 1034 [32.6%] vs 1602 of 4272 [37.5%]). Among participants included vs not included in the analysis, the prevalence of diabetes (276 of 1034 [26.7%] vs 983 of 4272 [23.0%]) and antihypertensive medication use (583 of 1034 [56.4%] vs 2051 of 4272 [48.0%]) was higher and the prevalence of current smoking was lower (102 of 1034 [9.9%] vs 598 of 4272 [14.0%]). Among participants included in the current analysis, the mean (SD) of each BP measure is given in Table 1. The prevalence of high daytime BP was 37.9%, high nighttime BP was 57.5%, and nondipping SBP was 72.6%.
Table 1. Characteristics of the 1034 Jackson Heart Study Participants Included in the Current Analysisa .
| Characteristic | Finding |
|---|---|
| Age, mean (SD), y | 58.9 (10.9) |
| Male sex | 337 (32.6) |
| BMI, mean (SD) | 31.2 (6.4) |
| Diabetes | 276 (26.7) |
| Less than high school education | 190 (18.4) |
| Current smoking | 102 (9.9) |
| History | |
| Stroke | 40 (3.9) |
| MI | 48 (4.6) |
| Total cholesterol level, mean (SD), mg/dL | 201.5 (39.6) |
| HDL-C level, mean (SD), mg/dL | 54.0 (15.0) |
| CRP level >3 mg/L | 490 (47.4) |
| eGFR <60 mL/min/1.73 m2 | 107 (10.3) |
| Albumin to creatinine ratio ≥30 mg/g | 108 (10.4) |
| Statin use | 162 (15.7) |
| Antihypertensive medication use | 583 (56.4) |
| Clinic blood pressure, mean (SD), mm Hg | |
| Systolic | 127.7 (15.8) |
| Diastolic | 74.3 (8.4) |
| Daytime BP, mean (SD), mm Hg | |
| SBP | 129.4 (13.5) |
| DBP | 77.6 (9.3) |
| High daytime BP | 392 (37.9) |
| Nighttime BP, mean (SD), mm Hg | |
| SBP | 121.3 (15.5) |
| DBP | 68.4 (9.5) |
| High nighttime BP | 595 (57.5) |
| Nocturnal SBP dipping, mean (SD), % | 6.3 (6.9) |
| Nondipping SBPb | 751 (72.6) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure; CRP, C-reactive protein; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; MI, myocardial infarction; SBP, systolic blood pressure.
SI conversion factors: To convert cholesterol and HDL-C levels to millimoles per liter, multiply by 0.2359.
Data are presented as number (percentage) unless otherwise indicated. High daytime BP was defined as daytime SBP of 135 mm Hg or higher or DBP of 85 mm Hg or higher. High nighttime BP was defined as nighttime SBP greater than 120 mm Hg or DBP greater than 70 mm Hg.
Defined as nighttime to daytime SBP ratio greater than 0.90.
Associations of Daytime and Nighttime BP Levels With CVD Events and All-Cause Mortality
During follow-up (median [interquartile range], 12.5 [11.1-13.6] years for CVD and 14.8 [13.7-15.6] years for all-cause mortality), 113 CVD events and 194 deaths occurred. Daytime and nighttime SBPs were each associated with increased risk for CVD and all-cause mortality in unadjusted analyses (Table 2). After multivariable adjustment, including for clinic SBP and DBP, the HRs for CVD events for each SD higher BP measure were 1.53 (95% CI, 1.24-1.88) for daytime SBP (per 13.5 mm Hg) and 1.48 (95% CI, 1.22-1.80) for nighttime SBP (per 15.5 mm Hg), and the HRs for all-cause mortality for each SD higher BP measure were 1.13 (95% CI, 0.97-1.33) for daytime SBP and 1.24 (95% CI, 1.06-1.45) for nighttime SBP. When daytime and nighttime SBPs were entered in the same model, the associations between daytime SBP and CVD events and between nighttime SBP and CVD events were attenuated (eTable 3 in the Supplement). The HR for all-cause mortality for each SD higher nighttime SBP was 1.24 (95% CI, 1.06-1.45), but no other associations were statistically significant.
Table 2. HRs for Cardiovascular Disease Events and All-Cause Mortality for Daytime and Nighttime SBPa.
| Model | Higher SBP, HR (95% CI) per 1 SD | |
|---|---|---|
| Daytime | Nighttime | |
| Cardiovascular Disease Events (n = 113) | ||
| Model 1 | 1.76 (1.48-2.10) | 1.81 (1.54-2.13) |
| Model 2 | 1.57 (1.30-1.89) | 1.57 (1.32-1.86) |
| Model 3 | 1.47 (1.22-1.77) | 1.44 (1.20-1.72) |
| Model 4 | 1.53 (1.24-1.88) | 1.48 (1.22-1.80) |
| All-Cause Mortality (n = 194) | ||
| Model 1 | 1.47 (1.29-1.67) | 1.64 (1.45-1.86) |
| Model 2 | 1.25 (1.08-1.44) | 1.33 (1.16-1.53) |
| Model 3 | 1.21 (1.05-1.40) | 1.29 (1.12-1.49) |
| Model 4 | 1.13 (0.97-1.33) | 1.24 (1.06-1.45) |
Abbreviations: HR, hazard ratio; SBP, systolic blood pressure.
Adjusted HRs (95% CIs) associated with a 1-SD higher daytime SBP and nighttime SBP levels are shown. The 1-SD increments are 13.5 mm Hg for daytime SBP and 15.5 mm Hg for nighttime SBP. Model 1 is unadjusted. Model 2 includes adjustment for age and sex. Model 3 includes adjustment for a composite risk score that consists of age, sex, body mass index, diabetes, educational level, smoking status, history of stroke, history of myocardial infarction, total cholesterol level, high-density lipoprotein cholesterol level, C-reactive protein level, estimated glomerular filtration rate less than 60 mL/min/1.73 m2, and statin and antihypertensive medication use. Model 4 includes adjustment for the composite risk score, clinic SBP, and clinic diastolic blood pressure.
Daytime and nighttime DBPs were each associated with increased risk for CVD and all-cause mortality in unadjusted analyses (eTable 4 in the Supplement). After multivariable adjustment, including for clinic SBP and DBP, the HRs for CVD events for each SD higher BP measure were 1.25 (95% CI, 1.02-1.51) for daytime DBP (per 9.3 mm Hg) and 1.30 (95% CI, 1.06-1.59) for nighttime DBP (per 9.5 mm Hg), and the HRs for all-cause mortality for each SD higher BP measure were 0.95 (95% CI, 0.81-1.10) for daytime DBP and 1.06 (95% CI, 0.90-1.24) for nighttime DBP. When daytime and nighttime DBPs were entered into the same model, the associations between daytime DBP and CVD events and between nighttime DBP and CVD events were attenuated.
The risk of CVD events increased across higher tertiles of daytime and nighttime SBP before and after multivariable adjustment (eTable 5 in the Supplement). A higher risk of CVD events for the highest tertile vs the lowest tertile of daytime and nighttime DBP was present after multivariable adjustment (eTable 6 in the Supplement).
There was evidence of an association of nighttime SBP and antihypertensive medication use with CVD events (P = .03 for interaction) (eTable 7 in the Supplement). The association of daytime SBP and antihypertensive medication use at baseline with CVD events was not statistically significant (P = .10 for interaction). The HRs for CVD events for each SD higher nighttime SBP measure were 1.68 (95% CI, 1.13-2.50) for participants not taking medication and 1.36 (95% CI, 1.07-1.72) for those taking medication (eTable 8 in the Supplement).
There were statistically significant increases in C statistics for CVD events for models that included daytime or nighttime SBP in addition to the composite risk score, clinic SBP, and clinic DBP vs models that consisted of the composite risk score, clinic SBP, and clinic DBP alone (eTable 9 in the Supplement). Changes in C statistics for all-cause mortality were not statistically significant for models that included vs those that did not include daytime or nighttime SBP or DBP in addition to the composite risk score, clinic SBP, and clinic DBP.
Associations of High Daytime and Nighttime BP With CVD Events and All-Cause Mortality
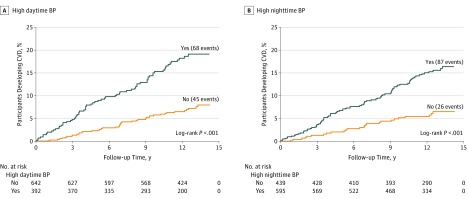
The cumulative incidence and incidence rate of CVD events and all-cause mortality were higher among participants with compared with those without high daytime BP and high nighttime BP (Figure and Table 3). High daytime BP and high nighttime BP were each associated with an increased risk of CVD events in unadjusted models and after multivariable adjustment. After multivariable adjustment, high daytime BP and high nighttime BP were not associated with an increased risk for all-cause mortality. When defined using the 2017 ACC/AHA BP guideline thresholds, the cumulative incidence and incidence rate of CVD events and all-cause mortality were higher among participants with high daytime BP and high nighttime BP compared with those without high daytime BP and high nighttime BP (eFigure in the Supplement). After multivariable adjustment, high daytime BP and high nighttime BP defined using the 2017 ACC/AHA BP guideline threshold were associated with CVD events, but no association was present for all-cause mortality (eTable 10 in the Supplement).
Figure. Cumulative Rate of Cardiovascular Disease (CVD) Events by High Nighttime and Daytime Blood Pressure (BP) Status .
The cumulative probability of CVD events for participants with and without high nighttime BP (A) and high daytime BP (B) were calculated using the Kaplan-Meier method. High daytime BP was defined as daytime systolic BP (SBP) of 135 mm Hg or higher or diastolic BP (DBP) of 85 mm Hg or higher. High nighttime BP was defined as nighttime SBP of 120 mm Hg or higher or DBP of 70 mm Hg or higher. Log-rank tests were used to calculate P values.
Table 3. HRs for Cardiovascular Disease Events and All-Cause Mortality for High Daytime and Nighttime BPa.
| Model | High BP, HR (95% CI) | |||
|---|---|---|---|---|
| Daytime | Nighttime | |||
| No (n = 642) | Yes (n = 392) | No (n = 439) | Yes (n = 595) | |
| Cardiovascular Disease Events | ||||
| No. of events | 45 | 68 | 26 | 87 |
| Rate (95% CI) | 5.9 (4.4-7.9) | 16.2 (12.8-20.6) | 5.0 (3.4-7.3) | 13.2 (10.7-16.3) |
| Model 1 | 1 [Reference] | 2.74 (1.88-4.00) | 1 [Reference] | 2.66 (1.72-4.13) |
| Model 2 | 1 [Reference] | 2.32 (1.58-3.39) | 1 [Reference] | 2.09 (1.34-3.26) |
| Model 3 | 1 [Reference] | 2.16 (1.47-3.18) | 1 [Reference] | 2.04 (1.30-3.19) |
| Model 4 | 1 [Reference] | 2.32 (1.53-3.51) | 1 [Reference] | 2.07 (1.29-3.32) |
| All-Cause Mortality | ||||
| No. of events | 95 | 99 | 59 | 135 |
| Rate (95% CI) | 10.5 (8.6-12.8) | 18.7 (15.4-22.8) | 9.5 (7.3-12.2) | 16.6 (14.0-19.7) |
| Model 1 | 1 [Reference] | 1.82 (1.37-2.41) | 1 [Reference] | 1.77 (1.30-2.40) |
| Model 2 | 1 [Reference] | 1.44 (1.09-1.91) | 1 [Reference] | 1.23 (0.91-1.68) |
| Model 3 | 1 [Reference] | 1.37 (1.02-1.82) | 1 [Reference] | 1.22 (0.88-1.67) |
| Model 4 | 1 [Reference] | 1.27 (0.93-1.72) | 1 [Reference] | 1.11 (0.80-1.54) |
Abbreviations: BP, blood pressure; HR, hazard ratio.
The rate is per 1000 person-years. Adjusted HRs (95% CIs) associated with high daytime BP and high nighttime BP are shown. High daytime BP was defined as daytime systolic BP of 135 mm Hg or higher or diastolic BP of 85 mm Hg or higher. High nighttime BP was defined as nighttime systolic BP of 120 mm Hg or higher or diastolic BP of 70 mm Hg or higher. Model 1 is unadjusted. Model 2 includes adjustment for age and sex. Model 3 includes adjustment for a composite risk score consisting of age, sex, body mass index, diabetes, educational level, smoking status, history of stroke, history of myocardial infarction, total cholesterol level, high-density lipoprotein cholesterol level, C-reactive protein level, estimated glomerular filtration rate less than 60 mL/min/1.73 m2, and statin and antihypertensive medication use. Model 4 includes adjustment for the composite risk score, clinic systolic BP, and clinic diastolic BP.
Associations of Nondipping With CVD Events and All-Cause Mortality
In unadjusted models and after multivariable adjustment, when nondipping SBP was grouped into 2 categories, there was no evidence of an association between nondipping SBP and CVD events (eTable 11 in the Supplement). Nondipping vs dipping SBP was associated with all-cause mortality in unadjusted models and after multivariable adjustment. In an unadjusted model and after multivariable adjustment, when nondipping SBP was grouped into 3 categories, reverse-dipping vs dipping SBP was not associated with CVD events and all-cause mortality (eTable 12 in the Supplement). Nondipping vs dipping SBP was associated with all-cause mortality in unadjusted models and after multivariable adjustment.
Sensitivity Analyses
After excluding 82 participants with a history of myocardial infarction and stroke at baseline, the HRs (95% CIs) for CVD events and all-cause mortality for daytime (2.26 [1.45-3.51] vs 12.9 [0.93-1.79] for CVD events vs all-cause mortality) and nighttime BP (1.76 [1.07-2.92] vs 0.97 [0.67-1.39] for CVD events vs all-cause mortality) were similar to those in the primary analyses (eTable 13 in the Supplement).
Discussion
In this community-based study of African American individuals, higher daytime and nighttime SBPs were each associated with an increased risk of CVD events and all-cause mortality. These associations were present in unadjusted models and after multivariable adjustment, including clinic BP and when modeling daytime and nighttime SBP as continuous and categorical variables. The increase in CVD risk associated with higher nighttime or daytime SBP was larger among participants not taking vs taking antihypertensive medication. Discrimination of participants with and without CVD events was improved beyond clinic-measured SBP and DBP levels by assessing daytime and nighttime SBP levels. Higher daytime and nighttime DBPs were each associated with an increased risk of CVD events in unadjusted models and after multivariable adjustment, including clinic BP and when modeling daytime and nighttime DBP as continuous and categorical variables. Nondipping BP and reverse-dipping BP were not associated with risk for CVD events after multivariable adjustment.
In the JHS, the correlation between daytime and nighttime SBP was high (Pearson r = 0.80). This finding suggests that people with high daytime SBP also have high nighttime SBP. The HRs for CVD events associated with daytime SBP and nighttime SBP were similar, with substantially overlapping 95% CIs. When daytime SBP and nighttime SBP were entered into the same model, the associations between daytime SBP and CVD events and between nighttime SBP and CVD events were attenuated, but nighttime SBP was statistically significantly associated with all-cause mortality. These findings suggest that among African American individuals, BP while awake and asleep may provide similar prognostic information about risk for CVD events but different information regarding all-cause mortality risk.
Few studies of BP on ABPM and outcomes have included participants from the United States or African American individuals,7,8,9,10 a racial group with a high prevalence of hypertension and hypertension-associated CVD risk.11 This issue is particularly relevant given that out-of-clinic BP measurement is recommended by the 2017 ACC/AHA BP guideline for the diagnosis and management of hypertension and that the data on ABPM used to support these recommendations are primarily based on white or Asian adults.24 In the current study, higher daytime and nighttime SBP levels were each associated with an increased risk of CVD events among participants taking and not taking antihypertensive medication. The HRs for the association of higher nighttime or daytime SBP with CVD events were higher among participants not taking vs taking antihypertensive medication. Antihypertensive medication use might attenuate the associations between nighttime or daytime BP and CVD events. Therefore, both daytime and nighttime BP levels may be important for assessing CVD risk among African Americans taking and not taking antihypertensive medication. However, conducting ABPM in the general population may be associated with practical challenges. In the United States, ABPM is not widely available and is primarily limited to specialty practices.29,30 This issue creates a barrier for conducting ABPM routinely to assess a person’s daytime and nighttime BP.
Thresholds used to define hypertensive daytime and nighttime BP are lower in the 2017 ACC/AHA BP guideline than in the 2005 AHA scientific statement on BP measurement (ie, daytime SBP/DBP≥130/80 mm Hg vs ≥135/85 mm Hg and nighttime SBP/DBP≥110/65 mm Hg vs ≥120/70 mm Hg).1,24 When defined by the 2017 ACC/AHA BP guidelines, high daytime BP and high nighttime BP were associated with increased risk for CVD events. Thresholds for defining high daytime and nighttime BP are based on observational studies, and randomized clinical trials are needed to determine the optimal thresholds used to define high daytime and nighttime BP among African American adults.31 Data are inconsistent regarding the effect of administration of antihypertensive drugs at bedtime rather than in the morning on daytime and nighttime BP on ABPM.32,33 Moreover, the data are sparse for the African American population.33,34 Further investigations are required to determine whether administration of antihypertensive drugs at bedtime vs in the morning—or a combination of both—is more effective for reducing CVD risk among African American individuals.
Strengths and Limitations
Strengths of this study include the well-characterized, community-based cohort of African American adults and the use of standardized data collection protocols in JHS. The JHS is among a few community-based studies that have performed ABPM among African American adults. In addition, the JHS actively followed up participants to identify CVD events and all-cause mortality, which were adjudicated using a standardized approach. There are several potential limitations to the current analysis. Only a subset of JHS participants underwent ABPM, and participants who underwent ABPM differed from those who did not undergo the procedure. Sleep apnea was not evaluated at baseline, and residual confounding may be present.35 We did not incorporate changes in clinic BP over time because most events occurred before visit 3. It is uncertain whether the increased CVD risk associated with high daytime or nighttime BP at baseline is explained by cumulative BP burden. Also, ABPM was performed only once in the JHS cohort; therefore, we were unable to assess whether changes in daytime and nighttime BP during follow-up were associated with outcomes.
Conclusions
Among African American individuals, higher daytime and nighttime SBPs were associated with an increased risk for CVD events and all-cause mortality independent of BP levels measured in the clinic. Measuring daytime and nighttime BP using ABPM during a 24-hour period may help identify African American individuals who have an increased CVD risk. These findings support the 2017 ACC/AHA BP guideline recommendation for use of ABPM for management of BP. Randomized clinical trials are needed to assess whether lowering daytime and nighttime BP with nonpharmacologic treatments and/or antihypertensive medications reduces the risk of CVD events beyond reductions in clinic BP.
eMethods. Supplementary methods
eTable 1. Number and percentage of participants included in the current analysis with missing data for variables (n = 1034)
eTable 2. Characteristics of JHS participants who were included in the current study and those who were not included
eTable 3. Hazard ratios for cardiovascular disease events and all-cause mortality for daytime SBP and nighttime SBP
eTable 4. Hazard ratios for cardiovascular disease events and all-cause mortality associated with daytime DBP and nighttime DBP
eTable 5. Hazard ratios for cardiovascular disease events by tertile of daytime SBP and nighttime SBP
eTable 6. Hazard ratios for cardiovascular disease events by tertile of daytime DBP and nighttime DBP
eTable 7. Heterogeneity in the association between each BP measure and outcome by antihypertensive medication use with the inclusion of multiplicative interaction terms
eTable 8. Hazard ratios for cardiovascular disease events for daytime SBP and nighttime SBP among participants taking and not taking antihypertensive medication
eTable 9. Changes in discrimination for cardiovascular disease events and all-cause mortality for daytime and nighttime BP
eTable 10. Hazard ratios for cardiovascular disease events and all-cause mortality for high daytime BP and high nighttime BP defined in the 2017 ACC/AHA High BP guidelines
eTable 11. Hazard ratios for cardiovascular disease events and all-cause mortality for dipping status in two categories (see footnote)
eTable 12. Hazard ratios for cardiovascular disease events and all-cause mortality for dipping status in three categories (see footnote)
eTable 13. Hazard ratios for cardiovascular disease events and all-cause mortality for daytime SBP and nighttime SBP among participants without a history of myocardial infarction and stroke (n = 952)
eFigure. Cumulative rate of CVD events by high nighttime BP status and high daytime BP status defined in the 2017 ACC/AHA BP guidelines
References
- 1.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111(5):697-716. doi: 10.1161/01.CIR.0000154900.76284.F6 [DOI] [PubMed] [Google Scholar]
- 2.Mancia G, Fagard R, Narkiewicz K, et al. ; Task Force Members . 2013 ESH/ESC Guidelines for the Management of Arterial Hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31(7):1281-1357. doi: 10.1097/01.hjh.0000431740.32696.cc [DOI] [PubMed] [Google Scholar]
- 3.Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med. 2006;354(22):2368-2374. doi: 10.1056/NEJMra060433 [DOI] [PubMed] [Google Scholar]
- 4.Yano Y, Kario K. Nocturnal blood pressure and cardiovascular disease: a review of recent advances. Hypertens Res. 2012;35(7):695-701. doi: 10.1038/hr.2012.26 [DOI] [PubMed] [Google Scholar]
- 5.Shimbo D, Abdalla M, Falzon L, Townsend RR, Muntner P. Role of ambulatory and home blood pressure monitoring in clinical practice: a narrative review. Ann Intern Med. 2015;163(9):691-700. doi: 10.7326/M15-1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clement DL, De Buyzere ML, De Bacquer DA, et al. ; Office versus Ambulatory Pressure Study Investigators . Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348(24):2407-2415. doi: 10.1056/NEJMoa022273 [DOI] [PubMed] [Google Scholar]
- 7.Boggia J, Li Y, Thijs L, et al. ; International Database on Ambulatory blood pressure monitoring in relation to Cardiovascular Outcomes (IDACO) investigators . Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet. 2007;370(9594):1219-1229. doi: 10.1016/S0140-6736(07)61538-4 [DOI] [PubMed] [Google Scholar]
- 8.Roush GC, Fagard RH, Salles GF, et al. ; ABC-H Investigators . Prognostic impact from clinic, daytime, and night-time systolic blood pressure in nine cohorts of 13,844 patients with hypertension. J Hypertens. 2014;32(12):2332-2340. doi: 10.1097/HJH.0000000000000355 [DOI] [PubMed] [Google Scholar]
- 9.Sega R, Facchetti R, Bombelli M, et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111(14):1777-1783. doi: 10.1161/01.CIR.0000160923.04524.5B [DOI] [PubMed] [Google Scholar]
- 10.Staessen JA, Thijs L, Fagard R, et al. ; Systolic Hypertension in Europe Trial Investigators . Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. JAMA. 1999;282(6):539-546. doi: 10.1001/jama.282.6.539 [DOI] [PubMed] [Google Scholar]
- 11.Benjamin EJ, Blaha MJ, Chiuve SE, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146-e603. doi: 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muntner P, Lewis CE, Diaz KM, et al. Racial differences in abnormal ambulatory blood pressure monitoring measures: results from the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Hypertens. 2015;28(5):640-648. doi: 10.1093/ajh/hpu193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Profant J, Dimsdale JE. Race and diurnal blood pressure patterns: a review and meta-analysis. Hypertension. 1999;33(5):1099-1104. doi: 10.1161/01.HYP.33.5.1099 [DOI] [PubMed] [Google Scholar]
- 14.McMullan CJ, Yano Y, Bakris GL, Kario K, Phillips RA, Forman JP. Racial impact of diurnal variations in blood pressure on cardiovascular events in chronic kidney disease. J Am Soc Hypertens. 2015;9(4):299-306. doi: 10.1016/j.jash.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 15.Thomas SJ, Booth JN III, Bromfield SG, et al. Clinic and ambulatory blood pressure in a population-based sample of African Americans: the Jackson Heart Study. J Am Soc Hypertens. 2017;11(4):204-212.e5. doi: 10.1016/j.jash.2017.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson DK, Kliewer W, Teasley N, Plybon L, Sica DA. Violence exposure, catecholamine excretion, and blood pressure nondipping status in African American male versus female adolescents. Psychosom Med. 2002;64(6):906-915. [DOI] [PubMed] [Google Scholar]
- 17.Taylor HA Jr, Wilson JG, Jones DW, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15(4 suppl 6):S6-4-17. [PubMed]
- 18.Thijs L, Hansen TW, Kikuya M, et al. ; IDACO Investigators . The International Database of Ambulatory Blood Pressure in relation to Cardiovascular Outcome (IDACO): protocol and research perspectives. Blood Press Monit. 2007;12(4):255-262. doi: 10.1097/MBP.0b013e3280f813bc [DOI] [PubMed] [Google Scholar]
- 19.Seals SR, Colantonio LD, Tingle JV, et al. Calibration of blood pressure measurements in the Jackson Heart Study. Blood Press Monit. 2019;24(3):130-136. doi: 10.1097/MBP.0000000000000379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravenell J, Shimbo D, Booth JN III, et al. Thresholds for ambulatory blood pressure among African Americans in the Jackson Heart Study. Circulation. 2017;135(25):2470-2480. doi: 10.1161/CIRCULATIONAHA.116.027051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Booth JN III, Diaz KM, Seals SR, et al. Masked hypertension and cardiovascular disease events in a prospective cohort of blacks: the Jackson Heart Study. Hypertension. 2016;68(2):501-510. doi: 10.1161/HYPERTENSIONAHA.116.07553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bromfield SG, Booth JN III, Loop MS, et al. Evaluating different criteria for defining a complete ambulatory blood pressure monitoring recording: data from the Jackson Heart Study. Blood Press Monit. 2018;23(2):103-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Brien E, Parati G, Stergiou G, et al. ; European Society of Hypertension Working Group on Blood Pressure Monitoring . European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31(9):1731-1768. doi: 10.1097/HJH.0b013e328363e964 [DOI] [PubMed] [Google Scholar]
- 24.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13-e115. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30(4):377-399. doi: 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 27.Arbogast PG, Kaltenbach L, Ding H, Ray WA. Adjustment for multiple cardiovascular risk factors using a summary risk score. Epidemiology. 2008;19(1):30-37. doi: 10.1097/EDE.0b013e31815be000 [DOI] [PubMed] [Google Scholar]
- 28.Hair JF, Anderson R, Tatham RL, Black WC. Multivariate Data Analysis. Upper Saddle River, NJ: Prentice Hall; 2006. [Google Scholar]
- 29.Kent ST, Shimbo D, Huang L, et al. Rates, amounts, and determinants of ambulatory blood pressure monitoring claim reimbursements among Medicare beneficiaries. J Am Soc Hypertens. 2014;8(12):898-908. doi: 10.1016/j.jash.2014.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kronish IM, Kent S, Moise N, et al. Barriers to conducting ambulatory and home blood pressure monitoring during hypertension screening in the United States. J Am Soc Hypertens. 2017;11(9):573-580. doi: 10.1016/j.jash.2017.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muntner P, Carey RM, Jamerson K, Wright JT Jr, Whelton PK. Rationale for ambulatory and home blood pressure monitoring thresholds in the 2017 American College of Cardiology/American Heart Association Guideline. Hypertension. 2019;73(1):33-38. doi: 10.1161/HYPERTENSIONAHA.118.11946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hermida RC, Ayala DE, Mojón A, Fernández JR. Decreasing sleep-time blood pressure determined by ambulatory monitoring reduces cardiovascular risk. J Am Coll Cardiol. 2011;58(11):1165-1173. doi: 10.1016/j.jacc.2011.04.043 [DOI] [PubMed] [Google Scholar]
- 33.Poulter NR, Savopoulos C, Anjum A, et al. Randomized crossover trial of the impact of morning or evening dosing of antihypertensive agents on 24-hour ambulatory blood pressure. Hypertension. 2018;72(4):870-873. doi: 10.1161/HYPERTENSIONAHA.118.11101 [DOI] [PubMed] [Google Scholar]
- 34.Bowles NP, Thosar SS, Herzig MX, Shea SA. Chronotherapy for hypertension. Curr Hypertens Rep. 2018;20(11):97. doi: 10.1007/s11906-018-0897-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies CW, Crosby JH, Mullins RL, Barbour C, Davies RJ, Stradling JR. Case-control study of 24 hour ambulatory blood pressure in patients with obstructive sleep apnoea and normal matched control subjects. Thorax. 2000;55(9):736-740. doi: 10.1136/thorax.55.9.736 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplementary methods
eTable 1. Number and percentage of participants included in the current analysis with missing data for variables (n = 1034)
eTable 2. Characteristics of JHS participants who were included in the current study and those who were not included
eTable 3. Hazard ratios for cardiovascular disease events and all-cause mortality for daytime SBP and nighttime SBP
eTable 4. Hazard ratios for cardiovascular disease events and all-cause mortality associated with daytime DBP and nighttime DBP
eTable 5. Hazard ratios for cardiovascular disease events by tertile of daytime SBP and nighttime SBP
eTable 6. Hazard ratios for cardiovascular disease events by tertile of daytime DBP and nighttime DBP
eTable 7. Heterogeneity in the association between each BP measure and outcome by antihypertensive medication use with the inclusion of multiplicative interaction terms
eTable 8. Hazard ratios for cardiovascular disease events for daytime SBP and nighttime SBP among participants taking and not taking antihypertensive medication
eTable 9. Changes in discrimination for cardiovascular disease events and all-cause mortality for daytime and nighttime BP
eTable 10. Hazard ratios for cardiovascular disease events and all-cause mortality for high daytime BP and high nighttime BP defined in the 2017 ACC/AHA High BP guidelines
eTable 11. Hazard ratios for cardiovascular disease events and all-cause mortality for dipping status in two categories (see footnote)
eTable 12. Hazard ratios for cardiovascular disease events and all-cause mortality for dipping status in three categories (see footnote)
eTable 13. Hazard ratios for cardiovascular disease events and all-cause mortality for daytime SBP and nighttime SBP among participants without a history of myocardial infarction and stroke (n = 952)
eFigure. Cumulative rate of CVD events by high nighttime BP status and high daytime BP status defined in the 2017 ACC/AHA BP guidelines