Key Points
Question
Have the frequency and long-term implications of clinically relevant pancreatic fistula after resection for pancreatic cancer changed in the era of neoadjuvant therapy?
Findings
In this cohort study of 346 patients receiving neoadjuvant therapy of overall 753 individuals with resected pancreatic cancers, the occurrence of clinically relevant pancreatic fistula was markedly reduced (3.6 times) compared with upfront resections, and classic clinically relevant pancreatic fistula factors—except for soft pancreatic texture—are no longer applicable. Yet, if clinically relevant pancreatic fistula occurs in this setting, long-term overall survival may be impaired.
Meaning
Despite being uncommon, clinically relevant pancreatic fistula following neoadjuvant therapy for pancreatic cancer may have a major and independent association with long-term survival, and patients might benefit from closer follow-up and further systemic interventions.
Abstract
Importance
In the past decade, the use of neoadjuvant therapy (NAT) has increased for patients with borderline and locally advanced pancreatic ductal adenocarcinoma (PDAC). Data on pancreatic fistula and related overall survival (OS) in this setting are limited.
Objective
To compare postoperative complications in patients undergoing either upfront resection or pancreatectomy following NAT, focusing on clinically relevant postoperative pancreatic fistula (CR-POPF) and potential associations with OS.
Design, Setting, and Participants
This retrospective cohort study was conducted on data from patients who underwent pancreatic resection for PDAC at the Massachusetts General Hospital from January 1, 2007, to December 31, 2017,
Exposures
Pancreatic cancer surgery with or without NAT.
Main Outcomes and Measures
Overall morbidity and CR-POPF rates were compared between NAT and upfront resection. Factors associated with CR-POPF were assessed with univariate and multivariate analysis. Survival data were analyzed by Kaplan-Meier curves and a Cox proportional hazards regression model.
Results
Of 753 patients, 364 were men (48.3%); median (interquartile range) age was 68 (61-75) years. A total of 346 patients (45.9%) received NAT and 407 patients (54.1%) underwent upfront resection. At pathologic examination, NAT was associated with smaller tumor size (mean [SD], 26.0 [15.3] mm vs 32.7 [14.4] mm; P < .001), reduced nodal involvement (102 [25.1%] vs 191 [55.2%]; P < .001), and higher R0 rates (257 [74.3%] vs 239 [58.7%]; P < .001). There were no significant differences in severe complication rate or 90-day mortality. The rate of CR-POPF was 3.6-fold lower in patients receiving NAT vs upfront resection (13 [3.8%] vs 56 [13.8%]; P < .001). In addition, factors associated with CR-POPF changed after NAT, and only soft pancreatic texture was associated with a higher risk of CR-POPF (38.5% vs 6.3%; P < .001). Survival analysis showed no differences between patients with or without CR-POPF after upfront resection (26 vs 25 months; P = .66), but after NAT, a worse overall survival rate was observed in patients with CR-POPF (17 vs 34 months; P = .002). This association was independent of other established predictors of overall survival by multivariate analysis (hazard ratio, 2.80; 95% CI, 1.44-5.45; P < .002).
Conclusions and Relevance
Neoadjuvant therapy may be associated with a significant reduction in the rate of CR-POPF. In addition, standard factors associated with CR-POPF appear to be no longer applicable following NAT. However, once CR-POPF occurs, it is associated with a significant reduction in long-term survival. Patients with CR-POPF may require closer follow-up and could benefit from additional therapy.
This cohort study examines the rate of postoperative pancreatic fistula in patients with pancreatic ductal adenocarcinoma receiving upfront resection or neoadjuvant therapy.
Introduction
In the past decade, the use of neoadjuvant treatment (NAT) has markedly increased for patients with borderline resectable and locally advanced pancreatic ductal adenocarcinoma (PDAC).1 This increase has enabled a large number of patients whose tumors were considered inoperable in the past to undergo pancreatic resection,2 and several studies have shown encouraging surgical outcomes,2,3,4 as well as improvement in pathologic findings.5,6
Postoperative pancreatic fistula (POPF) remains one of the most frequent and dangerous complications after pancreatic surgery.7 In the setting of upfront resection, POPF rates vary between 5% and 15% for proximal resections and are higher after distal pancreatectomy.8,9,10 Clinically relevant grade B and C fistulas (CR-POPF)11,12 in turn are associated with a significant increase of postpancreatectomy hemorrhage, severe infectious complications, and mortality rates.13,14,15 Several risk factors have been described to estimate the risk of POPF, such as soft pancreatic texture, small pancreatic duct diameter, and high intraoperative blood loss.16,17 However, these data have been validated almost exclusively in the context of upfront resection, and studies specifically addressing rate and factors associated with POPF in NAT cohorts are lacking.
The aim of this study was to assess the rate of CR-POPF in the era of NAT at our institution. The relevance of classic CR-POPF in patients receiving NAT was also assessed, as well as whether CR-POPF–related morbidity affected long-term outcomes after pancreatic cancer surgery.
Methods
Study Design and Patient Cohort
Adult patients who underwent a pancreatoduodenectomy or a distal pancreatectomy over an 11-year period (January 1, 2007, to December 31, 2017) at Massachusetts General Hospital were identified from a prospectively maintained institutional database. All patients with pathologic diagnosis of PDAC who underwent either neoadjuvant therapy and subsequent resection or upfront resection were included. Exclusion criteria were evidence of distant metastasis; PDAC arising from intraductal papillary mucinous neoplasms; other benign or malignant tumor diseases of the pancreas, including neuroendocrine carcinoma, acinar cell carcinoma, and distal cholangiocarcinoma; and total pancreatectomies. The study protocol was approved by the institutional review board at the Massachusetts General Hospital, which waived informed patient consent because of deidentification of data.
Clinical Management
Neoadjuvant Therapy
For most patients, FOLFIRINOX (folinic acid, fluorouracil, irinotecan, and oxaliplatin) was administered as the standard of care in those with locally advanced and borderline resectable PDAC, either followed, or not, by chemoradiotherapy with 50.4 Gy photon or 25 Gy proton radiotherapy.2 In addition, some clinical trials were ongoing during the study period, including different NAT regimens, such as short-course proton beam radiation with capecitabine or fluorouracil.18 Also included were patients who were referred to Massachusetts General Hospital after receiving different NAT regimens at outside institutions.
Surgical Approach
Patients with locally advanced and borderline resectable PDAC that did not progress during NAT were taken to the operating room for open exploration and surgical resection.19 A standard lymphadenectomy was performed according to the International Study Group on Pancreatic Surgery guidelines, including peripancreatic lymph-node stations as well as lymph-node clearance of the tumor facing the side of the superior mesenteric artery or the celiac trunk.20 Frozen sections were taken routinely from tumor-surrounding areas and additional vascular resections were performed when necessary, with the goal of achieving an R0 resection. Surgical drains were routinely left in all patients.
Adjuvant Therapy
For patients with upfront resection, a gemcitabine-based standard adjuvant chemotherapy was routinely administered.21 In addition, most patients who underwent upfront resection received radiotherapy as well, especially if an R1 resection was noted on the final pathologic report. After NAT, with the exception of the subgroup of patients who received only short-course chemoradiotherapy, there was no general recommendation for additional adjuvant chemotherapy, and most patients were referred for routine oncologic surveillance.
Data Acquisition
Patients’ clinicopathologic data were collected retrospectively from a prospective maintained database and from electronical medical records, including demographics, body mass index (BMI), preoperative (post-NAT) carbohydrate antigen (CA) 19-9 levels, placement of preoperative biliary stent, type and extent of surgery, intraoperative pancreatic texture and main pancreatic duct diameter, estimated intraoperative blood loss, intraoperative radiotherapy, and duration of surgery. Postoperative outcomes and treatment included the occurrence of any complications graded according to the Clavien-Dindo classification, 30-day and 90-day mortality, duration of postoperative hospital stay, readmission rate, and receipt of postoperative radiotherapy and/or chemotherapy. Postoperative pancreatic fistula was defined according to the updated International Study Group on Pancreatic Surgery guidelines into biochemical leakage if any measurable drain amylase was higher than 300 U/L after postoperative day 2, or grade B or C fistula depending on the duration of drainage (≥3 weeks), the clinical outcome of POPF, or the type of reintervention.12 Pathologic data included tumor size, tumor stage according to the 8th edition of the TNM classification,22 R-status based on a 1-mm tumor-free margin,23 the presence of perineural invasion, and lymphovascular invasion.
Follow-up was updated until August 31, 2018, through the medical records or phone interviews. Patients were followed up from the date of operation until their last oncologic surveillance or death.
Statistical Analysis
Dichotomous data are expressed as absolute numbers (relative percentages) and continuous data as mean (SD) or median and interquartile range (IQR). Continuous variables were assessed for normal distribution with the Shapiro-Wilk test. The t test or the Mann-Whitney test was used to compare parametric and nonparametric continuous variables, respectively. Nonrandom association for categorical variables was analyzed with the Fisher exact χ2 test.
The survival analysis was performed with the Kaplan-Meier method, and patients who did and did not experience CR-POPF were compared using the log-rank (Mantel-Cox) test. If a death was not reported during the follow-up period, patients were censored at the last available contact date. To assess the independent association between the occurrence of CR-POPF and overall survival (OS), a Cox proportional hazards regression model was constructed. Hazard ratios (HRs) are reported with 95% CIs. Positive resection margins, size of the tumor, degree of differentiation, nodal status, CA19-9 levels and receipt of adjuvant treatment were included in the multivariate model, together with the occurrence and severity of CR-POPF.
For each test, a 2-sided P value of .05 was considered significant. All statistical computations were performed using IBM SPSS, version 25 (IBM Corp).
Results
A total of 1102 patients underwent pancreatic surgery for primary pancreatic malignant tumors between 2007 and 2017 at the Massachusetts General Hospital. Of these patients, 342 individuals (31.0%) met the exclusion criteria and were removed from this study. Seven patients (<1%) were excluded owing to a completion pancreatectomy after a prior pancreatic head resection or distal pancreatectomy. Among the 753 patients who were available for the analysis with confirmed PDAC at pathologic examination, 364 were men (48.3%); median (interquartile range [IQR]) age was 68 (61-75) years. A total of 346 patients (45.9%) received NAT and 407 patients (54.1%) underwent upfront resection without any additional therapy prior to surgery. The median follow-up was 16 (IQR, 8-30) months for the entire cohort. Patient baseline characteristics and pathologic findings are summarized in Table 1. Patients with NAT compared with the upfront resection cohort were younger (66 [IQR, 59-72] vs 70 [IQR, 62-77] years; P < .001), had a lower mean (SD) BMI (calculated as weight in kilograms divided by height in meters squared) (25.8 [5.1] vs 26.9 [6.4]; P = .01) and had lower preoperative CA19-9 levels (median [IQR], 40 [ 13-139] vs 97 [23-403] U/mL; P < .001). Furthermore, patients receiving NAT were more frequently associated with favorable posttreatment pathologic characteristics, including smaller tumor size (mean [SD], 26.0 [15.3] vs 32.7 [14.4] mm; P < .001) or lower pT category (pT<3, 126 [36.4%] vs 32 [7.8%]; P < .001), negative lymph node status (191 [55.2%] vs 102 [25.2%]; P < .001), and higher R0 rates (257 [74.3%] vs 239 [58.7%]; P < .001). In contrast, patients who received NAT showed a higher frequency of preoperative biliary stenting (225 [65%] vs 234 [57.5%]; P = .04), longer operative times (mean [SD], 382 [111] vs 331 [118] minutes; P < .001), higher estimated intraoperative blood loss (median [IQR], 600 [400-1000] vs 500 ml [300-900] mL; P < .001), as well as a higher rate of vascular resections (71 [20.5%] vs 45 [11.1%]; P < .001). Among the NAT subgroup, most patients received FOLFIRINOX in combination with radiotherapy (185 [53.5%]); 125 patients (36.1%) received short-course chemoradiotherapy with concurrent capecitabine or fluorouracil, and the rest received gemcitabine-based treatment (25 [7.2%]) or other regimens (11 [3.2%]).
Table 1. Baseline and Pathologic Data.
| Variable | No. (%) | P Value | |
|---|---|---|---|
| UR (n = 407) | NAT (n = 346) | ||
| Men | 201 (49.4) | 163 (47.1) | .53 |
| Age, median (IQR), y | 70 (62-77) | 66 (59-72) | <.001 |
| BMI, mean (SD) | 26.9 (6.4) | 25.8 (5.1) | .01 |
| ASA >2a | 204 (50.1) | 153 (44.2) | .11 |
| Preoperative CA19-9, median (IQR), U/mL | 97.0 (23.0-403.0) | 40.0 (13.0-139.0) | <.001 |
| Biliary stent | 234 (57.5) | 225 (65.0) | .04 |
| Type of surgery | |||
| Pancreatoduodenectomy | 319 (78.4) | 285 (82.4) | .20 |
| Distal pancreatectomy | 88 (21.6) | 61 (17.6) | |
| Duration of surgery, mean (SD), min | 331 (118) | 382 (111) | <.001 |
| Resection | |||
| Vascular | 45 (11.1) | 71 (20.5) | <.001 |
| Extended | 15 (3.7) | 13 (3.8) | .96 |
| Intraoperative radiotherapy | 3 (0.7) | 73 (21.1) | <.001 |
| Estimated blood loss, median (IQR), mL | 500 (300-900) | 600 (400-1000) | <.001 |
| NAT regimen | |||
| Short-course CRT | NA | 125 (36.1) | NA |
| FOLFIRINOX | NA | 185 (53.5) | |
| Gemcitabine-based NAT | NA | 25 (7.2) | |
| Other | NA | 11 (3.2) | |
| AJCC stage | |||
| 0 | 0 | 19 (5.5) | <.001 |
| I-IIa | 103 (25.3) | 167 (48.3) | |
| IIb-III | 304 (74.7) | 158 (45.7) | |
| IV | 0 | 2 (0.5) | |
| Tumor stage | |||
| T0 | 0 | 19 (5.5) | <.001 |
| T1 | 5 (1.2) | 44 (12.7) | |
| T2 | 27 (6.6) | 63 (18.2) | |
| T3 | 367 (90.2) | 216 (62.4) | |
| T4 | 8 (2.0) | 4 (1.2) | |
| Tumor size, mean (SD), mm | 32.7 (14.4) | 26.0 (15.3) | <.001 |
| Nodal statusb | |||
| N0 | 102 (25.0) | 191 (55.2) | <.001 |
| N1 | 172 (42.3) | 112 (32.4) | |
| N2 | 133 (32.7) | 43 (12.4) | |
| R status | |||
| 0 | 239 (58.7) | 257 (74.3) | <.001 |
| 1 | 148 (36.4) | 86 (24.9) | |
| Tumor grading | |||
| G0 | 0 | 9 (2.6) | .009 |
| G1 | 27 (6.6) | 17 (4.9) | |
| G2 | 258 (63.4) | 171 (49.4) | |
| G3 | 115 (28.3) | 87 (25.1) | |
| G4 | 1 (0.2) | 1 (0.3) | |
| Invasion | |||
| Perineural | 378 (92.9) | 245 (70.8) | <.001 |
| Vascular | 278 (68.3) | 119 (34.4) | <.001 |
| Adjuvant treatment | 283 (69.5) | 161 (46.5) | <.001 |
Abbreviations: AJCC, American Joint Committee on Cancer; ASA, American Society of Anesthesiology; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CA19-9, carbohydrate antigen 19-9; CRT, chemoradiotherapy; FOLFIRINOX, folinic acid, fluorouracil, irinotecan, and oxaliplatin; IQR, interquartile range; NAT, neoadjuvant therapy; UR, upfront resection.
Level greater than 2 indicates ASA 3 or 4.
For nodal status, N0 indicates 0 positive lymph nodes; N1, 1-3 positive lymph nodes; and N2, more than 3 positive lymph nodes.
Rates of POPF in Patients Receiving NAT
No significant differences in the overall complication, major complication (Clavien-Dindo class ≥3), and 30-day and 90-day mortality rates were detected between the upfront resection and NAT groups (Table 2). Biochemical leakage was significantly reduced in patients with NAT compared with the upfront resection group (28 [8.1%] vs 78 [19.2%]; P < .001) as well as the rate of CR-POPF in the NAT group compared with the upfront resection group (13 [3.8%] vs 56 [13.8]; P < .001). Stratified by the type of surgery, patients undergoing distal pancreatectomy in the upfront resection group had a biochemical leakage rate of 26.1%, and in those undergoing pancreatoduodenectomy, the rate was 17.2% (P = .10). The comparative rates in the NAT cohort were 23.0% and 4.9% (P < .001). Regarding CR-POPF, the frequencies were 15.9% after distal pancreatectomy and 13.2% after pancreatoduodenectomy in the upfront resection group and 3.3% and 3.9% after distal pancreatectomy and pancreatoduodenectomy, respectively, in the NAT group.
Table 2. Intraoperative Characteristics and Surgical Outcomes.
| Variable | No. (%) | P Value | |
|---|---|---|---|
| UR (n = 407) | NAT (n = 346) | ||
| Soft pancreatic texture | 53 (13.0) | 26 (7.5) | .005 |
| Duct size, median (IQR), mm | 3 (3-5) | 4 (3-4) | .83 |
| POD drain removal, median (IQR), d | 5 (4-7) | 4 (2-5) | <.001 |
| Complications | |||
| Overall | 228 (56.0) | 181 (52.3) | .31 |
| Major | 70 (17.2) | 50 (14.5) | .28 |
| POPF | <.001 | ||
| Biochemical leakage | 78 (19.2) | 28 (8.1) | |
| CR-POPF | 56 (13.8) | 13 (3.8) | |
| Delayed gastric emptying | 62 (15.2) | 47 (13.6) | .52 |
| Biliary fistula | 12 (2.9) | 3 (0.9) | .04 |
| PPH | 13 (3.2) | 12 (3.5) | .83 |
| Complications | |||
| Pulmonary | 31 (7.6) | 13 (3.8) | .02 |
| Cardiac | 32 (7.9) | 16 (4.6) | .07 |
| Overall infectious | 115 (28.3) | 85 (24.6) | .25 |
| Sepsis | 23 (5.7) | 21 (6.1) | .81 |
| Intra-abdominal collections | 38 (9.3) | 19 (5.5) | .047 |
| Wound infection | 48 (11.8) | 44 (12.7) | .70 |
| Reintervention (nonsurgical) | 50 (12.3) | 33 (9.5) | .23 |
| Reoperation | 12 (2.9) | 8 (2.3) | .62 |
| LOS, median (IQR), d | 7 (6-9) | 6 (6-8) | .001 |
| Readmission | 74 (18.2) | 62 (17.9) | .93 |
| Mortality | |||
| 30 d | 9 (2.2) | 3 (0.9) | .14 |
| 90 d | 18 (4.4) | 12 (3.5) | .58 |
Abbreviations: CR-POPF, clinically relevant postoperative pancreatic fistula (grade B or C); IQR, interquartile range; LOS, length of postoperative hospital stay; NA, not applicable; NAT, neoadjuvant therapy; POPF, postoperative pancreatic fistula; PPH, postpancreatectomy hemorrhage; POD, postoperative day; UR, upfront resection.
In the NAT cohort, we detected a lower prevalence of soft pancreatic texture compared with the upfront resection cohort (26 [7.5%] vs 53 [13.0%]; P = .005). A lower rate of CR-POPF in the FOLFIRINOX cohort was observed in comparison with short-course chemoradiotherapy (2.7% vs 5.6%), although this finding was not statistically significant (eFigure in the Supplement). The intra-abdominal drains were removed earlier (median [IQR], 4 [2-5] vs 5 [4-7] days; P < .001), and the median (IQR) postoperative hospital stay was significantly shorter in the NAT group compared with the upfront resection cohort (6 [6-8] vs 7 [6-9]; days P = .001).
Change of Factors in POPF During NAT
Factors associated with the occurrence of CR-POPF were analyzed for the NAT and the upfront resection groups (Table 3). In the cohort of upfront resection, patients with high BMI (mean [SD], 28.7 [ 5.6] vs 26.6 [6.5]; P = .03), long duration of surgery (mean [SD], 401 [154] vs 320 [107] minutes; P < .001), small duct size (median [IQR], 3 [2-4] vs 4 [3-5] mm; P < .001), and high intraoperative estimated blood loss (median [IQR], 800 [ 500-1500] vs 500 [300-800] mL; P < .001) were more likely to develop a CR-POPF. None of these variables was associated with a higher incidence of CR-POPF in the NAT group, while the presence of soft pancreatic tissue significantly increased the risk of CR-POPF (38.5% vs 6.3%; P < .001).
Table 3. Factors Associated With CR-POPF in Upfront Resection and NAT .
| Variable | UR | P Value | NAT | P Value | ||
|---|---|---|---|---|---|---|
| No CR-POPF (n = 351) | CR-POPF (n = 56) | No CR-POPF (n = 333) | CR-POPF (n = 13) | |||
| Male | 172 (49.0) | 29 (51.8) | .70 | 159 (47.7) | 4 (30.8) | .23 |
| Age, median (IQR), y | 70 (62-77) | 68 (61-75) | .23 | 66 (59-72) | 71 (68-76) | .12 |
| BMI, mean (SD) | 26.6 (6.5) | 28.7 (5.6) | .03 | 25.8 (5.0) | 25.4 (5.5) | .79 |
| ASA >2, No. (%)a | 169 (48.1) | 34 (60.7) | .09 | 149 (44.9) | 4 (30.8) | .47 |
| Biliary stent, No. (%) | 203 (57.8) | 31 (55.4) | .78 | 217 (65.4) | 8 (61.5) | .79 |
| Type of surgery, No. (%) | .508 | .829 | ||||
| Pancreatoduodenectomy | 277 (78.9) | 42 (75.0) | .51 | 274 (82.3) | 11 (84.6) | .83 |
| Distal pancreatectomy | 74 (21.1) | 14 (25.0) | 59 (17.7) | 2 (15.4) | ||
| Duration of surgery, mean (SD), min | 320 (107) | 401 (154) | <.001 | 381 (113) | 408 (75) | .38 |
| Resection, No. (%) | ||||||
| Vascular | 38 (10.8) | 7 (12.5) | .71 | 67 (20.1) | 4 (30.8) | .35 |
| Extended | 12 (3.4) | 3 (5.4) | .48 | 12 (3.6) | 1 (7.7) | .45 |
| Soft pancreas | 46 (13.1) | 7 (12.5) | .38 | 21 (6.3) | 5 (38.5) | <.001 |
| Duct size, median (IQR), mm | 4 (3-5) | 3 (2-4) | .03 | 4 (3-4) | 3 (3-4) | .29 |
| EBL, median (IQR), mL | 500 (300-800) | 800 (500-1500) | <.001 | 600 (400-1000) | 625 (403-825) | .96 |
| NAT regimen, No. (%) | .310 | |||||
| Short-course CRT | NA | NA | 118 (35.4) | 7 (53.8) | .31 | |
| FOLFIRINOX | NA | NA | 180 (54.1) | 5 (38.5) | ||
| Gemcitabine-based NAT | NA | NA | 25 (7.5) | 0 | ||
| Other | NA | NA | 10 (3.0) | 1 (7.7) | ||
Abbreviations: ASA, American Society of Anesthesiology; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CR-POPF, clinically relevant postoperative pancreatic fistula (grade B or C); CRT, chemoradiotherapy; EBL, estimated blood loss; FOLFIRINOX, folinic acid, fluorouracil, irinotecan, and oxaliplatin; IQR, interquartile range; NA, not applicable; NAT, neoadjuvant therapy; UR, upfront resection.
Level greater than 2 indicates ASA 3 or 4.
POPF as a Prognostic Factor in Patients With NAT
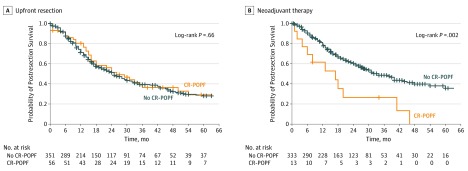
The occurrence of CR-POPF was associated with OS in both groups, using Kaplan-Meier curves (Figure). In the upfront resection group, no association of CR-POPF with OS was observed, with a median OS of 25 months in the nonfistula group compared with 26 months in patients with CR-POPF (P = .66). By contrast, patients in the NAT group experienced a significantly worse OS when a CR-POPF occurred, with a median OS of 34 months in patients without fistula compared with 17 months in patients with a CR-POPF (P = .002). The unfavorable association of CR-POPF with OS was independent of the occurrence of severe complications (HR, 2.08; 95% CI, 1.07-4.04; P = .03).
Figure. Clinically Relevant Pancreatic Fistula (CR-POPF) and Survival.
Survival after resection in patients who underwent upfront resection (A) or received neoadjuvant therapy (B). Vertical bars indicate censoring of patients alive at their last follow-up.
With use of a Cox proportional hazards regression model (Table 4), CR-POPF was shown to be a possible independent prognostic factor for OS (HR, 2.80; 95% CI, 1.44-5.45; P < .002), together with other well-established prognostic parameters including tumor size OS (HR, 1.02; 95% CI, 1.01-1.03; P < .001), lymph node involvement (for N0 vs N1: HR, 1.61; 95% CI, 1.07-2.42; P = .02 and N2 vs N0: HR, 2.05; 95% CI, 1.19-3.55; P = .01), R1 status (HR, 1.88; 95% CI, 1.28-2.76; P = .001), administration of adjuvant treatment (HR, 0.62; 95% CI, 0.42-0.93; P = .02), and high CA19-9 levels (HR, 2.74 U/mL; 95% CI, 1.70-4.42 U/mL; P < .001).
Table 4. Multivariate Analysis for OS in Patients Receiving NAT.
| Variable | HR (95% CI) | P Value |
|---|---|---|
| CR-POPF | 2.80 (1.44-5.45) | .002 |
| Tumor size | 1.02 (1.01-1.03) | <.001 |
| Tumor grading | NA | .35 |
| Nodal status | ||
| 1 | 1.61 (1.07-2.42) | .02 |
| 2 | 2.05 (1.19-3.55) | .001 |
| R1 status | 1.88 (1.28-2.76) | .001 |
| Adjuvant treatment | 0.62 (0.42-0.93) | .02 |
| CA19-9 ≥ 400 U/mL | 2.74 (1.70-4.42) | <.001 |
Abbreviations: CA19-9, carbohydrate antigen 19-9; CR-POPF, clinically relevant postoperative pancreatic fistula (grade B or C); HR, hazard ratio; NAT, neoadjuvant therapy; NA, not applicable; OS, overall survival.
Discussion
In the past decade, neoadjuvant treatment has increased for borderline resectable and locally advanced PDAC, yet its associations with postoperative outcomes remain largely unexplored. Although some studies have evaluated different NAT regimens and postoperative morbidity, definite conclusions cannot be drawn, as most observations come from small studies, including heterogeneous NAT regimens and without comparison with upfront resection groups.24 Larger cohort analyses have shown similar morbidity rates between NAT patients and primary resections,3,6,25 while previous data reported a significantly lower complication rate with FOLFIRINOX than upfront resections.5 The present study comparing 346 patients receiving NAT with a contemporaneous cohort of 407 patients with upfront resection of PDAC is consistent with prior reports. We observed an overall morbidity rate of 52% for the NAT group vs 56% in the upfront resection group, with a rate of severe complications of 14% and 17%, respectively. In addition, the length of hospital stay was generally 1 day shorter in patients receiving NAT, despite higher rates of intraoperative bleeding and longer operative time than patients with upfront resections. Moreover, a significantly reduced rate of pulmonary complications, postoperative collections, and procedure-related complications was detected in the NAT group.
Neoadjuvant therapy has been generally associated with a reduction in POPF occurrence, although reported POPF rates broadly range between 0% and 15%.26,27,28,29,30,31 Reasons for this variability may reside in the heterogeneity of neoadjuvant chemotherapy and/or radiotherapy schemes and the lack of a standardized CR-POPF definition across the studies. In our cohort, the occurrence of CR-POPF was 3.6 times lower in patients who received NAT compared with upfront resection, with rates of 3.8% vs 13.8%, respectively. These findings could seem contradictory, as patients who receive NAT experience longer operations, increased blood loss, and a higher rate of vascular resections, all of which have been associated in the past with an increased risk of CR-POPF.32 However, in the NAT group, we also observed a lower prevalence of soft pancreatic parenchyma, which historically is one of the major factors associated with CR-POPF. It has been described that NAT drives pathologic changes in the pancreatic gland, resulting in increased fibrosis and atrophy, which affects not only the neoplastic tissue, but also the remnant pancreas.33,34 Arguably, this protective factor successfully overruled the increased risk associated with the intraoperative POPF factors. We also found a different CR-POPF rate according to the type of NAT; specifically, the FOLFIRINOX regimen was associated with the lowest risk of POPF (2.7%) and the short-course chemoradiotherapy had the highest risk (5.6%), suggesting that duration and type of NAT have different effects on the pancreas.
Several fistula risk measures have been used in the past, with the one most often applied described by Callery et al,16 although validation studies failed to confirm high blood loss as a factor associated with the occurrence of CR-POPF.35,36 An alternative fistula risk score was published in 2019, which includes BMI, pancreatic texture, and pancreatic duct size.37 In our cohort of patients who underwent upfront resection, we validated some of these well-described factors associated with CR-POPF, such as high BMI, small pancreatic duct, high estimated intraoperative blood loss, and duration of surgery.38 However, we also found that these CR-POPF factors were no longer applicable after NAT, and that only soft pancreatic texture was independently associated with the occurrence of fistula. It can be argued that a firm pancreas may act as a surrogate marker for the response to NAT and a loss of that marker translates into a CR-POPF. Whether soft pancreatic texture is intrinsically associated with the gland or reflects a nonresponsive disease, and consequently a patient condition leading to higher risk of complication, is unclear.
To our knowledge, this is the first study specifically addressing whether CR-POPF is associated with patient long-term outcome after NAT. We found that the OS rate was halved in patients who experienced a CR-POPF compared with patients with no fistula. This outcome was also shown in multivariate analysis, where CR-POPF was an independent prognostic variable of worse survival, with a HR of 2.80, together with other well-established predictors of survival, such as tumor size, positive nodal status, positive resection margins, and high CA19-9 levels.
A recent study from our institution including patients with previous NAT reported that major complication occurring after pancreatoduodenectomy is significantly associated with reductions in long term survival in comparison with survival in patients without major complications.39 Yet, we observed an exclusive association between CR-POPF and survival, which was independent of the type and severity of other complications. We believe this finding may be representative of a diminished response to NAT, thereby resulting in a softer gland in conjunction with an expectedly lower survival rate. It has been described that an effective response to NAT translates into fibrotic remodeling of the pancreas, and that this response is associated with improved survival.4 It is also possible that an impaired immunologic status following NAT results in an even worse long-term outcome once CR-POPF occurs. Release of proinflammatory mediators has been described with large tumor burdens,40 and a recent study in esophagogastric cancer showed that patients with a high inflammatory score who were receiving NAT experienced a shorter overall and disease-free survival than those with a normal inflammatory status.41 In addition, during NAT, patients often experience hematologic abnormalities, such as neutropenia, with consequences for long-term survival that are poorly understood.42
Limitations
The present study has several limitations. First, the retrospective design cannot exclude confounders, such as the occurrence of any complications during NAT, the need for suspension of treatment, or the use of specific medications, such as corticosteroids or growth factors, which could have affected CR-POPF risk or OS. Moreover, we detected a low rate of CR-POPF, and therefore, no subgroup comparisons according to the severity of fistulas or the type of NAT were possible. In addition, no subgroup analysis of patients who received only neoadjuvant chemotherapy was performed, as the small sample size according to the fistula rate would have biased any statistical considerations. Also, despite the independent association between the fistula and OS, the indications for additional adjuvant treatment in our NAT group were not uniform.
Conclusions
Neoadjuvant therapy appears to provide significant protection from the development of CR-POPF, which is reduced by more than 60%. In addition, standard factors associated with CR-POPF appear to be no longer applicable following NAT. However, when CR-POPF occurs, it may severely impair long-term survival, independent of pathologic findings, receipt of adjuvant treatment, and CA19-9 levels. Therefore, patients with CR-POPF should undergo closer follow-up and perhaps be considered for additional chemotherapy. Future prospective validation studies are needed to confirm these findings.
eFigure. Occurrence of CR-POPF According to the Receipt and Type of NAT
References
- 1.Del Chiaro M, Søreide K. Trials and tribulations of neoadjuvant therapy in pancreatic cancer. Br J Surg. 2018;105(11):1387-1389. doi: 10.1002/bjs.11003 [DOI] [PubMed] [Google Scholar]
- 2.Michelakos T, Pergolini I, Fernández-del Castillo C, et al. Predictors of resectability and survival in patients with borderline and locally advanced pancreatic cancer who underwent neoadjuvant treatment with FOLFIRINOX. Ann Surg. 2019;269(4):733-740. doi: 10.1097/SLA.0000000000002600 [DOI] [PubMed] [Google Scholar]
- 3.Hackert T, Sachsenmaier M, Hinz U, et al. Locally advanced pancreatic cancer: neoadjuvant therapy with FOLFIRINOX results in resectability in 60% of the patients. Ann Surg. 2016;264(3):457-463. doi: 10.1097/SLA.0000000000001850 [DOI] [PubMed] [Google Scholar]
- 4.He J, Blair AB, Groot VP, et al. Is a pathological complete response following neoadjuvant chemoradiation associated with prolonged survival in patients with pancreatic cancer? Ann Surg. 2018;268(1):1-8. doi: 10.1097/SLA.0000000000002672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. 2015;261(1):12-17. doi: 10.1097/SLA.0000000000000867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marchegiani G, Andrianello S, Nessi C, et al. Neoadjuvant therapy versus upfront resection for pancreatic cancer: the actual spectrum and clinical burden of postoperative complications. Ann Surg Oncol. 2018;25(3):626-637. doi: 10.1245/s10434-017-6281-9 [DOI] [PubMed] [Google Scholar]
- 7.Loos M, Strobel O, Legominski M, et al. Postoperative pancreatic fistula: Microbial growth determines outcome. Surgery. 2018;164(6):1185-1190. doi: 10.1016/j.surg.2018.07.024 [DOI] [PubMed] [Google Scholar]
- 8.McMillan MT, Christein JD, Callery MP, et al. Comparing the burden of pancreatic fistulas after pancreatoduodenectomy and distal pancreatectomy. Surgery. 2016;159(4):1013-1022. doi: 10.1016/j.surg.2015.10.028 [DOI] [PubMed] [Google Scholar]
- 9.Ferrone CR, Warshaw AL, Rattner DW, et al. Pancreatic fistula rates after 462 distal pancreatectomies: staplers do not decrease fistula rates. J Gastrointest Surg. 2008;12(10):1691-1697. doi: 10.1007/s11605-008-0636-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt CM, Choi J, Powell ES, et al. Pancreatic fistula following pancreaticoduodenectomy: clinical predictors and patient outcomes. HPB Surg. 2009;2009:404520. doi: 10.1155/2009/404520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hackert T, Hinz U, Pausch T, et al. Postoperative pancreatic fistula: we need to redefine grades B and C. Surgery. 2016;159(3):872-877. doi: 10.1016/j.surg.2015.09.014 [DOI] [PubMed] [Google Scholar]
- 12.Bassi C, Marchegiani G, Dervenis C, et al. ; International Study Group on Pancreatic Surgery (ISGPS) . The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161(3):584-591. doi: 10.1016/j.surg.2016.11.014 [DOI] [PubMed] [Google Scholar]
- 13.Veillette G, Dominguez I, Ferrone C, et al. Implications and management of pancreatic fistulas following pancreaticoduodenectomy: the Massachusetts General Hospital experience. Arch Surg. 2008;143(5):476-481. doi: 10.1001/archsurg.143.5.476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMillan MT, Vollmer CM Jr, Asbun HJ, et al. The Characterization and prediction of ISGPF grade C fistulas following pancreatoduodenectomy. J Gastrointest Surg. 2016;20(2):262-276. doi: 10.1007/s11605-015-2884-2 [DOI] [PubMed] [Google Scholar]
- 15.Fuks D, Piessen G, Huet E, et al. Life-threatening postoperative pancreatic fistula (grade C) after pancreaticoduodenectomy: incidence, prognosis, and risk factors. Am J Surg. 2009;197(6):702-709. doi: 10.1016/j.amjsurg.2008.03.004 [DOI] [PubMed] [Google Scholar]
- 16.Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM Jr. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013;216(1):1-14. doi: 10.1016/j.jamcollsurg.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 17.McMillan MT, Soi S, Asbun HJ, et al. Risk-adjusted outcomes of clinically relevant pancreatic fistula following pancreatoduodenectomy: a model for performance evaluation. Ann Surg. 2016;264(2):344-352. doi: 10.1097/SLA.0000000000001537 [DOI] [PubMed] [Google Scholar]
- 18.Hong TS, Ryan DP, Borger DR, et al. A phase 1/2 and biomarker study of preoperative short course chemoradiation with proton beam therapy and capecitabine followed by early surgery for resectable pancreatic ductal adenocarcinoma. Int J Radiat Oncol Biol Phys. 2014;89(4):830-838. doi: 10.1016/j.ijrobp.2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fong ZV, Alvino DML, Fernández-Del Castillo C, et al. Reappraisal of staging laparoscopy for patients with pancreatic adenocarcinoma: a contemporary analysis of 1001 patients. Ann Surg Oncol. 2017;24(11):3203-3211. doi: 10.1245/s10434-017-5973-5 [DOI] [PubMed] [Google Scholar]
- 20.Tol JA, Gouma DJ, Bassi C, et al. ; International Study Group on Pancreatic Surgery . Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery. 2014;156(3):591-600. doi: 10.1016/j.surg.2014.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neoptolemos JP, Palmer DH, Ghaneh P, et al. ; European Study Group for Pancreatic Cancer . Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011-1024. doi: 10.1016/S0140-6736(16)32409-6 [DOI] [PubMed] [Google Scholar]
- 22.Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC Cancer Staging Manual: pancreas and hepatobiliary cancers. Ann Surg Oncol. 2018;25(4):845-847. doi: 10.1245/s10434-017-6025-x [DOI] [PubMed] [Google Scholar]
- 23.Strobel O, Hank T, Hinz U, et al. Pancreatic cancer surgery: the new R-status counts. Ann Surg. 2017;265(3):565-573. doi: 10.1097/SLA.0000000000001731 [DOI] [PubMed] [Google Scholar]
- 24.Verma V, Li J, Lin C. Neoadjuvant therapy for pancreatic cancer: systematic review of postoperative morbidity, mortality, and complications. Am J Clin Oncol. 2016;39(3):302-313. doi: 10.1097/COC.0000000000000278 [DOI] [PubMed] [Google Scholar]
- 25.Blair AB, Rosati LM, Rezaee N, et al. Postoperative complications after resection of borderline resectable and locally advanced pancreatic cancer: the impact of neoadjuvant chemotherapy with conventional radiation or stereotactic body radiation therapy. Surgery. 2018;163(5):1090-1096. doi: 10.1016/j.surg.2017.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer DH, Stocken DD, Hewitt H, et al. A randomized phase 2 trial of neoadjuvant chemotherapy in resectable pancreatic cancer: gemcitabine alone versus gemcitabine combined with cisplatin. Ann Surg Oncol. 2007;14(7):2088-2096. doi: 10.1245/s10434-007-9384-x [DOI] [PubMed] [Google Scholar]
- 27.Sahora K, Kuehrer I, Eisenhut A, et al. NeoGemOx: Gemcitabine and oxaliplatin as neoadjuvant treatment for locally advanced, nonmetastasized pancreatic cancer. Surgery. 2011;149(3):311-320. doi: 10.1016/j.surg.2010.07.048 [DOI] [PubMed] [Google Scholar]
- 28.Rose JB, Rocha FG, Alseidi A, et al. Extended neoadjuvant chemotherapy for borderline resectable pancreatic cancer demonstrates promising postoperative outcomes and survival. Ann Surg Oncol. 2014;21(5):1530-1537. doi: 10.1245/s10434-014-3486-z [DOI] [PubMed] [Google Scholar]
- 29.Takahashi H, Ogawa H, Ohigashi H, et al. Preoperative chemoradiation reduces the risk of pancreatic fistula after distal pancreatectomy for pancreatic adenocarcinoma. Surgery. 2011;150(3):547-556. doi: 10.1016/j.surg.2011.03.001 [DOI] [PubMed] [Google Scholar]
- 30.Czosnyka NM, Borgert AJ, Smith TJ. Pancreatic adenocarcinoma: effects of neoadjuvant therapy on post-pancreatectomy outcomes—an American College of Surgeons National Surgical Quality Improvement Program targeted variable review. HPB (Oxford). 2017;19(10):927-932. doi: 10.1016/j.hpb.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 31.Cooper AB, Parmar AD, Riall TS, et al. Does the use of neoadjuvant therapy for pancreatic adenocarcinoma increase postoperative morbidity and mortality rates? J Gastrointest Surg. 2015;19(1):80-86. doi: 10.1007/s11605-014-2620-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ecker BL, McMillan MT, Allegrini V, et al. Risk factors and mitigation strategies for pancreatic fistula after distal pancreatectomy: analysis of 2026 resections from the international, multi-institutional Distal Pancreatectomy Study Group. Ann Surg. 2019;269(1):143-149. doi: 10.1097/SLA.0000000000002491 [DOI] [PubMed] [Google Scholar]
- 33.Kalimuthu SN, Serra S, Dhani N, Chetty R. The spectrum of histopathological changes encountered in pancreatectomy specimens after neoadjuvant chemoradiation, including subtle and less-well-recognised changes. J Clin Pathol. 2016;69(6):463-471. doi: 10.1136/jclinpath-2016-203604 [DOI] [PubMed] [Google Scholar]
- 34.Chatterjee D, Katz MH, Rashid A, et al. Pancreatic intraepithelial neoplasia and histological changes in non-neoplastic pancreas associated with neoadjuvant therapy in patients with pancreatic ductal adenocarcinoma. Histopathology. 2013;63(6):841-851. doi: 10.1111/his.12234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shubert CR, Wagie AE, Farnell MB, et al. Clinical risk score to predict pancreatic fistula after pancreatoduodenectomy: independent external validation for open and laparoscopic approaches. J Am Coll Surg. 2015;221(3):689-698. doi: 10.1016/j.jamcollsurg.2015.05.011 [DOI] [PubMed] [Google Scholar]
- 36.Grendar J, Jutric Z, Leal JN, et al. Validation of fistula risk score calculator in diverse North American HPB practices. HPB (Oxford). 2017;19(6):508-514. doi: 10.1016/j.hpb.2017.01.021 [DOI] [PubMed] [Google Scholar]
- 37.Mungroop TH, van Rijssen LB, van Klaveren D, et al. ; Dutch Pancreatic Cancer Group . Alternative fistula risk score for pancreatoduodenectomy (a-FRS): design and international external validation. Ann Surg. 2019;269(5):937-943. doi: 10.1097/SLA.0000000000002620 [DOI] [PubMed] [Google Scholar]
- 38.Pratt WB, Callery MP, Vollmer CM Jr. Risk prediction for development of pancreatic fistula using the ISGPF classification scheme. World J Surg. 2008;32(3):419-428. doi: 10.1007/s00268-007-9388-5 [DOI] [PubMed] [Google Scholar]
- 39.Sandini M, Ruscic KJ, Ferrone CR, et al. Major complications independently increase long-term mortality after pancreatoduodenectomy for cancer [published online September 17, 2018]. J Gastrointest Surg. [DOI] [PubMed] [Google Scholar]
- 40.Falconer JS, Fearon KC, Plester CE, Ross JA, Carter DC. Cytokines, the acute-phase response, and resting energy expenditure in cachectic patients with pancreatic cancer. Ann Surg. 1994;219(4):325-331. doi: 10.1097/00000658-199404000-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jomrich G, Hollenstein M, John M, et al. The modified Glasgow prognostic score is an independent prognostic indicator in neoadjuvantly treated adenocarcinoma of the esophagogastric junction. Oncotarget. 2018;9(6):6968-6976. doi: 10.18632/oncotarget.24087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang XF, Huang WF, Nie J, Zhou Y, Tan DW, Jiang JH. Toxicity of chemotherapy regimens in advanced and metastatic pancreatic cancer therapy: A network meta-analysis. J Cell Biochem. 2018;119(7):5082-5103. doi: 10.1002/jcb.26266 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Occurrence of CR-POPF According to the Receipt and Type of NAT