Key Points
Question
Can sudden cardiac death risk in children with hypertrophic cardiomyopathy be predicted?
Findings
In this cohort study of 1024 consecutively evaluated children (age ≤16 years), a prognostic model was developed using preselected predictor variables identified from the literature. The model’s ability to predict risk at 5 years was internally validated using bootstrapping.
Meaning
This new, validated risk stratification model for sudden cardiac death risk in childhood hypertrophic cardiomyopathy may provide individualized estimates of risk at 5 years using readily obtained data on clinical risk factors; external validation studies are required to demonstrate the accuracy of this model's predictions in diverse patient populations.
Abstract
Importance
Sudden cardiac death (SCD) is the most common mode of death in childhood hypertrophic cardiomyopathy (HCM), but there is no validated algorithm to identify those at highest risk.
Objective
To develop and validate an SCD risk prediction model that provides individualized risk estimates.
Design, Setting, and Participants
A prognostic model was developed from a retrospective, multicenter, longitudinal cohort study of 1024 consecutively evaluated patients aged 16 years or younger with HCM. The study was conducted from January 1, 1970, to December 31, 2017.
Exposures
The model was developed using preselected predictor variables (unexplained syncope, maximal left-ventricular wall thickness, left atrial diameter, left-ventricular outflow tract gradient, and nonsustained ventricular tachycardia) identified from the literature and internally validated using bootstrapping.
Main Outcomes and Measures
A composite outcome of SCD or an equivalent event (aborted cardiac arrest, appropriate implantable cardioverter defibrillator therapy, or sustained ventricular tachycardia associated with hemodynamic compromise).
Results
Of the 1024 patients included in the study, 699 were boys (68.3%); mean (interquartile range [IQR]) age was 11 (7-14) years. Over a median follow-up of 5.3 years (IQR, 2.6-8.3; total patient years, 5984), 89 patients (8.7%) died suddenly or had an equivalent event (annual event rate, 1.49; 95% CI, 1.15-1.92). The pediatric model was developed using preselected variables to predict the risk of SCD. The model’s ability to predict risk at 5 years was validated; the C statistic was 0.69 (95% CI, 0.66-0.72), and the calibration slope was 0.98 (95% CI, 0.59-1.38). For every 10 implantable cardioverter defibrillators implanted in patients with 6% or more of a 5-year SCD risk, 1 patient may potentially be saved from SCD at 5 years.
Conclusions and Relevance
This new, validated risk stratification model for SCD in childhood HCM may provide individualized estimates of risk at 5 years using readily obtained clinical risk factors. External validation studies are required to demonstrate the accuracy of this model's predictions in diverse patient populations.
This cohort study develops a model to use in risk stratification for sudden cardiac death in children with hypertrophic cardiomyopathy.
Introduction
Sudden cardiac death (SCD) is the most common mode of death outside of infancy in childhood hypertrophic cardiomyopathy (HCM),1,2 with higher annual rates compared with those in adults with the disease.3 Current practice guidelines recommend primary prevention implantable cardioverter defibrillators (ICDs) in children based on the presence of clinical risk factors for SCD extrapolated mostly from observational adult studies,4,5 but this approach poorly discriminates risk in both adult and pediatric populations.6,7 A clinical risk tool (HCM Risk-SCD) that estimates the 5-year risk of SCD was developed8 and validated in adults with HCM.9,10,11,12 The aim of this study was to develop a similar pediatric SCD risk model using a large, international cohort and compare its performance with the adult model.
Methods
Study Population
The study cohort consisted of patients aged 1 to 16 years with HCM who were consecutively evaluated between January 1, 1970, and December 31, 2017, in 39 participating centers located in 17 countries (the International Paediatric Hypertrophic Cardiomyopathy Consortium; eTable 1 in the Supplement). A diagnosis of HCM was defined as a left-ventricular wall thickness greater than 2 SDs above the body surface area–corrected population mean (z score ≥2) that could not be explained solely by abnormal loading conditions or in accordance with published criteria for familial disease.5 Patients with prior ventricular fibrillation or sustained ventricular tachycardia (VT), known inborn errors of metabolism or syndromic causes of HCM (eg, RASopathy syndromes, Friedreich ataxia), presentation under 1 year, or less than 1 month of follow-up were excluded (eFigure 1 in the Supplement). The authors from each participating center guaranteed the integrity of data from their institution and had approval from a local ethics committee with waiver of informed consent.
Patient Assessment and Data Collection
Anonymized, noninvasive clinical data from a baseline evaluation were collected retrospectively, including demographics, cause of the disease, heart failure symptoms (New York Heart Association [NYHA]/Ross functional classification13), pedigree analysis, resting and ambulatory 12-lead electrocardiogram, and 2-dimensional Doppler and color transthoracic echocardiogram (from contemporaneously written reports). Patients had planned clinical reviews every 6 to 18 months. Data were collected independently at each participating center.
Clinical Outcomes
The primary study end point was a composite outcome of SCD or an equivalent event (aborted cardiac arrest, appropriate ICD therapy, or sustained VT associated with hemodynamic compromise).8,11,14,15 As in previous studies, ICD therapy was considered appropriate if the tachyarrhythmia was ventricular in origin.11,16,17 Sudden cardiac death was defined as a witnessed sudden death with or without documented cardiac failure, death within 1 hour of new symptoms, or nocturnal deaths with no antecedent history of worsening symptoms.18 Outcomes were ascertained by the treating cardiologist at each center.
Selection of Predictor Variables
A systematic review of the literature was performed in December 201519 to identify SCD risk factors with sufficient evidence to support their inclusion as predictor variables in the risk model (eTable 2 in the Supplement). Clinical risk factors were included as predictor variables if they had been examined in more than 2 published studies and independently associated with SCD in 2 or more univariable or multivariable survival analyses. Selection of predictor variables was not limited to studies with multivariable analyses owing to the limited evidence base available in pediatric HCM (all but 1 study included in the meta-analysis was retrospective, the majority had fewer than 150 participants, and most published studies used only univariable analyses).19 Candidate predictors are defined in Table 1.1,8,13,14,15,16,17,20,21,22,23 To account for somatic growth, maximal wall thickness and left atrial diameter measurements are expressed as z scores (defined as the number of SDs above or below the body surface area–corrected mean as a given measurement’s mean).24 The largest published reference populations for interventricular septal thickness22 and left atrial diameter23 were chosen following a review of the literature.
Table 1. Candidate Predictor Definitions.
| Candidate Predictor Variable | Definition | Coding |
|---|---|---|
| NYHA/Ross functional class | NYHA functional classification20/modified Ross heart failure classification for children13 at baseline evaluation | Binary (NYHA/Ross 1 = 0, NYHA/Ross ≥2 = 1) |
| Unexplained syncope | Defined as a transient loss of consciousness with no identifiable cause at or before first evaluation1,8,14,15 | Binary (no = 0, yes = 1) |
| Nonsustained ventricular tachycardia | ≥3 Consecutive ventricular beats at a rate of ≥120 beats/min lasting <30 s on ambulatory ECG monitoring (minimum duration 24 h) at or before first evaluation16,21 | Binary (no = 0, yes = 1) |
| Maximal wall thickness z score | Defined as the number of SDs from the population mean22; the 2-D measurement of maximal wall thickness (millimeters) is at baseline evaluationa | Continuous (z score) |
| Left atrial diameter z score | Defined as the number of SDs from the population mean23 the 2-D measurement of maximal left-atrial diameter (millimeters) is at baseline evaluationb | Continuous (z score) |
| Maximal LV outflow tract gradient | The maximum LV outflow tract gradient at rest or with Valsalva provocation using continuous wave Doppler from the apical 3- or 5-chamber views17c | Continuous (mm Hg) |
Abbreviations: 2-D, 2 dimensional; ECG, electrocardiogram; LV, left ventricular; NYHA, New York Heart Association.
Maximal wall thickness is the greatest thickness as measured by 2-D echocardiography in the parasternal short-axis views of the left ventricle in 4 places at the level of the mitral valve and papillary muscles (anterior and posterior septum, lateral and posterior wall) and in 2 places at the apical level (anterior and posterior septum).5
Left atrial diameter is determined by M mode or 2-D echocardiography in the parasternal long-axis plane.
Peak outflow tract gradient is determined using the modified Bernoulli equation: gradient = 4V2, where V is the peak aortic outflow velocity.
Statistical Analysis
Continuous variables are described as mean (SD) or median and interquartile range (IQR) as appropriate. Because the model was developed to predict 5-year SCD risk during childhood (age ≤16 years), the follow-up of patients was censored at age 22 years. The follow-up time for all patients was thus calculated from the date of their first evaluation to the date of reaching the study end point, death from another cause, or the date of their most recent evaluation prior to the end of the study period (December 2017 or age 22 years). Kaplan-Meier survival plots were used to describe the failure times.
Handling of Missing Data
Patients with more than 50% of the preselected predictors missing were excluded from model development. Logistic regression was used to identify predictors of missingness. The values for the missing predictors were imputed using multiple imputation techniques based on chained equations.25 The imputation model included all predictors of missingness, the outcome, all prespecified predictors of the risk model, and the estimate of the cumulative hazard function.26 A total of 49 imputed data sets were generated, and estimates obtained from the imputed data sets were combined using the Rubin rule.27
Development of the Pediatric Model
A minimum of 10 SCD or equivalent events are required per coefficient in the model to estimate the regression coefficients with adequate precision.28 This means that 50 SCD events would be required to allow estimation of the regression coefficients for the 5 preselected predictors with adequate precision. Additional events would be required for inclusion of nonlinear terms associated with the continuous predictors in the model.
All continuous predictors were centered around their mean values, and univariable Cox proportional hazards regression models were used to test the assumption of linearity with the outcome for each continuous predictor. The final model was developed using a Cox proportional hazards regression model including all 5 preselected predictors and quadratic terms for the continuous predictors where nonlinearity was found in the univariable analysis. The proportional hazards assumption was investigated using Schoenfeld residuals.29 A sensitivity analysis was performed by including the predictors of missingness in the final model. All regression models were fitted using robust SEs to account for clustering by center.30
The probability of SCD at t years for an individual patient can be calculated using the following equation, derived from the Cox proportional hazards regression model:
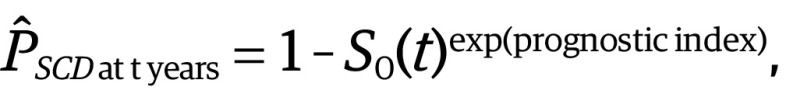 |
where SO(t) is the average survival probability at time t and the prognostic index is the sum of the products of the predictors and their coefficients.
Bootstrapping was used to evaluate the performance of the model since this is the most efficient validation procedure as all aspects of the model development are validated.31 For this purpose, 200 bootstrap samples were generated from each imputed data set and estimates were combined. Because the aim of the model is to predict 5-year SCD risk, patients were censored at 5 years from their first evaluation. The calibration slope was used to assess the degree of agreement between the observed and predicted hazards of SCD (a value close to 1 suggests good overall agreement).32 The C index (C-Uno33) was used to measure how well the model discriminated between high- and low-risk patients.34 A value of 1 indicates perfect discrimination and a value of 0.5 indicates no discrimination. The C index and calibration results presented are an average of the bootstrapped samples. Graphic comparisons of the observed and predicted risk of SCD at 5 years by risk groups (0%-<2%, 2%-<4%, 4%-<6%, and ≥6%) based on an imputed development sample are provided.
The model development process is summarized in eFigure 1 in the Supplement. Statistical analysis was performed using Stata Statistical Software, release 14 (StataCorp LP).
Results
Baseline Clinical Characteristics
The study cohort comprised 1024 patients from 39 centers with a median (IQR) age at baseline evaluation of 11 (7-14) years (eFigure 2A in the Supplement); 699 patients (68.3%) were boys. A family history of HCM was present in 534 of 1006 patients (53.1%). Baseline clinical characteristics are described in Table 2 and eTable 3 in the Supplement.
Table 2. Baseline Clinical Characteristicsa.
| Baseline Clinical Characteristic | No. (%) |
|---|---|
| Age, median (IQR), y | 11 (7-14) |
| Male sex | 699 (68.3) |
| Family history | |
| HCM (n = 1006) | 534 (53.1) |
| SCD (n = 1020) | 130 (12.8) |
| Unexplained syncope (n = 1023) | 102 (9.9) |
| NYHA/Ross classification (n = 1006) | |
| 1 | 783 (77.8) |
| 2 | 191 (19) |
| 3 | 29 (2.9) |
| 4 | 3 (0.3) |
| Medical therapy at baseline (n = 1021) | |
| None | 596 (58.4) |
| β-Blockers | 410 (40.2) |
| Amiodarone | 9 (0.9) |
| Other | 6 (0.6) |
| NSVT on ambulatory ECG (n = 856) | 55 (6.4) |
| MWT, mm | |
| No. | 997 |
| Mean (SD) | 17.1 (7.4) |
| z Score MWT | |
| No. | 906 |
| Mean (SD) | 11.1 (7.1) |
| LA diameter, mm | |
| No. | 712 |
| Mean (SD) | 33.4 (8.5) |
| z Score LA diameter | |
| No. | 675 |
| Mean (SD) | 1.9 (2.3) |
| LVOTg, max | |
| No. | 871 |
| Median (IQR) | 9 (6-22) |
Abbreviations: ECG, electrocardiogram; HCM, hypertrophic cardiomyopathy; IQR, interquartile range; LA, left atrium; LVOTg max, maximal left-ventricular outflow tract gradient; MWT, maximal wall thickness; NSVT, nonsustained ventricular tachycardia; NYHA, New York Heart Association; SCD, sudden cardiac death.
Total of 1024 patients unless otherwise indicated.
Clinical Course and Outcomes During Follow-up
Over a follow-up period of 5984 patient-years (median, 5.3 years; IQR, 2.6-8.3), 77 patients (7.5%) underwent a myectomy, 43 patients (4.2%) required a permanent pacemaker, and 21 patients (2.1%) underwent cardiac transplantation. A total of 267 patients (26.1%) received an ICD for primary (244 [91.4%]) or secondary (23 [8.6%]) prevention of SCD. Fifty-three patients (5.2%) died (SCD, 30 [56.6%]; heart failure, 9 [17.0%], heart failure related, 6 [11.3%]; other cardiovascular related, 3 [5.7%]; non–cardiovascular related, 2 [3.8%]; and unknown cause, 3 [5.7%]), with an annual mortality rate of 0.89 (95% CI, 0.68-1.16). Eighty-nine patients reached the SCD or equivalent end point within 5 years (SCD, 39 [43.8%]; aborted SCD, 16 [18.0%]; appropriate ICD discharge, 24 [27.0%]; and hemodynamically compromising, sustained VT, 10 [11.2%]), with an annual SCD end point rate of 1.49 (95% CI, 1.15-1.92) (eFigure 2B in the Supplement). Baseline clinical characteristics of patients with and without SCD or SCD equivalent end point and results from univariable regression analyses are reported in Table 3. NYHA class 2 or higher, unexplained syncope, nonsustained VT (NSVT), maximal wall thickness (MWT), and left atrium (LA) diameter were associated with SCD at the 5% significance level.
Table 3. Clinical Characteristics of Patients With and Without Sudden Cardiac Death End Points and Univariable Cox Proportional Hazards Regression Models.
| Characteristic | No. (%) | Hazard Ratio (95% CI) | P Value | ||
|---|---|---|---|---|---|
| Whole Cohort (N = 1024) | Patients With SCD End Points (n = 89) | Patients Without SCD End Points (n = 938) | |||
| Age, median (IQR) | 11 (7-14) | 10 (6-13) | 11 (7-14) | 1.05 (1.00-1.11) | .06 |
| Male sex | 699 (68.3) | 65 (73) | 634 (67.8) | 0.73 (0.50-1.17) | .20 |
| NYHA class >1 | 223 (22.2) | 28 (31.8) | 195 (21.2) | 1.70 (1.08-2.65) | .02 |
| Family history | |||||
| SCD | 130 (12.8) | 12 (13.5) | 118 (12.7) | 1.01 (0.55-1.85) | .98 |
| HCM | 534 (53.1) | 42 (48.3) | 492 (53.5) | 0.83 (0.55-1.27) | .39 |
| Unexplained syncope | 102 (9.9) | 16 (18) | 86 (9.2) | 2.06 (1.20-3.54) | .009 |
| NSVT | 55 (6.4) | 12 (16.4) | 43 (5.5) | 1.93 (1.03-3.61) | .04 |
| MWT, mean (SD), mm | 17.1 (7.4) | 20 (7.5) | 16.9 (7.3) | 1.05 (1.02-1.07) | <.001 |
| z Score MWT, mean (SD) | 11.1 (7.1) | 15 (7.5) | 10.7 (7) | 1.05 (1.02-1.08) | <.001 |
| MWT ≥30 mm | 81 (7.9) | 25 (31.7) | 164 (20.7) | 2.35 (1.33-4.18) | .004 |
| LA diameter, mean (SD), mm | 33.4 (8.5) | 36.5 (9.3) | 33 (8.3) | 1.05 (1.02-1.08) | .001 |
| z Score LA diameter, mean (SD) | 1.9 (2.3) | 3.2 (2.6) | 1.8 (2.2) | 1.19 (1.08-1.30) | <.001 |
| LVOTg max, median (IQR), mm Hg | 9 (6-22) | 12 (6-36) | 9 (6-20) | 1.00 (0.99-1.01) | .83 |
| LVOT ≥30 mm Hg | 189 (18.4) | 25 (31.7) | 164 (20.7) | 1.48 (0.92-2.38) | .11 |
Abbreviations: HCM, hypertrophic cardiomyopathy; LA, left atrium; IQR, interquartile range; LVOTg max, maximal left-ventricular outflow tract gradient; MWT, maximal wall thickness; NSVT, nonsustained ventricular tachycardia; NYHA, New York Heart Association; SCD, sudden cardiac death.
Model Development
The risk model was developed using the entire follow-up data and all events that occurred during the follow-up (ie, 1029 patients with 89 events.) A risk model was developed using the preselected variables (unexplained syncope, NSVT, LA diameter z score, MWT z score, and left-ventricular outflow tract [LVOT] gradient); the estimates of hazard ratios for the resulting model are reported in Table 4. Missing data for the preselected variables are summarized in eTable 4 in the Supplement. Complete data were available for 527 patients (51.5%); at least 1 predictor variable was missing in 48.5% of the sample. Including NYHA classification (the only additional predictor of missingness) as a predictor variable had little association with the estimates of the hazard ratios. The risk of SCD at 5 years for an individual patient with HCM can be calculated from the following equation as demonstrated in the HCM Risk-Kids calculator using Excel (Microsoft Corp) (depicted in the eAppendix in the Supplement).
| P(SCD at 5 years) = 1 − 0.949437808exp(prognostic index), |
where prognostic index = 0.2171364 • (MWT z score – 11.09) – 0.0047562 • (MWT z score2 – 174.12) + 0.130365 • (LA diameter z score – 1.92) + 0.429624 • unexplained syncope + 0.1861694 • NSVT – 0.0065555 • (maximal LVOT gradient – 21.8).
Table 4. Pediatric Sudden Cardiac Death Risk Prediction Model and Sensitivity Analyses for Predictor of Missingness.
| Predictor Variable | SCD Risk Prediction Model | Sensitivity Analysis: Model Including Predictors of Missingness | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| NSVT | 1.20 (0.53-2.76) | .66 | 1.16 (0.52-2.61) | .72 |
| LA diameter z score | 1.14 (1.03-1.26) | .01 | 1.13 (1.03-1.25) | .01 |
| MWT | ||||
| z Score | 1.24 (1.07-1.45) | .005 | 1.24 (1.07-1.44) | .005 |
| z Score2 | 0.995 (0.99-1.00) | .04 | 0.995 (0.99-1.00) | .04 |
| LVOT gradient | 0.99 (0.99-1.00) | .10 | 0.99 (0.99-1.00) | .11 |
| Unexplained syncope | 1.54 (0.79-2.98) | .20 | 1.52 (0.79-2.92) | .22 |
| NYHA | 1.15 (0.62-2.11) | .66 | ||
| Uno C statistic | 0.69 (0.66-0.72) | NA | 0.69 (0.66-0.72) | NA |
| Calibration slope | 0.98 (0.59-1.38) | NA | 0.96 (0.56-1.36) | NA |
Abbreviations: LA, left atrium; LVOT, left ventricular outflow tract; MWT, maximal wall thickness; NA, not applicable; NSVT, nonsustained ventricular tachycardia; NYHA, New York Heart Association; SCD, sudden cardiac death.
Validation
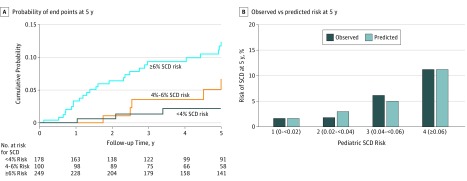
The performance of the model for predicting risk at 5 years was assessed using 1029 patients with 58 events. The C index was 0.69 (95% CI, 0.66-0.72) and calibration slope was 0.98 (95% CI, 0.59-1.38). Figure, B shows the comparison between the observed and predicted 5-year risk of SCD by clinical risk groups for 1 randomly selected imputed data set.
Figure. Performance of the HCM Risk-Kids Model.
A, Kaplan-Meier curve showing cumulative probability of sudden cardiac death (SCD) end points within 5 years of baseline evaluation by clinical risk groups calculated by the pediatric SCD risk model. Patients with complete data for the calculation of 5-year SCD risk estimates (n = 527) were classified into 3 risk groups (<4%, 4%-<6%, and ≥6%). B, Comparison of observed and predicted risk by clinical risk group of the pediatric SCD risk model (for 1 imputed data set). Vertical bars represent observed (dark blue) and model-based predicted (light blue) probability of SCD by 5 years. HCM indicates hypertrophic cardiomyopathy.
Clinical Implications
The clinical implications of the model were examined in 527 patients with 34 SCD end points who had complete data to allow calculation of 5-year SCD risk. The SCD end point was reached by 3 patients (1.7%) with a predicted risk lower than 4% (n = 178), 5 patients (5%) with a predicted risk of 4% to less than 6% (n = 100), and 26 patients (10.4%) with a predicted risk of 6% or greater (n = 249) (Figure, A). Using a 5-year SCD risk of 6% or greater to recommend primary prevention with ICD implantation would identify 26 of 34 SCD end points (76.5%), with ICD implantation in 223 of 493 patients (45.2%) not reaching SCD end points within 5 years. Using a 5-year SCD risk of 4% or greater to recommend primary prevention ICD implantation would identify 31 of 34 SCD end points (91.2%), with ICD implantation in 318 of 493 patients (64.5%) not reaching SCD end points within 5 years.
The pediatric model was developed using preselected variables to predict the risk of SCD. The model’s ability to predict risk at 5 years was validated; the C statistic was 0.69 (95% CI, 0.66-0.72), and the calibration slope was 0.98 (95% CI, 0.59-1.38). For every 10 ICDs implanted in patients with 6% or more of a 5-year SCD risk, 1 patient may potentially be saved from SCD at 5 years.
Comparison With Adult Risk Stratification Tool
The performance of the pediatric model to predict 5-year risk of SCD was compared with that of the adult risk stratification tool (HCM-Risk SCD). The adult model has modest discriminatory ability (C index, 0.67; 95% CI, 0.65-0.69) but does not predict risk accurately (calibration slope, 0.79; 95% CI, 0.43-1.15) for the pediatric cohort. It seems the risk of SCD is underestimated for all risk groups (eFigure 3 in the Supplement). Including age and family history of SCD in the pediatric model did not improve its performance (eTable 5 in the Supplement).
Discussion
To our knowledge, the model presented herein represents the first validated approach to risk stratification in childhood HCM and suggests that systematic risk evaluation can be used to guide ICD implantation in young patients with the disease. Compared with the prevalence in adults, HCM is a relatively uncommon disease in childhood and has a more diverse cause.1,35 Nevertheless, outside of infancy, the disease is caused mostly by mutations in sarcomere protein genes36,37 and results in SCD in a significant minority of children.3,38 Current approaches to risk stratification in childhood HCM have remained largely unchanged for more than 2 decades, with reliance on the assessment of a small number of clinical features (risk factors) to guide treatment decisions.4,5 A recent validation study of this approach to risk stratification has shown it to have limited discriminatory power (C index statistic, 0.62) with a positive predictive value of only 19%.6 In adults with HCM, there has been a shift toward quantitative risk prediction based on tools, such as HCM Risk-SCD,8 that form the basis of recommendations for ICD therapy based on absolute thresholds of risk. However, the HCM Risk-SCD tool is not recommended for use in childhood as patients younger than 16 years were specifically excluded in its development and echocardiographic variables were not corrected for body surface area, which led us to develop a new, pediatric-specific risk model. This study shows that, if applied to children, the existing adult model may underestimate the incidence of SCD for all risk groups and has a limited discriminatory power.
The new pediatric model that we have developed shows better discrimination between high- and low-risk patients with good calibration between the expected and observed risk. The performance is similar to that reported in adult cohorts for the adult model.11 Predictor variables were included only if previously associated with SCD in published studies with the result that family history of SCD and age at presentation were excluded. The lack of evidence in current literature supporting family history of SCD could be explained by a higher prevalence of de novo mutations in childhood, incomplete reporting of family history, or failure to adjust for family linkage. Although presentation of HCM in young adulthood has been linked with adverse outcomes,39 age was not included as a predictor variable as, outside of infancy,1 its role in prognosis remains unclear. In keeping with this rationale, including both age and family history of SCD as predictor variables did not improve the model’s performance. The effect of age may have been mitigated in the model by the fact that somatic growth in childhood was accounted for by using body surface area–corrected rather than absolute 2-dimensional echocardiographic measurements. Although 2 previous studies reported an increased risk of SCD in the presence of heart failure symptoms,40,41 inclusion of this variable did not improve the model’s performance.
To our knowledge, this study represents the largest population of childhood HCM with nonsyndromic disease published to date. The baseline demographics were similar to those seen in previous population-based studies1,2,35,40 with the exception of familial disease, which was more prevalent than previously reported in registry studies although in keeping with reports from reference centers.36 This higher prevalence of familial disease may be explained by the exclusion of syndromic disease (eg, RASopathy, inborn errors of metabolism, or Friedreich ataxia) or by family screening as the indication for initial evaluation but could also suggest that familial disease presenting during childhood is more common than usually appreciated. Compared with similar-sized adult cohorts, there was a lower prevalence of traditional risk factors (eg, family history of SCD, NSVT) yet a higher incidence of arrhythmic events (1.49% vs 0.6%11). In addition, risk factors with significant evidence in adult practice, such as family history of SCD and LVOT gradient, were not associated with arrhythmic events on univariable analysis in this cohort. The LVOT gradient appears to be inversely associated with the risk of SCD in this population. The finding that LVOT obstruction may be protective is in agreement with another recent, large pediatric population series but needs further exploration.42 Unexplained syncope, degree of hypertrophy, LA diameter, and NSVT showed the strongest association with the study outcome, although this finding was not significant at the 15% level for NSVT. These findings are in agreement with a recent meta-analysis19 and suggest that risk factors for SCD may differ between adult and pediatric cohorts.
The complete case analysis suggests that the model identifies the majority of patients at risk of an SCD event during the follow-up period. The identification of patients at risk of SCD was at the expense of ICD implantation in 45% of patients not yet reaching the end point during follow-up. However, as previous studies have demonstrated variable latency between ICD implantation and first appropriate therapy,43 these young patients may yet benefit from the decision to implant a device. Further refinement of the model presented could be achieved by exploring the role of novel risk factors for SCD in childhood HCM, including genetic data, late gadolinium enhancement on cardiac magnetic resonance imaging,44 and the resting 12-lead electrocardiogram, which, in a single study,45 has been suggested to accurately predict the risk of SCD.
In addition, because childhood is a time of significant somatic growth, the phenotype of a patient may evolve rapidly with a resulting change in the arrhythmic risk profile. Future studies exploring the changing role of individual clinical risk factors during childhood and use of serial clinical investigations in predicting risk would be valuable.
Limitations
Because childhood HCM is a rare disease and SCD is an uncommon event, a multicenter, retrospective, longitudinal design was necessary to develop a pediatric-specific model. This study is therefore limited by inherent problems of retrospective studies, in particular, missing data. The higher proportion of patients with at least 1 missing predictor compared with the adult development cohort (48.5% in the present study vs 21.7%8) may be explained by difficulties obtaining certain investigations in young patients (eg, ambulatory electrocardiogram) and the use of contemporaneously written echocardiographic reports.
Because missing data were associated with milder hypertrophy and the absence of heart failure symptoms, the complete case analysis is inherently biased toward those with more severe disease. This bias may explain the predominance of patients with a calculated 5-year risk score of 4% or greater (n = 349/527). This bias also suggests that clinicians are more likely to investigate thoroughly in the presence of severe disease.
Because the cohort was recruited longitudinally, the length of follow-up for individual patients varied, with a median length of follow-up of 5.3 years. The longevity of an ICD device is reported to be between 5 and 9 years,46,47 although children are known to be at increased risk for lead-related complications necessitating revision.14,43 The finite battery life and need for repeated device replacements, along with the lifetime burden of complications, needs to be carefully considered by clinicians when counseling patients and their parents on ICD implantation.
Although this study includes data collected across a wide time period, medical management of children with HCM has not changed significantly over this time, and studies have previously shown no era effect on survival.3 However, patients presenting in the earliest era (pre-1990) were more likely to be symptomatic for heart failure symptoms and have NSVT detected. Patients presenting in more recent years had lower absolute MWT and corresponding z score, but this difference did not reach statistical significance. The difference in MWT and corresponding z score may be the result of patients being diagnosed at an earlier time point in disease expression, possibly through family screening, although not at a younger age. Inherent to the study design, a survival bias may exist for all eras, as patients not surviving an out-of-hospital arrest are not represented. Future studies comparing those surviving an out-of-hospital arrest with those identified postmortem would be useful but was beyond the scope of this study.
The model should be used only in patients with similar clinical characteristics to the study cohort. In particular, it should not be used in patients presenting in infancy or with syndromic disease, inborn errors of metabolism, or neuromuscular disease.3,48 Future studies exploring the risk of SCD in these subgroups are required.
The number of patients undergoing invasive treatment of LVOT obstruction in this cohort was too small to model its association with SCD risk. However, obstructive disease was uncommon in this cohort, with only 18% having a gradient above 30 mm Hg.
Conclusions
We present what we believe to be the first validated risk stratification model for SCD in childhood HCM developed from a large, international cohort using readily collected data on clinical risk factors. The individualized estimates of risk could help clinicians to identify patients at highest risk and balance the risk of an arrhythmic event with prophylactic ICD implantation in conjunction with the patient and their parents or guardians. External validation studies are now required to demonstrate the accuracy of this model’s predictions in diverse patient populations. Consensus opinion of experts will be required to determine whether absolute thresholds for ICD recommendations are needed and, if so, where those thresholds should be set. Further refinement of this model could be achieved by including novel clinical risk predictors, such as cardiac magnetic resonance imaging and genetic data.
eFigure 1. Pediatric Risk Model for Sudden Cardiac Death (SCD) in Hypertrophic Cardiomyopathy (HCM Risk-Kids)
eFigure 2. Bar Chart Showing Age Distribution of Cohort
eFigure 3. Comparison of Observed and Predicted Risk by Clinical Risk Group for External Validation of Adult HCM-Risk SCD Model
eTable 1. Number of Patients Enrolled by Participating Center
eTable 2. Summary of Candidate Predictors Following Systematic Review of Literature
eTable 3. Baseline Clinical Characteristics by Era
eTable 4. Summary of Missing Data
eTable 5. Sensitivity Analysis: Model Including Age and Family History of SCD
eAppendix. HCM Risk-Kids Calculator
eReferences.
References
- 1.Colan SD, Lipshultz SE, Lowe AM, et al. Epidemiology and cause-specific outcome of hypertrophic cardiomyopathy in children: findings from the Pediatric Cardiomyopathy Registry. Circulation. 2007;115(6):773-781. doi: 10.1161/CIRCULATIONAHA.106.621185 [DOI] [PubMed] [Google Scholar]
- 2.Alexander PMA, Nugent AW, Daubeney PEF, et al. ; National Australian Childhood Cardiomyopathy Study . Long-term outcomes of hypertrophic cardiomyopathy diagnosed during childhood: results from a national population-based study. Circulation. 2018;138(1):29-36. doi: 10.1161/CIRCULATIONAHA.117.028895 [DOI] [PubMed] [Google Scholar]
- 3.Norrish G, Field E, Mcleod K, et al. Clinical presentation and survival of childhood hypertrophic cardiomyopathy: a retrospective study in United Kingdom. Eur Heart J. 2019;40(12):986-993. doi: 10.1093/eurheartj/ehy798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gersh BJ, Maron BJ, Bonow RO, et al. ; American College of Cardiology Foundation/American Heart Association Task Force on Practice; American Association for Thoracic Surgery; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Failure Society of America; Heart Rhythm Society; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons . 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg. 2011;142(6):e153-e203. doi: 10.1016/j.jtcvs.2011.10.020 [DOI] [PubMed] [Google Scholar]
- 5.Elliott PM, Anastasakis A, Borger MA, et al. ; Authors/Task Force Members . 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(39):2733-2779. doi: 10.1093/eurheartj/ehu284 [DOI] [PubMed] [Google Scholar]
- 6.Norrish G, Ding T, Field E, et al. A validation study of the European Society of Cardiology guidelines for risk stratification of sudden cardiac death in childhood hypertrophic cardiomyopathy. [published online June 1, 2019]. Europace. 2019;euz118. doi: 10.1093/europace/euz118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Mahony C, Tome-Esteban M, Lambiase PD, et al. A validation study of the 2003 American College of Cardiology/European Society of Cardiology and 2011 American College of Cardiology Foundation/American Heart Association risk stratification and treatment algorithms for sudden cardiac death in patients with hypertrophic cardiomyopathy. Heart. 2013;99(8):534-541. doi: 10.1136/heartjnl-2012-303271 [DOI] [PubMed] [Google Scholar]
- 8.O’Mahony C, Jichi F, Pavlou M, et al. ; Hypertrophic Cardiomyopathy Outcomes Investigators . A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM Risk-SCD). Eur Heart J. 2014;35(30):2010-2020. doi: 10.1093/eurheartj/eht439 [DOI] [PubMed] [Google Scholar]
- 9.Fernández A, Quiroga A, Ochoa JP, et al. Validation of the 2014 European Society of Cardiology sudden cardiac death risk prediction model in hypertrophic cardiomyopathy in a reference center in South America. Am J Cardiol. 2016;118(1):121-126. doi: 10.1016/j.amjcard.2016.04.021 [DOI] [PubMed] [Google Scholar]
- 10.Maron BJ, Casey SA, Chan RH, Garberich RF, Rowin EJ, Maron MS. Independent assessment of the European Society of Cardiology sudden death risk model for hypertrophic cardiomyopathy. Am J Cardiol. 2015;116(5):757-764. doi: 10.1016/j.amjcard.2015.05.047 [DOI] [PubMed] [Google Scholar]
- 11.O’Mahony C, Jichi F, Ommen SR, et al. International external validation study of the 2014 European Society of Cardiology guidelines on sudden cardiac death prevention in hypertrophic cardiomyopathy (EVIDENCE-HCM). Circulation. 2018;137(10):1015-1023. doi: 10.1161/CIRCULATIONAHA.117.030437 [DOI] [PubMed] [Google Scholar]
- 12.Vriesendorp PA, Schinkel AF, Liebregts M, et al. Validation of the 2014 European Society of Cardiology guidelines risk prediction model for the primary prevention of sudden cardiac death in hypertrophic cardiomyopathy. Circ Arrhythm Electrophysiol. 2015;8(4):829-835. doi: 10.1161/CIRCEP.114.002553 [DOI] [PubMed] [Google Scholar]
- 13.Ross RD. The Ross classification for heart failure in children after 25 years: a review and an age-stratified revision. Pediatr Cardiol. 2012;33(8):1295-1300. doi: 10.1007/s00246-012-0306-8 [DOI] [PubMed] [Google Scholar]
- 14.Moak JP, Leifer ES, Tripodi D, Mohiddin SA, Fananapazir L. Long-term follow-up of children and adolescents diagnosed with hypertrophic cardiomyopathy: risk factors for adverse arrhythmic events. Pediatr Cardiol. 2011;32(8):1096-1105. doi: 10.1007/s00246-011-9967-y [DOI] [PubMed] [Google Scholar]
- 15.Ziółkowska L, Turska-Kmieć A, Petryka J, Kawalec W. Predictors of long-term outcome in children with hypertrophic cardiomyopathy. Pediatr Cardiol. 2016;37(3):448-458. doi: 10.1007/s00246-015-1298-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monserrat L, Elliott PM, Gimeno JR, Sharma S, Penas-Lado M, McKenna WJ. Non-sustained ventricular tachycardia in hypertrophic cardiomyopathy: an independent marker of sudden death risk in young patients. J Am Coll Cardiol. 2003;42(5):873-879. doi: 10.1016/S0735-1097(03)00827-1 [DOI] [PubMed] [Google Scholar]
- 17.Maron MS, Olivotto I, Betocchi S, et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348(4):295-303. doi: 10.1056/NEJMoa021332 [DOI] [PubMed] [Google Scholar]
- 18.Elliott PM, Poloniecki J, Dickie S, et al. Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol. 2000;36(7):2212-2218. doi: 10.1016/S0735-1097(00)01003-2 [DOI] [PubMed] [Google Scholar]
- 19.Norrish G, Cantarutti N, Pissaridou E, et al. Risk factors for sudden cardiac death in childhood hypertrophic cardiomyopathy: a systematic review and meta-analysis. Eur J Prev Cardiol. 2017;24(11):1220-1230. doi: 10.1177/2047487317702519 [DOI] [PubMed] [Google Scholar]
- 20.Criteria Committee of the New York Heart Association; New York Heart Association . Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed New York, NY: Little Brown; 1994. [Google Scholar]
- 21.Maron BJ, Spirito P, Ackerman MJ, et al. Prevention of sudden cardiac death with implantable cardioverter-defibrillators in children and adolescents with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2013;61(14):1527-1535. doi: 10.1016/j.jacc.2013.01.037 [DOI] [PubMed] [Google Scholar]
- 22.Lopez L, Colan S, Stylianou M, et al. ; Pediatric Heart Network Investigators . Relationship of echocardiographic z scores adjusted for body surface area to age, sex, race, and ethnicity: the Pediatric Heart Network Normal Echocardiogram Database. Circ Cardiovasc Imaging. 2017;10(11):e006979. doi: 10.1161/CIRCIMAGING.117.006979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neilan TG, Pradhan AD, King ME, Weyman AE. Derivation of a size-independent variable for scaling of cardiac dimensions in a normal paediatric population. Eur J Echocardiogr. 2009;10(1):50-55. doi: 10.1093/ejechocard/jen110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailey BJ, Briars GL. Estimating the surface area of the human body. Stat Med. 1996;15(13):1325-1332. doi: [DOI] [PubMed] [Google Scholar]
- 25.van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18(6):681-694. doi: [DOI] [PubMed] [Google Scholar]
- 26.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377-399. doi: 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 27.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: Wiley Classics Library; 2004. [Google Scholar]
- 28.Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis; II: accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48(12):1503-1510. doi: 10.1016/0895-4356(95)00048-8 [DOI] [PubMed] [Google Scholar]
- 29.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239-241. doi: 10.1093/biomet/69.1.239 [DOI] [Google Scholar]
- 30.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48(4):817-838. doi: 10.2307/1912934 [DOI] [Google Scholar]
- 31.Harrell F. Regression Modeling Strategies. New York, NY: Springer; 2001. doi: 10.1007/978-1-4757-3462-1 [DOI] [Google Scholar]
- 32.Steyerberg EW. Clinical Prediction Modes: A Practical Approach to Development, Validation, and Updating. New York, NY: Springer; 2009. doi: 10.1007/978-0-387-77244-8 [DOI] [Google Scholar]
- 33.Uno H, Cai T, Pencina MJ, D’Agostino RB, Wei LJ. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med. 2011;30(10):1105-1117. doi: 10.1002/sim.4154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahman MS, Ambler G, Choodari-Oskooei B, Omar RZ. Review and evaluation of performance measures for survival prediction models in external validation settings. BMC Med Res Methodol. 2017;17(1):60. doi: 10.1186/s12874-017-0336-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nugent AW, Daubeney PE, Chondros P, et al. ; National Australian Childhood Cardiomyopathy Study . The epidemiology of childhood cardiomyopathy in Australia. N Engl J Med. 2003;348(17):1639-1646. doi: 10.1056/NEJMoa021737 [DOI] [PubMed] [Google Scholar]
- 36.Kaski JP, Syrris P, Esteban MT, et al. Prevalence of sarcomere protein gene mutations in preadolescent children with hypertrophic cardiomyopathy. Circ Cardiovasc Genet. 2009;2(5):436-441. doi: 10.1161/CIRCGENETICS.108.821314 [DOI] [PubMed] [Google Scholar]
- 37.Morita H, Rehm HL, Menesses A, et al. Shared genetic causes of cardiac hypertrophy in children and adults. N Engl J Med. 2008;358(18):1899-1908. doi: 10.1056/NEJMoa075463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bharucha T, Lee KJ, Daubeney PE, et al. ; NACCS (National Australian Childhood Cardiomyopathy Study) Investigators . Sudden death in childhood cardiomyopathy: results from a long-term national population-based study. J Am Coll Cardiol. 2015;65(21):2302-2310. doi: 10.1016/j.jacc.2015.03.552 [DOI] [PubMed] [Google Scholar]
- 39.Ho CY, Day SM, Ashley EA, et al. Genotype and lifetime burden of disease in hypertrophic cardiomyopathy: insights from the Sarcomeric Human Cardiomyopathy Registry (SHaRe). Circulation. 2018;138(14):1387-1398. doi: 10.1161/CIRCULATIONAHA.117.033200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lipshultz SE, Orav EJ, Wilkinson JD, et al. ; Pediatric Cardiomyopathy Registry Study Group . Risk stratification at diagnosis for children with hypertrophic cardiomyopathy: an analysis of data from the Pediatric Cardiomyopathy Registry. Lancet. 2013;382(9908):1889-1897. doi: 10.1016/S0140-6736(13)61685-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maurizi N, Passantino S, Spaziani G, et al. Long-term outcomes of pediatric-onset hypertrophic cardiomyopathy and age-specific risk factors for lethal arrhythmic events. JAMA Cardiol. 2018;3(6):520-525. doi: 10.1001/jamacardio.2018.0789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balaji S, DiLorenzo MP, Fish FA, et al. Risk factors for lethal arrhythmic events in children and adolescents with hypertrophic cardiomyopathy and an implantable defibrillator: an international multicenter study [published online April 23, 2019]. Heart Rhythm. 2019;S1547-5271(19)30369-8. doi: 10.1016/j.hrthm.2019.04.040 [DOI] [PubMed] [Google Scholar]
- 43.Kaski JP, Tomé Esteban MT, Lowe M, et al. Outcomes after implantable cardioverter-defibrillator treatment in children with hypertrophic cardiomyopathy. Heart. 2007;93(3):372-374. doi: 10.1136/hrt.2006.094730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Axelsson Raja A, Farhad H, Valente AM, et al. Prevalence and progression of late gadolinium enhancement in children and adolescents with hypertrophic cardiomyopathy. Circulation. 2018;138(8):782-792. doi: 10.1161/CIRCULATIONAHA.117.032966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ostman-Smith I, Wisten A, Nylander E, et al. Electrocardiographic amplitudes: a new risk factor for sudden death in hypertrophic cardiomyopathy. Eur Heart J. 2010;31(4):439-449. doi: 10.1093/eurheartj/ehp443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von Gunten S, Schaer BA, Yap SC, et al. Longevity of implantable cardioverter defibrillators: a comparison among manufacturers and over time. Europace. 2016;18(5):710-717. doi: 10.1093/europace/euv296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atallah J, Erickson CC, Cecchin F, et al. ; Pediatric and Congenital Electrophysiology Society (PACES) . Multi-institutional study of implantable defibrillator lead performance in children and young adults: results of the Pediatric Lead Extractability and Survival Evaluation (PLEASE) study. Circulation. 2013;127(24):2393-2402. doi: 10.1161/CIRCULATIONAHA.112.001120 [DOI] [PubMed] [Google Scholar]
- 48.Nugent AW, Daubeney PE, Chondros P, et al. ; National Australian Childhood Cardiomyopathy Study . Clinical features and outcomes of childhood hypertrophic cardiomyopathy: results from a national population-based study. Circulation. 2005;112(9):1332-1338. doi: 10.1161/CIRCULATIONAHA.104.530303 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Pediatric Risk Model for Sudden Cardiac Death (SCD) in Hypertrophic Cardiomyopathy (HCM Risk-Kids)
eFigure 2. Bar Chart Showing Age Distribution of Cohort
eFigure 3. Comparison of Observed and Predicted Risk by Clinical Risk Group for External Validation of Adult HCM-Risk SCD Model
eTable 1. Number of Patients Enrolled by Participating Center
eTable 2. Summary of Candidate Predictors Following Systematic Review of Literature
eTable 3. Baseline Clinical Characteristics by Era
eTable 4. Summary of Missing Data
eTable 5. Sensitivity Analysis: Model Including Age and Family History of SCD
eAppendix. HCM Risk-Kids Calculator
eReferences.