Abstract
Atherosclerotic cardiovascular disease is a leading cause of death and morbidity globally. Over the past several years, arterial inflammation has been implicated in the pathophysiology of athero-thrombosis, substantially confirming what pathologist Rudolf Virchow had observed in the 19th century. Lipid lowering, lifestyle changes, and modification of other risk factors have reduced cardiovascular complications of athero-thrombosis, but a substantial residual risk remains. In view of the pathogenic role of inflammation in athero-thrombosis, directly targeting inflammation has emerged as an additional potential therapeutic option; and some early promising results have been suggested by the Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS), in which canakinumab, a fully human monoclonal antibody targeting the pro-inflammatory and pro-atherogenic cytokine interleukin 1 beta, was shown to reduce cardiovascular events.
Keywords: Atherosclerosis, Inflammation, thrombosis
Introduction
Cardiovascular disease from atherosclerosis manifests as acute and chronic ischemic syndromes such as acute coronary syndromes, angina pectoris, claudication, ischemic strokes, congestive heart failure, and sudden and non-sudden cardiac death 1– 4 Atherosclerosis consists of build-up of plaque inside the intima of medium and large arteries, leading to chronic luminal narrowing or disruption of the plaque surface (plaque rupture or superficial erosion) with superimposed thrombosis and a subacute or acute luminal compromise. Most of the acute and life-threatening manifestations of atherosclerosis result from plaque disruption and thrombosis 1– 4.
Pathophysiology of atherosclerosis
Atherosclerotic plaques contain a variable mix of lipids, smooth muscle cells, extracellular matrix, calcium, and components of the immune system (both from innate and adaptive immunity) such as macrophages, dendritic cells, mast cells, natural killer cells, and T cells. In addition, increased plaque neovascularization and intraplaque hemorrhage are features of atherosclerotic plaques. Although the precise mechanism of athero-thrombosis remains incompletely understood, a number of risk factors that increase the likelihood of atherogenesis have been identified: these include dyslipidemia with elevated apolipoprotein B (apoB) 100–containing lipoproteins, low levels of high-density lipoprotein (HDL), hypertension, diabetes, smoking, central obesity and metabolic syndrome, advanced age, menopause, genetic factors and family history of premature coronary disease, chronic immune-inflammatory conditions (such as psoriasis, rheumatoid arthritis [RA], systemic lupus erythematosus, HIV, and Kawasaki’s syndrome), chronic infections, and radiation exposure 1– 5. Cardiovascular disease is now recognized as a major cause of premature mortality among patients with autoimmune chronic inflammatory conditions, and there is an urgent need to identify those who are at risk of cardiovascular ischemic events in order to optimize prevention and therapeutic intervention 6. In this regard, several clinical trials showed that methotrexate use is associated with a reduced risk of cardiovascular events in patients with RA. This suggests that reducing the inflammation in RA by using methotrexate not only improves disease-specific outcomes but also may reduce collateral damage such as atherosclerosis 7, 8.
Key role of lipids in atherogenesis
It is generally agreed that lipids play a key role in the initiation of atherosclerosis. Experimental observations have suggested that one of the earliest events in atherogenesis is the entry of atherogenic (apoB 100–containing) lipoproteins into the sub-endothelial space, where they interact with extracellular matrix components, leading to trapping of lipoproteins with subsequent aggregation and oxidative modification and then to generation of pro-inflammatory lipids 9, 10. These pro-inflammatory lipids lead to endothelial dysfunction manifesting with increased adhesivity of endothelium to circulating mononuclear cells which then are recruited into the sub-endothelium aided by the local production of inflammatory cytokines. Monocytes in the sub-endothelium mature into macrophages expressing scavenger receptors through which the lipids are engulfed, turning monocyte-derived macrophages into foam cells. Some foam cells are also derived from vascular smooth muscle cells. Monocyte-macrophages in the lesion secrete mediators that also recruit smooth muscle cells from the media; these smooth muscle cells migrate, proliferate, and secrete matrix proteins, contributing to build-up of plaque; some macrophages and dendritic cells present neoantigens to the T cells, creating a pro-inflammatory adaptive immune response that perpetuates inflammation in the plaque 1.
Critical role of inflammation in hyperlipidemia-induced atherogenesis
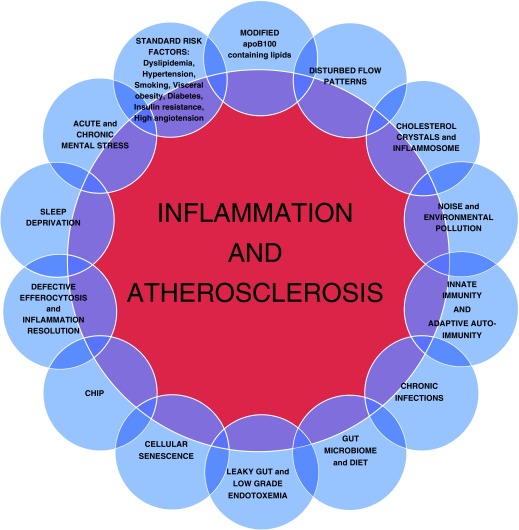
Experimental studies and many clinical observations have shown that hyperlipidemia is essential but not sufficient to produce atherosclerosis unless there is inflammation as well. Inflammation was, in fact, implicated in atherosclerosis by Virchow as far back as in 1858 11. Many cytokines and chemokines are involved in the development and progression of the atherosclerotic plaque. Some of them, such as colony-stimulating factor-1 (CSF-1) and monocyte chemoattractant protein-1 (MCP-1), whose partial or complete deletion dramatically reduces atherosclerosis in murine models despite severe hyperlipidemia, are important in the initial phases of plaque formation 12. Hyperlipidemia activates innate immunity by activating Toll-like receptor 2 (TLR-2) and TLR-4 pathways, leading to activation of inflammatory and pro-atherogenic genes in macrophages and endothelial cells 12. Disruption of lipid-induced innate immune signaling reduces atherosclerosis in hyperlipidemic murine models 12. Given the multifactorial nature of cardiovascular disease and the complexity of the inflammation pathways involved in atherosclerotic plaque development (as shown in Figure 1), the implications of findings in hyperlipidemic mice have to be carefully assessed when considering humans. In addition to apoB 100–containing lipoproteins, HDL may become pro-inflammatory and pro-atherogenic when it undergoes chemical modification by macrophage-derived myeloperoxidase or mast cell–derived proteases through acquisition of pro-inflammatory mediators (such as serum amyloid A and symmetrical dimethyl arginine) or loss of anti-inflammatory mediators (such as clusterin, paraoxonase, and apoA-1), creating a dysfunctional form of HDL which promotes atherosclerosis 13. Furthermore, mast cell–derived neutral proteases neutralize some of the critical anti-atherogenic functions of HDL. Thus, they degrade the pre-beta–HDL fraction, thereby blocking the ABCA1-dependent cholesterol efflux from foam cells 14. Moreover, the anti-inflammatory functions of apoA-1 on endothelial cells are lost upon its C-terminal cleavage by the human mast cell neutral protease chymase 15.
Figure 1. Different pathways for inflammation in athrosclerosis are depicted in this figure.
(CHIP, clonal hematopoetic mutations of indeterminate potential).
Thus, a large body of evidence implicates inflammation in the initiation and progression of atherosclerosis 1– 5, 12. Inflammation has also been implicated in outward remodeling that occurs with plaque formation, intraplaque neovascularization and plaque hemorrhage, matrix depletion with thinning of the fibrous cap through increased matrix proteolysis mediated by matrix-degrading enzymes, and eventually disruption of the fibrous cap leading to thrombosis 1– 5, 12. Plaque thrombogenicity has also been attributed to macrophage-derived tissue factor within the plaque 1– 5, 12. Thus, inflammation plays an important role at multiple steps in the evolution of athero-thrombosis.
Do other factors contribute to inflammation in atherosclerosis?
Local shear stress and flow dynamics
Atherosclerotic plaques tend to preferentially form at sites of low or oscillating shear stress, such as branch points and curvatures where flow patterns are disturbed; these disturbed flow patterns appear to promote entry of atherogenic lipoproteins by increasing their residence time; in addition, such disturbed flows promote a pro-inflammatory endothelial phenotype that is orchestrated by flow-sensitive transcription factors such as KLF2; inhibition of KLF2 by low shear stress promotes a pro-inflammatory phenotype contributing to atherogenesis and plaque inflammation 16. Murine studies have shown that athero-prone sites of normolipidemic mouse aorta contain cellular components priming these sites for enhanced inflammatory responses 17.
Cholesterol crystals and inflammasome activation
Cholesterol crystals, frequently present in atherosclerotic plaques, can activate the NLRP3 inflammasome pathway to induce secretion of pro-inflammatory and atherogenic cytokines like interleukin 1 beta (IL-1β) and IL-18 18– 22. Activation of NLRP3 inflammasome requires a priming signal which can be provided by neutrophil-derived extracellular traps and by oxidized lipids followed by the second signal provided by cholesterol crystals 19. Mitochondrial damage and dysfunction can also play important roles in activating NLRP3 inflammasome 21, 22.
Integrated stress response, inflammation, and atherosclerosis
Dyslipidemia, especially exposure to saturated fatty acids, induces endoplasmic reticulum stress, mitochondrial oxidative stress, and elF2 alpha phosphorylation which hyperactivates an integrated stress response (ISR), which in turn activates a local and systemic inflammatory response through activation of NLRP3 inflammasome: this process contributes to atherogenesis since inhibition of ISR at different nodes reduces atherosclerosis in hyperlipidemic murine models 22. Small-molecule ISR inhibitors could emerge as important anti-inflammatory agents for atherosclerosis and other chronic inflammatory conditions 22, 23.
Visceral obesity, insulin resistance, and type II diabetes mellitus
Several studies have shown that visceral adiposity is associated with organ-specific inflammation involving the adipose tissue, liver, pancreas, and arterial wall; visceral adipose tissue of diet-induced obese mice was demonstrated to be rich in T cells secreting interferon gamma (IFN-γ) at higher levels than lean controls. This inflammation is associated with insulin resistance and metabolic syndrome, eventually contributing to the development of type II diabetes mellitus 24– 28. Diabetes-associated dyslipidemia and pro-inflammatory state contribute to enhanced atherogenesis observed with diabetes 24– 28.
Smoking and environmental pollution
Active smoking and passive exposure to smoking are associated with increased atherogenesis and risk of acute vaso-occlusive cardiovascular events mediated in part by pro-inflammatory and pro-thrombotic effects of smoke exposure 29, which are associated in part with perturbations on lipid metabolism 30. Similarly, environmental pollutants may induce inflammation, enhancing risk of cardiovascular disease 29. The underlying mechanism in both cases may be due to the increased production of reactive oxygen species (ROS) exceeding the endogenous antioxidant capacity associated with an increase in the markers of inflammation 31, 32.
Hypertension and inflammation
Recent studies have implicated oxidative stress and innate and adaptive immunity in hypertension and hypertension-related end organ damage. Angiotensin II, salt retention, or increased mineralocorticoid activity activates innate immunity that precipitates or aggravates hypertension 33. These hypertensive stimuli also induce oxidative stress in antigen-presenting cells which lead to neoantigen formation 33. These neoantigens in turn lead to an adaptive immune response which can further damage end organs such as the kidney 33. Several experimental studies have implicated T helper 17 (Th17) cells and their cytokines in inflammation in hypertension, and IL-10 has a counterbalancing role by producing regulatory T (Treg) cells 33.
Adaptive autoimmunity and inflammation in atherosclerosis
An adaptive immune response to autoantigens, both humoral and cell-mediated, exists in animal models of atherosclerosis and in human subjects 1, 34, 35. T-cell activation occurs upon presentation of the antigen in the setting of an inflammatory state, resulting in clonal proliferation and in the differentiation of CD4 + T cells to Th1, Th2, or Th17 phenotype, depending on the cytokines secreted by the antigen-presenting cells 1, 34, 35. Both a pro-atherogenic inflammatory immune response mediated by Th1 and possibly Th17 and B-cell subsets and an athero-protective anti-inflammatory immune response mediated by Treg cells and B1 cells have been shown to exist and modulate atherosclerosis 1, 34, 35. Autoantigens that have been identified include antigens derived from both the protein and lipid components of apoB 100–containing lipoproteins, heat shock protein 60 (HSP 60), and beta glycoprotein 1, 34, 35. These observations have led to the concept of immunomodulatory therapies for atherosclerosis, which are being developed in various laboratories 34, 35.
Infections, atherosclerosis, and cardiovascular events
Several studies have implicated infections to either atherogenesis or precipitation of acute cardiovascular events 5, 36, 37. These infections include influenza, gingivitis, urinary tract infections, skin infections, HIV, pneumonia, and Helicobacter pylori infections 5, 36, 37. The link between infection and atherosclerosis has been attributed to direct infection of the vessel wall ( Chlamydia pneumoniae), indirect effects involving molecular mimicry, or systemic pro-inflammatory effects 5, 37. However, a number of large-scale randomized clinical trials targeting C. pneumoniae with antibiotics failed to reduce cardiovascular events 5. On the other hand, influenza vaccination has been shown to reduce cardiovascular events in a limited number of randomized clinical trials and in many observational studies 5. The cardioprotective effects of pneumococcal vaccines have not been as persuasively demonstrated 5.
Diet and gut microbe interaction
In recent years, gut microflora has been implicated in the pathogenesis of a number of diseases, including obesity, diabetes, hypertension, atherosclerosis–thrombosis, and neurodegenerative diseases 38. Several studies have suggested that certain dietary constituents such as phosphatidylcholine, choline, and carnitine are acted upon by enzyme trimethylamine lyase (TMA lyase) produced by gut microbes such as Clostridia, Shigella, Proteus, and Aerobacter to generate TMA which is converted into trimethylamine oxide (TMAO) by hepatic flavin mono-oxygenases. TMAO enhances foam cell formation by upregulating macrophage scavenger receptors which may contribute to its pro-atherogenic effects 38. In addition, TMAO enhances platelet activity and predisposes patients to thrombosis 38.
In human subjects, circulating TMAO levels correlate with the presence of coronary artery disease and the future risk of athero-thrombotic cardiovascular events 38. TMAO also has been shown to contribute to enhanced atherogenesis in murine models and enhanced platelet aggregation 38. In murine models, antibiotics directed at gut microflora reduce atherogenesis 38. Inhibition of TMA-generating microbial enzymes by dimethylbetane also reduces murine atherosclerosis 38. Consumption of a Mediterranean-type diet is also associated with lower circulating levels of TMAO 38 and this may account for the anti-inflammatory and health-promoting effects of a Mediterranean diet. Recently developed, non-toxic potent inhibitors of gut microbial TMA lyase (halomethylcholines) were shown to markedly inhibit platelet reactivity and thrombosis 38.
Leaky gut and low-grade endotoxemia
A number of studies have shown that low-grade endotoxemia due to leaky gut is present in human subjects under certain conditions and that, in animal models, such low-grade endotoxemia has pro-inflammatory effects and enhances atherosclerosis 39, 40.
Senescence-associated cellular secretory phenotype and inflammation
Senescent cells are characterized by short telomeres and other markers such as senescence-associated beta-glycosidase (SA-Beta Gal) p53, p21, and p16 ink4a 41. Experimental studies in murine models have shown the accumulation of senescent endothelial cells, macrophages, and smooth muscle cells in atherosclerotic plaques 42. Senescent cells express inflammatory cytokines in early stages of murine atherosclerosis and matrix-degrading enzymes in more advanced stages of atherosclerosis; both of these are implicated in atherogenesis and plaque instability 32. Depletion of these senescent cells reduces atherosclerosis and creates a more stable plaque composition, suggesting a causal role for senescent cells in inflammation, atherosclerosis, and plaque instability 41. These observations suggest that senolytic compounds, such as fisetin, that remove senescent cells may have athero-protective effects 42. Another important situation dealing with senescence and inflammation is chronic kidney disease. Uremia is typified by activation of innate immunity, which is characterized by activated monocytes and increased synthesis of pro-inflammatory cytokines (IL-6, tumor necrosis factor, and IL-1) 43– 45. In mice, chronic inflammation is related to cellular senescence, and senescent cells may upregulate and secrete pro-inflammatory cytokines as part of a senescence-associated secretory phenotype 46. This scenario is associated with progressive atherosclerosis and vascular calcification 45.
Somatic hematopoetic mutations and inflammation
Aging is associated with accumulation of somatic hematopoetic mutations in certain genes that contribute to increased risk of hematological cancers and also to increased cardiovascular mortality 47, 48. This phenomenon is also called clonal hematopoetic mutations of indeterminate potential (CHIP). Mutations in DNMT3A or TET2 genes, in particular, are associated with enhanced cardiovascular events 47, 48. Experimental observations in a murine model have demonstrated that enhanced atherosclerosis with TET2 mutations is likely due to increased activity of NLRP3 inflammasome in monocytes 47, 48. Such age-dependent somatic mutations may contribute to increased inflammation and cardiovascular risk in the elderly.
Impaired anti-inflammatory mechanisms
Acute inflammation generally resolves with time through the activity of a number of inflammation-resolving cellular and molecular mechanisms. These inflammation-resolving mechanisms involve lipid-derived pro-resolving humoral factors and cellular mechanisms. One of these cellular mechanisms involves clearance of apoptotic debris (efferocytosis) by macrophages mediated by several cellular receptors such as MerTK 49– 51. Thus, chronic persistent and smoldering inflammation may also result from failure of inflammation-resolving mechanisms in the host 49– 51. Molecules like CD47 that present a “don’t eat me” signal to the cells that clear apoptotic debris have been shown to be expressed in murine and human atherosclerotic plaques, and inhibition of CD47 stimulates efferocytosis and reduces atherosclerosis in murine models 50. Humoral mediators such as resolvins, protectins, and maresins are also involved in resolution of inflammation 49. Chronic inflammation likely results from an imbalance between pro-inflammatory and anti-inflammatory mediators; however, precise factors that modulate this delicate balance are poorly understood.
Acute and chronic mental stress and inflammation
Mental stress is known to play an adverse role in cardiovascular disease, but the mechanisms linking stress to atherosclerosis have remained elusive. Recent studies on mice have shown that acute and chronic mental stress induce a pro-inflammatory response in which the brain sends signals to the bone marrow and spleen, stimulating hematopoiesis and production of pro-inflammatory monocytes (Ly6 hi) that are recruited into atherosclerotic plaques, creating enhanced plaque inflammation 52, 53. Human studies have also provided support for the link between mental stress, inflammation, and atherosclerotic artery disease 54– 58. Interestingly, meditation has also been shown to reduce inflammatory markers and cardiovascular risk 56.
Sleep deprivation and fragmentation, inflammation, and atherosclerosis
Sleep fragmentation or deprivation is associated with increased cardiovascular risk in human subjects 59, 60. Recent experimental studies in murine models have shown that sleep fragmentation enhances atherosclerosis by suppressing the release of hypocretin (orexin) from the hypothalamus; suppression of hypocretin results in increased myeloid hematopoiesis and production of pro-inflammatory monocytes, likely by stimulating the release of CSF-1 by pre-neutrophilic precursors in the bone marrow 61. Non-invasive imaging has shown an increased subclinical atherosclerotic burden in subjects with inadequate or fragmented sleep 62. Obstructive sleep apnea (OSA) is recognized as an independent risk factor for atherosclerotic cardiovascular disease 63, 64. In patients with OSA, pro-inflammatory molecules, such as soluble intercellular adhesion molecule 1, soluble vascular adhesion molecule 1, and MCP-1, were detected at very high levels with direct correlation to the desaturation index 63, 64. A study with a rat model of recurrent obstructive apneas reported increased leukocyte–endothelial cell interactions characterized by a significant increase in the flux of leukocyte rolling, number of rolling leukocytes, and number of adherent leukocytes 65.
Summary and perspective
A large body of experimental and clinical observations highlights the role of inflammation in atherosclerosis and its complications (that is, plaque disruption and thrombosis). Some part of this inflammation is mediated through unhealthy lifestyles and conventional risk factors that can be addressed with aggressive lifestyle and risk factor modification. However, further incremental risk reduction may require agents that directly target inflammation. In keeping with these, a number of drugs targeting inflammation were tested in the clinic. The results, with the exception of those of the Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS), have largely been disappointing. Phospholipase inhibitors darapladib and varespladib, targeting pro-inflammatory phospholipases, failed to reduce cardiovascular events in randomized trials 66, 67. A mitogen-activated protein kinase inhibitor, losmapimod, also failed to reduce cardiovascular events 68. Because there appear to be multiple and redundant pathways for inflammation in athero-thrombosis, identification of the precise and optimal target for modification is relatively challenging. Recently, low-dose methotrexate was tested in the CIRT (cardiovascular inflammation reduction trial) and shown not to reduce cardiovascular events in high-risk patients 69. Interestingly, in this trial, low-dose Methotrexate also failed to reduce circulating inflammatory markers high-sensitivity C-reactive protein (hs-CRP) and IL-6 69. In contrast, methotrexate has been shown to reduce inflammatory markers when used in the setting of high inflammatory burden 7, 8, 70. On the other hand, preliminary studies of cardiovascular benefits of low-dose colchicine have been encouraging, and large-scale randomized clinical trials examining the cardiovascular benefits of low-dose colchicine are ongoing 71. A recent significant development in this arena was the landmark CANTOS trial, in which canakinumab, a monoclonal antibody to IL-1β, showed significant cardiovascular benefit without changes in circulating lipids, albeit at the expense of an increase in fatal infections 72. High cost, increased risk of serious infection, and a relatively modest clinical benefit with canakinumab will make it unfeasible for routine clinical use.
It is clear that the search for agents that selectively target adverse vascular inflammation without interfering with beneficial aspects of inflammation must continue 22, 34, 35. Several promising avenues of research, including different strategies, are in active development. Among these are the blockage of CD40-induced tumor necrosis factor receptor-associated factor (TRAF) signaling in macrophages 73 and triggering receptor expressed on myeloid cells 1 (TREM-1) 74, strategies that activate the inhibitory immune receptor CD31 75, or blocking CD47/SIRPA (signal regulatory protein alpha) signaling to promote inflammation resolution in plaques through enhanced efferocytosis 50. The CANTOS and CIRT trials showed important limitations related to immunosuppression. On the other hand, phospholipase inhibitors failed in reducing cardiovascular events. The optimal targets for modulation of inflammation need to be identified in order to develop anti-inflammatory therapies with high efficacy and safety. A potential and hopeful approach is the favorable modulation of atherosclerosis by vaccination by using antigens relevant to atherosclerosis. This would involve the development of antigen-specific antibodies or induction of antigen-specific Treg cells or other athero-protective immune responses. However, identification of the antigenic epitopes most relevant for atherosclerotic disease development in humans and some difficulties in vaccine design (for example, choice of the adjuvant, safety, and stability) are obstacles that will need to be overcome.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Magnus Bäck, Division of Valvular and Coronary Disease, Karolinska University Hospital, Stockholm, Sweden
Petri T Kovanen, Atherosclerosis Research Laboratory, Wihuri Research Institute , Helsinki, Finland
Avijit Lahiri, British Cardiac Research Trust , Prince Albert Road, London, NW8 7RE, UK
Jan Nilsson, Department of Clinical Sciences Malmö, Lund University, Lund, Sweden
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; peer review: 4 approved]
References
- 1. Libby P, Loscalzo J, Ridker PM, et al. : Inflammation, Immunity, and Infection in Atherothrombosis: JACC Review Topic of the Week. J Am Coll Cardiol. 2018;72(17):2071–81. 10.1016/j.jacc.2018.08.1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Falk E, Shah PK, Fuster V: Coronary plaque disruption. Circulation. 1995;92(3):657–71. 10.1161/01.CIR.92.3.657 [DOI] [PubMed] [Google Scholar]
- 3. Partida RA, Libby P, Crea F, et al. : Plaque erosion: a new in vivo diagnosis and a potential major shift in the management of patients with acute coronary syndromes. Eur Heart J. 2018;39(22):2070–6. 10.1093/eurheartj/ehx786 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 4. Shah PK: Molecular mechanisms of plaque instability. Curr Opin Lipidol. 2007;18(5):492–9. 10.1097/MOL.0b013e3282efa326 [DOI] [PubMed] [Google Scholar]
- 5. Pothineni NVK, Subramany S, Kuriakose K, et al. : Infections, atherosclerosis, and coronary heart disease. Eur Heart J. 2017;38(43):3195–201. 10.1093/eurheartj/ehx362 [DOI] [PubMed] [Google Scholar]
- 6. Ahearn J, Shields KJ, Liu CC, et al. : Cardiovascular disease biomarkers across autoimmune diseases. Clin Immunol. 2015;161(1):59–63. 10.1016/j.clim.2015.05.024 [DOI] [PubMed] [Google Scholar]
- 7. Westlake SL, Colebatch AN, Baird J, et al. : The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology (Oxford). 2010;49(2):295–307. 10.1093/rheumatology/kep366 [DOI] [PubMed] [Google Scholar]
- 8. Micha R, Imamura F, Wyler von Ballmoos M, et al. : Systematic Review and Meta-Analysis of Methotrexate Use and Risk of Cardiovascular Disease. Am J Cardiol. 2011;108(9):1362–70. 10.1016/j.amjcard.2011.06.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Skålén K, Gustafsson M, Rydberg EK, et al. : Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature. 2002;417(6890):750–4. 10.1038/nature00804 [DOI] [PubMed] [Google Scholar]
- 10. Ference BA, Ginsberg HN, Graham I, et al. : Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459–72. 10.1093/eurheartj/ehx144 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. Virchow R: Cellular pathology. John Churchill, London1858. Reference Source [Google Scholar]
- 12. Ait-Oufella H, Taleb S, Mallat Z, et al. : Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31(5):969–79. 10.1161/ATVBAHA.110.207415 [DOI] [PubMed] [Google Scholar]
- 13. Shah PK: Jekyll and Hyde of HDL: a lipoprotein with a split personality. Eur Heart J. 2013;34(46):3531–4. 10.1093/eurheartj/eht382 [DOI] [PubMed] [Google Scholar]
- 14. Lee-Rueckert M, Kovanen PT: The mast cell as a pluripotent HDL-modifying effector in atherogenesis: from in vitro to in vivo significance. Curr Opin Lipidol. 2015;26(5):362–8. 10.1097/MOL.0000000000000224 [DOI] [PubMed] [Google Scholar]
- 15. Nguyen SD, Maaninka K, Lappalainen J, et al. : Carboxyl-Terminal Cleavage of Apolipoprotein A-I by Human Mast Cell Chymase Impairs Its Anti-Inflammatory Properties. Arterioscler Thromb Vasc Biol. 2016;36(2):274–84. 10.1161/ATVBAHA.115.306827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dai G, Kaazempur-Mofrad MR, Natarajan S, et al. : Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci U S A. 2004;101:14871–6. 10.1073/pnas.0406073101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jongstra-Bilen J, Haidari M, Zhu SN, et al. : Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J Exp Med. 2006;203(9):2073–83. 10.1084/jem.20060245 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Duewell P, Kono H, Rayner KJ, et al. : NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464(7293):1357–61. 10.1038/nature08938 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Warnatsch A, Ioannou M, Wang Q, et al. : Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 2015;349(6245):316–20. 10.1126/science.aaa8064 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Grebe A, Hoss F, Latz E: NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ Res. 2018;122(12):1722–1740. 10.1161/CIRCRESAHA.118.311362 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Rajamäki K, Lappalainen J, Oörni K, et al. : Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5(7):e11765. 10.1371/journal.pone.0011765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Onat UI, Yildirim AD, Tufanli Ö, et al. : Intercepting the Lipid-Induced Integrated Stress Response Reduces Atherosclerosis. J Am Coll Cardiol. 2019;73(10):1149– 69. 10.1016/j.jacc.2018.12.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coll RC, Robertson AA, Chae JJ, et al. : A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21(3):248–55. 10.1038/nm.3806 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Weisberg SP, McCann D, Desai M, et al. : Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808. 10.1172/JCI19246 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Rocha VZ, Folco EJ, Sukhova G, et al. : Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res. 2008;103(5):467–76. 10.1161/CIRCRESAHA.108.177105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rocha VZ, Folco EJ, Ozdemir C, et al. : CXCR3 controls T-cell accumulation in fat inflammation. Arterioscler Thromb Vasc Biol. 2014;34(7):1374– 81. 10.1161/ATVBAHA.113.303133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Donath MY, Shoelson SE: Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98–107. 10.1038/nri2925 [DOI] [PubMed] [Google Scholar]
- 28. Shah PK: Innate immune pathway links obesity to insulin resistance. Circ Res. 2007;100(11):1531– 3. 10.1161/CIRCRESAHA.107.101104 [DOI] [PubMed] [Google Scholar]
- 29. Lüscher TF: Inflammation: the new cardiovascular risk factor. Eur Heart J. 2018;39(38):3483–7. 10.1093/eurheartj/ehy607 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Lietz M, Berges A, Lebrun S, et al. : Cigarette-smoke-induced atherogenic lipid profiles in plasma and vascular tissue of apolipoprotein E-deficient mice are attenuated by smoking cessation. Atherosclerosis. 2013;229(1):86–93. 10.1016/j.atherosclerosis.2013.03.036 [DOI] [PubMed] [Google Scholar]
- 31. Niemann B, Rohrbach S, Miller MR, et al. : Oxidative Stress and Cardiovascular Risk: Obesity, Diabetes, Smoking, and Pollution: Part 3 of a 3-Part Series. J Am Coll Cardiol. 2017;70(2):230–51. 10.1016/j.jacc.2017.05.043 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Miller MR, Shaw CA, Langrish JP: From particles to patients: Oxidative stress and the cardiovascular effects of air pollution. Future Cardiol. 2012;8(4):577–602. 10.2217/fca.12.43 [DOI] [PubMed] [Google Scholar]
- 33. Wenzel U, Turner JE, Krebs C, et al. : Immune Mechanisms in Arterial Hypertension. J Am Soc Nephrol. 2016;27(3):677–86. 10.1681/ASN.2015050562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chyu KY, Shah PK: In Pursuit of an Atherosclerosis Vaccine. Circ Res. 2018;123(10):1121–3. 10.1161/CIRCRESAHA.118.313842 [DOI] [PubMed] [Google Scholar]
- 35. Shah PK, Chyu KY, Dimayuga PC, et al. : Vaccine for atherosclerosis. J Am Coll Cardiol. 2014;64(25):2779–91. 10.1016/j.jacc.2014.10.018 [DOI] [PubMed] [Google Scholar]
- 36. Kearns A, Gordon J, Burdo TH, et al. : HIV-1-Associated Atherosclerosis: Unraveling the Missing Link. J Am Coll Cardiol. 2017;69(25):3084–98. 10.1016/j.jacc.2017.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Chen S, Shimada K, Crother TR, et al. : Chlamydia and Lipids Engage a Common Signaling Pathway That Promotes Atherogenesis. J Am Coll Cardiol. 2018;71(14):1553–70. 10.1016/j.jacc.2018.01.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brown JM, Hazen SL: Microbial modulation of cardiovascular disease. Nat Rev Microbiol. 2018;16(3):171–81. 10.1038/nrmicro.2017.149 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Wiesner P, Choi SH, Almazan F, et al. : Low doses of lipopolysaccharide and minimally oxidized low-density lipoprotein cooperatively activate macrophages via nuclear factor kappa B and activator protein-1: Possible mechanism for acceleration of atherosclerosis by subclinical endotoxemia. Circ Res. 2010;107(1):56–65. 10.1161/CIRCRESAHA.110.218420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stoll LL, Denning GM, Weintraub NL, et al. : Potential Role of Endotoxin as a Proinflammatory Mediator of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24(12):2227–36. 10.1161/01.ATV.0000147534.69062.dc [DOI] [PubMed] [Google Scholar]
- 41. Childs BG, Baker DJ, Wijshake T, et al. : Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science. 2016;354(6311):472–7. 10.1126/science.aaf6659 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Yousefzadeh MJ, Zhu Y, McGowan SJ, et al. : Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018;36:18–28. 10.1016/j.ebiom.2018.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Jurk D, Wilson C, Passos JF, et al. : Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat Commun. 2014;2:4172. 10.1038/ncomms5172 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Kato S, Chmielewski M, Honda H, et al. : Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3(5):1526–33. 10.2215/CJN.00950208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kooman JP, Kotanko P, Schols AM, et al. : Chronic kidney disease and premature ageing. Nat Rev Nephrol. 2014;10(12):732–42. 10.1038/nrneph.2014.185 [DOI] [PubMed] [Google Scholar]
- 46. Campisi J, d'Adda di Fagagna F: Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–40. 10.1038/nrm2233 [DOI] [PubMed] [Google Scholar]
- 47. Libby P, Ebert BL: CHIP (Clonal Hematopoiesis of Indeterminate Potential). Circulation. 2018;138(7):666–8. 10.1161/CIRCULATIONAHA.118.034392 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Ebert BL, Libby P: Clonal Hematopoiesis Confers Predisposition to Both Cardiovascular Disease and Cancer: A Newly Recognized Link Between Two Major Killers. Ann Intern Med. 2018;169(2):116–7. 10.7326/M18-0737 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Kasikara C, Doran AC, Cai B, et al. : The role of non-resolving inflammation in atherosclerosis. J Clin Invest. 2018;128(7):2713–23. 10.1172/JCI97950 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Kojima Y, Volkmer JP, McKenna K, et al. : CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature. 2016;536(7614):86–90. 10.1038/nature18935 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Bäck M, Yurdagul A, Jr, Tabas I, et al. : Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities Nat Rev Cardiol. 2019;16(7):389–406. 10.1038/s41569-019-0169-2 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Dutta P, Courties G, Wei Y, et al. : Myocardial infarction accelerates atherosclerosis. Nature. 2012;487(7407):325–9. 10.1038/nature11260 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Heidt T, Sager HB, Courties G, et al. : Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20(7):754–8. 10.1038/nm.3589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Han Y, Jing J, Tu S, et al. : ST elevation acute myocardial infarction accelerates non-culprit coronary lesion atherosclerosis. Int J Cardiovasc Imaging. 2014;30(2):253–61. 10.1007/s10554-013-0354-z [DOI] [PubMed] [Google Scholar]
- 55. Joshi NV, Toor I, Shah ASV, et al. : Systemic Atherosclerotic Inflammation Following Acute Myocardial Infarction: Myocardial Infarction Begets Myocardial Infarction. J Am Heart Assoc. 2015;4(9):e001956. 10.1161/JAHA.115.001956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Levine GN, Lange RA, Bairey-Merz CN, et al. : Meditation and Cardiovascular Risk Reduction: A Scientific Statement From the American Heart Association. J Am Heart Assoc. 2017;6(10): pii: e002218. 10.1161/JAHA.117.002218 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Hamer M, Kivimaki M, Lahiri A, et al. : Persistent cognitive depressive symptoms are associated with coronary artery calcification. Atherosclerosis. 2010;210(1):209–13. 10.1016/j.atherosclerosis.2010.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hamer M, O'Donnell K, Lahiri A, et al. : Salivary cortisol responses to mental stress are associated with coronary artery calcification in healthy men and women. Eur Heart J. 2010;31(4):424–9. 10.1093/eurheartj/ehp386 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 59. Hublin C, Partinen M, Koskenvuo M, et al. : Sleep and Mortality: A Population-Based 22-Year Follow-Up Study. Sleep. 2007;30(10):1245–53. 10.1093/sleep/30.10.1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cappuccio FP, Cooper D, D'Elia L, et al. : Sleep duration predicts cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32(12):1484–92. 10.1093/eurheartj/ehr007 [DOI] [PubMed] [Google Scholar]
- 61. McAlpine CS, Kiss MG, Rattik S, et al. : Sleep modulates haematopoiesis and protects against atherosclerosis. Nature. 2019;566(7744):383–387. 10.1038/s41586-019-0948-2 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 62. Domínguez F, Fuster V, Fernández-Alvira JM, et al. : Association of Sleep Duration and Quality With Subclinical Atherosclerosis. J Am Coll Cardiol. 2019;73(2):134–44. 10.1016/j.jacc.2018.10.060 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Song D, Fang G, Greenberg H, et al. : Chronic intermittent hypoxia exposure-induced atherosclerosis: A brief review. Immunol Res. 2015;63(1–3):121–30. 10.1007/s12026-015-8703-8 [DOI] [PubMed] [Google Scholar]
- 64. Lévy P, Pépin JL, Arnaud C, et al. : Obstructive sleep apnea and atherosclerosis. Prog Cardiovasc Dis. 2009;51(5):400–10. 10.1016/j.pcad.2008.03.001 [DOI] [PubMed] [Google Scholar]
- 65. Nácher M, Serrano-Mollar A, Farré R, et al. : Recurrent obstructive apneas trigger early systemic inflammation in a rat model of sleep apnea. Respir Physiol Neurobiol. 2007;155(1):93–6. 10.1016/j.resp.2006.06.004 [DOI] [PubMed] [Google Scholar]
- 66. O'Donoghue ML, Braunwald E, White HD, et al. : Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. JAMA. 2014;312(10):1006–15. 10.1001/jama.2014.11061 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Nicholls SJ, Kastelein JJ, Schwartz GG, et al. : Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial. JAMA. 2014;311(3):252–62. 10.1001/jama.2013.282836 [DOI] [PubMed] [Google Scholar]
- 68. O'Donoghue ML, Glaser R, Cavender MA, et al. : Effect of Losmapimod on Cardiovascular Outcomes in Patients Hospitalized With Acute Myocardial Infarction: A Randomized Clinical Trial. JAMA. 2016;315(15):1591–9. 10.1001/jama.2016.3609 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. Ridker PM, Everett BM, Pradhan A, et al. : Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N Engl J Med. 2019;380(8):752–62. 10.1056/NEJMoa1809798 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 70. Choi HK, Hernán MA, Seeger JD, et al. : Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359(9313):1173–7. 10.1016/S0140-6736(02)08213-2 [DOI] [PubMed] [Google Scholar]
- 71. Nidorf SM, Thompson PL: Why Colchicine Should Be Considered for Secondary Prevention of Atherosclerosis: An Overview. Clin Ther. 2019;41(1):41–8. 10.1016/j.clinthera.2018.11.016 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Ridker PM, Everett BM, Thuren T, et al. : Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377(12):1119–31. 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 73. Seijkens TTP, van Tiel CM, Kusters PJH, et al. : Targeting CD40-Induced TRAF6 Signaling in Macrophages Reduces Atherosclerosis. J Am Coll Cardiol. 2018;71(5):527–42. 10.1016/j.jacc.2017.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 74. Joffre J, Potteaux S, Zeboudj L, et al. : Genetic and Pharmacological Inhibition of TREM-1 Limits the Development of Experimental Atherosclerosis. J Am Coll Cardiol. 2016;68(25):2776–93. 10.1016/j.jacc.2016.10.015 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Groyer E, Nicoletti A, Ait-Oufella H, et al. : Atheroprotective effect of CD31 receptor globulin through enrichment of circulating regulatory T-cells. J Am Coll Cardiol. 2007;50(4):344–50. 10.1016/j.jacc.2007.04.040 [DOI] [PubMed] [Google Scholar]