Abstract
The field of aging research has progressed significantly over the past decades. Exogenously and endogenously inflicted molecular damage ranging from genotoxic to organellar damage drives the aging process. Repair mechanisms and compensatory responses counteract the detrimental consequences of the various damage types. Here, we discuss recent progress in understanding cellular mechanisms and interconnections between signaling pathways that control longevity. We summarize cell-autonomous and non-cell-autonomous mechanisms that impact the cellular and organismal aging process
Keywords: Ageing, longevity, DNA repair, autophagy, senescence, C. elegans
Throughout history, humankind has been preoccupied with longevity, death, and immortality, as evidenced by the first known epic, describing Gilgamesh’s futile quest for immortality. Death due to old age, however, appears to be rather rare in nature, as most species are confronted with various extrinsic sources of mortality, including predation, malnutrition, and life-threatening temperatures, all of which can limit the life span of individuals in their natural habitats. The vastly different life spans among closely related species 1, 2 were selected mainly via pressure exerted by extrinsic mortality risks that had to be balanced with the need for successful offspring generation. Some trees may persist thousands of years, whereas some insect species live for only a few days and other species, such as the small freshwater animal hydra, are thought to live indefinitely 3. Various primate species show considerable life span variations, ranging from around 10 to 60 years, even in protected environments. It is vigorously debated whether humans have a fixed maximum life span that plateaus at 115 years 4 or whether the mortality risk plateaus past 105 years 5, leaving open the theoretical possibility of immortality—provided there were an infinite number of 105-year-old individuals. In general, not all individuals of a given species reach the same age, even if they live in the same environment. This variation was most impressively demonstrated in a clonal Caenorhabditis elegans population that even under identical environmental conditions showed a stochastic life span distribution 6. Genome comparisons from species groups with different life spans have revealed evolutionary signatures in genes, some of which have been implicated in pathways associated with longevity regulation 7, 8. Among these are genes involved in DNA repair, splicesosome and RNA processing, cell cycle control and cell division, kynurenine metabolism, autophagy, wound healing, and hemostasis 7, 8. Such studies offer great potential for identifying aging modulators to enhance our understanding of the factors that govern the dynamics of life span determination.
Over the past three decades, environmental and metabolic factors as well as evolutionarily conserved pathways that influence life span have been identified ( Figure 1). Examples include several stress factors that, in excess, can negatively affect life span but that, in moderation, can trigger protective responses that lead to life span extension in a process called hormesis 9. For example, DNA damage is thought to accumulate in tissues during aging, as extrinsic and intrinsic sources of genotoxic stress lead to a wide array of DNA lesions, including oxidized DNA bases, apurinic sites, and DNA double-strand breaks (reviewed in 10). DNA damage drives the aging process via mechanisms ranging from interference with replication and transcription to the DNA damage response (DDR) that triggers apoptosis and cellular senescence 11. A range of congenital DNA repair defects lead to progeroid syndromes that are characterized by accelerated segmental aging phenotypes in humans 12, 13. Reactive oxygen species (ROS) have been prime suspects for accelerating the aging process; however, they can also trigger protective responses at low levels and are even necessary for certain life span extension phenotypes 14. For example, epigallocatechin-3-gallate (EGCG), a compound found in green tea ( Camellia sinensis L.), leads to ROS production and an extended median life span in C. elegans, but this effect is abrogated when the nematodes are treated with the ROS-neutralizing reducing agent N-acetylcysteine (NAC) 15. Similar positive life span and hormetic effects could be observed with low concentrations of other ROS inducers, such as naphthoquinones and arsenite, in a ROS-dependent manner 16, 17. Moreover, the influence of glycogen and glucose on the intracellular glutathione redox system, concomitant ROS scavenging, and life span reduction in long-lived daf-2 mutant C. elegans highlight the importance of ROS signaling and redox systems for life span control 18, 19. Strongly elevated ROS levels, however, induced by higher concentrations of paraquat shorten life span, presumably because of oxidative damage 15, 17, 19. A similar relationship can be observed regarding the nutritional state of animals, as severe nutrient and energy limitation can lead to death; however, calorie restriction (CR), dietary restriction (DR), or intermittent fasting has positive effects on life span in several model organisms, and modulation of metabolic parameters in a 2-year human trial showed potential benefits 20– 22. These life span extensions can also be triggered via modulation of molecular signaling pathways—for example, insulin-like growth factor (IGF) signaling—or via the inhibition of neuronal circuits involved in nutrient sensing 23. Based on information regarding the signaling mechanisms that mediate the life span–extending consequences of CR, pharmacological inhibitors such as the mammalian target of rapamycin (mTOR) inhibitor rapamycin were shown to be sufficient for life span extension in several model organisms, including mice 24. Currently, several efforts are underway to develop more specific TORC1 inhibitors to avoid the side effects associated with rapamycin treatment in humans, such as immunosuppression and impaired wound healing 25– 27.
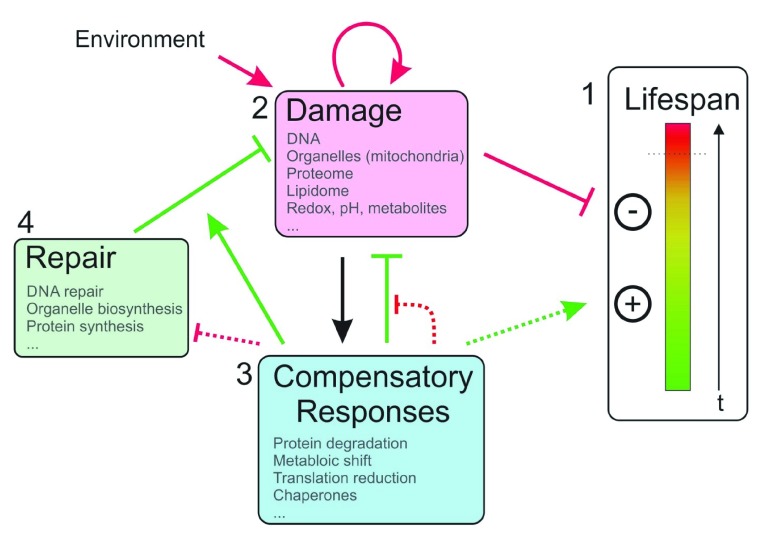
Figure 1. Life span determination.
(1) Organismal life starts out as a system in healthy homeostasis (green bar area), which becomes increasingly disorganized via deleterious effects (yellow/red bar area) until it reaches a threshold of system collapse and death (dotted line). Positive and negative effects determine the dynamics of this transition and therefore the life span of the individual. (2) Biological damage leading to disruption of systemic homeostasis can be triggered by environmental insults and internal metabolic processes, which can self-amplify and interact which each other. (3) Damage triggers compensatory responses that limit damage (green blunt arrow), facilitate damage repair (solid green arrow), and delay the complete disruption of homeostasis (dotted green arrow). Over time, compensatory responses exhaust their compensatory capacity and potentially limit repair resources (dotted red arrows). (4) Repair and re-synthesis of biological structures and components at least partially revert some types of damage. Green arrows denote positive effects on life span, red arrows denote negative effects on life span, and black arrows denote neutral or ambiguous effects.
Protein homeostasis, also called proteostasis, describes a compendium of processes that include proteasome-dependent protein degradation, several types of autophagy required for the degradation of biomolecules, protein aggregates and defective organelles (reviewed in 28), as well as downregulation of ribosomal protein translation 29, 30. Enhanced proteostasis mechanisms are often essential components of lifespan-extending pathways ( Figure 1). Inhibition of TOR signaling, for instance, reduces the initiation of protein translation, which might then alleviate proteotoxic stress, e.g., the age-related accumulation of protein aggregates 30. In addition, shifts in overall metabolism might cause or result from changes in proteostatic capacity 31. For example, mitochondria, which have important roles in energy generation and act as hubs for lipid metabolism and anabolic processes, have been implicated in lifespan regulation. Treatment with the natural compound urolithin A induces mitophagy and extends lifespan in C. elegans perhaps by eliminating dysfunctional mitochondria, thus linking autophagy and mitochondrial physiology to life span determination 32. Their involvement might be context-dependent, as mitochondrial stress responses, such as the mitochondrial unfolded protein response (UPR mt), can contribute to life span extension in C. elegans 23. In contrast, life span–extending mutations in the electron transport chain complex IV (COX-5B, previously referred to as CCO-1) or cytochrome c reductase (ISP-1) induce UPR mt marker expression; however, abrogating this induction via mutation of the transcription factor (TF) ATFS-1 does not ameliorate this life span extension 33. Zhou et al. demonstrated that the consequence of autophagy can also be context-dependent: whereas increased autophagy is necessary for many life span–extending paradigms (that is, CR), increased mitochondrial permeability can convert elevated autophagy into a life span–shortening process in C. elegans 34. This observation suggests that mitochondrial and autophagic functions may interact to modulate longevity.
A range of TFs and epigenetic and splicing modulators 35 that regulate stress responses and confer positive effects on life span have been identified. The discovery that attenuation of the insulin-like signaling (IIS) pathway extends life span in C. elegans ignited the field of the genetics of longevity. Importantly, IIS regulates life span via activation of the FOXO TF DAF-16. Since these seminal discoveries, numerous additional life span–regulating TFs have been identified. The HLH-30/TFEB TF mediates longevity via stress-induced regulation of autophagy-associated genes upon stress. Although these TFs have been characterized in isolation for quite some time, how they can cooperate with a variety of other TFs to regulate life span and stress responses has been recently delineated. Lin et al. suggested that DAF-16/FOXO and HLH-30/TFEB cooperate to regulate longevity and also have important independent functions under specific stress conditions 36. In contrast, the homeodomain TF CEH-60/PBX, whose role in development has been investigated, was recently shown to negatively affect life span in C. elegans by repressing DAF-16 activity 37. HLH-30 activity, which has positive effects on life span, can be enhanced via treatment with nuclear export blocker drugs 38 and modulators of intracellular calcium storage compartments 39. On the level of mRNA processing, the splicing factor SFA-1/SF1 has emerged as a requirement for the proper splicing of transcripts involved in lipid metabolism and other metabolic processes under conditions of life span–extending DR 35.
Epigenetic mechanisms are crucial for cell identity and function of differentiated cells, the differentiation of stem cells, and stem cell maintenance. So far, different adult stem cell systems have been identified which show aging-associated changes in epigenetic marks on chromatin and DNA (for example, in hematopoietic stem cells [HSCs], intestinal stem cells, and muscle stem cells [MuSCs]). Cell-intrinsic damage, accumulating during stem cell quiescence, may lead to epigenetic changes, while cell elimination is prevented through anti-apoptotic pathways (reviewed in 40). For example, these changes can lead to divergent deposition of H3K4me3 marks or action of Rad21/cohesin, resulting in impaired stem cell function and senescence signaling in aged MuSCs 41 or over-activation of inflammatory signaling in HSCs 42, respectively. Deficiencies in stem cell function, in turn, impair tissue homeostasis, function, and regeneration, leading to declining organismal health and most likely limiting life span. Moreover, epigenetic changes in aged stem cells might lead to increased incidences of aging-related diseases such as hematopoietic cancers by allowing the clonal selection of mutated HSCs 43– 45. Interestingly, transient epigenetic reprogramming via cyclic expression of the Yamanaka factors in mice showed signatures of improved health in a progeroid laminopathy mouse model 46.
It is becoming increasingly clear that in addition to cell-autonomous stress responses that modulate the intrinsic resilience of individual cells upon exposure to specific types of stress, signaling between cells and tissues can elicit such responses both locally and in distal tissues. The influence of these inter-tissue effects has been exemplified by heterochronic parabiosis and transplantation experiments, which suggest differences in circulating and local niche factors to dynamically alter tissue functionality and epigenetic states during aging 40, 47, 48. In particular, neuronal tissue has come into focus as a significant coordinator of life span–modulating processes; for example, neuronal autophagy (as well as autophagy in the intestine) can regulate life span–extending processes non-cell-autonomously 49. The immune system is an important non-cell-autonomous regulator that not only profoundly influences life span directly by preventing premature death due to infections but also protects organisms via cancer surveillance and removal of senescent cells. While the prowess of the immune system fades during aging through a process called immunosenescence 50, nuclear DNA damage, accumulating extranuclear DNA, and senescent cells fuel inflammation 51. Parts of the adaptive immune repertoire of individual aging humans have recently been characterized, and declines in the diversity of CD8 + (cytotoxic) T-cell and B-cell repertoires were observed 52, 53. Senescent cells, which adopt a distinct physiology (often via continuous DNA damage signaling 11), have themselves been implicated in impairing normal tissue function and promoting the development of a deleterious, chronic pro-inflammatory environment 54– 56. Targeting senescent cells has shown positive effects on immune function in mice and therefore appears to be a promising field of research to improve tissue aging in the elderly 57, including attempts to re-establish a balanced output of aging HSCs to regenerate lymphopoiesis during aging 58. In contrast, the senescence program might protect cells from transforming into cancer cells and has been implicated in tissue regeneration after skin injury 59. Together, these observations indicate that senescent cells serve dual roles in influencing life span: pro-longevity tumor suppression and tissue repair versus involvement in pro-aging inflammatory reactions.
As the important roles for the immune system and metabolism in life span modulation have been intensely studied for some time, the involvement of the microbiome has emerged more recently. Different microbiota compositions have been shown to modulate both the immune system and metabolism in positive and pathogenic ways. Studies on centenarians have suggested specific signatures in the gut microbiome in terms of its composition and diversity in long-lived humans 60, 61. For example, higher relative abundances of Akkermansia and Bifidobacterium, known health-associated microbes, are positively associated with exceptionally long-lived humans 60. Furthermore, microbial transfer experiments from young to middle-aged killifish, a short-lived model organism recently established for conducting research on vertebrate aging, demonstrated a positive effect of a young microbiome on life span 62.
Many stress factors that influence life span (for example, ROS, mitochondrial impairment, cellular redox imbalance, nutritional status, and protein translation) are intimately connected ( Figure 1). Thus, the delineation of clear cause-and-effect relationships between such factors and longevity is challenging. ROS, for example, can cause proteome stress 63 and lead to DNA damage 64 among other effects; however, it also stimulates protective (hormetic) responses 9. DNA damage, in turn, can lead to more ROS production 65, potentially also via consequential imbalances in the mitochondrial proteome. The DDR, which comprises checkpoint signaling and DNA repair pathways, protects cells from malignant transformation 66. The DDR can also lead to decreased general translation 67, which might alleviate proteostatic stress. The involvement of proteostasis in the DDR appears manifold, ranging from distinct roles during DNA repair to ensuring cellular homeostasis. DNA damage remains a central node in the network of these processes, as both exogenous and endogenous genotoxins constantly inflict DNA damage and because the DDR affects a vast range of metabolic and proteostatic responses 68. Interestingly, CR was shown to dramatically extend life span in nucleotide excision repair–deficient progeroid animals 69, suggesting a new perspective in the ongoing quest for therapies for congenital progeroid syndromes. Indeed, DAF-16–mediated stress responses to DNA damage in C. elegans can preserve tissue maintenance and function by elevating the tolerance to persistent DNA damage 70.
Interventional studies with the mTOR inhibitor rapamycin have had positive effects on delaying the onset of age-associated chronic disease markers and potentially negative effects in humans 71– 73. In addition to rapamycin, the drugs resveratrol and metformin have been used to modulate pathways involved in DR-mediated life span extension, and senolytic drugs (for example, dasatinib, quercetin 74, and fisetin 75) have been reported to differentially enhance apoptosis in senescent cells, depending on their original cell type. (Senolytics are reviewed in 76.) Dasatininb and quercetin were recently tested in a first human pilot study on idiopathic pulmonary fibrosis; however, the long-term effects of these treatments have not been assessed 77. These interventions might indeed provide potential therapeutic options for delaying the aging process; however, drugs for specifically enhancing DNA repair or alleviating DNA damage have not been developed yet. In contrast, the deletion of DDR components was shown to exert positive effects on tissue maintenance and life span in mice that prematurely aged because of telomere dysfunction 78– 80. These results suggest that modulating the DDR could provide interesting avenues for interventions for maintaining tissue homeostasis during aging.
In summary, recent progress has significantly expanded our knowledge of the various processes that modulate life span, including genetic regulators, stress responses, metabolism, cellular senescence, and inter-tissue communication. Stochastic effects, such as the occurrence of DNA damage, which can impact each one of those processes 68 could result in different individual aging trajectories, where hypothetical tipping points of declining tissue functionality are reached at different time points 81, 82. This might be one reason for the heterogeneity of individual life spans even in defined, homogenous model organism populations. Importantly, the interactions between longevity modulators are becoming increasingly apparent, highlighting the complexities underlying aging and life span determination. As multiple factors contribute to aging and the alleviation of age-related organismal deterioration, it might be necessary for future interventions to collectively target a range of longevity modulators—potentially even in a tissue- or cell type-specific manner—to extend the healthy life span in humans.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
K Lenhard Rudolph, Research Group on Stem Cell Aging, Leibniz Institute on Aging, Fritz Lipmann Institute (FLI), Jena, Germany
Jan Vijg, Department of Genetics, Albert Einstein College of Medicine, New York, USA
Evgeny Nudler, Department of Biochemistry and Molecular Pharmacology, New York University School of Medicine, New York, USA
Funding Statement
BS acknowledges funding from the Deutsche Forschungsgemeinschaft (SCHU 2494/3-1, SCHU 2494/7-1, CECAD, SFB 829, SFB 670, KFO 286, KFO 329, and GRK2407), the Deutsche Krebshilfe (70112899), and the European Cooperation in Science and Technology (COST) action (BM1408).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 3 approved]
References
- 1. Jones OR, Scheuerlein A, Salguero-Gómez R, et al. : Diversity of ageing across the tree of life. Nature. 2014;505(7482):169–73. 10.1038/nature12789 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 2. de Magalhães JP, Costa J: A database of vertebrate longevity records and their relation to other life-history traits. J Evol Biol. 2009;22(8):1770–4. 10.1111/j.1420-9101.2009.01783.x [DOI] [PubMed] [Google Scholar]
- 3. Nilsson Sköld H, Obst M: Potential for clonal animals in longevity and ageing studies. Biogerontology. 2011;12(5):387–96. 10.1007/s10522-011-9333-8 [DOI] [PubMed] [Google Scholar]
- 4. Dong X, Milholland B, Vijg J: Evidence for a limit to human lifespan. Nature. 2016;538(7624):257–9. 10.1038/nature19793 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 5. Barbi E, Lagona F, Marsili M, et al. : The plateau of human mortality: Demography of longevity pioneers. Science. 2018;360(6396):1459–61. 10.1126/science.aat3119 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 6. Herndon LA, Schmeissner PJ, Dudaronek JM, et al. : Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419(6909):808–14. 10.1038/nature01135 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Muntané G, Farré X, Rodríguez JA, et al. : Biological Processes Modulating Longevity across Primates: A Phylogenetic Genome-Phenome Analysis. Mol Biol Evol. 2018;35(8):1990–2004. 10.1093/molbev/msy105 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. Wirthlin M, Lima NCB, Guedes RLM, et al. : Parrot Genomes and the Evolution of Heightened Longevity and Cognition. Curr Biol. 2018;28(24):4001–4008.e7. 10.1016/j.cub.2018.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 9. Schumacher B: Transcription-blocking DNA damage in aging: a mechanism for hormesis. BioEssays. 2009;31(12):1347–56. 10.1002/bies.200900107 [DOI] [PubMed] [Google Scholar]
- 10. Moskalev AA, Shaposhnikov MV, Plyusnina EN, et al. : The role of DNA damage and repair in aging through the prism of Koch-like criteria. Ageing Res Rev. 2013;12(2):661–84. 10.1016/j.arr.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 11. Fumagalli M, Rossiello F, Mondello C, et al. : Stable Cellular Senescence Is Associated with Persistent DDR Activation. PLoS One. 2014;9(10):e110969. 10.1371/journal.pone.0110969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoeijmakers JH: DNA damage, aging, and cancer. N Engl J Med. 2009;361(15):1475–85. 10.1056/NEJMra0804615 [DOI] [PubMed] [Google Scholar]
- 13. Madabhushi R, Pan L, Tsai LH: DNA damage and its links to neurodegeneration. Neuron. 2014;83(2):266–82. 10.1016/j.neuron.2014.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scialò F, Sriram A, Fernández-Ayala D, et al. : Mitochondrial ROS Produced via Reverse Electron Transport Extend Animal Lifespan. Cell Metab. 2016;23(4):725–34. 10.1016/j.cmet.2016.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Xiong LG, Chen YJ, Tong JW, et al. : Epigallocatechin-3-gallate promotes healthy lifespan through mitohormesis during early-to-mid adulthood in Caenorhabditis elegans. Redox Biol. 2018;14:305–15. 10.1016/j.redox.2017.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Schmeisser S, Schmeisser K, Weimer S, et al. : Mitochondrial hormesis links low-dose arsenite exposure to lifespan extension. Aging Cell. 2013;12(3):508–17. 10.1111/acel.12076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hunt PR, Son TG, Wilson MA, et al. : Extension of lifespan in C. elegans by naphthoquinones that act through stress hormesis mechanisms. PLoS One. 2011;6(7):e21922. 10.1371/journal.pone.0021922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gusarov I, Nudler E: Glycogen at the Crossroad of Stress Resistance, Energy Maintenance, and Pathophysiology of Aging. BioEssays. 2018;40(9):e1800033. 10.1002/bies.201800033 [DOI] [PubMed] [Google Scholar]
- 19. Gusarov I, Pani B, Gautier L, et al. : Glycogen controls Caenorhabditis elegans lifespan and resistance to oxidative stress. Nat Commun. 2017;8:15868. 10.1038/ncomms15868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singh PP, Demmitt BA, Nath RD, et al. : The Genetics of Aging: A Vertebrate Perspective. Cell. 2019;177(1):200–20. 10.1016/j.cell.2019.02.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Redman LM, Smith SR, Burton JH, et al. : Metabolic Slowing and Reduced Oxidative Damage with Sustained Caloric Restriction Support the Rate of Living and Oxidative Damage Theories of Aging. Cell Metab. 2018;27(4):805–815.e4. 10.1016/j.cmet.2018.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Mattison JA, Colman RJ, Beasley TM, et al. : Caloric restriction improves health and survival of rhesus monkeys. Nat Commun. 2017;8:14063. 10.1038/ncomms14063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Denzel MS, Lapierre LR, Mack HID: Emerging topics in C. elegans aging research: Transcriptional regulation, stress response and epigenetics. Mech Ageing Dev. 2019;177:4–21. 10.1016/j.mad.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harrison DE, Strong R, Sharp ZD, et al. : Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–5. 10.1038/nature08221 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Cravedi P, Ruggenenti P, Remuzzi G: Sirolimus for calcineurin inhibitors in organ transplantation: contra. Kidney Int. 2010;78(11):1068–74. 10.1038/ki.2010.268 [DOI] [PubMed] [Google Scholar]
- 26. Benjamin D, Colombi M, Moroni C, et al. : Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10(11):868–80. 10.1038/nrd3531 [DOI] [PubMed] [Google Scholar]
- 27. Tang D, Tao S, Chen Z, et al. : Dietary restriction improves repopulation but impairs lymphoid differentiation capacity of hematopoietic stem cells in early aging. J Exp Med. 2016;213(4):535–53. 10.1084/jem.20151100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hansen M, Rubinsztein DC, Walker DW: Autophagy as a promoter of longevity: insights from model organisms. Nat Rev Mol Cell Biol. 2018;19(9):579–93. 10.1038/s41580-018-0033-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Molenaars M, Janssens GE, Santermans T, et al. : Mitochondrial ubiquinone-mediated longevity is marked by reduced cytoplasmic mRNA translation. Life Sci Alliance. 2018;1(5). 10.26508/lsa.201800082 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Solis GM, Kardakaris R, Valentine ER, et al. : Translation attenuation by minocycline enhances longevity and proteostasis in old post-stress-responsive organisms. eLife. 2018;7: pii: e40314. 10.7554/eLife.40314 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. López-Otín C, Galluzzi L, Freije JMP, et al. : Metabolic Control of Longevity. Cell. 2016;166(4):802–21. 10.1016/j.cell.2016.07.031 [DOI] [PubMed] [Google Scholar]
- 32. Ryu D, Mouchiroud L, Andreux PA, et al. : Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat Med. 2016;22(8):879–88. 10.1038/nm.4132 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Bennett CF, Vander Wende H, Simko M, et al. : Activation of the mitochondrial unfolded protein response does not predict longevity in Caenorhabditis elegans. Nat Commun. 2014;5:3483. 10.1038/ncomms4483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou B, Kreuzer J, Kumsta C, et al. : Mitochondrial Permeability Uncouples Elevated Autophagy and Lifespan Extension. Cell. 2019;177(2):299–314.e16. 10.1016/j.cell.2019.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Heintz C, Doktor TK, Lanjuin A, et al. : Splicing factor 1 modulates dietary restriction and TORC1 pathway longevity in C. elegans. Nature. 2017;541(7635):102–6. 10.1038/nature20789 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Lin XX, Sen I, Janssens GE, et al. : DAF-16/FOXO and HLH-30/TFEB function as combinatorial transcription factors to promote stress resistance and longevity. Nat Commun. 2018;9(1):4400. 10.1038/s41467-018-06624-0 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Dowen RH: CEH-60/PBX and UNC-62/MEIS Coordinate a Metabolic Switch that Supports Reproduction in C. elegans. Dev Cell. 2019;49(2):235–250.e7. 10.1016/j.devcel.2019.03.002 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Silvestrini MJ, Johnson JR, Kumar AV, et al. : Nuclear Export Inhibition Enhances HLH-30/TFEB Activity, Autophagy, and Lifespan. Cell Rep. 2018;23(7):1915–21. 10.1016/j.celrep.2018.04.063 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Wang C, Niederstrasser H, Douglas PM, et al. : Small-molecule TFEB pathway agonists that ameliorate metabolic syndrome in mice and extend C. elegans lifespan. Nat Commun. 2017;8(1):2270. 10.1038/s41467-017-02332-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ermolaeva M, Neri F, Ori A, et al. : Cellular and epigenetic drivers of stem cell ageing. Nat Rev Mol Cell Biol. 2018;19(9):594–610. 10.1038/s41580-018-0020-3 [DOI] [PubMed] [Google Scholar]
- 41. Schwörer S, Becker F, Feller C, et al. : Epigenetic stress responses induce muscle stem-cell ageing by Hoxa9 developmental signals. Nature. 2016;540(7633):428–32. 10.1038/nature20603 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Chen Z, Amro EM, Becker F, et al. : Cohesin-mediated NF-κB signaling limits hematopoietic stem cell self-renewal in aging and inflammation. J Exp Med. 2019;216(1):152–75. 10.1084/jem.20181505 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Busque L, Patel JP, Figueroa ME, et al. : Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nat Genet. 2012;44(11):1179–81. 10.1038/ng.2413 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Jaiswal S, Fontanillas P, Flannick J, et al. : Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–98. 10.1056/NEJMoa1408617 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Jaiswal S, Natarajan P, Silver AJ, et al. : Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med. 2017;377(2):111–21. 10.1056/NEJMoa1701719 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Ocampo A, Reddy P, Martinez-Redondo P, et al. : In Vivo Amelioration of Age-Associated Hallmarks by Partial Reprogramming. Cell. 2016;167(7):1719–1733.e12. 10.1016/j.cell.2016.11.052 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Villeda SA, Luo J, Mosher KI, et al. : The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477(7362):90–4. 10.1038/nature10357 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Conboy IM, Conboy MJ, Wagers AJ, et al. : Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760–4. 10.1038/nature03260 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Minnerly J, Zhang J, Parker T, et al. : The cell non-autonomous function of ATG-18 is essential for neuroendocrine regulation of Caenorhabditis elegans lifespan. PLoS Genet. 2017;13(5):e1006764. 10.1371/journal.pgen.1006764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K: Causes, consequences, and reversal of immune system aging. J Clin Invest. 2013;123(3):958–65. 10.1172/JCI64096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lan YY, Heather JM, Eisenhaure T, et al. : Extranuclear DNA accumulates in aged cells and contributes to senescence and inflammation. Aging Cell. 2019;18(2):e12901. 10.1111/acel.12901 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Yoshida K, Cologne JB, Cordova K, et al. : Aging-related changes in human T-cell repertoire over 20years delineated by deep sequencing of peripheral T-cell receptors. Exp Gerontol. 2017;96:29–37. 10.1016/j.exger.2017.05.015 [DOI] [PubMed] [Google Scholar]
- 53. de Bourcy CF, Angel CJ, Vollmers C, et al. : Phylogenetic analysis of the human antibody repertoire reveals quantitative signatures of immune senescence and aging. Proc Natl Acad Sci U S A. 2017;114(5):1105–10. 10.1073/pnas.1617959114 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Xu M, Tchkonia T, Ding H, et al. : JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S A. 2015;112(46):E6301–E6310. 10.1073/pnas.1515386112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bektas A, Schurman SH, Sen R, et al. : Aging, inflammation and the environment. Exp Gerontol. 2018;105:10–8. 10.1016/j.exger.2017.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bektas A, Schurman SH, Sen R, et al. : Human T cell immunosenescence and inflammation in aging. J Leukoc Biol. 2017;102(4):977–88. 10.1189/jlb.3RI0716-335R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Palacio L, Goyer ML, Maggiorani D, et al. : Restored immune cell functions upon clearance of senescence in the irradiated splenic environment. Aging Cell. 2019;18(4):e12971. 10.1111/acel.12971 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Wang J, Morita Y, Han B, et al. : Per2 induction limits lymphoid-biased haematopoietic stem cells and lymphopoiesis in the context of DNA damage and ageing. Nat Cell Biol. 2016;18(5):480–90. 10.1038/ncb3342 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 59. Demaria M, Ohtani N, Youssef SA, et al. : An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31(6):722–33. 10.1016/j.devcel.2014.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Biagi E, Franceschi C, Rampelli S, et al. : Gut Microbiota and Extreme Longevity. Curr Biol. 2016;26(11):1480–5. 10.1016/j.cub.2016.04.016 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Tuikhar N, Keisam S, Labala RK, et al. : Comparative analysis of the gut microbiota in centenarians and young adults shows a common signature across genotypically non-related populations. Mech Ageing Dev. 2019;179:23–35. 10.1016/j.mad.2019.02.001 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 62. Smith P, Willemsen D, Popkes M, et al. : Regulation of life span by the gut microbiota in the short-lived African turquoise killifish. eLife. 2017;6: pii: e27014. 10.7554/eLife.27014 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Berlett BS, Stadtman ER: Protein oxidation in aging and disease. Free Radic Biol Med. 2003;27:S4. [Google Scholar]
- 64. Cadet J, Wagner JR: DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb Perspect Biol. 2013;5(2): pii: a012559. 10.1101/cshperspect.a012559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gurkar AU, Robinson AR, Cui Y, et al. : Dysregulation of DAF-16/FOXO3A-mediated stress responses accelerates oxidative DNA damage induced aging. Redox Biol. 2018;18:191–9. 10.1016/j.redox.2018.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 66. Blackford AN, Jackson SP: ATM, ATR, and DNA-PK: The Trinity at the Heart of the DNA Damage Response. Mol Cell. 2017;66(6):801–17. 10.1016/j.molcel.2017.05.015 [DOI] [PubMed] [Google Scholar]
- 67. Loveless TB, Topacio BR, Vashisht AA, et al. : DNA Damage Regulates Translation through β-TRCP Targeting of CReP. PLoS Genet. 2015;11(6):e1005292. 10.1371/journal.pgen.1005292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Edifizi D, Nolte H, Babu V, et al. : Multilayered Reprogramming in Response to Persistent DNA Damage in C. elegans. Cell Rep. 2017;20(9):2026–43. 10.1016/j.celrep.2017.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. Vermeij WP, Dollé ME, Reiling E, et al. : Restricted diet delays accelerated ageing and genomic stress in DNA-repair-deficient mice. Nature. 2016;537(7620):427–31. 10.1038/nature19329 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 70. Mueller MM, Castells-Roca L, Babu V, et al. : DAF-16/FOXO and EGL-27/GATA promote developmental growth in response to persistent somatic DNA damage. Nat Cell Biol. 2014;16(12):1168–79. 10.1038/ncb3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Urfer SR, Kaeberlein TL, Mailheau S, et al. : A randomized controlled trial to establish effects of short-term rapamycin treatment in 24 middle-aged companion dogs. Geroscience. 2017;39(2):117–27. 10.1007/s11357-017-9972-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mannick JB, Del Giudice G, Lattanzi M, et al. : mTOR inhibition improves immune function in the elderly. Sci Transl Med. 2014;6(268): 268ra179. 10.1126/scitranslmed.3009892 [DOI] [PubMed] [Google Scholar]
- 73. Mannick J: TORC1 INHIBITION AS A POTENTIAL IMMUNOTHERAPY TO REDUCE INFECTIONS IN THE ELDERLY. Innov Aging. 2018;2(Suppl 1):545 10.1093/geroni/igy023.2011 [DOI] [Google Scholar]; F1000 Recommendation
- 74. Xu M, Pirtskhalava T, Farr JN, et al. : Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24(8):1246–56. 10.1038/s41591-018-0092-9 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Yousefzadeh MJ, Zhu Y, McGowan SJ, et al. : Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018;36:18–28. 10.1016/j.ebiom.2018.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 76. Kirkland JL, Tchkonia T, Zhu Y, et al. : The Clinical Potential of Senolytic Drugs. J Am Geriatr Soc. 2017;65(10):2297–301. 10.1111/jgs.14969 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 77. Justice JN, Nambiar AM, Tchkonia T, et al. : Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–63. 10.1016/j.ebiom.2018.12.052 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 78. Choudhury AR, Ju Z, Djojosubroto MW, et al. : Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nat Genet. 2007;39(1):99–105. 10.1038/ng1937 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 79. Schaetzlein S, Kodandaramireddy NR, Ju Z, et al. : Exonuclease-1 deletion impairs DNA damage signaling and prolongs lifespan of telomere-dysfunctional mice. Cell. 2007;130(5):863–77. 10.1016/j.cell.2007.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sperka T, Song Z, Morita Y, et al. : Puma and p21 represent cooperating checkpoints limiting self-renewal and chromosomal instability of somatic stem cells in response to telomere dysfunction. Nat Cell Biol. 2012;14(1):73–9. 10.1038/ncb2388 [DOI] [PubMed] [Google Scholar]
- 81. Sarnoski EA, Song R, Ertekin E, et al. : Fundamental Characteristics of Single-Cell Aging in Diploid Yeast. iScience. 2018;7:96–109. 10.1016/j.isci.2018.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 82. Song R, Sarnoski EA, Acar M: The Systems Biology of Single-Cell Aging. iScience. 2018;7:154–69. 10.1016/j.isci.2018.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation