Abstract
Background
It is known that one of the leading causes of morbidity in chronic kidney disease (CKD) is the anemic syndrome. Although the pathogenic mechanisms of anemia are multiple, erythropoietin deficiency appears as the dominant factor. Patients in hemodialysis (HD) have a high prevalence of protein energy wasting (PEW) that may explains the poor response to Erythropoietin (EPO).
Methods
Retrospective cohort study of patients on HD from January to December 2014. The participants were classified according to a diagnostic of PEW using the “Malnutrition Inflammation Score” (MIS) and bioimpedance analysis (BIA) measurement of body composition at the start of erythropoietin therapy and after 3 months of follow up. We performed descriptive statistics and analyzed the differences between groups with and without PEW considering their responsiveness. In addition, we calculated the relative risk of EPO resistance, considering p value < 0.05 as statistically significant.
Results
Sixty-one patients ended the follow up. Both groups were similar in basal hemoglobin, hematocrit and other hematopoiesis markers (p = NS). Patients without PEW have a decrease risk for poor response to treatment with EPO (RR = 0.562 [95% CI, 0.329–0.961-]) than those with PEW. Finally, hemoglobin concentrations were evaluated at baseline and every four weeks until week 12, finding a statistically significant improvement only in patients without PEW according MIS (p < 0.05).
Conclusions
PEW is an incremental predictor of poor responsiveness to EPO in HD patients, thus, it is important to consider correcting malnutrition or wasting for a favorable response to treatment with EPO.
Keywords: Protein energy wasting, Hemodialysis, Erythropoietin response
Background
One of the leading causes of morbidity in patients with advanced chronic kidney disease (CKD) is the presence of anemia. Although there are several pathogenic mechanisms for anemia in the CKD patient, Erythropoietin (EPO) deficiency appears as the dominant factor [1, 2]. There is a progressive decrease of the hematocrit (Hct); once the Glomerular Filtration Rate (GFR) falls below 60 ml/min /1.73m2; however, anemia is clinically assessable only after GFR has fallen to less than 30 ml/min/1.73m2 [3–5]. The addition of recombinant human erythropoietin (rHuEPO) to the treatment of anemia of renal origin has been the most important advance in this field. However, a percentage of patients responds poorly, or do not respond at all. The term “resistance” is meaningful here, since the serum concentrations of EPO required to obtain a response are extremely high, and the required dose of EPO is a predictor of mortality in this population [6, 7].
Definition of poor response
In general, in most practice guidelines, the recommended range of Hb is 10-12 g/dl [5]. To reach this level, it is generally sufficient to administer initial doses of 50–150 Units/kilogram/week (U/kg/week) and then maintenance doses of 40 U/kg/week, as long as a proper iron store are assured in the patient [8, 9].
Patients of change in hemoglobin (Hb) level < 2% were operationally considered to have had a poor response, as compared with those in the upper change in hemoglobin level (2 to 15% or more). We assessed the mean hemoglobin level at 4 weeks (early phase), as well as the mean dose received after 12 weeks [10].
Since a 2% increase in the Hb concentration is likely to be within the variability range of Hb values in individual patients, this value is considered as no increase.The ESA responsiveness index (ERI) was defined as the weekly weight-adjusted EPO dose (U/kg/week) divided by the hemoglobin level (g/dL) and calculated monthly to investigate resistance to EPO treatment [11].
The most common causes of EPO resistance, among other, are secondary hyperparathyroidism, chronic inflammatory processes, and malnutrition. While iron deficiency is the leading cause of failure to respond to treatment with EPO; it must not be forgotten that active inflammatory or infectious processes can temporarily block the response to iron therapy and, thereby, to EPO [12–14]. It is important to remember that the presence of anemia in the HD population, is considered one of the main causes of morbidity, closely related to a decrease in appetite and to a hypercatabolic state, all of which may induce a state of malnutrition and inflammation also known as protein-energy wasting (PEW) [15–17].
Recent literature suggests that PEW is an independent factor for EPO responsiveness when initiating treatment with erythropoiesis stimulating agents (ESA) [18–20]. With the presence of PEW, there is a less response to EPO therapy; however, results are not yet conclusive.
Methods
We conducted a retrospective cohort study including adult patients on HD from our Institute from January to December; ages > 18 years, with Kt/V > 1.2 and/or URR > 65%, serum ferritin > 100 ng/dl and transferrin saturation > 20%, and an individualized prescription of EPO. Patients were excluded if they had an immunologically mediated disease as lupus erythematosus and were in treatment with remission induction therapy (patients who after 3 months might recover renal function), if they had any metallic prosthesis that could interfere with bioelectrical impedance, if they had an active infectious disease, or if they had been in an emergency room or hospitalized at any point during the previous 3 months. Clinical, biochemical and treatment data, including EPO dose was obtained through medical records that are done monthly. Malnutrition Inflammation Score (MIS) and bio impedance vector analysis (BIVA) [22] are done routinely every 3 months in this population. All participants were followed up for 12 weeks. PEW was defined as a MIS [15] of > 7 [18, 21] or phase angle ≤5°.
Patients with an increase in hemoglobin level ≤ 2% is considered a non-significant change or poor response after the EPO treatment.
The nutritional status and body composition of the participants were analyzed by BIVA, using conventional measurements of bio impedance analysis (BIA); resistance (R), reactance (Xc) and Phase Angle; the data were then plotted using the method of bioelectrical impedance vectors normalized in Z-score, that is, Z (R) and Z (Xc) [22–24].
The protocol was approved by the Human Research and Ethics Committee of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán; (reference 1407). Data and material availability of the study will be provided if requested.
Statistical analysis
Data were summarized using proportions; means [±standard deviation (SD)] or medians (inter-quartile range) as appropriate using the Shapiro-Wilk for testing normality. To find differences between patients with or without PEW, we used Xi2, Mann-Whitney’s U test or Student’s t, according to their distribution. The correlation between phase angle by BIA or MIS and hemoglobin concentration was evaluated using Pearson’s or Spearman’s correlation coefficients. A test of repeated measures ANOVA or Kruskal Wallis was performed to compare changes in hemoglobin levels during the period of the study. We estimated the relative risk for poor response to treatment according to PEW diagnosis. To compare the confidence ellipses of the impedance vectors we used Hotelling’s T2 test. A p < 0.05 was considered as statistically significant. The data were analyzed using SPSS 20.0 (SPSS Inc., Chicago, IL, USA).
Results
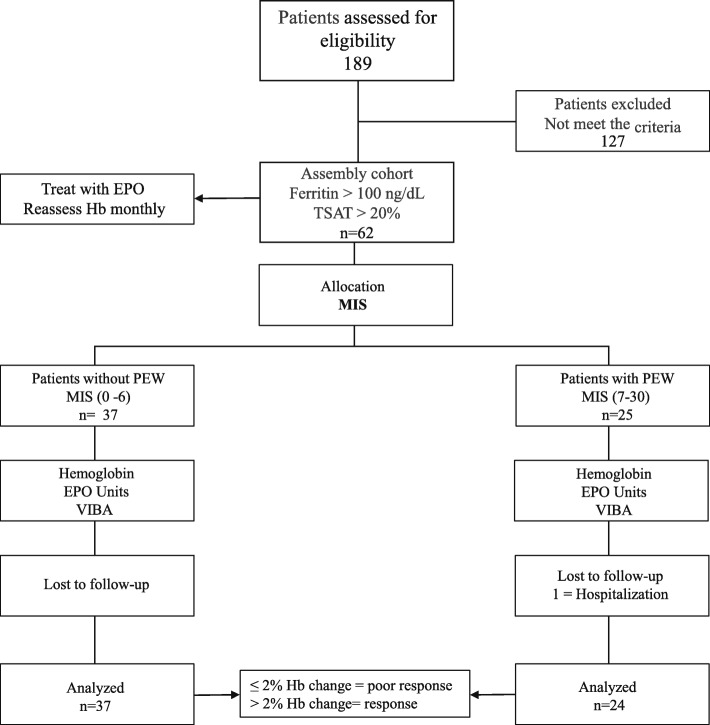
The medical records of 189 patients from our HD Clinic were evaluated retrospectively (Fig. 1). One hundred twenty-seven patients did not meet the inclusion criteria.
Fig. 1.
Flowchart showing the participation process
A total of 61 patients were included in the study, of which 37 had not PEW according MIS. General characteristics of the population are shown in Table 1. As expected, per definition of the two groups, statistically significant differences were found between the two groups in variables associated with nutritional status such as weight, body mass index (BMI), serum creatinine, serum albumin and potassium also we found that the patients with PEW had a higher ERI (IU/kg/week) (p = 0.04). Patients with PEW according to MIS had lower concentrations of serum creatinine than those without PEW. No significant differences in Hb, Hct, serum iron, transferrin saturation (TSAT), serum ferritin (Table 1). Patients that had low iron levels not necessarily were given iv iron, but we registered a 57.5% of patients that did received IV iron and the dose was at 100 mg weekly X 10 doses.
Table 1.
General characteristics of the population. Anthropometric and demographic characteristics
| VARIABLE (%) | without PEW n = 37 (60.6) |
PEW n = 24 (39.4) |
p value |
|---|---|---|---|
| Age (year) | 30 (20–77) | 28.5 (19–63) | 0.172 |
| Time on dialysis (months) | 8 (3–50) | 4.5 (3–32) | 0.044 |
| Weight (kg) | 57.5 (43–105) | 48 (35–87.7) | 0.015 |
| Height (meters) | 1.60 ± 0.08 | 1.57 ± 0.09 | 0.259 |
| BMI (kg/m2) | 23.27 ± 4.9 | 20.35 ± 3.3 | 0.015 |
| KT/V | 1.67 ± 0.34 | 1.77 ± 0.40 | 0.311 |
| URR % | 79.73 ± 6.02 | 80.24 ± 7.9 | 0.800 |
| Creatinine (mg/dl) | 10.8 (4.19–20.5) | 6 (2.52–15.84) | 0.001 |
| Sodium (mmol/L) | 138.19 ± 3.55 | 138.05 ± 3.46 | 0.324 |
| Potassium (mmol/L) | 5.128 ± 0.59 | 4.50 ± 0.89 | 0.009 |
| Phosphorus (μg/L) | 4.98 ± 1.76 | 4.27 ± 1.47 | 0.098 |
| Serum albumin (g/dl) | 3.46 ± 0.521 | 3.10 ± 0.47 | 0.008 |
| Glucose (mg/dl) | 112.22 (72–194) | 105 (72–597) | 0.696 |
| Uric acid (mg/dl) | 7.48 ± 1.51 | 7.36 ± 2.35 | 0.787 |
| Hemoglobin (g/dl) | 8.9 ± 1.78 | 9.34 ± 1.18 | 0.279 |
| Hematocrit(%) | 27.26 ± 5.5 | 28.37 ± 3.93 | 0.237 |
| PTH (pg/mL) | 498.4 (26–1825) | 226 (25.8–1005) | 0.046 |
| Serum Iron μg/dL | 75 (42–195– 104) | 76 (27–202) | 0.922 |
| Transferrin saturation % | 35.4 (20–61) | 32 (20–96.5) | 0.842 |
| Serum ferritin | 315.7 (100–1270) | 461.8 (118.8–1296.7) | 0.142 |
| Epo U/kg/week | 112 (32.5–276.5) | 136 (66.5–270) | 0.040 |
| Impedance characteristics | |||
| MEN | n = 11 | n = 9 | |
| Resistance R (Ohms) | 522.6 ± 102.3 | 588 ± 115.4 | 0.203 |
| Reactance Xc (Ohms) | 52.9 (28.4–81) | 50.5 (24.9–99.3) | 0.456 |
| Phase Angle (°) | 5.95 ± 1.27 | 4.88 ± 1.28 | 0.083 |
| Resistance/height(Ohms/meters) | 312.08 ± 64.15 | 457.7 ± 79 | 0.185 |
| Reactance/height (Ohms/meters) | 31.1 (16–48.2) | 29.7 (14.3–64.9) | 0.656 |
| WOMEN | n = 26 | n = 15 | |
| Resistance R (Ohms) | 638.15 ± 112 | 724.2 ± 154.5 | 0.072 |
| Reactance Xc (Ohms) | 62.9 (21.3–104) | 49 (23–68) | 0.076 |
| Phase Angle (°) | 5.2 ± 1.69 | 4.05 ± 1.33 | 0.020 |
| Resistance/height(Ohms/meters) | 406.7 ± 76.5 | 474.4 ± 106.1 | 0.041 |
| Reactance/height (Ohms/meters) | 40.7 (12.45–67.9) | 31.2 (15.7–44.9) | 0.127 |
Continuous variables are expressed as mean ± standard deviation, non-parametric as medians or inter-quartile ranges The categorical variables were compared using χ2 test and continuous variables were compared using the T or U Mann-Whitney test according to the variable
The BIA analysis of the studied population with and without PEW and subdivided by gender are shown in Table 1. Phase angle was higher in patients without PEW even though statistically significant differences were found only in women. In both genders, resistance is slightly lower in patients without PEW, indicating more lean body mass with statistically significant difference only in women (Table 1).
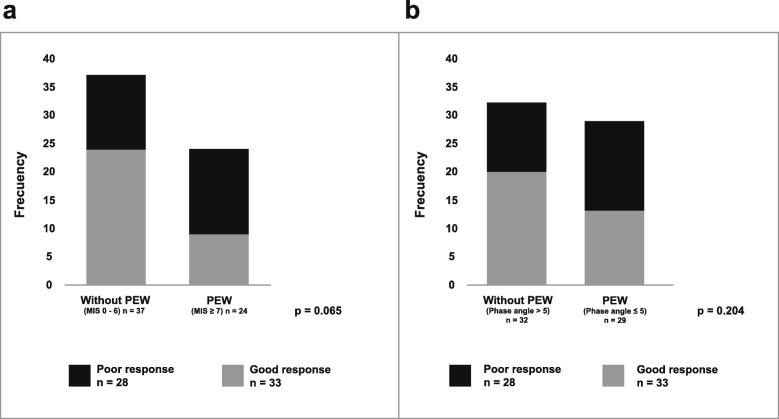
When analyzing the relationship between PEW by MIS score and the response to treatment with EPO (Fig. 2a), we found that 33 out of 61 patients responded satisfactorily, being the majority 72.7from the group of patients without PEW. In patients with PEW as defined by MIS, 53.6% had a poor response to EPO vs. only 46.4% of those patients without PEW showed hyporesponsivenes to EPO (p = 0.065). A non-significant change in the same direction was observed in patients with PEW defined by phase angle (33.3 vs 66.6%, p = 0.204) (Fig. 2b).
Fig. 2.
Relationship between the diagnosis PEW by MIS ≥7 (a) and Phase Angle ≤5 (b) with the response to treatment with EPO. (Xi2 test was performed to determine differences between groups)
After analyzing the characteristics of the patients according to the response to treatment with EPO, it was found that those patients with good response were those with the best nutritional status measured by MIS (p < 0.05), the median change of patients with good response it was 17.9% vs − 7.7% in those patients with a poor response (p < 0.001) Table 2.
Table 2.
Characteristics of the population according EPO response
| VARIABLE (%) | Response n = 33 |
Poor response n = 28 |
p value Final vs Final | ||||
|---|---|---|---|---|---|---|---|
| Basal | Final | ∆ | Basal | Final | ∆ | ||
| BMI (kg/m2) | 21.96 ± 3.9 | 21.7 ± 3.66 | 0.22 | 22 ± 5.18 | 21.2 ± 3.9 | − 0.14 | 0.674 |
| MIS score | 5 (1–14) | 4 (0–10)* | − 1.57 | 7 (1–13) | 6 (1–11) | 0 | 0.002 |
| Phase Angle (°) | 5.5 (2.1–7.6) | 5.5 (2.5–13)* | 0.74 | 4.7 (2.3–8.5) | 4.8 (2.2–8.1) | −0.26 | 0.1 |
| Hemoglobin (g/dl) | 8.8 ± 1.5 | 10.6 ± 1.7 * | Change% 17.9 (3.2–75.4) | 9.5 ± 1.6 | 8.5 ± 1.5 * | Change% − 7.7 (− 39.1–1.6) | 0.001 |
Continuous variables are expressed as mean ± standard deviation, non-parametric as medians or inter-quartile ranges
* p < 0.05 Basal vs Final
When estimating the relative risk (RR) for EPO response, we found that those patients without PEW by MIS (≤6) and by phase angle, (≥5), have a risk of response to treatment with EPO, (RR = 0.562 [CI 95% 0.329–0.961]) and (RR = 0.068 [CI95% 0.39–1.184]) as compared to those with PEW; however, the phase angle RR did not reach statistical significance.
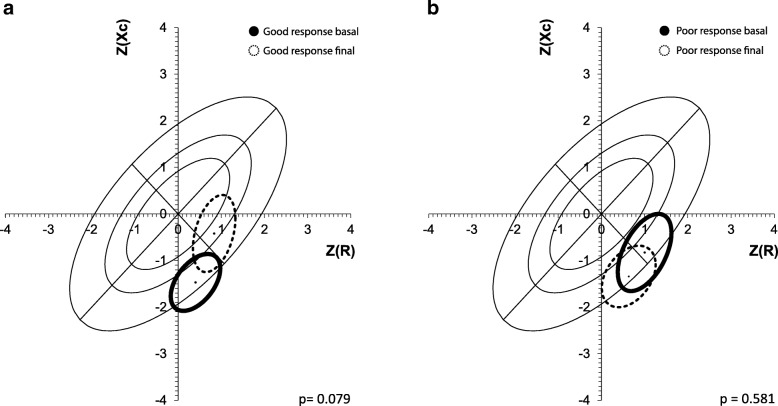
With respect to the impedance vector analysis, we found that patients with poor response to EPO, showed a shift on the low–right side of the short axis of the vector outside the 75th percentile of the tolerance ellipses, indicating a certain trend to cachexia, although we did not find a statistically significant difference (Hottelling T2 Test; p < 0.58), (Fig. 3b). Patients with good response to EPO improved their body composition, migrating towards the center of tolerance ellipses indicating better dry weight and body mass although we did not find a statistically significant difference (p = 0.079), (Fig. 3a).
Fig. 3.
Relationship between BIVA and treatment with EPO according to the good (a) and poor (b) response
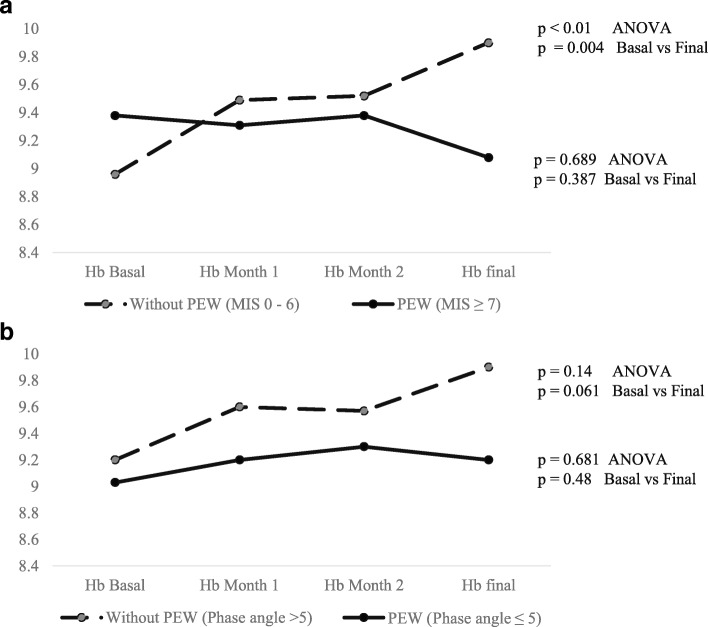
Finally, hemoglobin levels were evaluated at baseline and every four weeks until week 12, finding a statistically significant improvement only in patients without PEW diagnosed by MIS (Fig. 4a) (p < 0.01) and phase angle (Fig. 4b).
Fig. 4.
Longitudinal comparisons of Hb concentrations during the study period according MIS (a) and Phase Angle (b)
Discussion
Treatment of anemia of patients with CKD has significantly changed over 20 years ago with the addition of recombinant human erythropoietin. Over 90% of patients that receive EPO respond to treatment; however, many factors may block or diminish the response to this hormone replacement. Once iron deficiency and active bleeding are excluded, the diagnosis of EPO resistance is established, and a multiplicity of factors could be responsible for this [25].
The most commonly reported causes of lack of response to EPO treatment are iron deficiency, secondary hyperparathyroidism, chronic inflammatory processes, and malnutrition. Iron deficiency appears as the most frequent cause of failure to respond to treatment with EPO; however, it must not be forgotten that active inflammatory or infectious processes can temporarily block the response to iron therapy and therefore, to EPO [12–14]. That is why an evaluation of PEW is always relevant in this population. The concept of PEW, proposed by the International Society of Renal Nutrition and Metabolism, refers to a condition that encompasses the multiple nutritional and catabolic alterations that occur in chronic kidney disease and that are associated with morbidity and mortality. Although insufficient food intake due to lack of appetite and dietary restrictions contribute to PEW, other factors contribute to the development of this syndrome, such as alterations induced by uremia, increases in energy expenditure, persistent inflammation, acidosis, and multiple endocrine disorders that cause a state of hyper-metabolism, leading to excessive catabolism of muscle and fat [16].
Several studies have assessed the response to EPO according to various factors; however, very few studies have had as main objective to assess the nutritional status of the subjects, measuring it instead through isolated variables. Among the studies that assess nutritional status through isolated variables is one published by Kudoh et al. who assessed the effect of carnitine as therapy for overcoming the resistance to EPO; they found that less than 50% of studied patients responded to treatment with EPO plus carnitine, showing a serum albumin concentration of 3.7 ± 0.2 vs 3.6 ± 0.02 g/dL in poor responders, although these differences were not statistically significant [26]. However, the present study do not found a differences statistically significant according albumin and EPO response (3.2 ± 0.41 vs 3.4 ± 0.63 p = 259). A cross-sectional study by Locatelli et al. that aimed to assess the response to treatment with EPO, found that patients with a lower Index of Response to Erythropoietin (IRE) had a better nutritional status as assessed by BMI (24.9 ± 4.3 vs 21.3 ± 3.8 m/kg2, p < 0.001) and albumin (4.2 ± 0.4 vs 4.1 ± 0.5 g/dl p < 0.001) [7], contrary to what we observed in this study, in which patients who did respond to treatment had a similar BMI than those who did not respond satisfactorily to treatment (21.96 ± 3.9 vs 22 ± 5.18 p = 0.645)). Nevertheless, it is worth noting that Locatelli et al. ranked their results according to the IRE, whereas in this study the outcome variable was the response, or lack of response, to treatment according to the diagnosis of PEW.
Only a few studies have had the main purpose of assessing the relationship between PEW and the response to treatment with EPO, such as that conducted by Kalantar et al., in which the main goal was to find the association between serum ferritin and markers of inflammation, nutrition, and iron in hemodialysis. They found that patients with lower Hb levels (10.9 ± 1.7 vs 10.3 ± 1.18 g/dL p < 0.4) were also the ones with worse nutritional status, but the differences were not significant. They also included the MIS score as a variable, finding that patients with malnutrition by Subjective global assessment (SGA) according to were also those with the highest MIS score (SGA 1 = MIS 4.0 ± 1.2, SGA 2 = MIS 7.7 ± 1.8 and SGA 3 = 13.4 ± 3.0) [27]. This study served as the basis for establishing the cutoff point in MIS with respect to the diagnosis of PEW (MIS 0–6 = no PEW vs MIS ≥7 = PEW) in this research. In another study, the same group of researchers led by Kalantar et al. evaluated the effect of PEW on the poor response to treatment with EPO in patients undergoing HD. They suggested that the presence of low concentrations of cholesterol, prealbumin, and iron, among others, has a major effect on the poor response to treatment (measured by IRE), since they found that those with a lower IRE had a lower MIS score, while a higher IRE corresponded to higher total scores (5.8 ± 3.4 vs 7.5 ± 4.0 p < 0.001). Regarding the BMI, they reported that patients with lower IRE had a better BMI compared to those with a higher IRE, who had a lower BMI (27.7 ± 7.6 vs 25.3 ± 5.5) [19].
Recently, a study of 754 HD patients by Rattanasompattikul et al., with complete nutritional and inflammatory markers data, considered wasting as an independent factor for resistance to EPO. They found that elevated levels of inflammatory markers and low levels of nutritional markers were independent predictors of a decreased response to EPO [18]. MIS scores lower than 6.4 were associated with a poor response to treatment with EPO (p < 0.001). We describe similar results in our study, where the cutoff for diagnosis of PEW was MIS ≥ 7.
The pro-inflammatory cytokines are potent inhibitors of erythropoiesis in vivo and in vitro [4]. The inhibition of erythropoiesis by cytokines such as TNF-α and IFN-γ is also important in the development of resistance to EPO, as well as to Interleukin-6 (IL-6) and C-reactive protein (CRP). These cytokines participate in the genesis of anemia through different mechanisms: accelerating the apoptosis of erythroid precursors, decreasing the number of EPO receptors in them, producing a relative decrease in the synthesis of EPO by the kidneys, increasing the synthesis of hepcidin and inducing erythrophagocytosis [26, 28–30]. One limitation of our study is that we did not have any data about CRP or other inflammation markers, as inflammation is a clear confounder as it often associated with both EPO poor response and PEW.
Since these cytokines have a similar sensitivity for the diagnosis of inflammation as MIS, we decided to make an independent evaluation of the predictions of MIS. Patients diagnosed without PEW (0–6 points of the sum of items MIS) have a 95% decrease risk of poor response to treatment with EPO than those with PEW (RR = 0.068 [CI95% 0.39–1.184]).
Another way to evaluate PEW and cachexia (severe form of PEW) is based on bioelectrical impedance vectors, or the components of the body’s electrical properties (resistance, reactance, and phase angle) [22, 23, 31–34]. Colin et al. found that patients with a phase angle of 4.65 ± 1.23° had a Hb of 12.33 g/dl ± 0.67 g/dl and an Hct of 37.28% ± 2.49 g/dl, while those patients with a phase angle of 5.48 ± 1.17° had an Hb concentration of 15.09 ± 1.55 g/dl and an Hct concentration of 45.01 ± 4.70%; these differences were statistically significant for each indicator (p < 0.001). In addition, the final measurement showed that those patients with a phase angle greater than 5 responded satisfactorily to treatment with EPO, while those with a lower phase angle did not respond to the treatment. Patients with resistance to EPO had a vector pattern indicating a trend to cachexia (p < NS), while patients who responded to treatment had a normal vector pattern. The major limitation of our study is that it was a retrospective, single center study, and the relatively small sample size.
Conclusions
The results of this study suggest that PEW is an incremental predictor of poor responsiveness to EPO in HD patients. It is important to consider that all patients should have a careful nutritional and body composition assessment, to implement an exhaustive intervention if required and thus favor an adequate response to the EPO and consequently reduce the presence of anemia. However, it is necessary to do more studies with larger sample size to be able to verify this hypothesis, and control with measurement of inflammation.
Acknowledgements
We are grateful for the Hemodialysis Unit Staff and the Dietitians of the Renal Nutrition and Nephrology Department for providing the facilities to carry out this work).
Abbreviations
- BIA
Bioimpedance analysis
- BIVA
Bioimpedance vector analysis
- BMI
Body mass index
- CKD
Chronic kidney disease
- CRP
C-reactive protein
- EPO
Erythropoietin
- ERI
ESA responsiveness index
- ESA
Erythropoiesis stimulating agents
- GFR
Glomerular Filtration Rate
- Hb
Hemoglobin
- Hct
Hematocrit
- HD
Hemodialysis
- IFN-γ
Interferon-γ
- IL-1
Interleukin-1
- IL-6
Interleukin-6
- IRE
Index of Response to Erythropoietin
- MIS
Malnutrition Inflammation Score
- PEW
Protein-energy wasting
- R
Resistance
- rHuEPO
Recombinant human erythropoietin
- SGA
Subjective global assessment
- TNF –β
Tumor necrosis factor-β
- TNF-α
Tumor necrosis factor alpha
- TSAT
Transferrin saturarion
- U/kg/week
Units/kilogram/week
- Xc
Reactance
Authors’ contributions
AJGO Participated in the research generation, collection, and analysis of the data and in writing the paper. RCR and VRA participated in the analysis and interpretation of the data and revision of the manuscript. OVV participated in the revision of the data and manuscript. AEC: Participated in study conception and design, revision and analysis of the data and revision and approval of the final version of the manuscript. All authors read and approved the final manuscript.
Funding
No economic financing was obtained for this investigation.
Availability of data and materials
All the authors agree to provide the analysis database as well as any material in case the editorial committee requires it.
Ethics approval and consent to participate
This protocol was approved by the Human Research and Ethics Committee of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (reference 1407).
Not applicable because it is a retrospective study.
Consent for publication
Not applicable.
Competing interests
All authors declare not having any conflict of interest related to this investigation.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ailema González-Ortiz, Email: ailejgo@gmail.com.
Ricardo Correa-Rotter, Email: correa-rotter@gmail.com.
Armando Vázquez-Rangel, Email: drarmandovazquez@hotmail.com.
Olynka Vega-Vega, Email: olynkavega@gmail.com.
Ángeles Espinosa-Cuevas, Phone: +52-56550382, Email: angespinosac@gmail.com, Email: angeles.espinosac@incmnsz.mx.
References
- 1.Méndez-Durán Antonio, Francisco Méndez-Bueno J., Tapia-Yáñez Teresa, Montes Angélica Muñoz, Aguilar-Sánchez Leticia. Epidemiología de la insuficiencia renal crónica en México. Diálisis y Trasplante. 2010;31(1):7–11. doi: 10.1016/S1886-2845(10)70004-7. [DOI] [Google Scholar]
- 2.López-Cervantes M, Rojas-Rusell M, Tirado-Gómez LL, Durán-Arenas JL, Pacheco-Domínguez RL, Venad. Enfermedad renal cronica y su atencion mediante tratamiento sustitutivo en México [Internet]. Vol. 1, Facultad de Medicina, Universidad Nacional Autónoma de México. México D.F.; 2009. 21–54 p.
- 3.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63:793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 4.Hodges VM, Rainey S, Lappin TR, Maxwell AP. Pathophysiology of anemia and erythrocytosis. Crit Rev Oncol. 2007;64:139–158. doi: 10.1016/j.critrevonc.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 5.KDIGO Anemia Work Group KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int Suppl. 2012;2:279–335. doi: 10.1038/kisup.2012.37. [DOI] [Google Scholar]
- 6.Wanner Christoph, Brenner Susanne. CREDENCE and DELIGHT deliver on renal benefits. Nature Reviews Nephrology. 2019;15(8):459–460. doi: 10.1038/s41581-019-0171-2. [DOI] [PubMed] [Google Scholar]
- 7.Roger SD, Locatelli F, Woitas RP, Laville M, Tobe SW, Provenzano R, et al. C.E.R.A. once every 4 weeks corrects anaemia and maintains haemoglobin in patients with chronic kidney disease not on dialysis. Nephrol Dial Transplant [Internet]. 2011 Dec 1;26(12):3980–6. 10.1016/j.otsr.2019.04.012. [DOI] [PMC free article] [PubMed]
- 8.Locatelli F, Andrulli S, Memoli B, Maffei C, Del Vecchio L, Aterini S, et al. Nutritional-inflammation status and resistance to erythropoietin therapy in haemodialysis patients. Nephrol Dial Transplant. 2006;21(4):991–998. doi: 10.1093/ndt/gfk011. [DOI] [PubMed] [Google Scholar]
- 9.Donato H, Ferro H. [human recombinant erythropoietin therapy]. Medicina (B Aires) [internet]. 2006;66(1):51–69. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16555732. [PubMed]
- 10.Solomon SD, Uno H, Lewis EF, Eckardt K-U, Lin J, Burdmann EA, et al. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med [Internet] 2010;363(12):1146–1155. doi: 10.1056/NEJMoa1005109. [DOI] [PubMed] [Google Scholar]
- 11.Santos EJF, Hortegal EV, Serra HO, Lages JS, Salgado-Filho N, dos Santos AM. Epoetin alfa resistance in hemodialysis patients with chronic kidney disease: a longitudinal study. Brazilian J Med Biol Res. 2018;51(7):1–7. doi: 10.1590/1414-431x20187288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalantar-Zadeh K, Lee GH, Miller JE, Streja E, Jing J, Robertson JA, et al. Predictors of Hyporesponsiveness to erythropoiesis-stimulating agents in hemodialysis patients. Am J Kidney Dis [Internet]. 2009;53(5):823–834. doi: 10.1053/j.ajkd.2008.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujikawa T, Ikeda Y, Fukuhara S, Akiba T, Akizawa T, Kurokawa K, et al. Time-dependent resistance to erythropoiesis-stimulating agent and mortality in hemodialysis patients in the Japan Dialysis outcomes and practice patterns study. Nephron Clin Pract [Internet] 2012;122(1–2):24–32. doi: 10.1159/000346740. [DOI] [PubMed] [Google Scholar]
- 14.Smrzova Andrea, Horak Pavel, Skacelova Martina, Hermanova Zuzana, Langova Katerina, Zadrazil Josef, Novotny Dalibor. Intima media thickness measurement as a marker of subclinical atherosclerosis in SLE patient. Biomedical Papers. 2014;158(3):404–411. doi: 10.5507/bp.2013.054. [DOI] [PubMed] [Google Scholar]
- 15.González-Ortiz AJ, Arce-Santander CV, Vega-Vega O, Correa-Rotter R, Espinosa-Cuevas M de LA. Assessment of the reliability and consistency of the “Malnutrition Inflammation Score” (MIS) in Mexican adults with chronic kidney disease for diagnosis of protein-energy wasting syndrome (PEW). Nutr Hosp [Internet]. 2014 Oct 4;31(3):1352–8. 10.3305/nh.2015.31.3.8173. [DOI] [PubMed]
- 16.Carrero JJ, Stenvinkel P, Cuppari L, Ikizler TA, Kalantar-Zadeh K, Kaysen G, et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM) J Ren Nutr [Internet]. 2013;23(2):77–90. doi: 10.1053/j.jrn.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int [Internet] 2008;73(4):391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 18.Rattanasompattikul M, Molnar MZ, Zaritsky JJ, Hatamizadeh P, Jing J, Norris KC, et al. Association of malnutrition-inflammation complex and responsiveness to erythropoiesis-stimulating agents in long-term hemodialysis patients. Nephrol Dial Transplant [Internet]. 2013;28(7):1936–1945. doi: 10.1093/ndt/gfs368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalantar-Zadeh K, McAllister CJ, Lehn RS, Lee GH, Nissenson AR, Kopple JD. Effect of malnutrition-inflammation complex syndrome on EPO hyporesponsiveness in maintenance hemodialysis patients. Am J Kidney Dis [Internet]. 2003;42(4):761–773. doi: 10.1016/S0272-6386(03)00915-6. [DOI] [PubMed] [Google Scholar]
- 20.Kalantar-Zadeh K, Braglia A, Chow J, Kwon O, Kuwae N, Colman S, et al. An anti-inflammatory and antioxidant nutritional supplement for Hypoalbuminemic hemodialysis patients: a pilot/feasibility study. J Ren Nutr [Internet] 2005;15(3):318–331. doi: 10.1016/j.jrn.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis [Internet] 2001;38(6):1251–1263. doi: 10.1053/ajkd.2001.29222. [DOI] [PubMed] [Google Scholar]
- 22.Piccoli A, Codognotto M, Piasentin P, Naso A. Combined evaluation of nutrition and hydration in dialysis patients with bioelectrical impedance vector analysis (BIVA) Clin Nutr [Internet] 2014;33(4):673–677. doi: 10.1016/j.clnu.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Espinosa Cuevas MÁ, Rivas Rodríguez L, González Medina EC, Atilano Carsi X, Miranda Alatriste P, Correa RR. Vectores de impedancia bioeléctrica para la composición corporal en población mexicana. Rev Investig Clínica. 2007;59(1):15–24. [PubMed] [Google Scholar]
- 24.Espinosa Cuevas MA, Navarrete Rodriguez G, Villeda Martinez ME, Atilano Carsi X, Miranda Alatriste P, Tostado Gutiérrez T, et al. Body fluid volume and nutritional status in hemodialysis: vector bioelectric impedance analysis. Clin Nephrol. [PubMed]
- 25.Gunnell J, Yeun JY, Depner TA, Kaysen GA. Acute-phase response predicts erythropoietin resistance in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis [Internet]. 1999;33(1):63–72. doi: 10.1016/S0272-6386(99)70259-3. [DOI] [PubMed] [Google Scholar]
- 26.Kudoh Y, Aoyama S, Torii T, Chen Q, Nagahara D, Sakata H, et al. Long-term effects of Oral L-carnitine supplementation on Anemia in chronic hemodialysis. Cardiorenal Med [Internet] 2014;4(1):53–59. doi: 10.1159/000360865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalantar-Zadeh K, Rodriguez RA, Humphreys MH. Association between serum ferritin and measures of inflammation, nutrition and iron in haemodialysis patients. Nephrol Dial Transplant [Internet]. 2004;19(1):141–149. doi: 10.1093/ndt/gfg493. [DOI] [PubMed] [Google Scholar]
- 28.Molnar MZ, Czira ME, Rudas A, Ujszaszi A, Haromszeki B, Kosa JP, et al. Association between the malnutrition-inflammation score and post-transplant anaemia. Nephrol Dial Transpl. 2011;26:2000–2006. doi: 10.1093/ndt/gfq690. [DOI] [PubMed] [Google Scholar]
- 29.Stenvinkel P, Ketteler M, Johnson RJ, Lindholm B, Pecoits-Filho R, Riella M, et al. IL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia--the good, the bad, and the ugly. Kidney Int. 2005;67(4):1216–1233. doi: 10.1111/j.1523-1755.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- 30.Stenvinkel P. The role of inflammation in the anaemia of end-stage renal disease. Nephrol Dial Transplant [Internet] 2001;16(Suppl 7):36–40. doi: 10.1093/ndt/16.suppl_7.36. [DOI] [PubMed] [Google Scholar]
- 31.Ellis KJ. Human Body Composition: In Vivo Methods. Physiol Rev. 2000;80(2). 10.2214/AJR.19.21424. [DOI] [PubMed]
- 32.Colín-Ramírez E, Castillo-Martínez L, Orea-Tejeda A, Asensio Lafuente E, Torres Villanueva F, Rebollar González V, et al. Body composition and echocardiographic abnormalities associated to anemia and volume overload in heart failure patients. Clin Nutr [Internet]. 2006;25(5):746–757. doi: 10.1016/j.clnu.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Vega A, Ruiz C, Abad S, Quiroga B, Velázquez K, Yuste C, et al. Body composition affects the response to erythropoiesis-stimulating agents in patients with chronic kidney disease in dialysis. Ren Fail [Internet] 2014;36(7):1073–1077. doi: 10.3109/0886022X.2014.917937. [DOI] [PubMed] [Google Scholar]
- 34.Piccoli A, Rossi B, Pillon L. A new method for monitoring, body fluid variation by bioimpednace anañysis: the RXc graph. Kidney Int. 1994;46:534–539. doi: 10.1038/ki.1994.305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the authors agree to provide the analysis database as well as any material in case the editorial committee requires it.