Abstract
Volatile organic compounds (VOCs) of antagonistic yeasts are considered as environmental safe fumigants to promote the resistance and quality of strawberry (Fragaria ananassa). By GC‐MS assays, VOCs of Hanseniaspora uvarum (H. uvarum) fumigated strawberry fruit showed increased contents of methyl caproate (5.8%), methyl octanoate (5.1%), and methyl caprylate (10.9%) in postharvest cold storage. Possible mechanisms of H. uvarum VOCs involved in regulations of the defense‐related enzymes and substances in strawberry were investigated during postharvest storage in low temperature and high humidity (2 ± 1°C, RH 90%–95%). Defense‐related enzymes assays indicated H. uvarum VOCs stimulated the accumulation of CAT, SOD, POD, APX, PPO, and PAL and inhibited biosynthesis of MDA in strawberry fruit under storage condition. Moreover, the expression levels of related key enzyme genes, such as CAT, SOD, APX42, PPO, and PAL6, were consistently increased in strawberry fruit after H. uvarum VOCs fumigation.
Keywords: defense‐related enzymes, GC‐MS, Hanseniaspora uvarum, strawberry, volatile organic compounds
1. INTRODUCTION
Fungal infections bring serious loss in strawberry production, especially during field and postharvest phases (Paulus, 1990). Many studies reported successful preventing of postharvest decay with chemical fungicides (El‐Mougy, El‐Gamal, & Abdalla, 2008; Sallato, Torres, Zoffoli, & Latorre, 2007). To date, there is much concern about fungicide residue in agricultural product, and biocontrol has been considered as a more acceptable method for controlling postharvest diseases (Karunaratne, 2011; Kilani‐Feki et al., 2016; Mohamed & Saad, 2009; Pretorius, Van, & Clarke, 2015). Multiple biocontrol agents (BCAs) have been isolated from environment, and they were shown to facilitate the control of postharvest decay of strawberry fruit (El Ghaouth & Wilson, 2002; Menel, Faten, & Moktar, 2012; Wei, Mao, & Tu, 2014; Zhang et al., 2007), suggesting possible application of BCAs in strawberry postharvest storage. Some yeast strains with potential biocontrol ability directly inhibit the growth of fungal pathogens on strawberry by antagonism pattern (Wei et al., 2014; Zhang et al., 2007). Different mechanisms were involved in antagonistic microorganism‐mediated fungal pathogen growth inhibition, and volatile organic compounds (VOCs) have been suggested to act as a functional molecular factor to interfere fungal pathogen growth (Asari, Matzén, Petersen, Bejai, & Meijer, 2016; Sánchez‐Fernández et al., 2016). It was also reported that VOCs treatment may control postharvest fungal disease and increase the storage period of strawberry fruit (Archbold, Hamiltonkemp, Barth, & Langlois, 1997).
Plant resistance could be triggered by various biotic and abiotic stresses and was known as induced system resistance (ISR; Choudhary & Johri, 2009; Heil & Bostock, 2002). Plenty of studies indicated BCAs decreasing the fruit postharvest decay by enhancing defense‐related enzyme activities. A study on peach showed antagonistic yeast strains, including P. membranifaciens, C. laurentii, Candida guilliermondii, and Rhodotorula glutinis, can stimulate activities of catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD; Xu, Qin, & Tian, 2008). In another study on apple fruit, it showed Aureobasidium pullulans induced the activities of chitinase, glucanase, and peroxidase in fruit tissue after 24 hr (Ippolito, Elghaouth, Wilson, & Wisniewski, 2000).
One of those antagonistic yeast strain that inhibits the growth of B. cinerea on strawberry fruit is H. uvarumi, probably by nutrients and space competition, host defense induction, morphology change of hyphae, and synthesis of secondary metabolites (Cai, Yang, Xiao, Qin, & Si, 2015; Qin et al., 2013). Preharvest application of H. uvarum on strawberry fruit was able to stimulate the enzymes associated with active oxygen metabolism, such as POD, polyphenol oxidase (PPO), SOD, phenylalanine ammonialyase (PAL), and CAT (Cai et al., 2015).
Although it is relatively efficient to control postharvest diseases by applying biocontrol yeast strains directly on strawberry fruit, BCAs treatment might increase the risk of microbe contamination during marketing stage. Thus, VOCs treatment has a broader prospect instead of directly spraying BCAs culture on strawberry fruit surface in postharvest stage. However, little is known about these specific mechanisms of controlling strawberry postharvest diseases of by VOCs.
In previous study, VOCs produced by H. uvarum showed effective inhibition to the growth of B. cinerea in vitro (Cai et al., 2015). Here, we explore the further functions of VOCs produced by H. uvarum by testing possible alterations in strawberry volatile emissions, as well as the variations of defense‐related enzymes and substances in strawberry fruit during postharvest storage period.
2. MATERIALS AND METHODS
2.1. H. uvarum strain and culture
Hanseniaspora uvarum (Genbank accession number: JX125041) was isolated from the surface of strawberry and maintained in PDA medium (200 g/L potato, 20 g/L dextrose, 15 g/L agar).
Hanseniaspora uvarum strain was grown in PDB liquid medium (200 g/L potato, 20 g/L dextrose) at 28°C for 24 hr in a gyratory shaker at 200 g. The strain culture was centrifuged at 6,000 g for 15 min at 4°C, and the yeast strain precipitate was then adjusted to final concentration of 1 × 109 CFU/ml with sterile distilled water with 0.05% Tween‐20. Five hundred microliter of yeast suspension was inoculated on the PDA dishes for the preparation of VOCs treatment.
2.2. Fruit and VOCs treatment
Fruit of Fragaria ananassa “Hong Yan” was harvested in early morning from a farm in Yuhua district, Nanjing city, Jiangsu province, China. The strawberry fruit was then transported to laboratory condition immediately within 2 hr. A total of 300 strawberry fruits in similar shape and size and without physical injuries or microorganisms infection were randomly selected.
Hanseniaspora uvarum VOCs treatment on strawberry fruit was conducted in sealed glass desiccators (9 cm by 27 cm, down diameter by up diameter, with a hollow clapboard inside) as previously described (Qin, Xiao, Cheng, Zhou, & Si, 2017). Eight uncovered Petri dishes with H. uvarum culture on PDA medium were placed on the bottom of the glass desiccator (use noninoculated PDA medium as negative control), and fresh strawberries were placed on the hollow clapboard. All desiccators were sealed by parafilm and incubated at 2°C for 3 days. Then, all treated strawberry fruits were placed in normal storage condition (2 ± 1°C, RH 90%–95%) with appropriate aeration system. Strawberry fruit samples were collected for further biochemical assessment after 0, 3, 8, 13, 18, and 25 days, respectively.
2.3. Detection of defense‐related enzymes in strawberry fruit
The enzyme activities of strawberry fruit were measured following the methods described in previous experiment with some modifications (Cai et al., 2015). Strawberry fruit samples (2.0 g for each treatment) were homogenized in 8 ml ice‐cold phosphate buffer (50 mmol/L, pH 7.8, containing 1% polyvinylpyrrolidone [PVP]) and then centrifuged at 12,000 g for 20 min at 4°C, and supernatant was collected as crude extract. Each strawberry fruit treatment had three parallel lines, and the test was repeated twice.
For POD activity measurement, reaction buffer (1 ml 0.1 mol/L acetic acid buffer, 1 ml crude extract, 1 ml 2‐methoxyphenol, 0.4 ml 0.75% H2O2) was mixed with crude extract of strawberry fruit. Each variation (0.001) of the mixture absorbance at 460 nm within 2 min represents 1 unit of the enzyme activity. The specific activity was expressed as units per gram of fresh weight.
The SOD activity of strawberry fruit was evaluated by following procedures. The reaction buffer (2.0 ml 50 mmol/L, pH 7.8 phosphate buffer, 0.2 ml 30 mmol/L L‐methionine, 0.2 ml 750 mol/L nitrotetrazolium blue chloride, 0.3 ml 20 µmol/L riboflavin) was gently mixed with 0.5 ml crude extract, and reaction mixture was then exposed by fluorescent lamp (400 lx) for 10 min. Each enzyme unit of SOD activity was defined as 50% inhibition rate of the photochemical reaction displayed by absorbance at 560 nm, and the reaction mixture without exposure treatment was considered as blank. The specific activity was expressed as units per gram of fresh weight.
For CAT activity measurement, reaction buffer (3 ml sodium phosphate buffer, 0.4 ml 0.75% H2O2) was mixed with 0.2 ml crude extract. Each variation (0.001) of the mixture absorbance at 240 nm within 2 min represents 1 unit of the CAT enzyme activity. The specific activity was expressed as units per gram of fresh weight.
For APX activity measurement, reaction buffer (3 ml sodium phosphate buffer, 0.2 ml 0.01 mol/L L‐ascorbic acid, 0.2 ml 0.75% H2O2) was gently mixed with 0.2 ml crude extract. Each variation (0.001) of the mixture absorbance at 290 nm within 2 min represents 1 unit of the APX enzyme activity. The specific activity was expressed as units per gram of fresh weight.
For activity of PAL and PPO, content measurement of MDA, and superoxide anion in strawberry fruit, the procedures were as described in previous study (Cai et al., 2015).
2.4. Volatile compound analysis on strawberry fruit
Top parts of three strawberry fruits for each treatment were collected and placed in headspace sampling tubes. GC‐MS analysis was then conducted as following steps. A 2‐cm fused‐silica fiber coated with divinylbenzene/carboxen/polydimethylsiloxane 50/20 μm (DBV/CAR/PDMS) was inserted into the side of the sampling tubes via a silicone septum after 15 min of headspace equilibration. Volatile compounds were collected from headspace sampling tubes for 30 min and then desorbed into GC injector for 5 min at 250°C. Separation was achieved on an HP‐Innowas fused‐silica capillary column. The GC oven temperature program consisted of 40°C for 2.5 min, raised from 40 to 200 at 5°C/min, and then to 240°C for 5 min at 10°C/min. Helium was used as carrier gas with a constant column flow rate of 0.004 mol/hr. Compounds exiting the column were ionized via electron impact at 70 eV and scanned with a quadrupole mass spectrometer with a m/z range between 30 and 300 Th. The volatile profile of each sample was reported as absolute peak area.
2.5. Real‐time PCR analysis of mRNA abundance
Fresh strawberry fruit for defense relevant gene test was treated with H. uvarum VOCs for 1, 2, and 3 days, and then, strawberry samples (0.1 g for each) were collected and ground into powder with liquid N2. All the microbe treatments followed the method described in fruit and VOCs treatment section. Total RNA was extracted using 1 ml TRIZOL reagent (Invitrogen Co.) and was treated with RNase‐free DNAse (Takara Co.) to remove genomic DNA. Reverse transcription of RNA was performed with 1 μg of total RNA using M‐MLV reverse transcriptase, according to the manufacturer's protocol (Invitrogen Co.). The primers used were listed in Table 1. A constitutively expressed gene (18S rRNA in Fragaria x ananassa) was a reference gene in quantitative real‐time PCR analysis. Three replicates were performed for each treatment.
Table 1.
Sequence of the primers used for genes expression in strawberry
| Gene | Genebank | Forward primer sequence (5′–3′) | Reverse primer sequence (5′–3′) |
|---|---|---|---|
| PAL6 | HM641823.1 | GTGAAAGAAGCGAAGAAGG | GAAGCTCGGAGCAGTATG |
| CAT | KC433883.1 | GTCTTCTTCGTCCGTGAT | GTAGTTCCCAGCAGCAAT |
| PPO | EU523113.1 | GGAGGCGGAGCAATAGAGAC | AGCCAGACCCCCTCAATACT |
| APX42 | AF159630.1 | CACAAGGAACGGTCTGGATT | CGCAGCGTATTTCTCAACAA |
| SOD | – | ATGGTGCTCCTGAAGATGA | TAGAGTGTGGTCCAGTGAG |
| 18S rRNA | X15590.1 | TGTGAAACTGCGAATGGCTCATTAA | GAAGTCGGGATTTGTTGCACGTATT |
2.6. Statistical analysis
All statistical analyses were performed in the SAS Software (version 8.2; SAS Institute). Comparison of means was performed by Duncan's multiple range tests. Statistical significance was assessed at the level of p < 0.05.
3. RESULTS
3.1. Effects of H. uvarum VOCs on gray mold decay of strawberry during cold storage
Hanseniaspora uvarum is an effective BCA in preventing postharvest diseases (Cai et al., 2015), and its VOCs also showed positive effects in prolonging shelf life of strawberry fruit (Qin et al., 2017). Further experiments were conducted to understand the functions of H. uvarum VOCs in controlling strawberry postharvest diseases. Gray mold decay occurs during low‐temperature storage of strawberry fruit, usually caused by physical damage and infections of fungal pathogen spores, such as Botrytis cinera on fruit surface. In Figure 1a, H. uvarum VOCs inhibited the in vitro growth of B. cinerea on PDA plate in 7 days (Figure 1a, right), while the hyphae of B. cinerea had spread on whole plate within 3 days without treatment (Figure 1a, left). Figure 1b, which showed the appearance of H. uvarum VOCs treated or nontreated strawberry fruit at 25 dpt (day post‐treatment), suggests the decay severity of H. uvarum VOC‐treated strawberry fruits was obviously lower than that of nontreated fruit, and H. uvarum VOCs was able to restrain the spontaneous happening of gray mold decay symptom on strawberry fruit in cold storage condition. These results obviously showed that the H. uvarum VOCs treatment can maintain the quality of strawberry during cold storage period.
Figure 1.
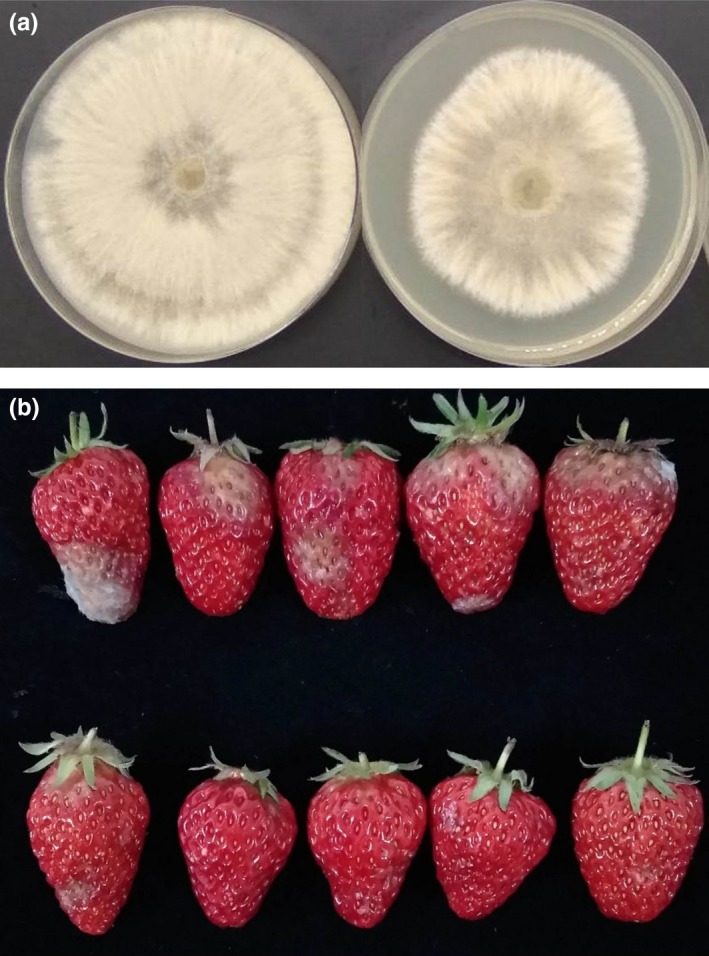
Effects of H. uvarum volatile organic compounds (VOCs) to B. cinera and strawberry fruit. (a) H. uvarum VOCs obviously inhibit in vitro growth of B. cinera in 7 days. (b) Changes in appearance of H. uvarum VOC‐treated strawberry fruit (down) and control group (up) after cold storage for 25 days. Three biological replicates were performed for each assay with similar results
3.2. H. uvarum VOCs altered content of organic esters in strawberry volatile emissions
The contents of organic esters are main flavor factors of strawberry fruit, which derived from multiple esterification reactions during strawberry postharvest storage. According to the results of GC‐MS analysis, both control group and H. uvarum VOCs treatment showed increased contents of organic esters in strawberry fruit VOCs, and the increments of several specific organic esters were higher in H. uvarum VOCs treatment (Figure 2). Table 2 lists all detected strawberry volatile emissions of strawberry fruit after 5 days of room temperature storage. By H. uvarum VOCs treatment, the strawberry fruit released 5.8% more of methyl acetate, 5.1% more of methyl caprylate, and 10.9% more of ethyl octanoate after 5 days compared with strawberry fruit before storage (Table 2). In addition, nontreated strawberry fruit showed slightly lower increments of methyl acetate, methyl caprylate, and ethyl octanoate (3.7%, 4.4%, and 8.9%, respectively, in Table 2). These results indicated that H. uvarum VOCs treatment might improve the flavor of strawberry fruit by increasing the contents of specific esters during postharvest storage.
Figure 2.
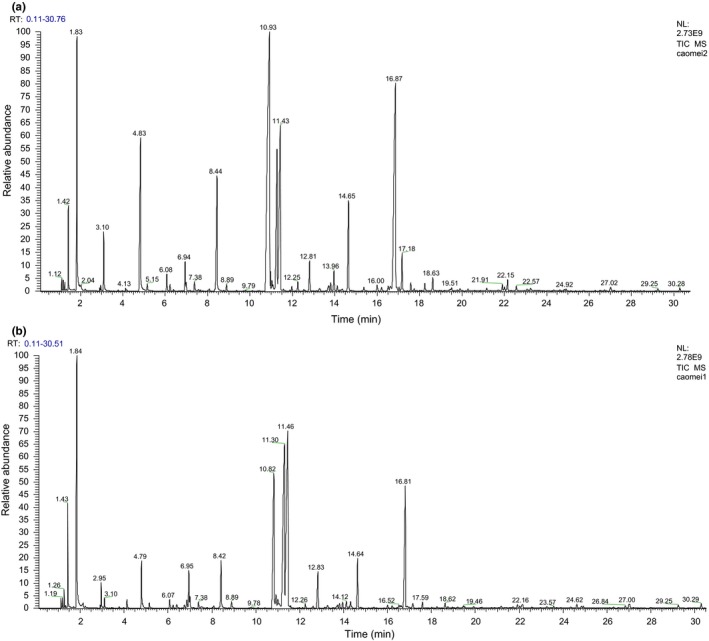
GC‐MS analysis on strawberry volatile emissions 5 days after H. uvarum volatile organic compounds (VOCs) (a) and mock treatments (b). Three biological replicates were performed for each assay with similar results
Table 2.
Strawberry volatile organic compounds tested 5 days after H. uvarum VOCs treatment and their peak area percentages
| RT (retention time) | Compound name | Molecular formula | Peak area percentage | ||
|---|---|---|---|---|---|
| CAS (registration number) | CK | H. uvarum | |||
| 1.12 | Nitrogen oxide | N2O | 20621‐02‐7 | 0.18 | 0.64 |
| 1.19 | 2‐aminoethyl alcohol | C2H7NO | 141‐43‐5 | 0.89 | 0.39 |
| 1.27 | Ethyl alcohol | C2H6O | 64‐17‐5 | 1.2 | 0.39 |
| 1.42 | Methyl n‐acetylglycine | C5H9NO3 | 1117‐77‐7 | 7.6 | 4.1 |
| 1.84 | Ethyl acetate | C4H8O2 | 141‐78‐6 | 17.7 | 12.9 |
| 2.90 | Ethyl propionate | C5H10O2 | 105‐37‐3 | 1.8 | 0.26 |
| 3.10 | Methyl butyrate | C5H10O2 | 623‐42‐7 | 0.7 | 3.2 |
| 3.78 | Ethyl isobutyrate | C6H12O2 | 97‐62‐1 | 0.7 | 0.13 |
| 3.97 | Ethyl butyrate | C6H12O2 | 105‐54‐4 | 0.18 | 0.13 |
| 4.13 | Isobutyl acetate | C6H12O2 | 110‐19‐0 | 0.18 | 0.26 |
| 4.19 | Methyl 2‐methylbutyrate | C6H12O2 | 868‐57‐5 | – | 0.39 |
| 4.79/4.82 | Ethyl butyrate | C6H12O2 | 105‐54‐4 | 3.5 | 0.13 |
| 5.15 | Butyl acetate | C6H12O2 | 123‐86‐4 | – | 0.13 |
| 5.87 | Propyl butyrate | C11H14O2 | 105‐66‐8 | – | 0.26 |
| 6.07 | Ethyl 2‐methylbutyrate | C7H14O2 | 7452‐79‐1 | 0.7 | 0.77 |
| 6.23 | Ethyl isovalerate | C7H14O2 | 108‐64‐5 | 0.36 | 0.39 |
| 6.95 | Isoamyl acetate | C7H14O2 | 123‐92‐2 | 2.7 | 1.3 |
| 7.38 | Isoamyl acetate | C7H14O2 | 123‐92‐2 | 0.53 | 0.64 |
| 8.41/8.44 | Methyl hexanoate | C7H14O2 | 106‐70‐7 | 3.7 | 5.8 |
| 8.76 | Methyl hex‐3‐enoate | C7H12O2 | 2396‐78‐3 | 0.36 | 0.13 |
| 8.89 | Ethyl tiglate | C7H14O2 | 5837‐78‐5 | 0.53 | 0.39 |
| 9.78 | Methyl ester | C7H12O2 | 13894‐63‐8 | 0.18 | 0.26 |
| 10.10 | 2‐Pentanol | C8H16O2 | 108‐84‐9 | 0.18 | 7.1 |
| 10.80/10.82 | Ethyl caproate | C8H16O2 | 123‐66‐0 | 9.8 | 12.9 |
| 11.08 | (E)‐2‐Hexen‐1‐ol | C8H14O2 | 2497‐18‐9 | 1.8 | 3.9 |
| 11.27/11.29 | 2‐Pentanol | C8H16O2 | 108‐84‐9 | 11.5 | 6.4 |
| 11.42/11.45 | Octyl acetate | C10H20O2 | 112‐14‐1 | 12.8 | 8.3 |
| 12.82 | Methoxy‐2,5‐dimethyl‐3(2H)‐furanone | C6H8O2 | 4077‐47‐8 | 3.5 | 1.9 |
| 13.72 | Propyl hexanoate | C9H18O2 | 626‐77‐7 | 0.53 | – |
| 14.65 | Methyl octanoate | C9H18O2 | 111‐11‐5 | 4.4 | 5.1 |
| 16.81/16.86 | Ethyl caprylate | C10H20O2 | 106‐32‐1 | 8.9 | 10.9 |
| 17.59 | Butyric anhydride | C8H14O3 | 106‐31‐0 | 0.53 | – |
| 18.62 | phenethyl acetate | C10H12O2 | 103‐45‐7 | 0.18 | 0.64 |
| 19.20 | 1‐nonanecarboxylic acid | C10H20O2 | 0.18 | – | |
| 19.46 | Tridecane | C13H28 | 629‐50‐5 | 0.18 | 0.13 |
| 19.51 | Ethyl nonanoate | C11H22O2 | 123‐29‐5 | 0.36 | 0.26 |
| 19.92 | Nonyl acetate | C11H22O2 | 143‐13‐5 | 0.18 | 0.13 |
| 22.15 | Ethyl caprate | C12H24O2 | 110‐38‐3 | 0.36 | 0.64 |
| 24.92 | 2,4‐bis(1,1‐dimethylethyl)‐6‐methyl‐ | C15H24O | 616‐55‐7 | 0.18 | 0.26 |
| 27.01 | 1,1,1‐trimethyl‐,1,1′,1″‐triester with boric acid (H3BO3) | C9H28Si4 | 4325‐85‐3 | 0.18 | 0.26 |
| 29.25 | Heptadecane | C17H36 | 629‐78‐7 | 0.18 | 0.26 |
3.3. Effects of H. uvarum VOCs on defense‐related enzymes and substances of strawberry during cold storage
Microbe VOCs were reported for inducing the synthesis of plant defense‐related enzymes during biocontrol process in many reports (Giorgio, Stradis, Cantore, & Iacobellis, 2015; Raza, Ning, Yang, Huang, & Shen, 2016). To explore the role of H. uvarum VOCs further, we measured the contents of different defense‐related enzymes and substances on strawberry fruit treated by H. uvarum VOCs in cold storage period.
In assays of APX activity in strawberry fruit, the APX enzyme activities of H. uvarum VOC‐treated fruit were slightly higher than nontreatment group on the 3, 8, 13, and 25 dpt. The peak value of APX enzyme activity was observed on day 13 in H. uvarum VOC‐treated fruit which is approximately 600 U/(min g kg−1), and the APX activity of treated fruit was significantly higher (p < 0.05) than that of the nontreatment group on 18 dpt (Figure 3a). As shown in Figure 3b, under the cold storage condition, SOD accumulation peaked at 13 dpt in strawberry fruit of both H. uvarum VOCs treatment and nontreatment groups, and the SOD enzyme activity of H. uvarum VOC‐treated fruit was significantly higher (p < 0.05) than nontreatment group at 3 and 13 dpt. In the strawberry fruit during storage period, POD activity reached a maximum at 8 dpt in both H. uvarum VOCs treatment and nontreatment groups, and the POD activity of treatment group was induced approximately 1.2‐fold than that of the nontreatment group (Figure 3c). In Figure 3d, it was shown that the CAT activity of H. uvarum VOC‐treated fruit was significantly higher (p < 0.05) than that of the control group from 3 to 25 dpt, and the CAT activity in H. uvarum VOCs treatment group has been increased by 16.18% compared with nontreatment group at the end of storage.
Figure 3.
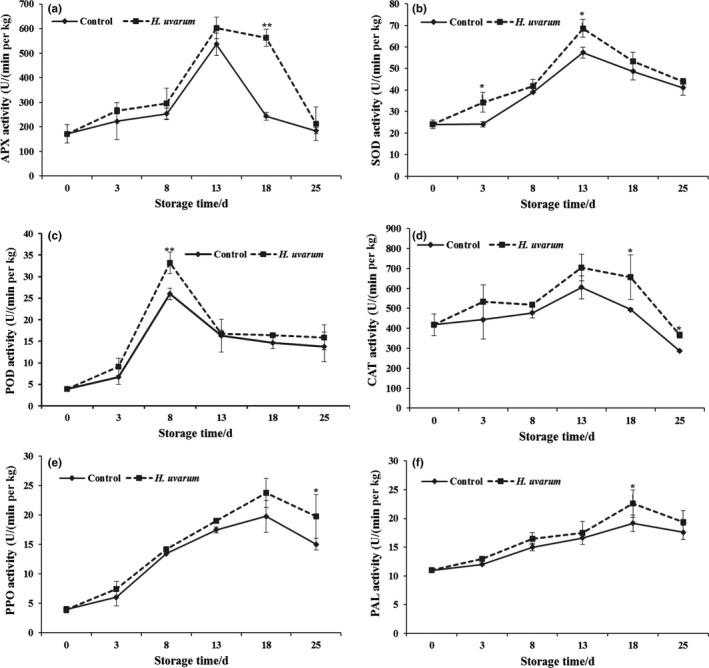
Effect of fumigation with H. uvarum volatile organic compounds (VOCs) on defense‐related enzymes in strawberry fruit during cold storage. (a) APX, (b) SOD, (c) POD, (d) CAT, (e) PPO, (f) PAL. Each value is the mean for three independent replicates. Asterisks indicate statistical differences compared to control according to Duncan's multiple range test at p ≦ 0.05 level. The vertical bar indicates the standard error. Three biological replicates were performed for each assay with similar results
Under the condition of H. uvarum VOCs treatment, the PPO activity in strawberry fruits was slightly higher than that in nontreatment group from 0 to 13 dpt (Figure 3e). The PPO activity reached the peak at 18 dpt in both H. uvarum VOCs treatment and nontreatment groups and was approximately 1.2‐fold in treated strawberry fruit compared with nontreated strawberry fruit. The PPO activity of H. uvarum VOCs treatment group was significantly (p < 0.05) higher than that of nontreatment group at the end of storage period (18 and 25 dpt; Figure 3e). In consistent with PPO activity, the results of PAL activity assay displayed similar tendency. The peak values of PPO activity in both H. uvarum VOCs treatment and nontreatment groups were observed at 18 dpt, and the PAL activity of treatment group was significantly (p < 0.05) higher than that of control group at the 18 dpt in cold storage condition (Figure 3f).
Next, we examined whether the H. uvarum VOCs treatment is able to trigger the change of MDA and superoxide anion production contents in strawberry fruit during storage period. Figure 4a showed MDA contents change in strawberry fruit during cold storage condition up to 25 dpt. The peak values of MDA contents were observed at 13 dpt in both H. uvarum VOCs treatment group and nontreatment group. The MDA content accumulation in H. uvarum VOCs treatment group was significantly lower (p < 0.05) than that of the nontreatment group at 18 and 25 dpt. In Figure 4b, the superoxide anion production was significantly increased in H. uvarum VOC‐treated strawberry fruit in 3 dpt (p < 0.01) and 8 dpt (p < 0.05). After 13 dpt, the superoxide anion production in treated strawberry fruit kept in the same level with nontreated fruit (Figure 4b) indicates the influence of H. uvarum VOCs on superoxide anion production in strawberry fruit is more like in early stage of postharvest storage.
Figure 4.
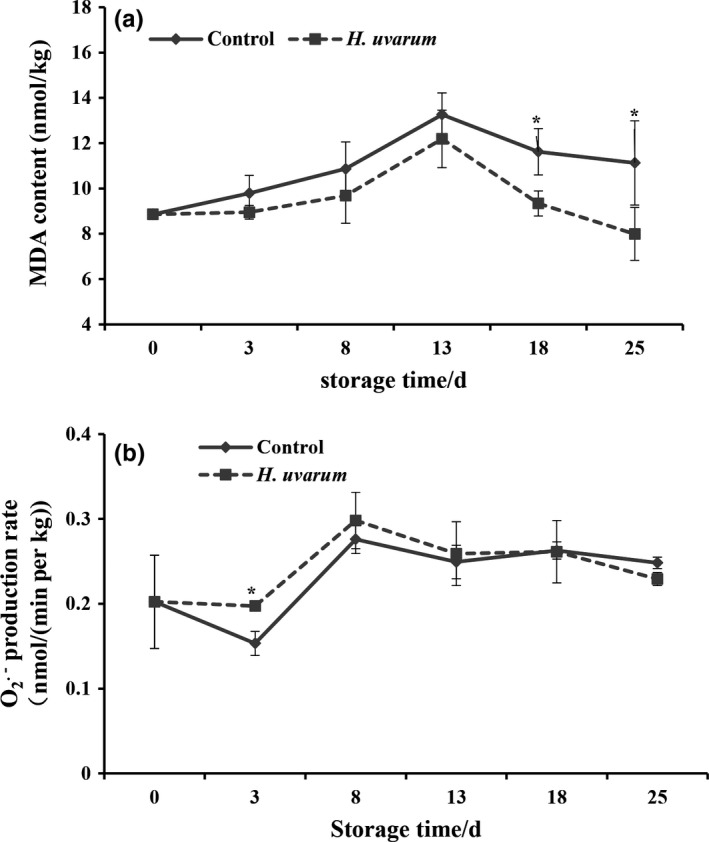
Effect of fumigation with H. uvarum volatile organic compounds (VOCs) on MAD content (a) and O2− production rate (b) in strawberry fruit during cold storage. Each value is the mean for three independent replicates. Asterisks indicate statistical differences compared to control according to Duncan's multiple range test at p ≦ 0.05 level. The vertical bar indicates the standard error, and three biological replicates were performed for each assay with similar results
3.4. Effects of H. uvarum VOCs on expression levels of defense‐related enzyme genes in strawberry
Expression levels of defense‐related enzyme genes were analyzed in strawberry fruit on 1, 2, and 3 days during H. uvarum VOCs fumigation. In this study, relative expression levels of key enzyme genes involved in biosynthesis of APX, SOD, CAT, PPO, and PAL in strawberry fruit were shown in Figure 5. Basically, expression levels of SOD, PPO, and PAL were significantly induced after 1 day of H. uvarum VOCs treatment in Figure 5b,d,e. Expression levels of APX, SOD, CAT, and PPO were significantly induced after 2 days of H. uvarum VOCs treatment in Figure 5a–e. All tested genes, including APX, SOD, CAT, PPO, and PAL, were significantly induced after 3 days of H. uvarum VOCs treatment in Figure 5a–e.
Figure 5.

Effect of fumigation with H. uvarum volatile organic compounds (VOCs) on expression levels of defense‐related enzyme genes. Expression levels of SOD (a), CAT (b), PAL (c), PPO (d), and APX42 (e) were detected in strawberry fruit during cold storage. Each value is the mean for three independent replicates. Asterisks indicate statistical differences compared to control according to Duncan's multiple range test at p ≦ 0.05 level. The vertical bar indicates the standard error, and three biological replicates were performed for each assay with similar results
4. DISCUSSION
The proposed involvement of the H. uvarum volatile production in maintaining quality of strawberry fruit in cold storage period is consistent with its known roles in controlling gray mold caused by B. cinerea by directly applying H. uvarum cells on fruit (Cai et al., 2015; Qin et al., 2017).
Since strawberry is one of the popular fruits with high economic value, studies of reducing postharvest loss and maintaining fruit quality of strawberry attract wide attentions. Previous studies demonstrated some functions of H. uvarum in controlling the postharvest diseases of strawberry, such as inhibiting of B. cineare infection. Possible explanations are as follows: H. uvarum was able to compete nutrient and reproduction space with other microbes on strawberry surface, and some specific chemicals produced by H. uvarum cells could suppress conidial germination and hyphal growth of B. cinerea (Cai et al., 2015). Thus, it was important to examine the effects of possible productions of H. uvarum on strawberry fruit during storage condition. A study of Qin et al. (2017) indicated that H. uvarum VOCs directly inhibit in vitro fungal growth and prolong the shelf life of strawberry fruit. Yet, they have not investigated further effects and mechanisms of H. uvarum VOCs.
One of the aims of this study is to demonstrate whether H. uvarum VOCs treatment could alter specific contents in strawberry volatile emissions. Our results showed that varieties of organic esters were produced in strawberry during postharvest storage, which makes most of the contributions to the strawberry's flavor. Our previous data indicated that volatile emissions of “Hong Yan” mostly were methyl caproate, methyl butyrate, ethyl butyrate, and butyl acetate, which is similar with presented results in Table 2. It is interesting to see that H. uvarum VOCs treatment could increase some of the specific organic esters in strawberry fruit in postharvest storage, which might help improve the flavor and commercial value.
Furthermore, APX is known as a key enzyme in the ascorbic acid–glutathione cycle reaction, which can remove H2O2 in plants; SOD is one of the important active oxygen scavenging enzymes in plants and delays the aging process of fruit; CAT is one of the main removal enzymes of H2O2 in plants and reduces the toxic effects of H2O2 and other free radicals on plant cells; POD has the capability to remove H2O2 from plants and delays fruit senescence; and PAL is responsible for the biosynthesis of p‐coumaric acid derivatives, phytoalexin, and lignins that contribute to plant defense systems (Gill & Tuteja, 2010). Collectively, the levels of SOD, CAT, POD, APX, and PAL activities are the physiological characteristics to analyze and quantify the strawberry host resistance against pathogen infection. Our results proved that VOCs produced by H. uvarum could significantly induce the accumulation of defense‐related enzymes, such as SOD, POD, CAT, APX, PAL, and PPO. Importantly, the ability to improve contents of defense‐related enzymes is normally related to induction of system resistance in plants (Zhu & Ma, 2007), which suggests that H. uvarum VOCs could also be a potential microbe‐associated molecular patterns and function in the early perception status of the ISR of H. uvarum.
As an elicitor for improving the resistance of strawberry fruit by inducing the activities of antioxidant enzymes, our study also manifested that H. uvarum VOCs could reduce the contents of MDA in strawberry fruit throughout the storage period (Figure 4a), whereas the content of superoxide anion production rate was merely lower than nontreated strawberry fruit at the end of storage period (25 dpt, Figure 4b). MDA is the product of lipid peroxidation in plant cell membrane, as well as the generated rate of superoxide anion, which directly reacts with the degree of fruit ripening. Therefore, these two parameters partially represent the degree of damage to plant tissue (Macarisin, Droby, Bauchan, & Wisniewski, 2010). Thus, higher level of SOD, CAT, POD, APX, PAL, and APX activities and lower level of MDA content in strawberry fruit treated with H. uvarum VOCs indicate that VOCs of H. uvarum could trigger effective antioxidant defense system and probably delay senescence on strawberry fruit during postharvest storage.
Previous researches mostly focused on study of preventing fruit decay and fungal infections by microbe VOCs fumigation. Alström (2001) first proved VOCs produced by soil bacteria inhibit the growth of Verticillium dahliae effectively (Alström, 2001). Chaurasia et al. (2005) indicated that VOCs of Bacillus subtilis have antagonistic effect on the pathogenic fungi and cause six pathogenic fungus spore and mycelium deformity (Chaurasia et al., 2005). Study of Arrarte, Garmendia, Rossini, Wisniewski, and Vero (2017) indicated VOCs produced by Antarctic strains of Candidasake control postharvest Penicillium and gray mold of apple (Arrarte et al., 2017). Huang et al. (2011) reported that VOC produced by Candida intermedia strain C410 suppressed the conidial germination and mycelial growth of B. cinerea and effectively controlled gray mold disease on strawberry (Huang et al., 2011). Moreover, they investigated the specific species of C. intermedia VOCs, such as esters, alcohols, alkenes, alkanes, alkynes, organic acids, ketones, and aldehydes, in which 1,3,5,7‐cyclooctatetraene and 3‐methyl‐1‐butanol were the most abundant. In another study, a total of 28 species were detected in H. uvarum VOCs, in which ethyl acetate and 1,3,5,7‐cyclooctatetraene were the most abundant (Qin et al., 2017). Indeed, there is a gap in our knowledge if microbe VOCs had positive effects on fruit quality during storage period.
Collectively, our study showed the VOCs produced by H. uvarum could improve the efficiency of scavenging active oxygen, slow down the process of strawberry peroxide, and delay the fruit senescence during the cold storage period. In addition, unlike traditional BCAs treatment, VOCs treatment on strawberry fruit does not require direct contact with fruit surface, which benefits them act as bio‐fumigants. We confirmed the functions of H. uvarum VOCs for regulating defense‐related enzymes and substances of reactive oxygen metabolism in strawberry in strawberry fruit. We also found that the H. uvarum VOCs could increase the contents of specific esters, such as methyl caproate, methyl octanoate, and methyl caprylate, in strawberry volatile emissions during postharvest storage. In our future study, it would be particularly interesting to understand the gene‐level regulation of H. uvarum VOCs on strawberry fruit and identify predominant functional ingredient in H. uvarum VOCs.
5. CONCLUSIONS
The present study showed that treatment with VOCs produced by the antagonist H. uvarum increased specific esters in strawberry volatile emissions during postharvest storage. Furthermore, the study indicated that treatment with VOCs can enhance the activity of defense‐related enzymes and inhibit the accumulation of MDA content and the production rate of superoxide anion. These results also showed that VOCs maintain the strawberry quality via inducing the expression of defense‐related enzyme genes. The molecular mechanism of VOCs treatment to increase strawberry resistance has not been elucidated yet. The future work should pay attention to investigate which signal pathway was induced on strawberry fruit treated with H. uvarum VOCs.
CONFLICT OF INTEREST
It should be understood that none of the authors have any financial or scientific conflict of interest with regard to the research described in this manuscript.
ETHICAL APPROVAL
This study does not involve any human or animal testing.
ACKNOWLEDGMENTS
This study was supported by Science and Technology Department of Jiangsu Province (No BE2010385) and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions and National College Students' innovation and entrepreneurship (No. 201310307046).
Wang L, Dou G, Guo H, et al. Volatile organic compounds of Hanseniaspora uvarum increase strawberry fruit flavor and defense during cold storage. Food Sci Nutr. 2019;7:2625–2635. 10.1002/fsn3.1116
Luyao Wang and Guoxia Dou equally contributed to this work.
Contributor Information
Chunhao Jiang, Email: chjiang@njau.edu.cn.
Hongmei Xiao, Email: xhm@njau.edu.cn.
REFERENCES
- Alström, S. (2001). Characteristics of bacteria from oilseed rape in relation to their biocontrol activity against Verticillium dahliae . Journal of Phytopatholoy, 149, 57–64. 10.1046/j.1439-0434.2001.00585.x [DOI] [Google Scholar]
- Archbold, D. D. , Hamiltonkemp, T. R. , Barth, M. M. , & Langlois, B. E. (1997). Identifying natural volatile compounds that control gray mold (Botrytis cinerea) during postharvest storage of strawberry, blackberry, and grape. Journal of Agricultural Food Chemistry, 45, 4032–4037. [Google Scholar]
- Arrarte, E. , Garmendia, G. , Rossini, C. , Wisniewski, M. , & Vero, S. (2017). Volatile organic compounds produced by antarctic strains of candida sake play a role in the control of postharvest pathogens of apples. Biological Control, 109, 14–20. 10.1016/j.biocontrol.2017.03.002 [DOI] [Google Scholar]
- Asari, S. , Matzén, S. , Petersen, M. A. , Bejai, S. , & Meijer, J. (2016). Multiple effects of Bacillus amyloliquefaciens volatile compounds: Plant growth promotion and growth inhibition of phytopathogens. Fems Microbiology Ecology, 92, 6. [DOI] [PubMed] [Google Scholar]
- Cai, Z. , Yang, R. , Xiao, H. , Qin, X. , & Si, L. (2015). Effect of preharvest application of Hanseniaspora uvarum on postharvest diseases in strawberries. Postharvest Biology and Technology, 100, 52–58. 10.1016/j.postharvbio.2014.09.004 [DOI] [Google Scholar]
- Chaurasia, B. , Pandey, A. , Palni, L. M. , Trivedi, P. , Kumar, B. , & Colvin, N. (2005). Diffusible and volatile compounds produced by an antagonistic Bacillus subtilis strain cause structural deformations in pathogenic fungi in vitro. Microbiological Research, 160, 75–81. 10.1016/j.micres.2004.09.013 [DOI] [PubMed] [Google Scholar]
- Choudhary, D. K. , & Johri, B. N. (2009). Interactions of Bacillus spp. and plants with special reference to induced systemic resistance (ISR). Microbiological Research, 164, 493–513. 10.1016/j.micres.2008.08.007 [DOI] [PubMed] [Google Scholar]
- El Ghaouth, A. , & Wilson, C. (2002). Candida saitoana compositions for biocontrol of plant postharvest decay. U.S. patent 6,419,922. [Google Scholar]
- El‐Mougy, N. , El‐Gamal, N. , & Abdalla, M. (2008). The use of fungicide alternatives for controlling postharvest decay of strawberry and orange fruits. Journal of Plant Protection Research, 48, 385–395. 10.2478/v10045-008-0048-z [DOI] [Google Scholar]
- Gill, S. S. , & Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry, 48, 909 10.1016/j.plaphy.2010.08.016 [DOI] [PubMed] [Google Scholar]
- Giorgio, A. , Stradis, A. D. , Cantore, P. L. , & Iacobellis, N. S. (2015). Biocide effects of volatile organic compounds produced by potential biocontrol rhizobacteria on Sclerotinia sclerotiorum . Frontiers in Microbiology, 6, 1–13. 10.3389/fmicb.2015.01056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil, M. , & Bostock, R. M. (2002). Induced systemic resistance (ISR) against pathogens in the context of induced plant defences. Annals of Botany, 89, 503–512. 10.1093/aob/mcf076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, R. , Li, G. Q. , Zhang, J. , Yang, L. , Che, H. J. , Jiang, D. H. , & Huang, H. C. (2011). Control of postharvest botrytis fruit rot of strawberry by volatile organic compounds of Candida intermedia . Phytopathology, 101, 859. [DOI] [PubMed] [Google Scholar]
- Ippolito, A. , Elghaouth, A. , Wilson, C. L. , & Wisniewski, M. (2000). Control of postharvest decay of apple fruit by Aureobasidium pullulans and induction of defense responses. Postharvest Biology and Technology, 19, 265–272. 10.1016/S0925-5214(00)00104-6 [DOI] [Google Scholar]
- Karunaratne, A. M. (2011). Biocontrol mechanisms employed by PGPR and strategies of microbial antagonists in disease control on the postharvest environment of fruits In Maheshwari D. (Ed.), Bacteria in agrobiology: Crop ecosystems (pp. 131–163). Berlin, Heidelberg: Springer. [Google Scholar]
- Kilani‐Feki, O. , Khedher, S. B. , Dammak, M. , Kamoun, A. , Jabnoun‐Khiareddine, H. , Daami‐Remadi, M. , & Tounsi, S. (2016). Improvement of antifungal metabolites production by Bacillus subtilis v26 for biocontrol of tomato postharvest disease. Biological Control, 95, 73–82. 10.1016/j.biocontrol.2016.01.005 [DOI] [Google Scholar]
- Macarisin, D. , Droby, S. , Bauchan, G. , & Wisniewski, M. (2010). Superoxide anion and hydrogen peroxide in the yeast antagonist–fruit interaction: A new role for reactive oxygen species in postharvest biocontrol? Postharvest Biology and Technology, 58, 194–202. 10.1016/j.postharvbio.2010.07.008 [DOI] [Google Scholar]
- Menel, K. , Faten, K. , & Moktar, H. (2012). Combining biocontrol agent and high oxygen atmosphere, to reduce postharvest decay of strawberries. African Jouranl of Microbiology Research, 6, 5179–5187. [Google Scholar]
- Mohamed, H. , & Saad, A. (2009). The biocontrol of postharvest disease (Botryodiplodia theobromae) of guava (Psidium guajava l.) by the application of yeast strains. Postharvest Biology and Technology, 53, 123–130. 10.1016/j.postharvbio.2009.04.001 [DOI] [Google Scholar]
- Paulus, A. O. (1990). Fungal diseases of strawberry. HortScience, 25, 885–889. 10.21273/HORTSCI.25.8.885 [DOI] [Google Scholar]
- Pretorius, D. , Van, R. J. , & Clarke, K. G. (2015). Enhanced production of antifungal lipopeptides by Bacillus amyloliquefaciens for biocontrol of postharvest disease. New Biotechnology, 32, 243 10.1016/j.nbt.2014.12.003 [DOI] [PubMed] [Google Scholar]
- Qin, X. , Xiao, H. , Cheng, X. , Zhou, H. , & Si, L. (2017). Hanseniaspora uvarum prolongs shelf life of strawberry via volatile production. Food Microbiology, 63, 205–212. 10.1016/j.fm.2016.11.005 [DOI] [PubMed] [Google Scholar]
- Qin, X. J. , Xiao, H. M. , Liu, L. , Gao, J. X. , Wang, X. X. , & Yang, R. (2013). Effects of Hanseniaspora uvarum integrated with salicylic acid or sodium bicarbonate on postharvest decay of grapes. Advance Materials Research, 781, 1780–1785. [Google Scholar]
- Raza, W. , Ning, L. , Yang, L. , Huang, Q. , & Shen, Q. (2016). Response of tomato wilt pathogenralstonia solanacearumto the volatile organic compounds produced by a biocontrol strain Bacillus amyloliquefaciens sqr‐9. Scientific Report, 6, 24856 10.1038/srep24856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallato, B. V. , Torres, R. , Zoffoli, J. P. , & Latorre, B. A. (2007). Effect of boscalid on postharvest decay of strawberry caused by Botrytis cinerea and Rhizopus stolonifer . Spanish Journal of Agricultural Research, 5, 67 10.5424/sjar/2007051-224 [DOI] [Google Scholar]
- Sánchez‐Fernández, R. E. , Diaz, D. , Duarte, G. , Lappe‐Oliveras, P. , Sánchez, S. , & Macías‐Rubalcava, M. L. (2016). Antifungal volatile organic compounds from the Endophyte nodulisporium sp. Strain gs4d2ii1a: A qualitative change in the intraspecific and interspecific interactions with Pythium aphanidermatum . Microbial Ecology, 71, 347–364. 10.1007/s00248-015-0679-3 [DOI] [PubMed] [Google Scholar]
- Wei, Y. , Mao, S. , & Tu, K. (2014). Effect of preharvest spraying Cryptococcus laurentii on postharvest decay and quality of strawberry. Biological Control, 73, 68–74. 10.1016/j.biocontrol.2014.02.016 [DOI] [Google Scholar]
- Xu, X. , Qin, G. , & Tian, S. (2008). Effect of microbial biocontrol agents on alleviating oxidative damage of peach fruit subjected to fungal pathogen. International Journal of Food Microbiology, 126, 153–158. 10.1016/j.ijfoodmicro.2008.05.019 [DOI] [PubMed] [Google Scholar]
- Zhang, H. , Wang, L. , Dong, Y. , Jiang, S. , Cao, J. , & Meng, R. (2007). Postharvest biological control of gray mold decay of strawberry with Rhodotorula glutinis . Biological Control, 40, 287–292. [Google Scholar]
- Zhu, S. , & Ma, B. (2007). Benzothiadiazole‐ or methyl jasmonate‐induced resistance to Colletotrichum musae in harvested banana fruit is related to elevated defense enzyme activities. Journal of Pomology and Horticultural Science, 82, 500–506. [Google Scholar]


