Abstract
Background
Eradication of minimal residual disease (MRD), at the end of Fludarabine-Cyclophosphamide-Rituximab (FCR) treatment, is a validated surrogate marker for progression-free and overall survival in chronic lymphocytic leukaemia. But such deep responses are also associated with severe immuno-depletion, leading to infections and the development of secondary cancers.
Methods
We assessed, blood MRD and normal immune cell levels at the end of treatment, in 162 first-line FCR patients, and analysed survival and adverse event.
Results
Multivariate Landmark analysis 3 months after FCR completion identified unmutated IGHV status (HR, 2.03, p = 0.043), the level of MRD reached (intermediate versus low, HR, 2.43, p = 0.002; high versus low, HR, 4.56, p = 0.002) and CD4 > 200/mm3 (HR, 3.30, p < 0.001) as factors independently associated with progression-free survival (PFS); neither CD8 nor NK counts were associated with PFS. The CD4 count was associated with PFS irrespective of IGHV mutational status, but only in patients with detectable MRD (HR, 3.51, p = 0.0004, whereas it had no prognostic impact in MRD < 10− 4 patients: p = 0.6998). We next used a competitive risk model to investigate whether immune cell subsets could be associated with the risk of infection and found no association between CD4, CD8 and NK cells and infection.
Conclusions
Consolidation/maintenance trials based on detectable MRD after FCR should investigate CD4 T-cell numbers both as a selection and a response criterion, and consolidation treatments should target B-cell/T-cell interactions.
Keywords: Chronic lymphocytic Leukaemia, Minimal residual disease, CD4 T-cells, Immunosuppression, Chemo-immunotherapy
Background
In chronic lymphocytic leukaemia (CLL), chemo-immunotherapy (CIT) with fludarabine, cyclophosphamide and rituximab (FCR) is now well established as a standard of care for young treatment-naive, fit patients without TP53 locus alterations (mutations and/or deletions) and with normal renal function [1, 2]. When compared to new generation targeted signalling inhibitors, FCR induces very prolonged remission periods in a subset of patients with IGHV mutations (IGHV-M), with three independent long-term follow-up studies reporting a > 10 year progression-free survival (PFS), specifically in patients in whom minimal residual (MRD) cannot be detected (< 10− 4) after treatment completion [3–5]. In a pooled analysis from randomised trials, FCR treatment of patients without IGHV mutations (IGHV-UM) resulted in a median PFS of only 42.9 months, with the absence of a plateau on the PFS curve and an attenuation of the advantages of reaching an undetectable MRD status [6]. In the context of CIT, the evaluation of MRD is of utmost importance because patients with undetectable MRD after treatment still achieve better PFS and overall survival (OS) than those with detectable MRD [7–12]. The quantification of MRD is however not recommended beyond the context of clinical trials [13, 14].
A number of factors are known to be associated with the depth of MRD response achieved by CIT (TP53 mutation and/or deletion 17p [del17p], high β2-microglobulin levels, or complex karyotype). Conversely, we have a limited understanding of the factors that influence an almost universal relapse in IGHV-UM patients, despite achieving undetectable MRD status [6]. Indeed, there is a lack of clinical factors that can accurately improve the prognostic power of eradicating MRD [15]. Since bystander immune cells such as CD4 T-cells promote CLL survival/proliferation in tumour niches before FCR [16], we hypothesised that normal lymphocyte levels may influence the duration of PFS independently of the MRD status achieved after completion of therapy. Since FCR also induces profound and durable lymphopenia, we correlated these measurements to the well-described risk of developing secondary malignancies and/or serious infectious events [17].
Methods
Study population
Between January 01, 2005 and February 29, 2016, 162 patients receiving frontline FCR for CLL in two institutions (IUCT-Oncopôle, Toulouse and Institut Bergonié, Bordeaux, France) were enrolled in our study. Patients’ clinical and biological data were retrieved from medical charts. In addition to complete blood counts, flow cytometry analyses were performed on peripheral blood samples at the end of treatment (EOT, i.e. 3 months after the last course of FCR) to monitor both normal immune reconstitution (CD4, CD8, NK) and MRD levels. MRD was quantified by 8-colour flow cytometry, with a sensitivity of at least 10− 4, using a combination (MRD antibody cocktail) comprising CD81-FITC (BD Pharmingen), CD43-PE (Beckman Coulter), CD79b-PerCP Cy5.5 (BD Biosciences), CD5-PC7 (Beckman Coulter), CD22-APC (BD Biosciences), CD20-AA700 (Biolegend), CD45-APC-H7 (BD Biosciences) and CD19-BV510 (BD Biosciences). One to five hundred microliters of fresh blood were incubated with the MRD antibody cocktail for 15 min, then red cells were lysed (with BD lysis buffer) for 15 min and washed twice. Flow cytometry analysis of a minimum of 105 leucocytes was carried out on a Navios instrument with Kaluza software (Beckman Coulter). Residual CLL cell gating and quantification was assessed according to the ERIC recommendations [18–20].
Definition of outcomes
Progression-free survival (PFS) was calculated from the first day of the first cycle of FCR (D1C1) to either relapse (per IwCLL2008 recommendations) or death, from any cause [13]. Overall survival (OS) was calculated from D1C1 FCR to death, from any cause. At the end of treatment (EOT, i.e. 3 months after the last course of FCR), the overall response rate was classed as either complete clinical response (clinical CR), complete response with incomplete bone marrow recovery (CRi), partial response (PR), or failure. This response assessment differed from the IwCLL2008 criteria, in that bone marrow biopsies are not warranted beyond the context of clinical trials in France; this explains why we used the term “clinical CR” instead of complete response (CR). MRD levels were classified as undetectable (< 10− 4), intermediate (10− 4 to 10− 2) and high (≥10− 2), as defined by the German CLL study group in the CLL8 and CLL10 trials [7, 21].
Opportunistic infections were described as follows: herpes zoster, Pneumocystis pneumonia, CMV disease, infection-driven hemophagocytic lymphohistiocytosis, invasive fungal infection, Toxoplasma gondii infection, malignant external otitis, progressive multifocal leukoencephalopathy, hepatitis B re-activation (in patients who were previously both anti-hepatitis B core and anti-hepatitis B surface antigen positive), and chronic hepatitis E infection. Severe infections were defined as any infection leading to hospitalisation (irrespectively of a common terminology criteria grade). Patients received primary prophylaxis with trimethoprim-sulfamethoxazole and valaciclovir in > 90% of cases (stopped 6 months after EOT evaluation in most cases [22]).
Statistical analyses
Continuous variables were presented as the median with a range (min-max) and categorical variables were summarised by frequencies and percentages. EOT CD4 counts were evaluated as a binary covariable with a threshold of 200/mm3, typically used to guide infection prophylaxis in HIV patients [23], but also in routine haematology practice. NK, CD8 and monocyte count cut-offs used were based on the median count at EOT.
The chi-square or Fisher’s exact test was used to compare categorical variables. Survival rates were estimated by Kaplan-Meier, with 95% confidence intervals (95%CI). Patients that were still alive were censored at the cut-off date or at their last available follow-up. Univariate and multivariate analyses were performed using the Logrank test and the Cox proportional hazards model; Hazard Ratios (HR) were estimated with 95% confidence intervals. Landmark analyses were performed at 9 months after initiation of treatment, to assess the impact of variables evaluated post-treatment on OS and PFS. Cumulative incidences of opportunistic and/or serious infections were estimated using a competing risks model, with relapse and death considered as competing events. Univariate analyses were performed using the Fine and Gray model and sub Hazard Ratios were estimated with a 95%CI. All tests were two-sided and p values < 0.05 were considered statistically significant. All analyses were conducted with STATA v13 (Stata Corporation, College Station, TX, USA) and R (3.4.3).
Results
Pre-therapy cohort characteristics
Patients’ characteristics are summarised in Table 1. Patients were males in 69.1% of cases. The median age was 61.5 years and Binet stage was B/C in 79% of patients. Other known prognostic variables included: 11q deletion in 22.2%, 17p deletion in 3.9%, IGHV-UM status in 63.4%, β2-microglobulin > 3.5 mg/L in 78.3%, complex karyotype in 21.6%, NOTCH1 mutations in 16.2%, and SF3B1 mutations in 8.8% of patients. The majority (75.9%) of patients received 6 cycles of FCR, and 98.1% received at least 4 cycles.
Table 1.
Patients’ pre-treatment characteristics
| Pre-FCR characteristics | n (%) or median [min-max] |
|---|---|
| Gender | |
| •Men | 112 (69.1) |
| •Women | 50 (30.9) |
| Age category | |
| •>65 years | 58 (35.8) |
| •≤65 years | 104 (64.2) |
| Binet stage | |
| •Binet A | 34 (21.3) |
| •Binet B | 85 (53.1) |
| •Binet C | 41 (25.6) |
| •Missing | 2 |
| Time from diagnosis to FCR (months) | 22.1 [0.03-203.00] |
| Lymphocytes (G/L) (n=114) | 90.6 [1.30-888.0] |
| β2-microglobulin | |
| •≤3.5 mg/L | 15 (21.7) |
| •>3.5 mg/L | 54 (78.3) |
| •Missing | 93 |
| LDH value | |
| •LDH≤ULN | 52 (45.6) |
| •LDH>ULN | 62 (54.4) |
| •Missing | 48 |
| IGHV mutation status | |
| •IGHV-M | 48 (36.6) |
| •IGHV-UM | 83 (63.4) |
| •Missing | 31 |
| Del13q | |
| •Del13q | 49 (37.4) |
| •No del13q | 82 (62.6) |
| •Missing | 31 |
| Trisomy 12 | |
| •Trisomy 12 | 32 (24.4) |
| •No trisomy 12 | 99 (75.6) |
| •Missing | 31 |
| Del11q | |
| •Del11q | 34 (22.2) |
| •No del11q | 119 (77.8) |
| •Missing | 9 |
| Del6q | |
| •Del6q | 14 (12.8) |
| •No del6q | 95 (87.2) |
| •Missing | 53 |
| t(14;18)(q32;q21) | |
| •t(14;18)(q32;q21) | 5 (4.6) |
| •No t(14;18)(q32;q21) | 104 (95.4) |
| •Missing | 53 |
| Del17p | |
| •Del17p | 6 (3.9) |
| •No del17p | 146 (96.1) |
| •Missing | 10 |
| Complex karyotype | |
| •Complex karyotype | 87 (78.4) |
| •No complex karyotype | 24 (21.6) |
| •Missing | 51 |
| TP53 mutation | |
| •TP53 mutation | 2 (2.9) |
| •No TP53 mutation | 68 (97.1) |
| •Missing | 92 |
| NOTCH1 mutation | |
| •NOTCH1 mutation | 11 (16.2) |
| •No NOTCH1 mutation | 57 (83.8) |
| •Missing | 94 |
| SF3B1 mutation | |
| •SF3B1 mutation | 6 (8.8) |
| •No SF3B1 mutation | 62 (91.2) |
| •Missing | 94 |
| MYD88 mutation | |
| •MYD88 mutation | 1 (1.5) |
| •No MYD88 mutation | 66 (98.5) |
| •Missing | 95 |
| BRAF mutation | |
| •No BRAF mutation | 67 (100) |
| •Missing | 85 |
Response rates, PFS and MRD assessment
The overall response rate was 98.8%, with 96.2% of patients achieving clinical CR/CRi. An EOT MRD assessment was available for 147 patients, of these 65.3% achieved undetectable MRD, 27.2% achieved intermediate levels, and 7.5% had high levels. After a median follow-up (FU) of 60.5 months (95%CI [54.0–71.5]), 46.3% of patients relapsed or died, with a median PFS of 65.7 months (95%CI [54.5–74.7]). In the univariate Cox model, baseline characteristics associated with shorter PFS were IGHV-UM (HR, 2.55 [1.42–4.59], p = 0.0012), del17p and/or TP53 mutation (HR, 3.87 [1.34–11.22], p = 0.0072) and del11q (HR, 2.19 [1.35–3.56], p = 0.0012) (Table 2). As expected from previous studies, EOT MRD levels were associated with PFS (intermediate versus low HR, 2.64 [1.55–4.50] p = 0.0004, high versus low HR, 6.95 [3.24–14.92], p < 0.0001) (Fig. 1A). With regards to IGHV mutational status, in IGHV-M patients, the 5-year PFS rate was 87.7% in MRD undetectable versus 35.9% in MRD detectable, against 51.2% versus 31.9% in IGHV-UM patients respectively (Fig. 1B-1C). Notably, patients with detectable MRD levels had comparable 5-year PFS rates irrespective of their IGHV mutational status.
Table 2.
Factors associated with progression-free survival (PFS) by univariate and multivariate analysis
| PFS | univariate analysis | multivariate analysis | ||
|---|---|---|---|---|
| Variables | HR [95%CI] | p | HR [95%CI] | p |
| 11q deletion (n = 153) | ||||
| No (ref) | 1.00 | 1.00 | ||
| Yes | 2.19 [1.35; 3.56] | 0.0012 | 1.74 [0.94; 3.21] | 0.076 |
| IGHV mutation status (n = 131) | ||||
| Mutated (ref) | 1.00 | 1.00 | ||
| Unmutated | 2.55 [1.42; 4.59] | 0.0012 | 2.03 [1.02; 4.04] | 0.043 |
| EOT MRDa (n = 131) | ||||
| Low (ref) | 1.00 | 1.00 | ||
| Intermediate | 2.64 [1.55; 4.50] | 2.43 [1.39; 4.27] | 0.002 | |
| High | 6.95 [3.24; 14.92] | < 0.0001 | 4.56 [1.76;11.79] | 0.002 |
| EOT CD4a (/mm3, n = 132) | ||||
| ≤ 200 (ref) | 1.00 | 1.00 | ||
| > 200 | 2.28 [1.35; 3.86] | 0.0016 | 3.30 [1.79; 6.06] | < 0.001 |
| EOT NKa (/mm3, n = 109) | ||||
| ≤ 100 (ref) | 1.00 | – | – | |
| > 100 | 1.03 [0.60; 1.78] | 0.9101 | ||
| EOT CD8a (/mm3, n = 132) | ||||
| ≤ 150 (ref) | 1.00 | – | – | |
| > 150 | 1.02 [0.62; 1.66] | 0.9418 | ||
| EOT monocytesa (/mm3, n = 114) | ||||
| ≤ 400 (ref) | 1.00 | – | – | |
| > 400 | 1.30 [0.77; 2.19] | 0.3257 | ||
aindicates Landmark analysis at 9 months
Fig. 1.
PFS of the different EOT MRD level groups, and according to IGHV mutational status. (A) PFS of the different MRD level groups at EOT in the whole population (both p < 0.0001 for low versus intermediate and low versus high levels). (B) PFS according to EOT MRD status (detectable versus undetectable) in IGHV-unmutated patients (p = 0.0206). (C) PFS according to EOT MRD status (detectable versus undetectable) in IGHV-mutated patients (p = 0.0002). EOT: end of treatment, MRD: minimal residual disease
Normal immune cells subsets and PFS
At EOT, the median counts of CD4, CD8 T-cells, monocytes and NK lymphocytes were 154, 153, 418 and 114/mm3 respectively. A level of CD4 ≤ 200/mm3 was observed in 64.2% of patients. In Landmark analyses, EOT CD4 > 200/mm3 was associated with an increased risk of relapse (median PFS 39.3 months versus 67.4 months if EOT CD4 ≤ 200/mm3, HR, 2.28 [1.35–3.86] p = 0.0016) (Fig. 2A)). PFS was not associated with EOT CD8 T-cell levels (≤150/mm3 versus > 150/mm3 p = 0.9418), nor with EOT monocyte levels (≤400/mm3 versus > 400/mm3 p = 0.3257), nor with NK cells (≤100/mm3 versus > 100/mm3 p = 0.9101). Reaching a low EOT CD4 T-cell count was associated with a trend towards a better PFS in IGHV-M patients (5-year PFS of 76.2% versus 42.8%, HR, 2.81 [0.92–8.54], p = 0.0576), and with a much greater PFS in IGHV-UM patients (median PFS of 63.7 versus 30.7 months, HR, 4.09 [2.00–8.39], p < 0.0001, Fig. 2B-2C). In multivariate Landmark analysis (Table 2), the following variables were associated with PFS: IGHV-UM (HR, 2.03 [1.02–4.04], p = 0.043), EOT CD4 > 200/mm3 (HR; 3.30 [1.79–6.06], p < 0.001) and EOT MRD (intermediate versus low, HR, 2.43 [1.39–4.27], p = 0.002; high versus low, HR, 4.56 [1.76–11.79], p = 0.002).
Fig. 2.
PFS of the different EOT CD4 levels and according to IGHV mutational status in the whole population. (A) PFS curves according to EOT CD4 status in the whole population (p = 0.0016). (B) PFS curves according to EOT CD4 status in patients with IGHV-unmutated status (p < 0.0001) (C) PFS curves according to EOT CD4 status in patients with mutated IGHV (p = 0.0576)
PFS and CD4 counts in different MRD subgroups
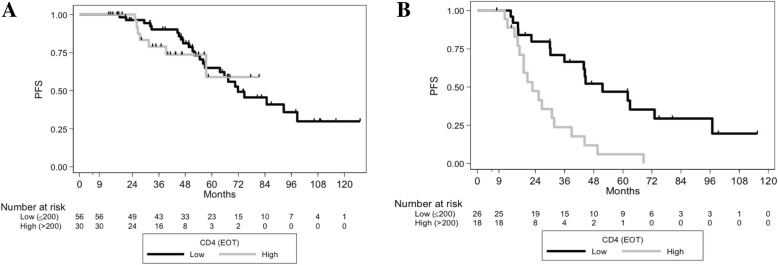
As the CD4 count was found to be an independent parameter which more accurately redefined PFS according to IGHV mutational status, we next sought to investigate its association with PFS in the undetectable and detectable MRD subgroups (due to the very small number of patients with high EOT MRD [n = 10], we pooled these patients with the intermediate EOT MRD patients). In the low MRD group (n = 86), patients with EOT CD4 ≤ 200/mm3 had 5-year PFS of 65% versus 59% if CD4 > 200/mm3 (p = 0.6998, Fig. 3A). Conversely, in cases with detectable MRD levels at EOT (n = 44), patients with EOT CD4 ≤ 200/mm3 had a 5-year PFS of 47.03% versus 5.93% if EOT CD4 > 200/mm3 (HR, 3.51 95%CI [1.68–7.32], p = 0.0004) (Fig. 3B). Taken together, these results suggest that the EOT CD4 count may help clinicians to more accurately predict PFS in patients with detectable MRD levels following FCR treatment.
Fig. 3.
PFS according to EOT CD4, and according to EOT MRD levels. (A) PFS curves according to EOT CD4 status in patients with undetectable (< 10−4) EOT MRD (p = 0.6998). (B) PFS curves according to EOT CD4 status in patients with detectable (≥10− 4) EOT MRD (p = 0.0004)
Overall survival (OS) and toxicities after FCR
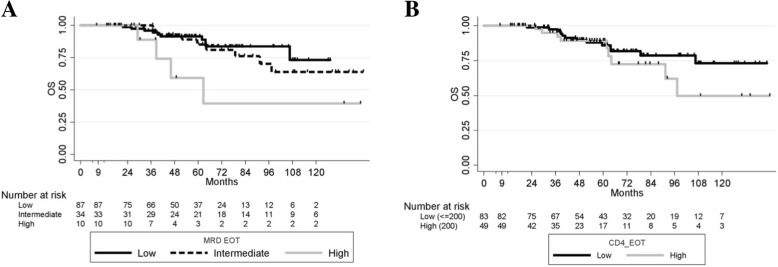
Twenty-five patients (15.4%) died. Five-year OS was 87.7% (95%CI [80.34–92.50]). In Landmark univariate analyses, only a high versus a low level of MRD at EOT was associated with OS (intermediate versus low, HR, 1.45 [0.57–3.72] p = 0.435, high versus low, HR, 3.96 [1.23–12.74], p = 0.021), whereas the EOT CD4 cell count was not found associated with OS (HR, 1.62 [0.69–3.81], p = 0.2631) (Fig. 4). During FU, 20 patients (12.3%) developed a secondary cancer within a median time of 40 months from D1C1 FCR (range, 6–111), and 10 patients (6.2%) developed a Richter transformation (RT) within a median time of 59.5 months from D1C1 FCR (Table 3). Due to the small number of patients with secondary cancers as the first event (n = 10), we could not investigate the association of EOT CD4, CD8 and NK cell counts with the incidence of those events; nevertheless, of these 10 patients, 4 had EOT CD4 > 200/mm3, 4 had EOT NK > 100/mm3 and 5 had EOT CD8 > 150/mm3, a proportion rather similar to that measured in the entire cohort.
Fig. 4.
OS according to EOT MRD, and according to EOT CD4 count. (A) OS according to level of EOT MRD (for low versus intermediate, p = 0.435 and low versus high levels, p = 0.021). (B) OS according to EOT CD4 status in the whole population (p = 0.263)
Table 3.
Other cancers which developed during follow-up. AML indicates acute myeloid leukaemia.
| Cancer | Number |
|---|---|
| Secondary haematologic | |
| AML/myelodysplasia | 2 |
| Anaplastic large cell lymphoma | 1 |
| Multiple myeloma | 1 |
| Erdheim-Chester disease | 1 |
| Richter transformation | |
| Diffuse large B-cell lymphoma | 9 |
| Hodgkin lymphoma | 1 |
| Solid tumours | |
| Non-melanoma skin cancer | 4 |
| Lung cancer | 4 |
| Prostate cancer | 2 |
| Colorectal cancer | 2 |
| Oropharyngeal cancers | 2 |
| Breast cancer | 1 |
Twenty-five patients developed a serious infection, within a median time of 15 months from D1C1 FCR (range, 2–112), and thirty-five patients developed an opportunistic infection, within a median time of 14 months from D1C1 FCR (range, 2–94). When performing a Landmark analysis at 9 months from D1C1 FCR, the cumulative incidence of serious and/or opportunistic infection was 4.6% at 12 months and 14.9% at 24 months. The competing risk analysis (Table 4) did not detect any association between EOT levels of NK, CD8 or CD4 and serious and/or opportunistic infections. Figure 5 represents the cumulative risk of serious and/or opportunistic infections and relapse or death with the EOT CD4 T-cell count in the entire studied population.
Table 4.
Univariate analyses of factors associated with severe and/or opportunistic infections. Landmark competing risk analysis at 9 months. sHR indicates sub-Hazard Ratio
| Infections | Relapse/death | |||
|---|---|---|---|---|
| Variables | sHR [95%CI] | p | sHR [95%CI] | p |
| EOT MRD status (n = 125) | ||||
| < 10−4 (ref) | 1.00 | 1.00 | ||
| ≥ 10−4 | 0.72 [0.29; 1.82] | 0.492 | 3.02 [1.68; 5.45] | < 0.001 |
| EOT CD4 (/mm3, n = 125) | ||||
| ≤ 200 (ref) | 1.00 | 1.00 | ||
| > 200 | 0.97 [0.40; 2.38] | 0.948 | 2.34 [1.26; 4.33] | 0.007 |
| EOT NK (/mm3, n = 119) | ||||
| ≤ 100 (ref) | 1.00 | 1.00 | ||
| > 100 | 0.51 [0.21; 1.22] | 0.128 | 1.07 [0.58; 1.96] | 0.837 |
| EOT CD8 (/mm3, n = 125) | ||||
| ≤ 150 | 1.00 | 1.00 | ||
| > 150 | 1.84 [0.79; 4.30] | 0.158 | 1.06 [0.59; 1.89] | 0.843 |
Fig. 5.

Cumulative incidence of severe and/or opportunistic infections, and of relapse/death according to EOT CD4. Threshold of 200/mm3. High EOT CD4 were associated with higher risk of death/relapse/Richter transformation (HR, 2.34 [1.26–4.33], p = 0.007), whereas no association was found between EOT CD4 and severe and/or opportunistic infections (HR, 0.97 [0.40–2.38], p = 0.948)
Landmark competing risk analysis at 9 months. sHR indicates the sub-Hazard Ratio.
Discussion
We report results obtained from a large series of patients receiving frontline FCR in the routine practice of two large regions of southwestern France, with a median follow up of over 5 years. Our population was rather similar to that of the CLL8 study and other cohorts, but also included older patients and patients with more advanced disease [1, 4, 21]. We first confirmed the general clinical importance, of achieving a low MRD level at EOT, which extends the relevance of assessing MRD well beyond that of clinical trials. We observed a plateau in the PFS curves of IGHV-M patients who achieved a low MRD level endpoint, and also the universal relapse pattern of IGHV-UM patients despite eradicating MRD in peripheral blood. In an attempt to better understand this unique feature, we investigated whether normal lymphocyte counts could redefine the prognosis in distinct subgroups of patients. We found that the post-therapy CD4 count was associated with a different prognosis depending on the IGHV status, and that this also extended to patients with detectable MRD at EOT. The CD4 count was however not associated with infections, even though this parameter is generally routinely used in clinical practice to determine the start/hold timing of prophylactic measures (with trimethoprim-sulfamethoxazole and/or valaciclovir).
Since no plateau was observed in the PFS curves of low CD4 IGHV-UM patients, it is very unlikely that this parameter alone could explain the relapse pattern observed in these patients. But in the detectable MRD group, a high CD4 count post-FCR was able to identify a subgroup of patients with a median PFS of only 24 months (a widely accepted definition of FCR-refractory disease [2]). Hence, the CD4 count could help identify patients who may benefit from a consolidation after FCR, especially if the drug modulates T-cells numbers and effects (such as lenalidomide [24–29] or ibrutinib [30–33]). Our previous series was the first to illustrate an effect on CD4 T-cell count following FCR treatment in CLL [34]. A thorough analysis of the phenotype of these T-cells revealed that most were CD4+ CD25+ CD127- FoxP3+ (and as such likely to belong to the T regulatory subset, our unpublished data), which have previously been reported to mediate a CLL-supportive effect in vitro and in vivo [16, 35–37]. Another single-centre retrospective study found that absolute lymphocyte count < 1000/μl three months post-FCR was associated with OS and event-free survival, without MRD data and without analysing the lymphocyte subsets (thus they could not determine the clonal nature of these lymphocytes [38]). In addition to reflecting the pharmacodynamic activity of FCR, we consider lympho-depletion as a more complex, dynamic period of lymphocyte recovery with inter-clonal competitions. It would be surprising that a 3-drug regimen dose effect would be restricted to the CD4 subset (and not to CD8 or NK lymphocytes). Since the prognostic benefits of CD4 T-cells in our study were only observed in patients with detectable residual CLL cells, this argues for a bystander effect rather than just a dose effect. It would be interesting to further investigate CD4 effects in CLL, and to observe whether patients with low EOT CD4 already presented with low CD4 prior to FCR treatment; this could help clinicians identify patients with a high probability of reaching low EOT CD4 after CIT, and thus help select patients who would benefit the most from CIT, which would be a useful distinction to make as FCR is currently being compromised by other first-line therapeutic strategies [39]. Since our research focussed on identifying patients who would benefit from maintenance therapy after completing FCR, we did not perform this type of analysis; neither did we perform sequential lymphocyte subset counts during FCR therapy, as has been previously reported in the case of sequential MRD measurements taken during FCR therapy [40].
Furthermore, by highlighting the clinical relevance of CLL cell interactions with their microenvironment in relation to PFS, our research may pave the way for the investigation of associations between other amenable factors (such as CD40 or IL4) and PFS [41, 42]; this kind of research could help clinicians to optimise the tools and timing (before, during or after FCR completion), to exploit the complex interactions between CLL and normal immune cells.
Our cohort confirmed the high rate of infection, previously observed, during the first two years following FCR (Fig. 5) [17]. It is therefore perhaps not surprising that a low EOT CD4 count was not associated with an increased risk of infection, which means that a CD4 cell count is not useful to manage anti-viral or microbial prophylaxes, in clinical practice (the 200/mm3 threshold for discontinuing prophylactic measures was first suggested by HIV-treating physicians, but has never been validated in onco-heamatology patients [22, 23]). Monitoring of NK cells may be more informative to predict possible infectious complications in these patients (we indeed found a trend between low EOT NK cells and infectious events). Some authors have recently suggested a protective role of NK cells in CLL, not in terms of progression of disease, but in terms of OS, corroborating our observation [43]. However, these authors did not study the influence of NK cells on infections, nor the impact of NK cells after frontline CIT. Secondary cancer rates in our cohort were found to be comparable to those reported in the literature [4, 5], but only in terms of Richter transformation: it is noteworthy that our rate of myelodysplastic syndromes/AML was unusually low (2/162) when compared to the MDACC FCR300 cohort (14/300), but our follow-up duration was much shorter. This cannot be explained by dose intensity of FCR, since the French oral FC regimen is slightly over-dosed compared to intravenous FC. In the latter series, 59/300 patients developed solid tumours (28 non-melanoma skin cancers), as compared to 15/162 patients in our cohort. No correlations with EOT lymphocyte counts could be drawn from our analyses.
Conclusion
Our data suggests that in real-life clinical practice, CD4 cell counts should be assessed after completing FCR, not to stop prophylaxes, but as an opportunity to discuss our patient’s recruitment into a clinical trial assessing maintenance, or to mitigate our multiple concerns about prognostication, response durations and/or infectious risks. This parameter is easily available in most centres, but does not replace MRD as the best post-therapy evaluation tool (it is not the “MRD of the poor”). We think there is a window of opportunity to develop post-FCR T-cell targeted (not only B-cell-targeted with antiCD20 antibodies) strategies aiming at eradicating B/T-cell interactions driving subsequent clinical relapses.
Acknowledgements
The authors thank their colleagues working in the Onco-Occitanie network.
Abbreviations
- CIT
chemo-immunotherapy
- CLL
chronic lymphocytic leukaemia
- CR
complete response
- CRi
complete response with incomplete bone marrow recovery
- EOT
end of treatment
- FCR
fludarabine-cyclophosphamide-rituximab
- FU
follow-up
- HIV
human immunodeficiency virus
- HR
hazard ratio
- IGHV-M
mutated IGHV status
- IGHV-UM
unmutated IGHV status
- IWCLL
international workshop on chronic lymphocytic leukaemia
- MRD
minimal residual disease
- OS
overall survival
- PFS
progression-free survival
- PR
partial response
Author’s contributions
LY and AQM designed the research, MG, FD, MP, FV, LO, BF performed the research and collected data, EM, TF performed the statistical analyses, MG, EM, LY wrote the manuscript. All authors read and approved the final manuscript.
Funding
This work was partly supported by the grant “Investissement d’Avenir” ANR-11-PHUC-001 of the French National Research Agency. The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
Written informed consent was obtained from all patients in accordance with the Declaration of Helsinki, allowing the collection of clinical and biological data in an anonymized database. Our Institutional Review Board (University Hospital of Toulouse –Office of Research, Development and Innovation) approved our retrospective study with informed consent for MRD analyses.
Consent for publication
Not applicable (research on patients’ data).
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet Lond Engl. 2010;376:1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 2.Eichhorst B, Robak T, Montserrat E, Ghia P, Hillmen P, Hallek M, et al. Chronic lymphocytic leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol. 2015;26(Suppl 5):v78–v84. doi: 10.1093/annonc/mdv303. [DOI] [PubMed] [Google Scholar]
- 3.Rossi D, Terzi-di-Bergamo L, De Paoli L, Cerri M, Ghilardi G, Chiarenza A, et al. Molecular prediction of durable remission after first-line fludarabine-cyclophosphamide-rituximab in chronic lymphocytic leukemia. Blood. 2015;126:1921–1924. doi: 10.1182/blood-2015-05-647925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson PA, Tam CS, O’Brien SM, Wierda WG, Stingo F, Plunkett W, et al. Fludarabine, cyclophosphamide, and rituximab treatment achieves long-term disease-free survival in IGHV-mutated chronic lymphocytic leukemia. Blood. 2016;127:303–309. doi: 10.1182/blood-2015-09-667675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer K, Bahlo J, Fink AM, Goede V, Herling CD, Cramer P, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127:208–215. doi: 10.1182/blood-2015-06-651125. [DOI] [PubMed] [Google Scholar]
- 6.Chai-Adisaksopha C, Brown JR. FCR achieves long-term durable remissions in patients with IGHV-mutated CLL. Blood. 2017;130:2278–2282. doi: 10.1182/blood-2017-07-731588. [DOI] [PubMed] [Google Scholar]
- 7.Böttcher S, Ritgen M, Fischer K, Stilgenbauer S, Busch RM, Fingerle-Rowson G, et al. Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: a multivariate analysis from the randomized GCLLSG CLL8 trial. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30:980–988. doi: 10.1200/JCO.2011.36.9348. [DOI] [PubMed] [Google Scholar]
- 8.Kovacs G, Robrecht S, Fink AM, Bahlo J, Cramer P, von Tresckow J, et al. Minimal residual disease assessment improves prediction of outcome in patients with chronic lymphocytic leukemia (CLL) who achieve partial response: comprehensive analysis of two phase III studies of the German CLL study group. J Clin Oncol Off J Am Soc Clin Oncol. 2016. [DOI] [PubMed]
- 9.Kwok M, Rawstron AC, Varghese A, Evans PAS, O’Connor SJM, Doughty C, et al. Minimal residual disease is an independent predictor for 10-year survival in CLL. Blood. 2016;128:2770–2773. doi: 10.1182/blood-2016-05-714162. [DOI] [PubMed] [Google Scholar]
- 10.Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101–1110. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- 11.Fischer K, Cramer P, Busch R, Böttcher S, Bahlo J, Schubert J, et al. Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: a multicenter phase II trial of the German chronic lymphocytic leukemia study group. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30:3209–3216. doi: 10.1200/JCO.2011.39.2688. [DOI] [PubMed] [Google Scholar]
- 12.Bouvet E, Borel C, Obéric L, Compaci G, Cazin B, Michallet A-S, et al. Impact of dose intensity on outcome of fludarabine, cyclophosphamide, and rituximab regimen given in the first-line therapy for chronic lymphocytic leukemia. Haematologica. 2013;98:65–70. doi: 10.3324/haematol.2012.070755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the international workshop on chronic lymphocytic leukemia updating the National Cancer Institute-working group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131:2745–2760. doi: 10.1182/blood-2017-09-806398. [DOI] [PubMed] [Google Scholar]
- 15.Boettcher S, Ritgen M, Fischer K, Stilgenbauer S, Busch R, Fingerle-Rowson GR, et al. Minimal residual disease (MRD) re-growth kinetics are an independent predictor for progression free survival (PFS) in chronic lymphocytic leukemia (CLL) and are related to biologically defined CLL-subgroups– results from the CLL8 trial of the German CLL study group (GCLLSG). Blood. 2011;118:1777–7.
- 16.Bagnara D, Kaufman MS, Calissano C, Marsilio S, Patten PEM, Simone R, et al. A novel adoptive transfer model of chronic lymphocytic leukemia suggests a key role for T lymphocytes in the disease. Blood. 2011;117:5463–5472. doi: 10.1182/blood-2010-12-324210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tam CS, O’Brien S, Wierda W, Kantarjian H, Wen S, Do K-A, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rawstron AC, Villamor N, Ritgen M, Böttcher S, Ghia P, Zehnder JL, et al. International standardized approach for flow cytometric residual disease monitoring in chronic lymphocytic leukaemia. Leukemia. 2007;21:956–964. doi: 10.1038/sj.leu.2404584. [DOI] [PubMed] [Google Scholar]
- 19.Rawstron AC, Böttcher S, Letestu R, Villamor N, Fazi C, Kartsios H, et al. Improving efficiency and sensitivity: European research initiative in CLL (ERIC) update on the international harmonised approach for flow cytometric residual disease monitoring in CLL. Leukemia. 2013;27:142–149. doi: 10.1038/leu.2012.216. [DOI] [PubMed] [Google Scholar]
- 20.Rawstron AC, Fazi C, Agathangelidis A, Villamor N, Letestu R, Nomdedeu J, et al. A complementary role of multiparameter flow cytometry and high-throughput sequencing for minimal residual disease detection in chronic lymphocytic leukemia: an European research initiative on CLL study. Leukemia. 2016;30:929–936. doi: 10.1038/leu.2015.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eichhorst B, Fink A-M, Bahlo J, Busch R, Kovacs G, Maurer C, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17:928–942. doi: 10.1016/S1470-2045(16)30051-1. [DOI] [PubMed] [Google Scholar]
- 22.Maertens J, Cesaro S, Maschmeyer G, Einsele H, Donnelly JP, Alanio A, et al. ECIL guidelines for preventing Pneumocystis jirovecii pneumonia in patients with haematological malignancies and stem cell transplant recipients. J Antimicrob Chemother. 2016;71:2397–2404. doi: 10.1093/jac/dkw157. [DOI] [PubMed] [Google Scholar]
- 23.Phair J, Muñoz A, Detels R, Kaslow R, Rinaldo C, Saah A. The risk of Pneumocystis carinii pneumonia among men infected with human immunodeficiency virus type 1. Multicenter AIDS cohort study group. N Engl J Med. 1990;322:161–165. doi: 10.1056/NEJM199001183220304. [DOI] [PubMed] [Google Scholar]
- 24.Fink AM, Bahlo J, Sandra R, Al-Sawaf O, Aldaoud A, Hebart H, et al. Lenalidomide maintenance after front line therapy substantially prolongs progression free survival in high risk CLL: interim results of a phase 3 study (CLL M1 study of the German CLL study group). Blood. 2016;128:229–9.
- 25.Chanan-Khan AA, Zaritskey A, Egyed M, Vokurka S, Semochkin S, Schuh A, et al. Lenalidomide maintenance therapy in previously treated chronic lymphocytic leukaemia (CONTINUUM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Haematol. 2017;4:e534–e543. doi: 10.1016/S2352-3026(17)30168-0. [DOI] [PubMed] [Google Scholar]
- 26.Shanafelt TD, Ramsay AG, Zent CS, Leis JF, Tun HW, Call TG, et al. Long-term repair of T-cell synapse activity in a phase II trial of chemoimmunotherapy followed by lenalidomide consolidation in previously untreated chronic lymphocytic leukemia (CLL) Blood. 2013;121:4137–4141. doi: 10.1182/blood-2012-12-470005. [DOI] [PubMed] [Google Scholar]
- 27.Ramsay AG, Clear AJ, Fatah R, Gribben JG. Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: establishing a reversible immune evasion mechanism in human cancer. Blood. 2012;120:1412–1421. doi: 10.1182/blood-2012-02-411678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kater AP, Tonino SH, Egle A, Ramsay AG. How does lenalidomide target the chronic lymphocytic leukemia microenvironment? Blood. 2014;124:2184–2189. doi: 10.1182/blood-2014-05-578286. [DOI] [PubMed] [Google Scholar]
- 29.Strati P, Keating MJ, Burger JA, O’Brien SM, Wierda WG, Estrov Z, et al. Consolidation treatment with lenalidomide following front-line or salvage chemoimmunotherapy in chronic lymphocytic leukemia. Haematologica. 2017;102:e494–e496. doi: 10.3324/haematol.2017.171561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubovsky JA, Beckwith KA, Natarajan G, Woyach JA, Jaglowski S, Zhong Y, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122:2539–2549. doi: 10.1182/blood-2013-06-507947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niemann CU, Herman SEM, Maric I, Gomez-Rodriguez J, Biancotto A, Chang BY, et al. Disruption of in vivo chronic lymphocytic leukemia tumor-microenvironment interactions by Ibrutinib--findings from an investigator-initiated phase II study. Clin Cancer Res Off J Am Assoc Cancer Res. 2016;22:1572–1582. doi: 10.1158/1078-0432.CCR-15-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Podhorecka M, Goracy A, Szymczyk A, Kowal M, Ibanez B, Jankowska-Lecka O, et al. Changes in T-cell subpopulations and cytokine network during early period of ibrutinib therapy in chronic lymphocytic leukemia patients: the significant decrease in T regulatory cells number. Oncotarget. 2017;8:34661–34669. doi: 10.18632/oncotarget.16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Long M, Beckwith K, Do P, Mundy BL, Gordon A, Lehman AM, et al. Ibrutinib treatment improves T cell number and function in CLL patients. J Clin Invest. 2017;127:3052–3064. doi: 10.1172/JCI89756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ysebaert L, Gross E, Kühlein E, Blanc A, Corre J, Fournié JJ, et al. Immune recovery after fludarabine-cyclophosphamide-rituximab treatment in B-chronic lymphocytic leukemia: implication for maintenance immunotherapy. Leukemia. 2010;24:1310–1316. doi: 10.1038/leu.2010.89. [DOI] [PubMed] [Google Scholar]
- 35.Lad D, Hoeppli R, Huang Q, Garcia R, Xu L, Toze C, et al. Regulatory T-cells drive immune dysfunction in CLL. Leuk Lymphoma. 2017:1–4. [DOI] [PubMed]
- 36.Rissiek A, Schulze C, Bacher U, Schieferdecker A, Thiele B, Jacholkowski A, et al. Multidimensional scaling analysis identifies pathological and prognostically relevant profiles of circulating T-cells in chronic lymphocytic leukemia. Int J Cancer. 2014;135:2370–2379. doi: 10.1002/ijc.28884. [DOI] [PubMed] [Google Scholar]
- 37.Mpakou VE, Ioannidou H-D, Konsta E, Vikentiou M, Spathis A, Kontsioti F, et al. Quantitative and qualitative analysis of regulatory T cells in B cell chronic lymphocytic leukemia. Leuk Res. 2017;60:74–81. doi: 10.1016/j.leukres.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Joffe E, Ariela Arad N, Bairey O, Fineman R, Ruchlemer R, Rahimi-Levene N, et al. Persistently low lymphocyte counts after FCR therapy for chronic lymphocytic leukemia are associated with longer overall survival. Hematol Oncol. 2018;36:128–135. doi: 10.1002/hon.2444. [DOI] [PubMed] [Google Scholar]
- 39.Shanafelt TD, Wang V, Kay NE, Hanson CA, O’Brien SM, Barrientos JC, et al. A Randomized Phase III Study of Ibrutinib (PCI-32765)-Based Therapy Vs. Standard Fludarabine, Cyclophosphamide, and Rituximab (FCR) Chemoimmunotherapy in Untreated Younger Patients with Chronic Lymphocytic Leukemia (CLL): A Trial of the ECOG-ACRIN Cancer Research Group (E1912). Blood. 2018;132(Suppl 1):LBA–4–LBA–4.
- 40.Strati P., Keating M. J., O'Brien S. M., Burger J., Ferrajoli A., Jain N., Tambaro F. P., Estrov Z., Jorgensen J., Challagundla P., Faderl S. H., Wierda W. G. Eradication of bone marrow minimal residual disease may prompt early treatment discontinuation in CLL. Blood. 2014;123(24):3727–3732. doi: 10.1182/blood-2013-11-538116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smallwood D. T., Apollonio B., Willimott S., Lezina L., Alharthi A., Ambrose A. R., De Rossi G., Ramsay A. G., Wagner S. D. Extracellular vesicles released by CD40/IL-4-stimulated CLL cells confer altered functional properties to CD4+ T cells. Blood. 2016;128(4):542–552. doi: 10.1182/blood-2015-11-682377. [DOI] [PubMed] [Google Scholar]
- 42.Aguilar-Hernandez M. M., Blunt M. D., Dobson R., Yeomans A., Thirdborough S., Larrayoz M., Smith L. D., Linley A., Strefford J. C., Davies A., Johnson P. M. W., Savelyeva N., Cragg M. S., Forconi F., Packham G., Stevenson F. K., Steele A. J. IL-4 enhances expression and function of surface IgM in CLL cells. Blood. 2016;127(24):3015–3025. doi: 10.1182/blood-2015-11-682906. [DOI] [PubMed] [Google Scholar]
- 43.Wang Wen-Ting, Zhu Hua-Yuan, Wu Yu-Jie, Xia Yi, Wu Jia-Zhu, Wu Wei, Liang Jin-Hua, Wang Li, Fan Lei, Li Jian-Yong, Xu Wei. Elevated absolute NK cell counts in peripheral blood predict good prognosis in chronic lymphocytic leukemia. Journal of Cancer Research and Clinical Oncology. 2018;144(3):449–457. doi: 10.1007/s00432-017-2568-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.