Abstract
Background
The clinical benefit of immunotherapeutic approaches against cancer has been well established although complete responses are only observed in a minority of patients. Combination immunotherapy offers an attractive avenue to develop more effective cancer therapies by improving the efficacy and duration of the tumor-specific T-cell response. Here, we aimed at deciphering the mechanisms governing the response to PD-1/PD-L1 checkpoint blockade to support the rational design of combination immunotherapy.
Methods
Mice bearing subcutaneous MC-38 tumors were treated with blocking PD-L1 antibodies. To establish high-dimensional immune signatures of immunotherapy-specific responses, the tumor microenvironment was analyzed by CyTOF mass cytometry using 38 cellular markers. Findings were further examined and validated by flow cytometry and by functional in vivo experiments. Immune profiling was extended to the tumor microenvironment of colorectal cancer patients.
Results
PD-L1 blockade induced selectively the expansion of tumor-infiltrating CD4+ and CD8+ T-cell subsets, co-expressing both activating (ICOS) and inhibitory (LAG-3, PD-1) molecules. By therapeutically co-targeting these molecules on the TAI cell subsets in vivo by agonistic and antagonist antibodies, we were able to enhance PD-L1 blockade therapy as evidenced by an increased number of TAI cells within the tumor micro-environment and improved tumor protection. Moreover, TAI cells were also found in the tumor-microenvironment of colorectal cancer patients.
Conclusions
This study shows the presence of T cell subsets in the tumor micro-environment expressing both activating and inhibitory receptors. These TAI cells can be targeted by combined immunotherapy leading to improved survival.
Electronic supplementary material
The online version of this article (10.1186/s40425-019-0700-3) contains supplementary material, which is available to authorized users.
Keywords: Mass cytometry, Combinatorial immunotherapy, T cells
Introduction
Immunotherapy has become an important treatment option for cancer patients. Especially, clinical trials with antibodies that block the interaction between the inhibitory receptor PD-1, expressed on previously activated T cells, with its broadly expressed ligand PD-L1, resulted in unprecedented clinical response rates for patients with advanced cancer [1–3]. Despite these encouraging results, still only a fraction of patients show durable responses, whereas the majority of treated patients show no beneficial clinical response [1, 4]. Therefore, there is a need for more effective treatment regimens, like combinatorial immunotherapies, which offer an attractive avenue to improve the efficacy and the duration of the tumor specific T-cell response.
Both CD8+ and CD4+ T cells can mount responses against many human cancer types, especially those with higher mutational burden [5]. Studies have shown that T cells are however partially inhibited by PD-1/PD-L1 interactions [6] and releasing this constraint by blocking the PD-1 pathway can to some extent reinvigorate T cells leading to clinical benefit in a number of cancer patients [7]. However, tumor-specific T cells are also restrained by several other inhibitory mechanisms [8, 9], which put forward the premise that PD-1/PD-L1-based monotherapies could be enhanced so that the majority of patients will have durable clinical benefit. Indeed, recent studies reported co-treatment regimens to PD-1 blockade [10–13]. In depth mechanistic studies of PD-1/PD-L1 blockade in vivo may lead to rational design of improved co-treatment protocols.
Identification of biomarkers related to immunotherapeutic response and resistance could support the rational design of complementary therapies in which the additional targeting of those biomarkers would lead to more effective cancer therapies. Identification of the relevant responding cell types to therapy reveals insight into the underlying immunological mechanisms of on-going clinical response, as well as into the development of adaptive resistance during such a therapy. Here we used high-dimensional, single-cell mass cytometry and a customized bioinformatics pipeline Cytofast [14] to generate an in-depth analysis of the tumor-infiltrating immune cells upon PD-L1-based treatment. Our aim was to identify responsiveness-associated targets to improve immunotherapy. We discovered unique CD4+ and CD8+ T cell subsets that increased after anti-PD-L1 immunotherapy and were characterized by expression of both activating and inhibitory receptors, hence we defined these cells as TAI cells. By therapeutic targeting of the activating and inhibitory receptors on the TAI cells in vivo, significant improvement of immunotherapy was shown, correlating with an increase of the CD8+ TAI cells in the tumor micro-environment (TME). TAI cells were also present within tumor-infiltrated immune cells from mismatch repair-deficient (MMRd) colorectal cancer patients. Together, our data show the importance of the TAI cells and their possible targetability to induce tumor regression in colorectal cancer.
Methods
Mice
C57BL/6 J mice were purchased from The Jackson Laboratory. All animal experiments were approved by the Animal Experiments Committee of LUMC and were executed according to the animal experimentation guidelines of the LUMC in compliance with the guidelines of Dutch and European committees.
Staining and acquisition for CyTOF mass cytometry
Metal conjugated antibodies were purchased from Fluidigm or conjugated to unlabeled antibodies in-house. All non-platinum conjugations were performed using X8 polymer as per manufacturer’s protocol (Fluidigm) and were performed at 100 μg scale. Conjugation with 208 Bismuth was performed using a protocol adapted from M. Spitzer [15]. All in-house conjugated antibodies were diluted to 0.5 mg/ml in antibody stabilizer supplemented with 0.05% sodium azide (Candor Biosciences). Appropriate antibody dilution was determined by serial dilution staining to minimize background and optimize detection of positively expressing populations.
CyTOF data were acquired and analyzed on-the-fly, using dual-count mode and noise-reduction on. All other settings were either default settings or optimized with tuning solution, as instructed by Fluidigm Sciences. After data acquisition, the mass bead signal was used to normalize the short-term signal fluctuations with the reference EQ passport P13H2302 during the course of each experiment and the bead events were removed [16].
CyTOF mass cytometry data analysis
To isolate immune cells from the tumor, solid tumors were excised after a flushing step to remove the blood from TME. Exclusion criteria were ulceration of tumors, incomplete or unsuccessful flushing (determined by an unexpected high numbers of B cells in the TME). Single-cell suspensions were then prepared by mechanical and enzymatic (collagenase D and DNase, Sigma-Aldrich) dissociation, followed by density gradient centrifugation on an 100% / 70% / 40% / 30% Percoll (GE Healthcare) gradient.
After staining cells according to van Unen et al. [17], we analyzed live immune cells from the TME. We set our gating strategy to live single cells, positive for CD45, and excluded reference beads. For further analysis, live CD45+ gated files were sample-tagged, their marker expression arcsinh5 transformed and subjected to dimensionality reduction analyzes in Cytosplore [18]. All markers were taken in account to process the clustering analysis except PD-L1, which is a marker used only as a quality control to check the efficacy of PD-L1 blocking antibodies. The PD-L1 blocking antibody we used (clone MIH5, rat-anti-mouse, IgG2a subtype) binds to FcyRIIb and FcyRIII but not to FcyRI and FcyRIV, and is not able to mediate specific killing or depletion [19]. By staining with the same antibody clone, PD-L1 downmodulation was determined to show the effectiveness of the provided therapeutic antibodies to block PD-L1 binding.
Pooled samples from control and PD-L1 treated groups were analyzed by hierarchical stochastic neighbourhood embedding (HSNE) [20] based on approximated t-distributed stochastic neighbourhood embedding (A-tSNE) [21]. The default perplexity and iterations of the HSNE analysis were 30 and 1.000, respectively. If some clusters showed a similar phenotype, they were manually merged in Cytosplore. For further data exploration, CD4+ T cell, CD8+ T cell, CD19+ B cell, CD11b+ myeloid cell lineages were analyzed separately. Downstream analysis was performed by Cytofast [14] and Cytofworkflow [22].
Diffusion map
Diffusion map was generated with R using the cytofkit package [23] by displaying only CD3+ metaclusters identified by PhenoGraph [24] as a confirmation method of the HSNE clustering.
Reference standard comparison
Reference standard samples were compared with each other by calculating the similarity between their respective t-SNE maps. We used the Jensen-Shannon (JS) divergence to quantify the similarity between t-SNE maps. After converting t-SNE maps into two-dimensional probability density functions, the similarity between two maps is quantified as the JS divergence between their corresponding probability density functions. We used the base 2 logarithm in the JS divergence computation, which results in a continuous range of JS divergence values between 0 (for identical distributions) and 1 (for fully disjoint distributions), the algorithm being provided by E.D. Amir [25]. The average overlap frequency (AOF) is determined as described by E.D. Amir [26]
Flow cytometry
Mouse
Single-cell suspensions were prepared from TME [27] obtained from untreated or PD-L1 treated mice by an incubation of 15 min with collagenase and DNase IV (Roche) and by mincing the tumor tissue through a 70-μm cell strainer (BD Bioscience). Live cells were washed with RPMI-1640 supplemented with 8% FBS and P/S and once with FACS buffer. Subsequently, samples were incubated with Fc block mouse (2%) and mouse serum (5%) for 10 min, then stained with antibodies (Additional file 1: Table S1A) for 30 min at 4 °C in the dark and finally rinsed two times with PBS containing 0.5% BSA solution. Samples were acquired using the LSR Fortessa (BD Biosciences) and results analyzed with FlowJo and Cytosplore software.
Granzyme B staining of tumor-infiltrated T cells
MC-38 tumors were injected subcutaneously in C57BL/6 J mice, consecutively treated with 200 μg PD-L1 at three different timepoints (10, 13 and 16 days after tumor inoculation). At day 8 post treatment, tumors were excised and single-cell suspensions were generated as described above. Cells were next stimulated overnight in vitro with MC-38 tumor cells with a concentration of Brefeldine A of 4 μg/mL. Cells were then cell surface stained with antibodies to CD45, CD3, CD8, CD4, PD-1 and CD39 followed by intracellular Granzyme B staining after fixation. The phenotype was assessed by flow cytometry using the LSR Fortessa and results were analyzed with FlowJo.
Human studies
Cryopreserved colorectal tumor digests (excision and single cell suspension preparation by mechanical dissociation followed by slow freezing in 10% DMSO) were thawed and mashed through 70 μm filters into RPMI-1640 supplemented with 8% FBS and P/S. Live cells were washed once with RPMI-1640 with 8% FBS and P/S and once with FACS buffer. Two staining reactions of 1 × 106 cells per tumor sample were analyzed. All samples were then incubated with 2% of each bovine, murine, rat, hamster, and rabbit serum PBS with human TruStain FcX (Biolegend, 422,302) at 4 °C for 10 min. Samples were processed for surface staining (Additional file 1: Table S1B) and analyzed using a similar protocol as described for processing, staining and analyzing murine tumor samples. All specimens were anonymized and handled according to the ethical guidelines described in the Code for Proper Secondary Use of Human Tissue in the Netherlands of the Dutch Federation of Medical Scientific Societies.
In vivo murine tumor experiments
MC-38 colon adenocarcinoma cells were injected at a dose of 0.3 × 106 cells subcutaneously (s.c.) in the right flank. Antibodies blocking LAG-3 and PD-L1 were injected intraperitoneally and agonistic anti-ICOS antibodies were given subcutaneously, next to the tumor. Tumor diameter was measured every 2 to 3 days with a calliper and reported as volume using the formula (w × h × l) x (π/6).
Statistical analyses
Statistical analyses were performed using R software or Prism (GraphPad). Unpaired two-tailed t-tests were used for subset abundance comparisons.
Results
Efficacy of PD-L1 blockade parallels with an increase in tumor infiltrating CD8+ T cells over time
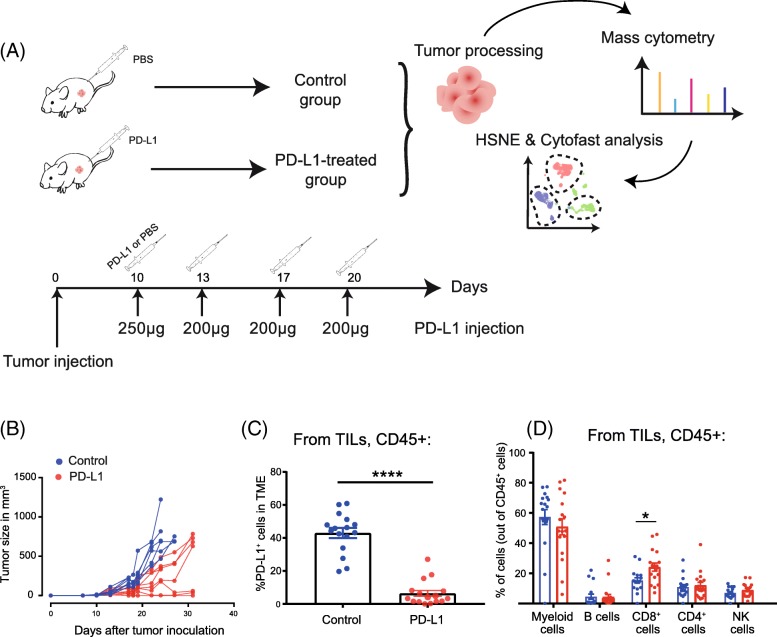
To examine the effect of PD-L1 blocking therapy, we used the colorectal adenocarcinoma mouse model MC-38. Mice were inoculated with MC-38 tumor cells, and when tumors were established after 10 days (tumor volume of 30–40 mm3), mice were treated with PD-L1 blockade therapy or left untreated (control group) (Fig. 1A). To identify biomarkers that respond to immunotherapy with PD-L1 blockade, we set up a CyTOF mass cytometry panel for in-depth phenotypic characterization of tumor-infiltrated lymphocytes (TILs) in preclinical tumor models, which allows kinetic dissection of anti-tumor immune responses. The panel consisted of 38 cell surface markers and was designed to identify the major lymphoid and myeloid subsets and to ascertain the differentiation and activation status of these subsets (Additional file 1: Figure S1). We isolated immune cells from the tumor 8 days after start of immunotherapy and stained the single cell suspensions followed by mass cytometry acquisition of 3.5 million cells in total. In parallel, tumor growth was measured to assess the therapeutic benefit of the PD-L1 blockade treatment. Treated animals displayed a significant delay in tumor progression or even had complete tumor eradication (Fig. 1B). To determine the effectiveness of the provided therapeutic antibodies to block PD-L1 binding, the cell surface expression of PD-L1 in the TME was assessed by staining with the same antibody clone (i.e. MIH5). Indeed, the PD-L1 expression on CD45+ tumor-infiltrated immune cells from the treated group was significantly decreased compared to control animals (Fig. 1C).
Fig. 1.
PD-L1-blocking treatment induces delay of MC-38 tumor growth. (a) Schematic of CyTOF mass cytometry experiment investigating the effect of PD-L1 antibody treatment on the TME. Mice were challenged with MC-38 tumor cells and subsequently tumor-bearing mice were treated either with PBS (n = 16 mice) or PD-L1 blocking antibodies (n = 16 mice). Tumors were isolated and analyzed by mass cytometry (CyTOF). Cluster identification was performed with HSNE and downstream analysis was done with Cytofast. (b) Tumor growth curves of individual mice in the control group (mock injected with PBS, blue lines) and PD-L1-treated group (red lines). (c) Frequency of CD45+ PD-L1+ cells in the TME 8 days after therapy starts displayed on a per-mouse basis with mean ± SEM. (d) Overview of the immune cell composition in the TME shown in percentage of cells on a per-mouse basis with mean ± SEM (n = 16 mice per group)
To monitor the robustness of the measurement, we included reference standard acquisitions and used the Jensen-Shannon (JS) divergence calculation to determine similarity between samples. The results yielded consistency between the measurements with low JS distance, meaning high similarities between samples (Additional file 1: Figure S2A). We also tested the quality of our staining by using the Average Overlap Frequency (AOF), a metric to evaluate and quantify the robustness of staining and clustering quality in high-dimensional data [26]. Importantly, all the markers involved in the cluster identification of CD3+ cells (e.g. CD4, CD8, PD-1, ICOS, etc.) showed an AOF < 0.3, which indicates a valid staining of the samples and a clear separation between negative and positive signals (Additional file 1: Figure S2B). Together, these data showed a stable and reliable sample acquisition with limited inter-sample variation.
An overview of the main tumor-infiltrating immune cells identified by mass cytometry showed a higher proportion of CD8+ T cells in the PD-L1 treated group (24.1%) compared to the control group (16.1%) 8 days after first injection (Fig. 1D). Simultaneously, the frequency of the CD11b+ myeloid compartment decreased after PD-L1 blockade. Thus, PD-L1 blockade empowers the increase of CD8+ T cells and limits the infiltration of myeloid cells in the TME.
PD-L1 treatment increases selectively CD8+ T-cell subsets expressing both activating and inhibitory receptors
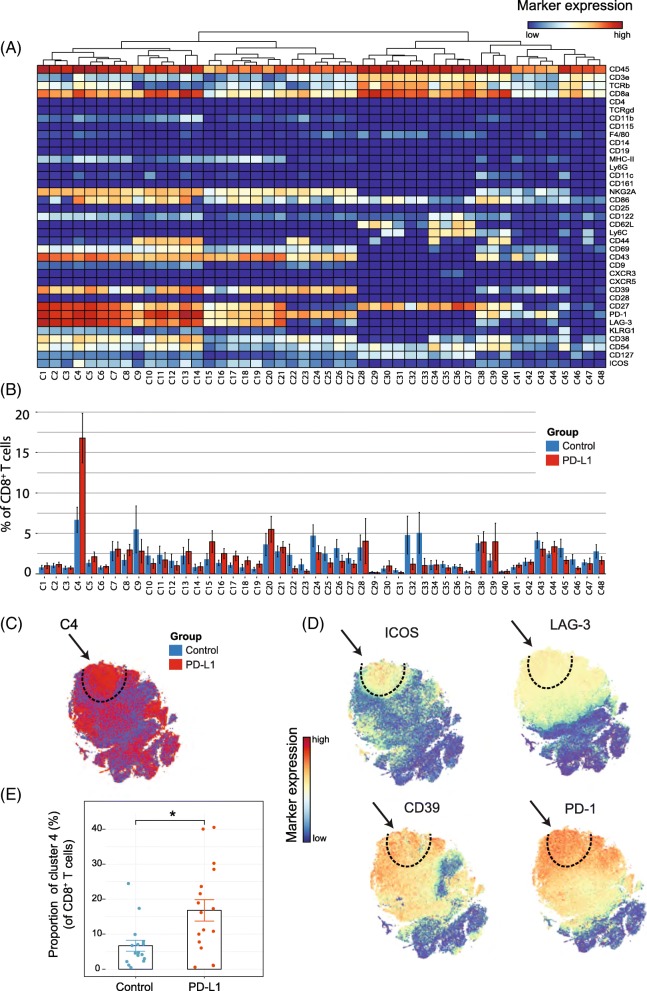
Because treatment with anti-PD-L1 has major effects on the expansion of the CD8+ T-cell compartment, we analyzed in detail the CD8+ TIL subset at this timepoint and identified 48 different CD8+ T-cell subsets (Fig. 2A). t-SNE clustering allowed distinction between naïve (e.g. cluster C28 expressing CD62L, CD27), effector (e.g. cluster C13 and C14 expressing CD54, CD38, CD27, CD44) and central-memory subsets (e.g. cluster C34 expressing CD54, CD62L, CD44, CD27). Remarkably, one cluster (cluster C4) displayed both activating (ICOS, CD69, CD43) and inhibitory receptors (PD-1, LAG-3, NKG2A). To visualize the distribution of each identified cluster, we displayed the abundance of each subset per treatment group (Fig. 2B). The t-SNE map overlaid with the expression of specific markers showed that the cluster C4 subset could be defined by the inhibitory molecule LAG-3 and the costimulatory receptor ICOS. Essentially, co-expression of ICOS and LAG-3 was highly specific to the PD-L1 blockade treated group (Fig. 2C, D). Further characterization of this subset also demonstrated up-regulation of the ectonucleotidase CD39, the early activation marker CD69, the inhibitory NKG2A receptor, and the activation/exhaustion cell surface marker PD-1. The CD8+ T-cell subset expressing both the activating and inhibitory molecules, referred hereafter as TAI cells, represented approximately 17% of all the CD8+ T cells across individual mice in the PD-L1 blockade group compared to 7% in the control group (Fig. 2E). Next, we validated the presence of CD8+ TAI cells by flow cytometry. We isolated TILs from the TME and stained for the markers ICOS, LAG-3, CD69, CD39 and PD-1. The CD8+ TAI subset (CD8+, LAG-3+, CD39+, PD-1+, ICOS+) population could indeed be identified, and was more abundant following PD-L1 blockade therapy (mean = 22%, sd = 16%, n = 6) than in the untreated group (mean = 9%, sd = 8%, n = 6; p-value = 0.03 by Student’s t-test). In addition, we confirmed our findings in the MCA205 sarcoma model. We identified the CD8+ TAI cells by flow cytometry and observed that PD-L1 treatment increased this subset as compared to the control untreated group (Additional file 1: Figure S3A).
Fig. 2.
Identification of CD8+ T-cell clusters in Tumor Infiltrating T-cell populations (a) Heatmap of all CD8+ T-cell clusters identified at day 8 after the start of the PD-L1 treatment. Data shown is based on t-SNE plots, and is pooled from the control and PD-L1 treated group. Level of ArcSinh5-transformed expression marker is displayed by a rainbow scale. Dendrogram on the top represents the hierarchical similarity between the identified clusters. (b) Average and SEM in percentage of each CD8+ T-cell cluster among the CD8+ T-cell population of control (blue bars) and PD-L1 group (red bars). (c) t-SNE plot of respectively 0.32 × 106 and 0.35 × 106 CD8+ T cells from control (blue) and PD-L1 treated (red) group. (d) Same t-SNE plots as above now showing the level of expression marker by a rainbow scale. The arrow identifies the cluster of interest C4 (having a shared CD8+ LAG3+ ICOS+ phenotype). (e) Bar graph showing the mean frequency of cluster 4 (± SEM, unpaired t-test). Individual mice belonging to the control (blue) and PD-L1 treated (red) group are indicated
Identification of TAI cell subsets in the tumor-infiltrated CD4+ T-cell compartment
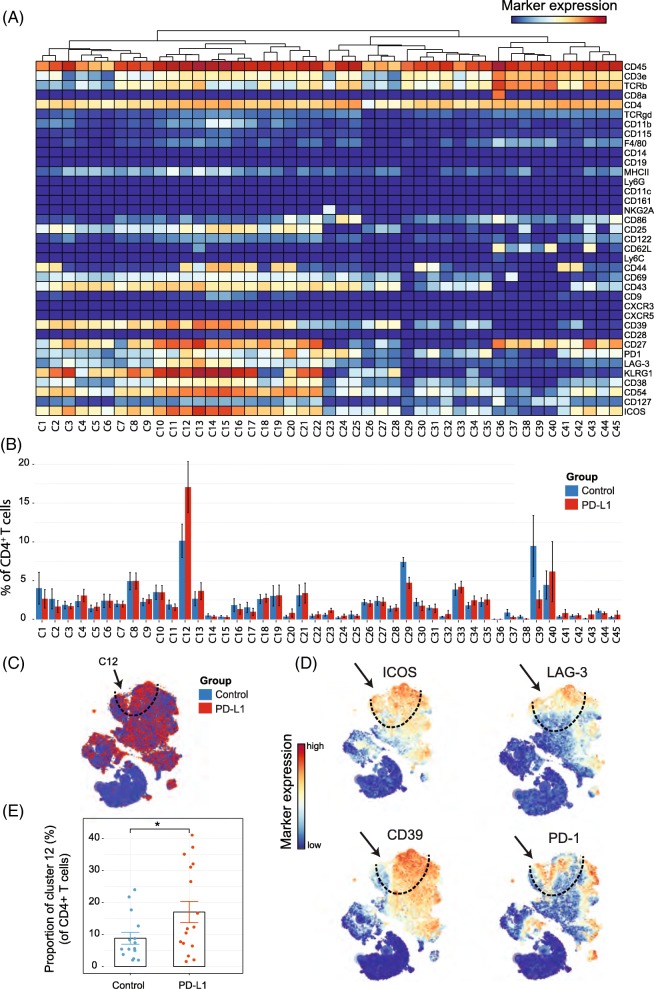
We next analyzed whether PD-L1 blockade therapy-specific subsets were also apparent in the CD4+ T-cell compartment. The t-SNE algorithm identified 45 CD4+ T-cell subsets revealing the heterogeneous profile of the CD4+ T cells (Fig. 3A, B). Notably, as for the CD8+ T cells, one subset was identified that correlated with PD-L1 treatment (cluster C12) and displayed the activating molecule ICOS and the inhibitory molecule LAG-3. In addition, these CD4+ TAI cells expressed CD27, CD39, CD43, CD44, CD54, KLRG1 and PD-1. The t-SNE map overlaid with the expression of specific markers showed that these subsets could also be defined by LAG-3, ICOS and CD39 and the co-expression of those markers was highly specific to the PD-L1 treated group (Fig. 3C, D). The TAI subset of CD4+ T cells was also significantly more abundant, representing about 17% of the total CD4+ T-cell population within the tumor infiltrated immune cells of the treated group compared to 8% in the control group (Fig. 3E). Also, in the MCA205 tumor model, the CD4+ TAI cells were identified and were increased by PD-L1 treatment (Additional file 1: Figure S3B).
Fig. 3.
Identification of CD4+ T-cell clusters in Tumor Infiltrating T-cell populations (a) Heatmap of all CD4+ T-cell clusters identified at day 8 after the start of the PD-L1 treatment. Data shown is based on t-SNE plots, and is pooled from the control and PD-L1 treated group. Level of ArcSinh5-transformed expression marker is displayed by a rainbow scale. Dendrogram on the top represents the hierarchical similarity between the identified clusters. (b) Average and SEM in percentage of each CD4+ T-cell cluster among the CD4+ T-cell population of control (blue bars) and PD-L1 group (red bars). (c) t-SNE plot of respectively 0.23 × 106 and 0.25 × 106 CD4+ T cells from control (blue) and PD-L1 treated (red) group. (d) Same t-SNE plots as above now showing the level of expression marker by a rainbow scale. The arrow identifies the cluster of interest 12 (having a shared CD4+ LAG3+ ICOS+ phenotype)
(e) Bar graph showing the mean frequency of cluster 12 (± SEM, unpaired t-test). Individual mice belonging to the control (blue) and PD-L1 treated (red) group are indicated.
Differentiation relationships of the identified PD-L1 treatment-associated T-cell subsets
To corroborate the results obtained from the previous t-SNE analysis regarding the PD-L1 treatment-associated T-cell subsets, we used the PhenoGraph algorithm to identify cell clusters and their differentiation status [24]. Similar T-cell metaclusters as those depicted by t-SNE earlier were indeed identified (Fig. 4A). The CD4 and CD8 T-cell lineages could be distinguished into a resting phenotype (called CD44low), an activated intermediate phenotype without inhibitory marker expression (called CD44int), and the TAI cells expressing both inhibitory and activation molecules (called TAI). To investigate the relationship between those metaclusters identified by PhenoGraph, we used the diffusion map algorithm [28].
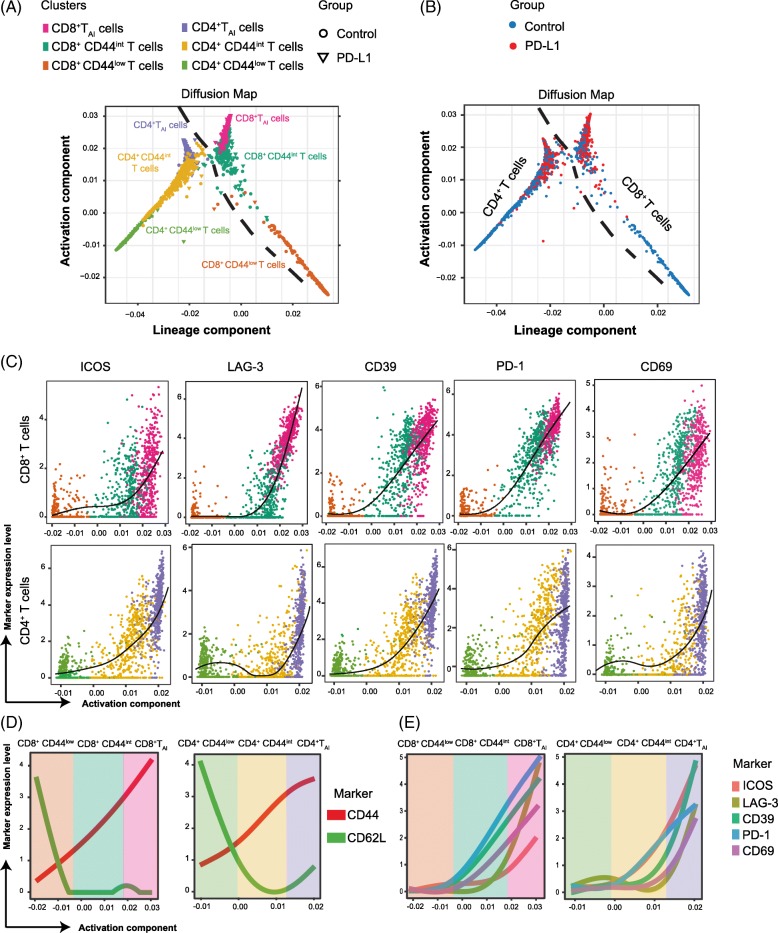
Fig. 4.
Diffusion maps of the identified CD4+ and CD8+ subsets in the control and treated group. (a) Two-dimensional diffusion map of the CD4+ and CD8+ T cells present in the tumor at day 8 after the first PD-L1 treatment. Three different CD4+ and CD8+ T cell metaclusters have been identified by PhenoGraph. Continuity of the pattern reveals relationship between the different represented metaclusters (n = 5 mice per group). (b) Diffusion map of the CD4+ and CD8+ TAI cell displayed by group origin (PBS in blue and PD-L1 in red). (c) Diffusion map of the CD4+ and CD8+ TAI cell displayed by marker expression ICOS, LAG-3, CD39, PD-1 and CD69. (d) Expression levels of CD44 and CD62L on the metaclustered CD4+ and CD8+T cell populations. (e) Expression levels of ICOS, LAG-3, CD39, PD-1 and CD69 on the metaclustered CD4+ and CD8+T cell populations
The two represented components defined gradual trends of variation (Fig. 4A) correlated with signatures for lineage and activation. Both CD4+ and CD8+ T cells could be distinguished on the diffusion map, showing the independent differentiation lineages of CD4+ and CD8+ T cells. The TAI cells (CD39+, PD-1+, LAG-3+, ICOS+), more frequent in the PD-L1 treated group (Fig. 4B), could be derived from an intermediate phenotype, which was CD44int. Thus, due to PD-L1 blockade treatment, T cells further differentiate into the more activated TAI phenotype.
We next analyzed the level of expression of the individual activating and inhibitory molecules that were modulated upon anti-PD-L1 therapy. By displaying the diffusion map with the expression level (Fig. 4C), we observed that the expression of ICOS, LAG-3 and CD39 started to be upregulated on intermediate phenotypes but maximum expression of these molecules was reached on both CD4+ and CD8+ TAI cells.
A summary of the phenotype of the three different clusters studied is represented by the evolution of the markers CD62L and CD44 (Fig. 4D). While PD-1 expression was more prominent on CD8+ TAI cells, ICOS was more abundantly expressed on CD4+ TAI cells (Fig. 4E). The inhibitory and activating markers NKG2A, CD38 and CD43 were also found to be upregulated on the CD8+ TAI cell subset (data not shown).
Early induction of CD4+ TAI and CD8+ TAI cells upon PD-L1 blocking
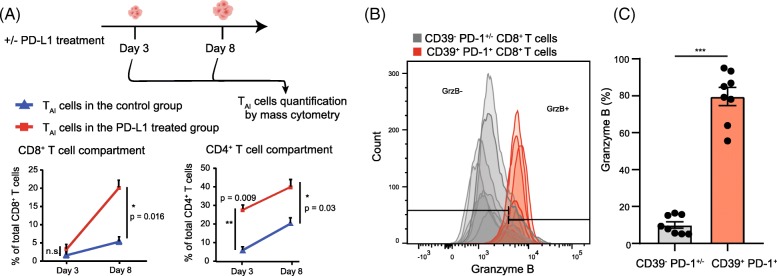
PD-L1 blocking treatment enhanced CD4+ and CD8+ TAI cell subsets in the TME 8 days post therapy. To determine if the expansion of these compartments occurred already early after treatment, we analyzed the TME at day 3 post-treatment (i.e. 13 days after tumor inoculation). The expansion of the CD4+ TAI cells started at an earlier stage, 3 days post-therapy, and continued over time. The presence of the CD8+ TAI cells could also be observed 3 days after the start of the treatment, but these cells significantly increased over time (Fig. 5A). Essentially, the vast majority of the CD39+ PD1+ CD8+ T cells that are present in the TME produce copious amounts of granzyme B, revealing their cytotoxic potential (Fig. 5B).
Fig. 5.
Quantification and cytotoxic capacity of the TAI cells in the TME (a) Average percentage (and SEM) of TAI cells within the CD8+ (left panel) and CD4+ (right panel) T-cell compartment at day 3 and 8 post PD-L1 blockade therapy in MC-38 tumor challenged mice. (b) Granzyme B expression of CD8+ T cell subsets at day 8 post PD-L1 treatment in MC-38 tumor bearing mice. The grey shaded histograms represent the CD39− PD-1+/− CD8+ T cells and the red shaded histograms depict the CD39+ PD-1+ CD8+ TAI cells of individual mice. (c) The percentage of granzyme B+ cells among the CD39+ PD-1+ CD8+ TAI cells after 8 days of PD-L1 treatment in the MC-38 tumor model compared to the CD39− PD-1+/− CD8 T cells
Rational design of combinatorial immunotherapy targeting activating and inhibitory receptors
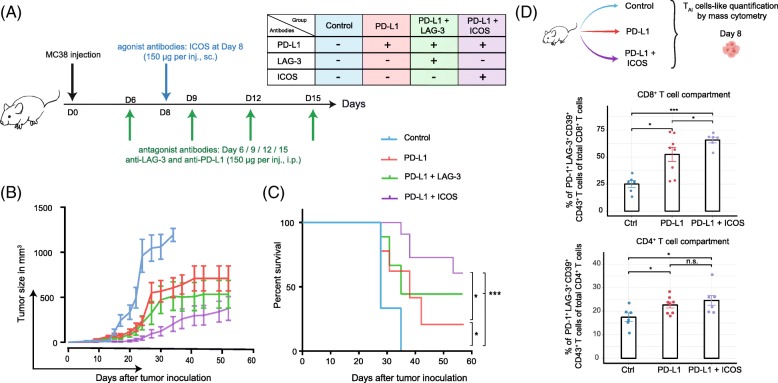
The data above indicate that the activity of anti-PD-L1 treatment could be mediated via the expansion of CD4+ and CD8+ TAI cells that express activating receptors and inhibitory receptors. We assessed if we could further enhance the functionality of the TAI cells by combining the PD-L1 blockade treatment with antibodies targeting inhibitory and stimulatory molecules. For the proof-of-principle, we performed co-treatment studies with blocking antibodies to the inhibitory receptor LAG-3 and with agonistic antibodies to ICOS during PD-L1 blockade (Fig. 6A).
Fig. 6.
Correlation between the presence of TAI cells in the TME and tumor growth. (a) Regimen scheme of (combinatorial) antibody treatment after tumor injection. (b) Comparison of tumor growth between control group (n = 9), PD-L1 antibody treated group (n = 9), PD-L1 and ICOS antibody treated group (n = 11), PD-L1 and LAG-3 antibody treated group (n = 10). (c) Survival curves for each treatment mentioned above. (d) Study of the tumor-microenvironment after control (n = 6), single therapy (PD-L1, n = 8) or combinatorial therapy (PD-L1 and ICOS, n = 6) of the CD8+ TAI-like cells (left panel) and CD4+ TAI-like cells (right panel) at day 8 (unpaired t-test) displayed on a per-mouse basis with mean ± SEM
PD-L1 blockade therapy in combination with LAG-3 blockade resulted in enhanced survival and tumor growth delay. Co-treatment with agonistic ICOS antibody improved PD-L1 blockade therapy even further (Fig. 6B-C, Additional file 1:Figure S4).
Next, we aimed to examine whether induction of TAI cells is linked to the improved survival rate observed in the PD-L1 plus ICOS targeting combination therapy. At day 8 after single and combined therapy, we analyzed the TME, and specifically analyzed the TAI cell abundance in each tumor. Because, in vivo treatment with ICOS antibodies prevents ex vivo staining for ICOS, we defined the TAI cells with the markers PD-1, CD39 and CD43. The percentage of CD8+ TAI cells was significantly higher in the PD-L1 blockade treated group compared to the control group. Importantly, significantly higher percentages of CD8+ TAI cells were observed in mice treated by the combined ICOS and PD-L1 targeted therapy compared to control or PD-L1 blockade treated mice. Expansion of CD4+ TAI cells upon single and combinatorial therapy was equivalent (Fig. 6D). Thus, combinatorial therapy targeting ICOS and PD-L1 expands CD8+ TAI cells and relates to improved survival of the treated mice.
Identification of TAI cells in human colorectal cancer
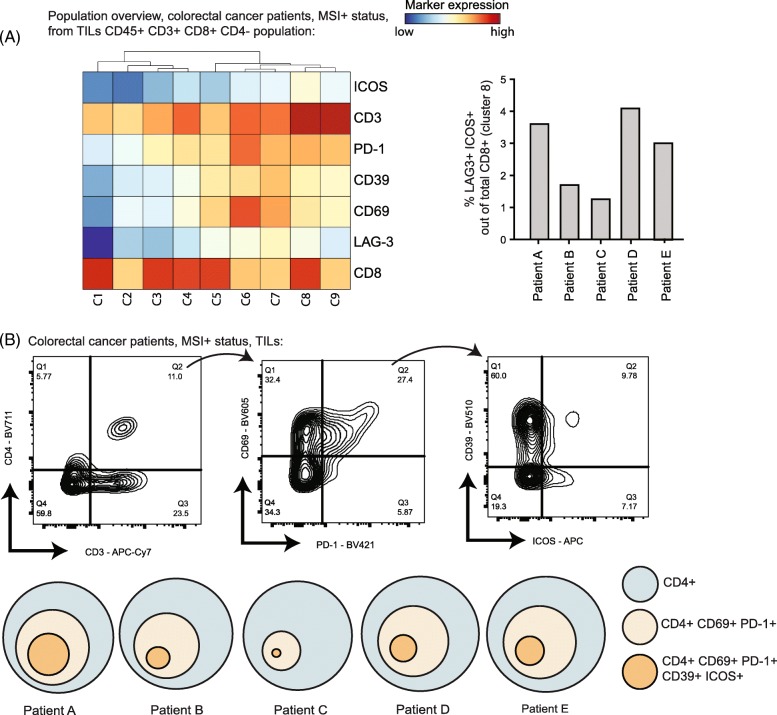
To extrapolate our findings in preclinical models to clinical settings, we questioned whether the TAI cells were present within tumor-infiltrated immune cell populations in human tumors. We investigated the phenotype of the TILs in colorectal tumors of five patients, who have not undergone any immunotherapy. To reflect the immunogenicity of the MC-38 model, we selected MMRd colorectal cancer patients [29]. We designed our flow cytometry panel to characterize putative TAI subsets within the tumor infiltrated CD8+ and CD4+ T cells. Hence, we included the activating receptors ICOS and CD69, also the inhibitory receptors like LAG-3 and CD39. We depicted the CD8+ T cells phenotypic diversity by gating on CD45+ CD8+ CD4− cells and showed that a subset (cluster 8) with a similar phenotype (CD69+ ICOS+ and LAG-3+) as identified in mice tumors could be found in human tumors (Fig. 7A). The CD4+ T cell pool in human tumors contained a substantial fraction of cells with a CD69+ PD1+ phenotype, and within this population a CD39+ ICOS+ subset could be identified (Fig. 7B). Together, these results established that in tumors of mice and humans, CD4+ and CD8+ TAI cell subsets are present.
Fig. 7.
Identification of the TAI cell subset in humans. (a) Heatmap of CD8+ T-cell phenotypes (pre-gated on CD45+ CD3+ CD4− cells) in tumors of 5 human colorectal cancer (MMRd) patients. Dendrogram above shows the hierarchical similarity between the identified clusters. Right panel shows the frequency of CD8+ LAG3+ ICOS+ cells (cluster 8) among total CD8+ T cells across the 5 patients. (b) Gating strategy to identify the CD4+ CD69+ PD1+ CD39+ ICOS+ population in human colorectal cancer. The abundance proportional to circle area is shown
Discussion
Variance of clinical outcomes upon checkpoint blocking immunotherapy like PD-L1 antibody treatment reflects the diversity of the anti-tumor immune response. In the current work, we identified the expansion of CD4+ and CD8+ T-cell subsets that strikingly co-expressed both inhibitory markers, like PD-1 and LAG-3, and activating markers like ICOS. These subsets, named TAI cells, expanded over time, starting 3 days after therapy and were still visible 8 days after the start of the therapy. Since the PD-L1 blocking antibody we used does not induce antibody-dependent cell-mediated cytoxicity [19], the expansion of the TAI cells is most likely caused by blocking the PD-1 signaling pathways rather than e.g. depleting PD-L1+ cells or reactions to the antibody itself.
The TAI cells appear to play a central role in mediating tumor rejection, despite the expression of inhibitory receptors. The variance seen in response to PD-L1 therapy could be explained by a variable expansion of TAI cells in the TME and needs to be further explored. Our unbiased high dimensional immunophenotyping of the TME provides a deeper insight on the immune changes triggered by immune checkpoint blockade. By identifying a precise expansion of specific subsets in the TME, this strategy enabled us to rationally design immunotherapeutic combination treatments. We were able to enhance the anti-tumor efficacy of PD-L1 blocking therapy by combining it with an agonist ICOS therapy or an antagonist LAG-3 therapy. The TAI cells identified in our murine models shared a similar phenotype with colorectal cancer patients and therefore a similar effect of the combination therapy could be expected. Hence, this detection of the TAI cells in human tumors could thus pave the path to clinically target these cells in colorectal cancer by e.g. combined PD-1/PD-L1 and ICOS targeted immunotherapy. We surmise that TIL analysis by mass cytometry might be a powerful tool for personal-guided combinatorial therapy for each individual patient.
Our mass cytometry panel only screened for certain immunomodulatory molecules of the CD28 superfamily. Upregulation of other molecules, as has been reported for CTLA-4 [30] or BTLA, might have occurred but were not analyzed due to the limitation of the number of markers in our designed mass cytometry panel. On the other hand, we have included other markers like LAG-3, CD39, CD38, NKG2A, CD43, CD54, ICOS, KLRG1, which have never been analyzed at once in mass cytometry on ex vivo TILs. A large percentage of the TAI cells may be tumor-reactive and have encountered tumor-specific antigenic peptides (e.g. neo-antigens). The granzyme B expression within the TAI cells underlines this and is consistent with previous work showing that CD39 expression is a marker for cancer-related CD8+ T cells in the TME [31]. Consistently, CD8+ T cells expressing PD-1 have also been shown to be more reactive against tumors [32].
Our study is in line with previous studies on other tumor models like the T3 methylcholanthrene-induced sarcomas showing that inhibitory markers like PD-1 and TIM-3 and activating receptors like ICOS are co-expressed on tumor-specific T cells [33]. In addition, it was found that the expansion of CD8+ T cells expressing PD-1 improves the efficacy of adoptive T-cell therapy [34] and T cells co-expressing CD39 and PD-1 or LAG-3 and PD-1 were found to expand after anti-PD-1 therapy [7, 35].
Remarkably, in a viral setting, CD8+ T cells that provide the proliferative burst after PD-1 therapy are expressing ICOS [36], suggesting that the TAI cell expansion in the TME relies on the co-expression of ICOS and PD-1 markers. PD-1 and ICOS are also co-expressed on T cells in human bladder tumors [37]. Our results can also explain the positive correlation between higher ICOS expression and a better overall survival in colorectal cancer patients [38]. Together, this is strengthening the relevance of targeting the PD-1+ ICOS+ TAI cells by the above-mentioned dual therapy targeting PD-L1 and ICOS. Interestingly, ICOS appears to be relatively higher expressed on CD4+ TAI cells than on CD8+ TAI cells, which we aim to further explore. The TAI cells expanding after PD-L1 blocking therapy also co-expressed LAG-3, which might explain the better efficiency of the combination of targeting PD-L1 and LAG-3. These findings are coherent with what has been previously reported in other studies [39, 40].
The TAI cells are intratumorally present at an early stage, irrespective of the treatment and respond to immunotherapy as shown by an increase in the TME across time. This suggests that the TAI cells are an identifiable unique subset among T cells, existing before immunotherapy, which can be further expanded by treatment. Tracking these cells in the TME warrants further investigation and would inform about their origin and the plasticity of their phenotype.
The expansion kinetics of the CD4+ TAI cells compared to the CD8+ TAI cells after PD-L1 treatment are dissimilar. In both relative abundance and absolute numbers CD4+ TAI cells are already strongly expanded at day 3 after treatment in contrast to CD8+ TAI cells, while at day 8 the CD8+ TAI cells are more expanded. This is in line with a restored early helper function of the CD4 compartment to stimulate expansion of effector CD8+ T cells. Immunotherapy in the MC-38 model is fully dependent on CD8+ T cells [41]. Indeed, after 8 days of PD-L1 treatment, regression of tumor size becomes apparent. We could confirm that similar tumor infiltrating T-cell subsets exist in colorectal cancer patients. CD4+ TAI subsets co-expressing inhibitory PD-1 and activating ICOS as well as CD39 and CD69 were detectable in freshly resected colon tumor from MMRd colorectal cancer patients known to express neo-epitopes due to accumulated point-mutations. It would be interesting to study these TAI subpopulations in patients upon treatment with checkpoint therapy or other immunotherapies.
The relevance of targeting simultaneously inhibitory and activating molecules is already transposed in humans. For example, three clinical trials ongoing (NCT02904226, NCT02723955 and NCT02520791) are meant to study the effect of anti-ICOS as monotherapy or in combination with anti-PD-1. Our preclinical study suggests the synergistic effect of ICOS together with a blocking PD-L1 therapy. A systematic immunophenotyping of the TME should enable a better prediction of response to immunotherapy and a progress in development of rational immunotherapeutic strategies.
Conclusion
This study described the expansion of a treatment-related cell subset, named TAI cells, which co-express activating and inhibitory molecules. In preclinical mouse models, both CD4+ and CD8+ TAI cells were higher in abundance in the TME upon PD-L1 therapy. Co-targeting the inhibitory receptor LAG-3 or the activating receptor ICOS on the TAI cells further enhanced this subset and resulted in improved tumor immunity. TAI cells were also present in human colorectal tumors. We surmise that targeting the inhibitory and activating receptors on these TAI cells could lead to enhanced tumor immunity.
Additional file
: Figure S1. Mass cytometry panel and marker expression. Figure S2. Quality control assessment of the data generated by mass cytometry. Figure S3. Identification of CD4+ and CD8+ TAI cells in the MCA205 sarcoma model. Figure S4. Synergy of combination immunotherapy. Table S1. FACS panels used in the study. (DOCX 2099 kb)
Acknowledgements
We would like to thank Sandra Laban for providing technical support, Tamim Abdelaal for his assistance in computational analysis, Camilla Labrie, Elham Beyranvand Nejad and Anke Redeker for their technical support in performing animal experiments and CyTOF processing, Sjoerd van der Burg for his critical review of the manuscript, Ferenc Scheeren for the characterisation of the PD-L1 antibodies (clone MIH5), and the LUMC flow cytometry facility for their support during CyTOF acquisition.
Abbreviations
- AOF
Average Overlap Frequency
- A-tSNE
Approximated t-distributed Stochastic Neighbor Embedding
- HSNE
Hierarchical Stochastic Neighbor Embedding
- MMRd
MisMatch Repair-deficient
- TILs
Tumor Infiltrated Lymphocytes
- TME
Tumor Micro-Environment
- t-SNE
t-distributed Stochastic Neighbor Embedding
Authors’ contributions
GB conceived the study, performed the experiments, analyzed the data and wrote the manuscript. AY, EvdG, SvD and MC helped with tumor material processing and animal experiments. TH, VvU, FK, and AV developed Cytosplore and HSNE applications. NFM provided human tumor material. RA and FO designed the experiments, initiated and supervised the project and wrote the manuscript. All authors discussed the results and commented the manuscript. All authors read and approved the final manuscript.
Funding
The authors acknowledge funding from the European Commission (Horizon 2020 MSCA grant under proposal number 675743; project acronym ISPIC) and from the Dutch Cancer Society (UL 2015–7817).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Ethics approval and consent to participate
The inclusion of patients has been conducted in accordance with the ethical principles set out in the Declaration of Helsinki. Patient inclusion was done with approval of the Leiden Medical Centre Medical Ethical Committee. Written informed consent was obtained prior to data collection.
Consent for publication
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
Competing interests
The authors declare no potential financial or commercial conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ramon Arens and Ferry Ossendorp contributed equally to this work.
Contributor Information
Ramon Arens, Phone: +31 715266704, Email: R.Arens@lumc.nl.
Ferry Ossendorp, Phone: +31 715263434, Email: F.A.Ossendorp@lumc.nl.
References
- 1.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 3.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545(7652):60–65. doi: 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canale FP, Ramello MC, Nunez N, Furlan CLA, Bossio SN, Serran MG, et al. CD39 expression defines cell exhaustion in tumor-infiltrating CD8(+) T cells. Cancer Res. 2018;78(1):115–128. doi: 10.1158/0008-5472.CAN-16-2684. [DOI] [PubMed] [Google Scholar]
- 9.Jiang Y, Li Y, Zhu B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015;6:e1792. doi: 10.1038/cddis.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchan Sarah L., Fallatah Mohannad, Thirdborough Stephen M., Taraban Vadim Y., Rogel Anne, Thomas Lawrence J., Penfold Christine A., He Li-Zhen, Curran Michael A., Keler Tibor, Al-Shamkhani Aymen. PD-1 Blockade and CD27 Stimulation Activate Distinct Transcriptional Programs That Synergize for CD8+T-Cell–Driven Antitumor Immunity. Clinical Cancer Research. 2018;24(10):2383–2394. doi: 10.1158/1078-0432.CCR-17-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lichtenegger FS, Rothe M, Schnorfeil FM, Deiser K, Krupka C, Augsberger C, et al. Targeting LAG-3 and PD-1 to enhance T cell activation by antigen-presenting cells. Front Immunol. 2018;9:385. doi: 10.3389/fimmu.2018.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207(10):2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messenheimer David J., Jensen Shawn M., Afentoulis Michael E., Wegmann Keith W., Feng Zipei, Friedman David J., Gough Michael J., Urba Walter J., Fox Bernard A. Timing of PD-1 Blockade Is Critical to Effective Combination Immunotherapy with Anti-OX40. Clinical Cancer Research. 2017;23(20):6165–6177. doi: 10.1158/1078-0432.CCR-16-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beyrend G, Stam K, Hollt T, Ossendorp F, Arens R. Cytofast: a workflow for visual and quantitative analysis of flow and mass cytometry data to discover immune signatures and correlations. Comput Struct Biotechnol J. 2018;16:435–442. doi: 10.1016/j.csbj.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spitzer MH, Carmi Y, Reticker-Flynn NE, Kwek SS, Madhireddy D, Martins MM, et al. Systemic immunity is required for effective Cancer immunotherapy. Cell. 2017;168(3):487–502. doi: 10.1016/j.cell.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finck R, Simonds EF, Jager A, Krishnaswamy S, Sachs K, Fantl W, et al. Normalization of mass cytometry data with bead standards. Cytometry A. 2013;83(5):483–494. doi: 10.1002/cyto.a.22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Unen V, Li N, Molendijk I, Temurhan M, Hollt T, van der Meulen-de Jong AE, et al. Mass cytometry of the human mucosal immune system identifies tissue- and disease-associated immune subsets. Immunity. 2016;44(5):1227–1239. doi: 10.1016/j.immuni.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Hollt T, Pezzotti N, van Unen V, Koning F, Eisemann E, Lelieveldt B, et al. Cytosplore: interactive immune cell phenotyping for large single-cell datasets. Comput Graph Forum. 2016;35(3):171–180. doi: 10.1111/cgf.12893. [DOI] [Google Scholar]
- 19.van der Schoot JMS, Fennemann FL, Valente M, Dolen Y, Hagemans IM, Becker AMD, Le Gall CM, van Dalen D, Cevirgel A, van Bruggen JAC, Engelfriet M, Caval T, Bentlage AEH, Fransen MF, Nederend M, Leusen JHW, Heck AJR, Vidarsson G, Figdor CG, Verdoes M, Scheeren FA, et al. Functional diversification of hybridoma-produced antibodies by CRISPR/HDR genomic engineering. Sci Adv. 2019;5:eaaw1822. [DOI] [PMC free article] [PubMed]
- 20.van Unen V, Hollt T, Pezzotti N, Li N, Reinders MJT, Eisemann E, et al. Visual analysis of mass cytometry data by hierarchical stochastic neighbour embedding reveals rare cell types. Nat Commun. 2017;8(1):1740. doi: 10.1038/s41467-017-01689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pezzotti N, Lelieveldt BPF, Van Der Maaten L, Hollt T, Eisemann E, Vilanova A. Approximated and user steerable tSNE for progressive visual analytics. IEEE Trans Vis Comput Graph. 2017;23(7):1739–1752. doi: 10.1109/TVCG.2016.2570755. [DOI] [PubMed] [Google Scholar]
- 22.Nowicka Malgorzata, Krieg Carsten, Weber Lukas M., Hartmann Felix J., Guglietta Silvia, Becher Burkhard, Levesque Mitchell P., Robinson Mark D. CyTOF workflow: Differential discovery in high-throughput high-dimensional cytometry datasets. F1000Research. 2017;6:748. doi: 10.12688/f1000research.11622.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen H, Lau MC, Wong MT, Newell EW, Poidinger M, Chen J. Cytofkit: a Bioconductor package for an integrated mass cytometry data analysis pipeline. PLoS Comput Biol. 2016;12(9):e1005112. doi: 10.1371/journal.pcbi.1005112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levine JH, Simonds EF, Bendall SC, Davis KL, el AD A, Tadmor MD, et al. Data-driven phenotypic dissection of AML reveals progenitor-like cells that correlate with prognosis. Cell. 2015;162(1):184–197. doi: 10.1016/j.cell.2015.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amir ED, Davis KL, Tadmor MD, Simonds EF, Levine JH, Bendall SC, et al. viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat Biotechnol. 2013;31(6):545–552. doi: 10.1038/nbt.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amir El-ad David, Guo Xinzheng V., Mayovska Oksana, Rahman Adeeb H. Average Overlap Frequency: A simple metric to evaluate staining quality and community identification in high dimensional mass cytometry experiments. Journal of Immunological Methods. 2018;453:20–29. doi: 10.1016/j.jim.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Beyranvand Nejad E, van der Sluis TC, van Duikeren S, Yagita H, Janssen GM, van Veelen PA, et al. Tumor eradication by cisplatin is sustained by CD80/86-mediated Costimulation of CD8+ T cells. Cancer Res. 2016;76(20):6017–6029. doi: 10.1158/0008-5472.CAN-16-0881. [DOI] [PubMed] [Google Scholar]
- 28.Angerer P, Haghverdi L, Buttner M, Theis FJ, Marr C, Buettner F. destiny: diffusion maps for large-scale single-cell data in R. Bioinformatics. 2016;32(8):1241–1243. doi: 10.1093/bioinformatics/btv715. [DOI] [PubMed] [Google Scholar]
- 29.Banerjea A, Hands RE, Powar MP, Bustin SA, Dorudi S. Microsatellite and chromosomal stable colorectal cancers demonstrate poor immunogenicity and early disease recurrence. Color Dis. 2009;11(6):601–608. doi: 10.1111/j.1463-1318.2008.01639.x. [DOI] [PubMed] [Google Scholar]
- 30.Wei Spencer C., Levine Jacob H., Cogdill Alexandria P., Zhao Yang, Anang Nana-Ama A.S., Andrews Miles C., Sharma Padmanee, Wang Jing, Wargo Jennifer A., Pe’er Dana, Allison James P. Distinct Cellular Mechanisms Underlie Anti-CTLA-4 and Anti-PD-1 Checkpoint Blockade. Cell. 2017;170(6):1120-1133.e17. doi: 10.1016/j.cell.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simoni Yannick, Becht Etienne, Fehlings Michael, Loh Chiew Yee, Koo Si-Lin, Teng Karen Wei Weng, Yeong Joe Poh Sheng, Nahar Rahul, Zhang Tong, Kared Hassen, Duan Kaibo, Ang Nicholas, Poidinger Michael, Lee Yin Yeng, Larbi Anis, Khng Alexis J., Tan Emile, Fu Cherylin, Mathew Ronnie, Teo Melissa, Lim Wan Teck, Toh Chee Keong, Ong Boon-Hean, Koh Tina, Hillmer Axel M., Takano Angela, Lim Tony Kiat Hon, Tan Eng Huat, Zhai Weiwei, Tan Daniel S. W., Tan Iain Beehuat, Newell Evan W. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018;557(7706):575–579. doi: 10.1038/s41586-018-0130-2. [DOI] [PubMed] [Google Scholar]
- 32.Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, et al. PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. J Clin Invest. 2014;124(5):2246–2259. doi: 10.1172/JCI73639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fehlings M, Simoni Y, Penny HL, Becht E, Loh CY, Gubin MM, et al. Checkpoint blockade immunotherapy reshapes the high-dimensional phenotypic heterogeneity of murine intratumoural neoantigen-specific CD8+ T cells. Nat Commun. 2017;8(1):562. doi: 10.1038/s41467-017-00627-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandez-Poma SM, Salas-Benito D, Lozano T, Casares N, Riezu-Boj JI, Mancheno U, et al. Expansion of tumor-infiltrating CD8+ T cells expressing PD-1 improves the efficacy of adoptive T-cell therapy. Cancer Res. 2017;77(13):3672–3684. doi: 10.1158/0008-5472.CAN-17-0236. [DOI] [PubMed] [Google Scholar]
- 35.Edwards Jarem, Wilmott James S., Madore Jason, Gide Tuba Nur, Quek Camelia, Tasker Annie, Ferguson Angela, Chen Jinbiao, Hewavisenti Rehana, Hersey Peter, Gebhardt Thomas, Weninger Wolfgang, Britton Warwick J., Saw Robyn P.M., Thompson John F., Menzies Alexander M., Long Georgina V., Scolyer Richard A., Palendira Umaimainthan. CD103+Tumor-Resident CD8+T Cells Are Associated with Improved Survival in Immunotherapy-Naïve Melanoma Patients and Expand Significantly During Anti–PD-1 Treatment. Clinical Cancer Research. 2018;24(13):3036–3045. doi: 10.1158/1078-0432.CCR-17-2257. [DOI] [PubMed] [Google Scholar]
- 36.Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537(7620):417–421. doi: 10.1038/nature19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong Yien Ning Sophia, Joshi Kroopa, Khetrapal Pramit, Ismail Mazlina, Reading James L., Sunderland Mariana Werner, Georgiou Andrew, Furness Andrew J.S., Ben Aissa Assma, Ghorani Ehsan, Oakes Theres, Uddin Imran, Tan Wei Shen, Feber Andrew, McGovern Ursula, Swanton Charles, Freeman Alex, Marafioti Teresa, Briggs Timothy P., Kelly John D., Powles Thomas, Peggs Karl S., Chain Benjamin M., Linch Mark D., Quezada Sergio A. Urine-derived lymphocytes as a non-invasive measure of the bladder tumor immune microenvironment. The Journal of Experimental Medicine. 2018;215(11):2748–2759. doi: 10.1084/jem.20181003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Luo Y, Qin SL, Mu YF, Qi Y, Yu MH, et al. The clinical impact of ICOS signal in colorectal cancer patients. Oncoimmunology. 2016;5(5):e1141857. doi: 10.1080/2162402X.2016.1141857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72(4):917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang R-Y, Eppolito C, Lele S, Shrikant P, Matsuzaki J, Odunsi K. LAG3 and PD1 co-inhibitory molecules collaborate to limit CD8+ T cell signaling and dampen antitumor immunity in a murine ovarian cancer model. Oncotarget. 2015;6(29):27359–27377. doi: 10.18632/oncotarget.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kleinovink JW, Marijt KA, Schoonderwoerd MJA, van Hall T, Ossendorp F, Fransen MF. PD-L1 expression on malignant cells is no prerequisite for checkpoint therapy. Oncoimmunology. 2017;6(4):e1294299. doi: 10.1080/2162402X.2017.1294299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
: Figure S1. Mass cytometry panel and marker expression. Figure S2. Quality control assessment of the data generated by mass cytometry. Figure S3. Identification of CD4+ and CD8+ TAI cells in the MCA205 sarcoma model. Figure S4. Synergy of combination immunotherapy. Table S1. FACS panels used in the study. (DOCX 2099 kb)
Data Availability Statement
All data generated or analyzed during this study are included in this published article.