Abstract
Background
The metabolic syndrome (MetS) is a clustering of abdominal obesity, diabetes and prediabetes, high cholesterol and high blood pressure, that confers an increased risk of cardiovascular disease. There is limited data on incidence of MetS from South Asia. This study investigated incidence and risk factors for new onset MetS in an urban adult Sri Lankan population.
Methods
Subjects (selected by age-stratified random sampling from the Ragama Medical Officer of Health area) were screened initially in 2007 (35–64 years) and re-evaluated in 2014 (42–71 years). On both occasions they were assessed by structured interview, anthropometric measurements, liver ultrasound, and biochemical/serological tests. MetS was diagnosed on International Diabetes Federation (IDF-2006) criteria. Total body fat (TBF) and visceral fat percentage (VFP) were measured in 2014, using body impedance method. Incidence and factors at baseline, associated with new onset MetS, were investigated among those who presented for re-evaluation.
Results
2985 (99.1%) [1636 (54.8%) women (54.8%); median age (IQR) 53 (47–59) years] from the initial cohort in 2007 had complete data. 2148 (71.9%) [1237 (57.6%) women; median age (IQR) 60 (54–66) years] attended follow-up. 949 of them [701 (73.9%) women; median age (IQR) 60 (54–65) years] had MetS (prevalence 47.2%, 95% CI 45.0–49.4%). Of 1246 who did not have MetS in 2007, 265 [178 (67.1%) women, median age (IQR) 57 (51–64) years] had developed MetS after 7 years (annual incidence 3.5% (95% CI 2.4–4.5%). Females (OR = 4.9, 95% CI 3.4–7.4), BMI > 23 kg/m2 in 2007 (OR = 1.6 per unit increase, 95% CI 1.5–1.7), weight gain (by 2–5% OR = 2.0, 95% CI 1.1–3.5; by > 5% OR = 2.2, 95% CI 1.4–3.4), and increase in waist circumference (by 2–5% OR = 7.0, 95% CI 4.0–12.2; by > 5% OR = 13.4, 95% CI 8.3–22.4) from baseline and presence of non-alcoholic fatty liver disease (NAFLD) in 2007 (OR = 1.70, 95% CI 1.04–2.76) were associated new onset MetS. Those with MetS had abnormal VFP and TBF in 2014 [P < 0.001].
Conclusion
In this study, annual incidence of MetS was 3.5%. Female gender, BMI > 23 kg/m2 and NAFLD in 2007 and increase in weight and waist circumference from baseline were significantly associated with new onset MetS. Obesity was the best predictor of future MetS.
Keywords: Incidence, Metabolic syndrome, Obesity, Diabetes, Hypertension, Dyslipidemia, Risk factors, Sri Lanka
Background
The metabolic syndrome (MetS) is a clustering of abdominal obesity, diabetes and prediabetes, high cholesterol and high blood pressure [1]. Since each component of the MetS is an independent risk factor for cardiovascular disease (CVD), when these components occur together the risk of CVD also increases exponentially. Individuals with MetS are twice as likely to die from cardiovascular or cerebrovascular disease, three times as likely to have a myocardial infarction or stroke, and five times more likely to develop type 2 diabetes, compared to those without MetS [2]. Therefore, there are medical and economic imperatives for early identification of individuals likely to develop MetS in later life, since lifestyle interventions and treatment may prevent the development of future diabetes or CVD.
According to estimates of the International Diabetes Federation (IDF) almost one quarter of the world’s adults have MetS [1]. The prevalence of MetS is increasing in most countries, in association with the twin global epidemics of obesity and diabetes. While worldwide prevalence is estimated at 20–25% [3], in the Asia–Pacific region it is 12–37% [4], and in South Asia it is approximately 26% [5]. Previous studies have estimated a MetS prevalence of 24.3% among adults in Sri Lanka [6].
While there are many reports on the prevalence of MetS, data on incidence are scarce. Only a few community based studies have reported on incident MetS in Asia; incidence in West Asia is estimated at 5.5% [7], and in South Korea it is 4.9% [8]. Despite high prevalence of the condition, there are no reports on the incidence or risk factors associated with new onset MetS in South Asian populations.
The Ragama Health Study (RHS) is a large community-based cohort study on non-communicable diseases [9]. It is a collaborative study between the National Centre for Global Health and Medicine, Tokyo, Japan and the Faculty of Medicine, University of Kelaniya, Ragama, Sri Lanka. It was initially conducted in the Ragama Medical Officer of Health area of the Gampaha district in 2007. The study population was adults aged 35–64 years, chosen by age-stratified random sampling from electoral lists. This cohort was re-evaluated in 2014 as part of a 7-year follow up study. We previously reported a prevalence of 38.9% for MetS from the 2007 RHS initiation cohort [10]. The aim of the present study was to assess the incidence and risk factors of new-onset MetS in the same cohort after 7 years of follow-up.
Methods
This study was part of an on-going, large, community-based cohort follow up study: the Ragama Health Study (RHS) [9]. Ethical approval for the study was obtained from the Ethical Review Committee of the Faculty of Medicine, University of Kelaniya, Ragama, Sri Lanka. (Approval No—P/38/09/2006 and P/169/08/2014). The study was conducted in the Ragama Medical Officer of Health (MOH) administrative area situated 18 km north of the commercial capital, Colombo. Ragama has urban characteristics and a multi-ethnic population. The study population consisted of resident adults, originally selected by age-stratified random sampling from electoral lists. The target population was screened initially in 2007 (aged 35–64 years) and subsequently invited back after 7 years (aged 42–71 years) for re-evaluation in 2014.
On both occasions all participants were assessed using a structured interview, clinical and anthropometric measurements, liver ultrasound, and biochemical and serological tests. Details of screening of the inception cohort are described elsewhere [9]. Trained medical personnel interviewed the participants at re-evaluation, obtaining information on socio-demographic variables and lifestyle habits, including diet, physical activity (PA) and alcohol consumption. Past medical records of the subjects were analysed and recorded. Height was measured using a wall-mounted stadiometer to the nearest 0.1 cm and weight was measured (in light indoor clothing) using a digital scale to the nearest 0.1 kg. Waist circumference (WC) was measured at the midpoint between the inferior border of the ribcage and the superior iliac crest, and hip circumference was measured at the level of the trochanters. Waist and hip circumferences were measured to the nearest 0.1 cm using an inelastic measuring tape. Blood pressure was measured from the right upper limb in the sitting position using an Omron 705CP automatic blood pressure monitor and mean value of two readings taken 5 min apart was recorded.
Change in WC was classified as reduction (> 5% of initial measurement), no change (reduction ≤ 5% to increase < 5%) and increase (≥ 5% of initial measurement). Change in weight was classified as loss (> 5%), no change (loss ≤ 5% to gain < 5%), gain ≥ 5% and gain ≥ 10%.
Total body fat (TBF) and visceral fat percentage (VFP) were measured, during re-evaluation in 2014, with a body composition monitor using a proven bioelectrical impedance method (Omron HBF-362 body composition monitor, Omron Healthcare, Lake Forrest, Illinois, United States). Abnormal TBF was defined for females as > 32% and for males as > 25%, while abnormal VFP for both females and males was defined as > 10%. MetS was diagnosed on International Diabetes Federation (IDF 2006) criteria [1].
A 10-mL sample of venous blood was obtained from each subject. This was used to determine glycosylated hemoglobinA1c (HbA1c), fasting serum triglycerides (TG) and high density lipo-proteins (HDL), serum alanine aminotransferase activity (ALT) and hepatitis B and C serology [hepatitis B surface antigen (HBsAg) and anti-hepatitis C virus antibodies (anti-HCV) using CTK Biotech ELISA kits]. All subjects underwent ultrasonography of the liver with a 5-MHz 50 mm convex probe (MindrayDP-10 Ultrasound Diagnostic Systems, Mindray Medical International Limited, Shenzhen, China). Five trained medical officers carried out liver ultrasonography. Non-alcoholic fatty liver disease (NAFLD) was diagnosed on established ultrasound criteria for fatty liver, in the absence of any secondary cause for fatty liver [9].
Data were entered in Epi Info 7 (Centres for Disease Control and Prevention, Atlanta, GA, USA) and logical and random checks were done. Statistical analysis was done using R programming language version 3.5.1. Continuous and categorical data were described using median and inter quantile range (IQR) and frequencies (percentages), respectively. Groups comparison were done using Wilcoxon Rank Sum test and Pearson’s Chi-square-test. Variables that are associated with new onset of MetS were identified in a two-step process; initially, individual variables were screened using Wilcoxon rank sum test and Pearson’s Chi-square-test and variables which showed association at P = 0.2 level were considered as the potential variables for model fitting. Subsequently, these variables were considered to develop a generalized linear model to identify the variables that were associated with new onset MetS. Model fit was assessed with residual plots and ratio between residual deviance and residual degrees of freedom. P < 0.05 was considered as significant.
Results
2985/3012 (99.1%) [1636-women (54.8%); median age (IQR) 53 (47–59) years] of the original study participants had complete data for analysis. 2148/2985 (71.9%) of the original cohort attended follow-up [1237 (57.5%) women; median age (IQR) 60 (54–66) years]. Initial and follow-up cohorts were similar, except for fewer males and more participants having central obesity in the follow-up cohort (Table 1).
Table 1.
Profile of the participants in 2007 who attended and did not attend follow up in 2014
| Attended follow-up in 2014 | Did not attend follow-up 2014 | P value | |
|---|---|---|---|
| n = 2148 | n = 837 | ||
| Males (%) | 910 (42.4) | 439 (52.4) | < 0.001 |
| Median age (IQR) | 53.0 (47.0–59.0) | 53.0 (46.0–59.7) | 0.630 |
| Central obesity | 1213 (56.5%) | 405 (48.4%) | < 0.001 |
| Median FBS (IQR) | 104.0 (96.0–117.0) | 104.5 (97.0–124.0) | 0.035 |
| Median SBP (IQR) | 132.0 (119.0–147.0) | 132.0 (120.0–147.0) | 0.461 |
| Median DBP (IQR) | 78.0 (71.0–87.0) | 79.0 (72.0–87.9) | 0.340 |
| Median TG (IQR) | 115.0 (85.0–162.0) | 121.0 (86.0–161.0) | 0.226 |
| Median HDL (IQR) | 50.0 (48.0–52.0) | 50.0 (48.0–52.0) | 0.759 |
IQR inter quantile range, FBS fasting blood sugar, SBP systolic blood pressure, DBP diastolic blood pressure, TG triglyceride, HDL high density lipoprotein
949 participants [701 females (73.9%); median age (IQR) 60 (54–65) years] had MetS in 2014. Overall prevalence of MetS in 2014 was 47.2% (95% CI 45.0–49.4%), with a prevalence of 28.6% (95% CI 25.6–1.6%) among males and 61.4% (95% CI 58.6–64.2%) among females.
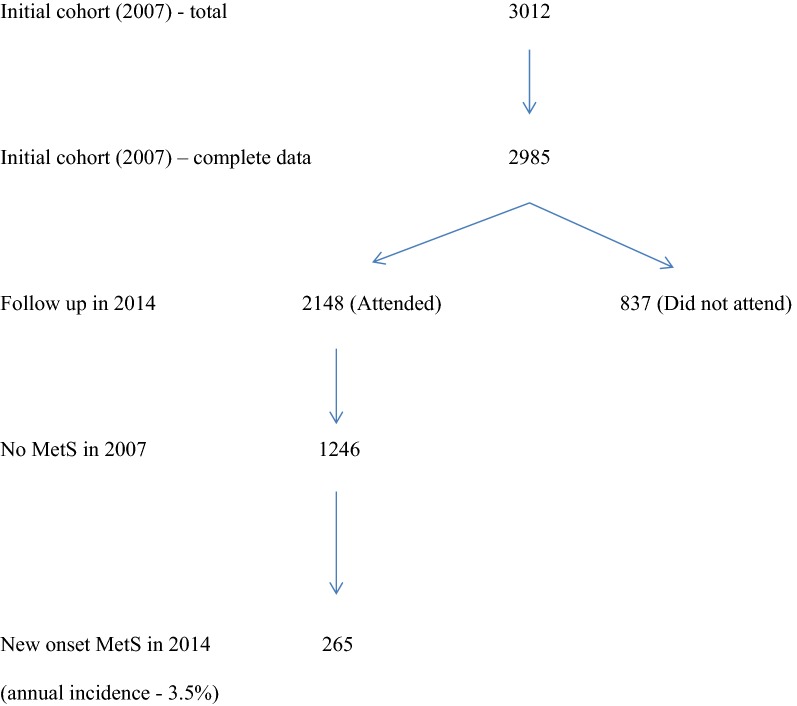
Out of 1246 participants who did not have MetS in 2007 and attended follow up in 2014, 265 [178 females (67.1%), median age (IQR) 57 (51–64) years] had developed new-onset MetS after 7 years, giving an annual incidence rate of 3.5% (95% CI 2.4–4.5%) (Fig. 1).
Fig. 1.
Study population
Table 2 shows a comparison between those with and without new-onset MetS in 2014, with regard to their characteristics at baseline (2007). Those who developed new-onset MetS were likely to be female, and have a higher BMI, central obesity and NAFLD in 2007. Any increase in weight and waist circumference from baseline was also significantly more likely to be associated with development of new-onset MetS.
Table 2.
Characteristics of participants in 2007 and the presence and absence of new-onset MetS in 2014
| Characteristic in 2007 | New-onset MetS in 2014 n = 265 |
No MetS in 2014 n = 875 |
P value |
|---|---|---|---|
| Females (%) | 178 (67.2%) | 292 (35.1%) | < 0.001 |
| Median age (IQR) | 50.0 (44.0–57.0) | 53.0 (47.0–58.0) | 0.037 |
| BMI > 23 kg/m2 | 174 (65.9%) | 211 (25.4%) | < 0.001 |
| Increase in weight (%) | |||
| < 2 | 87 (33.1%) | 485 (58.5%) | < 0.001 |
| 2–5 | 132 (50.2%) | 220 (26.5%) | < 0.001 |
| > 5 | 44 (16.7%) | 124 (15.0%) | < 0.001 |
| Central obesity | 101 (38.2%) | 87 (10.5%) | < 0.001 |
| Increase in waist (%) | |||
| < 2 | 59 (22.3%) | 548 (66.0%) | < 0.001 |
| 2–5 | 52 (19.6%) | 109 (13.1%) | < 0.001 |
| > 5 | 154 (58.1%) | 174 (20.9%) | < 0.001 |
| Raised plasma glucose | 206 (77.7%) | 707 (84.6%) | 0.013 |
| Raised blood pressure | 104 (39.4%) | 350 (42.2%) | 0.468 |
| Raised triglycerides | 58 (21.9%) | 188 (22.6%) | 0.861 |
| Reduced HDL cholesterol | 63 (24.4%) | 138 (16.7%) | 0.011 |
| NAFLD in 2007 | 72 (27.2%) | 100 (12.0%) | < 0.001 |
IQR inter quantile range, BMI body mass index, HDL high density lipoprotein, NAFLD non-alcoholic fatty liver disease
Parameter estimates of the fitted generalized model for new-onset MetS is given in Table 3. New-onset MetS was associated with female gender, BMI > 23 kg/m2 and NAFLD in 2007, weight gain and increase in waist circumference from 2007 to 2014. Females compared to males (OR = 4.9, 95% CI 3.4–7.4), those with BMI > 23 kg/m2 (OR = 1.6 per unit increase, 95% CI 1.5–1.7) and NAFLD (OR = 1.70, 95% CI 1.04–2.76) in 2007 had a higher risk of developing MetS.
Table 3.
Parameter estimates of the fitted generalized linear model for incident MetS
| Parameter | Estimate | Std. error | Z value | P value |
|---|---|---|---|---|
| Intercept | − 14.36 | 1.03 | − 13.90 | < 0.001 |
| Females compared to males | 1.60 | 0.20 | 7.99 | < 0.001 |
| BMI > 23 kg/m2 in 2007 | 0.46 | 0.04 | 11.57 | < 0.001 |
| Increase in weight | ||||
| 2–5% compared to < 2% | 0.69 | 0.29 | 2.39 | 0.017 |
| > 5% compared to < 2% | 0.77 | 0.23 | 3.36 | < 0.001 |
| > 5% compared to < 2–5% | 0.09 | 0.28 | 0.31 | 0.756 |
| Increase in waist circumference (reference level < 2%) | ||||
| 2–5% compared to < 2% | 1.94 | 0.28 | 6.83 | < 0.001 |
| > 5% compared to < 2% | 2.61 | 0.26 | 10.22 | < 0.001 |
| > 5% compared to < 2–5% | 0.67 | 0.25 | 2.65 | 0.008 |
| NAFLD 2007 | 0.53 | 0.25 | 2.14 | 0.03 |
BMI body mass index, NAFLD non-alcoholic fatty liver disease
Compared to those with a weight gain of less than 2% from baseline in 2007, participants who gained 2–5% weight (OR = 2.0, 95% CI 1.1–3.5) and more than 5% weight (OR = 2.2, 95% CI 1.4–3.4) had higher risk of developing MetS. However, there was no difference in the risk between those who gained 2–5% and more than 5% weight (OR = 1.1, 95% CI 0.6–1.9). Compared to those who had waist circumference increase less than 2% from baseline in 2007, participants who had an increase of 2–5% (OR = 7.0, 95% CI 4.0–12.2) and more than 5% (OR = 13.4, 95% CI 8.3–22.4) had a higher risk of developing MetS. Risk of MetS was higher among those who had more than 5% increase in waist circumference since 2007, compared to those who had an increase of 2–5% (OR = 2.0, 95% CI 1.2–3.2).
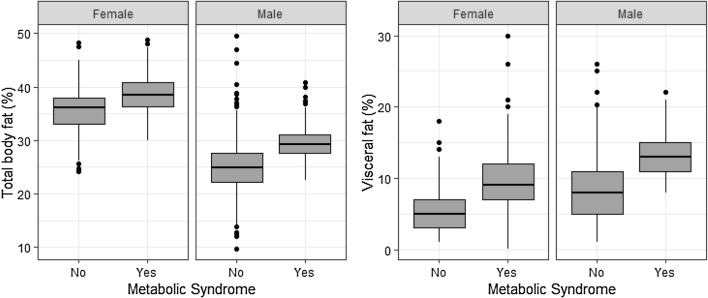
Both males and females with MetS had a higher median (IQR) total body fat percentage in 2014 compared to those without [females: 38.5% (36.3–40.9%) vs. 36.2% (33.0–37.9%), P < 0.001; males: 29.2% (27.6–31.2%) vs. 24.9% (22.2–27.7%), P < 0.001]. Those with new-onset MetS had a higher percentage of total body fat (77.9% vs. 60.0%, P < 0.001), and a higher visceral fat content (51.9% vs. 18.4%, P < 0.001), compared to those without new-onset MetS (Fig. 2).
Fig. 2.
Distribution of total body fat percentage and visceral fat percentage among males and females with respect to MetS status
Discussion
In this community cohort follow-up study, the annual incidence of MetS was 3.5%. Female gender, BMI > 23 kg/m2 and NAFLD in 2007 and increase in weight and waist circumference from baseline were predictive of new-onset MetS. Abnormal TBF and VPF in 2014 were also significantly more likely in those with new-onset MetS.
In 2007 we reported a community prevalence of 38.9% for MetS in this urban, adult Sri Lankan population [10]. By 2014, 28.6% of men and 61.4% of women had MetS, giving a combined prevalence of 47.2%, which is almost half the study population. This is a higher community prevalence of MetS than previously reported from the region: Asia–Pacific 11.9–37.1% [4], South-Asia 26.1% [5] and Sri Lanka 26.1–34.8% [6], and may be explained by the increasing mean age of the study population by 2014 with a cumulative effect for development of MetS. The prevalence of MetS appears to vary according to the degree of urbanization, lifestyle and other socio-cultural parameters, such as income and level of education. Recent data indicate that approximately a third of the urban population in many large cities in South Asia have MetS [11, 12]. The MetS prevalence rates of other regions from previous reports are also comparatively lower than what is reported here: Africa 12.5–62.5% [13], Central America 23.0–35.1% [14], Europe 11.6–26.3% [15], Middle East 13.6–36.3% [16], South America 18.8–43.3% [17], and worldwide 20–25% [3].
There are previously reported studies on incidence of MetS from some parts of Asia. Hwang et al. [8] followed up a cohort of 1095 subjects in semi-urban Korea for 5 years and reported a cumulative MetS incidence of 3.5%, which is similar to the incidence reported in our study. Hwang et al. also noted that the presence of components of MetS at baseline were predictive of future development of MetS, a feature also seen in our study. In a study of 2858 Iranian adults Hadaegh et al. [7] reported a MetS incidence of 5.5%, over 9.3 years of follow up. In this study too predictors of developing incident MetS included individual components of MetS. Ours is the first to report the incidence and risk factors for MetS from a community based, cohort follow-up study in a South Asian population. Our reported incidence of 3.5% is comparable to previous reports in selected Asian populations.
In a more recent study from Europe, Van Ancum et al. reported about predictors of MetS in community-dwelling adults aged 55–85 years in the Netherlands over a 3-year period [18]. A higher BMI was predictive of developing MetS in this longitudinal cohort of 218 participants, although incidence was high at 30%.
In the inception population in 2007, we reported that the presence of MetS was associated with older age, female sex, worsening glycaemic control and obesity [10]. At re-assessment 7 years later, female sex, BMI > 23 kg/m2, NAFLD and increase in weight and waist circumference strongly predicted the development of new onset MetS. In addition, increased TBF and VPF were also associated with new-onset MetS. Therefore, obesity, whether central or general, appears to be the pivotal risk factor for the development of MetS.
Our study showed an association between the presence of NAFLD in 2007 and the development of MetS in 2014. Previous studies have also shown an association between NAFLD and the presence of MetS, and NAFLD has been proposed as a novel criterion for the diagnosis of MetS [12]. NAFLD may be the hepatic manifestation of MetS and insulin resistance is likely to be the main pathogenetic mechanism. Hyperinsulinemia is probably the consequence rather than cause of NAFLD and NAFLD can be considered an independent predictor of cardiovascular disease [19].
The strengths of our study are in its design as a true community-based, prospective, cohort follow-up with a reasonable number attending follow-up after 7 years. Furthermore, almost all demographic characteristics were similar in the inception and follow-up cohorts. There were, however, several limitations in our study. One was that information on TBF and VPF were not available at baseline for risk assessment. Another was that our follow-up sample consisted of mainly older adults. However, it is well-known that prevalence of MetS increases with older age and the dominant components may also differ from those seen in younger populations [20]. Although the diagnosis of NAFLD was based on accepted criteria (rather than surrogate markers such as hepatic enzymes), inter-observer variability of the operators was not formally assessed. Due to the unreliability of the collected data related to dietary habits and physical activity, we could not investigate the effects of diet and exercise on the development of MetS in this population. This is a major limitation of this study, which we hope to address during the next follow-up of the study cohort.
Conclusion
This study demonstrates that the incidence and prevalence of MetS in urban Sri Lankan adults are high and is comparable to those reported in other parts of Asia [21]. The strong association of incident MetS with obesity and its markers suggests that preventive strategies should be aimed at control of obesity in the community. Early identification obesity coupled with emphasis on healthy lifestyle, diet and regular physical activity at community level is the best approach to prevent the development of MetS.
Acknowledgements
We thank all those who have continuously supported the Ragama Health Study. We especially thank the participants of the study for their continued cooperation.
Abbreviations
- ALT
serum alanine aminotransferase
- anti-HCV
anti-hepatitis C virus antibodies
- BMI
body mass index
- BP
blood pressure
- CI
confidence interval
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- FBS
fasting blood sugar
- HbA1c
glycosylated hemoglobinA1
- HBsAg
hepatitis B surface antigen
- HDL
high density lipoprotein
- IDF
International Diabetes Federation
- IQR
inter quantile range
- MetS
metabolic syndrome
- MOH
Medical Officer of Health
- NAFLD
non-alcoholic fatty liver disease
- OR
odds ratio
- PA
physical activity
- RHS
Ragama Health Study
- SBP
systolic blood pressure
- TBF
total body fat
- TG
triglyceride
- VFP
visceral fat percentage
- WC
waist circumference
Authors’ contributions
SdeS, MAN, AP and HJdeS conceptualized and designed the study. AK, SdeS, MAN, RW and HJdeS were involved in the establishment of the Ragama Health Study cohort. AK, DK, ADS and AD were involved in data collection. AP and DE analyzed the data assisted by SdeS and MAN. SdeS, MAN, DE and HJdeS prepared and revised the manuscript. All authors read and approved the final manuscript.
Funding
We gratefully acknowledge Grants from the Ministry of Higher Education of Sri Lanka and National Center for Global Health and Medicine, Tokyo, Japan for conduct of this study. The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Ethical approval was obtained from the Ethics Review Committee, Faculty of Medicine, University of Kelaniya. Informed written consent was obtained from all participants in 2007 and 2014.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. 2006;23(5):469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 2.Stern MP, Williams K, González-Villalpando C, Hunt KJ, Haffner SM. Does the metabolic syndrome improve identification of individuals at risk of type 2 diabetes and/or cardiovascular disease? Diabetes Care. 2004;27(11):2676–2681. doi: 10.2337/diacare.27.11.2676. [DOI] [PubMed] [Google Scholar]
- 3.International Diabetes Federation. The IDF consensus worldwide definition of the metabolic syndrome. 2015. 16/9/2016; http://www.idf.org/metabolic-syndrome. Accessed 25 Dec 2016.
- 4.Ranasinghe P, Mathangasinghe Y, Jayawardena Y, Hills AP, Misra A. Prevalence and trends of metabolic syndrome among adults in the Asia-Pacific region: a systematic review. BMC Public Health. 2017;17:101. doi: 10.1186/s12889-017-4041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aryal N, Wasti SP. The prevalence of metabolic syndrome in South Asia: a systematic review. Int J Diabetes Dev Ctries. 2016;36(3):255–262. doi: 10.1007/s13410-015-0365-5. [DOI] [Google Scholar]
- 6.Katulanda P, Ranasinghe P, Jayawardana R, Sheriff R, Matthews DR. Metabolic syndrome among Sri Lankan adults: prevalence, patterns and correlates. Diabetol Metab Syndr. 2012;4(1):24. doi: 10.1186/1758-5996-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hadaegh F, Hasheminia M, Lotfaliany M, Mohebi R, Azizi F, Tohidi M. Incidence of metabolic syndrome over 9 years follow-up; the importance of sex differences in the role of insulin resistance and other risk factors. PLoS ONE. 2013;8(9):e76304. doi: 10.1371/journal.pone.0076304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang JH, Kam S, Shin JY, Kim JY, Lee KE, Kwon GH, Chun BY, Chae SC, Yang DH, Park HS, Hwang TY. Incidence of metabolic syndrome and relative importance of five components as a predictor of metabolic syndrome: 5-year follow-up study in Korea. J Korean Med Sci. 2013;28(12):1768–1773. doi: 10.3346/jkms.2013.28.12.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dassanayake AS, Kasthuriratne A, Rindrajith S, et al. Prevalence and risk factors for non-alcoholic fatty liver disease among adults in an urban Sri Lankan population. J Gastroenterol Hepatol. 2009;24:1284–1288. doi: 10.1111/j.1440-1746.2009.05831.x. [DOI] [PubMed] [Google Scholar]
- 10.Chackrewarthy S., Gunasekera D., Pathmeswaren A., Wijekoon C. N., Ranawaka U. K., Kato N., Takeuchi F., Wickremasinghe A. R. A Comparison between Revised NCEP ATP III and IDF Definitions in Diagnosing Metabolic Syndrome in an Urban Sri Lankan Population: The Ragama Health Study. ISRN Endocrinology. 2013;2013:1–7. doi: 10.1155/2013/320176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandit K, Goswami S, Ghosh S, Mukhopadhyay P, Chowdhury S. Metabolic syndrome in South Asians. Indian J Endocrinol Metab. 2012;16(1):44–55. doi: 10.4103/2230-8210.91187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Souza MR, Diniz MdF, Medeiros-Filho JE, Araújo MS. Metabolic syndrome and risk factors for non-alcoholic fatty liver disease. Arq Gastroenterol. 2012;49(1):89–96. doi: 10.1590/S0004-28032012000100015. [DOI] [PubMed] [Google Scholar]
- 13.Okafor CI. The metabolic syndrome in Africa: current trends. Indian J Endocrinol Metab. 2012;16(1):56–66. doi: 10.4103/2230-8210.91191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong-McClure RA, et al. Prevalence of metabolic syndrome in Central America: a cross-sectional population-based study. Rev Panam Salud Publica. 2015;38(3):202–208. [PubMed] [Google Scholar]
- 15.van Vliet-Ostaptchouk JV, et al. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: a collaborative analysis of ten large cohort studies. BMC Endocr Disord. 2014;14:9. doi: 10.1186/1472-6823-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sliem HA, et al. Metabolic syndrome in the Middle East. Indian J Endocrinol Metab. 2012;16(1):67–71. doi: 10.4103/2230-8210.91193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marquez-Sandoval F, et al. The prevalence of metabolic syndrome in Latin America: a systematic review. Public Health Nutr. 2011;14(10):1702–1713. doi: 10.1017/S1368980010003320. [DOI] [PubMed] [Google Scholar]
- 18.Van Ancum JM, Jonkman NH, van Schoor NM, et al. Predictors of metabolic syndrome in community-dwelling older adults. PLoS ONE. 2018;13(10):e0206424. doi: 10.1371/journal.pone.0206424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vanni E, Bugianesi E, Kotronen A, De Minicis S, Yki-Järvinen H, Svegliati-Baroni G. From the metabolic syndrome to NAFLD or vice versa? Dig Liver Dis. 2010;42:320–330. doi: 10.1016/j.dld.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Kolovou GD, Anagnostopoulou KK, Salpea KD, Mikhailidis DP. The prevalence of metabolic syndrome in various populations. Am J Med Sci. 2007;333(6):362–371. doi: 10.1097/MAJ.0b013e318065c3a1. [DOI] [PubMed] [Google Scholar]
- 21.Ranasinghe P, Mathangasinghe Y, Jayawardena R, Hills AP, Misra A. Prevalence and trends of metabolic syndrome among adults in the Asia-Pacific region: a systematic review. BMC Public Health. 2017;17(1):101. doi: 10.1186/s12889-017-4041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.