Abstract
Background
High temperature is a major environmental stress that limits plant growth and agriculture productivity. Mitogen-activated protein kinases (MAPKs) are highly conserved serine and threonine protein kinases that participate in response to diverse environmental stresses in plants. A total of 16 putative SlMAPK genes are identified in tomato, and SlMAPK3 is one of the most extensively studied SlMAPKs. However, the role of SlMAPK3 in response to heat stress is not clearly understood in tomato plants. In this study, we performed functional analysis of SlMAPK3 for its possible role in response to heat stress.
Results
qRT-PCR analyses revealed that SlMAPK3 relative expression was depressed by heat stress. Here, wild-type (WT) tomato plants and CRISPR/Cas9-mediated slmapk3 mutant lines (L8 and L13) were used to investigate the function of SlMAPK3 in response to heat stress. Compared with WT plants, slmapk3 mutants exhibited less severe wilting and less membrane damage, showed lower reactive oxygen species (ROS) contents, and presented higher both activities and transcript levels of antioxidant enzymes, as well as elevated expressions of genes encoding heat stress transcription factors (HSFs) and heat shock proteins (HSPs).
Conclusions
CRISPR/Cas9-mediated slmapk3 mutants exhibited more tolerance to heat stress than WT plants, suggesting that SlMAPK3 was a negative regulator of thermotolerance. Moreover, antioxidant enzymes and HSPs/HSFs genes expression were involved in SlMAPK3-mediated heat stress response in tomato plants.
Electronic supplementary material
The online version of this article (10.1186/s12870-019-1939-z) contains supplementary material, which is available to authorized users.
Keywords: Tomato plants, SlMAPK3, Heat tolerance, ROS, Antioxidant enzymes, HSPs, HSFs
Background
High temperature, which causes heat stress, has become an increasingly serious agricultural problem in many regions of the world as a result of global warming [1]. Under heat stress, plant cells always show membrane damage, reactive oxygen species (ROS) overproduction and metabolic disturbance that further limit crop productivity and quality [2]. Tomato (Solanum lycopersicum) is a globally popular horticultural commodity with great economic importance, which also functions as a model plant species widely used in plant science, since it shows highly susceptible to diverse environmental stresses, such as drought, salinity, chilling, and heat.
In plants, mitogen activated protein kinase (MAPK) cascade have been reported to participate in signal transduction including plant development, hormone regulation, disease resistance, and stress responses [3]. There is increasing evidence that MAPK cascades play a vital role in mediating diverse cellular signaling network by transmitting extracellular stimuli to intracellular responses, which positively regulates gene expression and protein functions under various abiotic stresses, ultimately resulting in adaptive responses to environmental stresses [4]. The basic MAPK signaling modules are composed by an interlinked cascade of three consecutively acting protein kinases: MAPKK kinases (MAPKKKs), MAPK kinases (MAPKKs), and MAPKs, which are sequentially activated by phosphorylation. Previous studies reported that MAPK genes expression are significantly induced in response to heat treatment [5], and AtMAPK6 in Arabidopsis thaliana [6], ZmMAPK1 in maize [7], MnMAPK1 in Mulberry [8], and SlMAPK1 in tomato [9], have been shown to participate in heat stress response. However, there is a lack of report concerning the involvement of SlMAPK3 in heat stress response.
In the tomato genome, 16 putative SlMAPK family genes have been identified, which can be clustered into four major groups (A–D) considering the similar exon-intron structures [4]. To date, SlMAPK3, a member in group A, is one of the most extensively studied SlMAPKs in tomato. It has been well documented that SlMAPK3 plays an essential role in mediating a diversity of biotic and abiotic stress responses, including herbivorous insects [10], fungus [11], wounding [12], chilling [13], and drought [14]. Our previous studies revealed that knockout of SlMAPK3 in tomato plant resulted in reduced drought tolerance and decreased disease resistance to Botrytis cinerea [11, 14]. However, the specific role of SlMAPK3 in response to heat stress is not understood in tomato plants.
Oxidative damage on cell membranes has been implicated as a common event under abiotic stress that is assessed by the increase in both MDA content and ion leakage level [15]. Besides, oxidative damage can be ascribed to the overproduction of ROS, and both the formation and the scavenging of ROS are important for maintaining the steady state levels of ROS. The NADPH oxidase encoded by respiratory burst oxidase homolog (RBOH) genes is the major source of ROS in plants [16–18]. Previous studies documented that suppressed transcript level of SlRBOH1 compromised BR-induced activation of SlMAPK1/2 and SlMAPK3, and silencing of either SlMAPK1 or SlMAPK2 reduced SlRBOH1 transcript level and H2O2 accumulation [16, 17]. Moreover, SlMAPKs have been reported to participate in the regulation of defense response against abiotic stress by scavenging excess ROS [9, 14, 19]. For example, knockout of SlMAPK1 enhanced tolerance to heat stress by elevating antioxidant enzymes activities, which are crucial in ROS scavenging [9]. Our previous study showed that slmapk3 mutants were sensitive to drought stress, with lower antioxidant enzymes activities and higher H2O2 content [14]. However, the relationship between ROS and SlMAPK3-mediated heat tolerance still remains unclear.
In plants, heat shock proteins (HSPs), including HSP100, HSP90, HSP70, HSP60, and small HSPs (smHSPs) [20], are generally considered as important molecular chaperones that contribute to maintain and/or restore protein homeostasis, which are crucial for plant survival under heat stress [21]. In addition, heat stress transcription factors (HSFs) are responsible for heat stress-induced gene expression [22]. Examples of this is that, under heat stress, HSFs can regulate HSPs genes expression by binding to heat stress elements (HSE: 5′-AGAAnnTTCT-3′) that are presented in the promoters of HSPs, ultimately inducing the responsiveness of downstream genes to heat stress [22]. It was previously reported that under heat stress, AtMAPK6 could phosphorylate HSFA2, and the phosphorylated-HSFA2 played an important role in response to heat stress [6]. In tobacco, a heat-activated MAP kinase (HAMK) functioned as a regulator in heat response, and heat induced expression of HSF1/2 required the existence of MAPKK [23]. Thus, there might be correlations between HSPs/HSFs and MAPK-associated signaling pathways under heat stress.
In this study, relative expression of SlMAPK3 was examined after different high temperature treatments in tomato plants, and CRISPR/Cas9-mediated slmapk3 mutants (L8 and L13) were applied to investigate the role of SlMAPK3 in response to heat stress. Our current results demonstrated that knockout of SlMAPK3 could enhance heat tolerance, reduce ROS accumulation, and upregulate several HSPs/HSFs genes expressions in tomato plants, which implied that SlMAPK3 acted as a negative regulator of defense response to heat stress.
Results
Analysis of expression patterns of SlMAPK3 under different temperature conditions
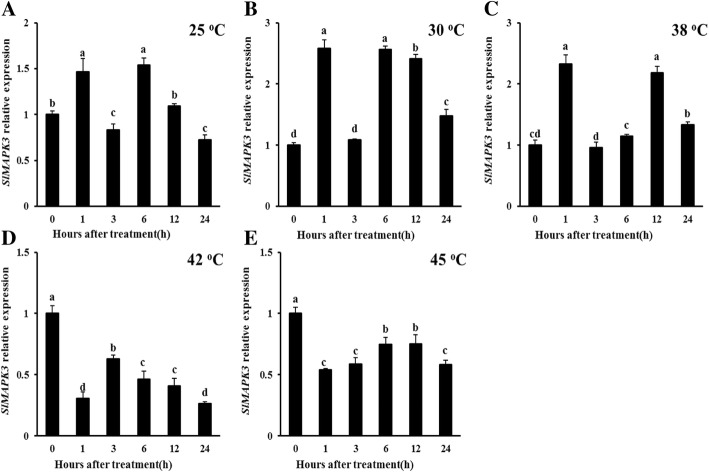
Transcript levels of SlMAPK3 at different temperatures (25, 30, 38, 42, and 45 °C) were investigated by qRT-PCR (Fig. 1, P < 0.05). Our results showed that SlMAPK3 relative expressions were increased at 25, 30, and 38 °C, which showed fluctuating changes (Fig. 1a, b and c, P < 0.05). On the contrary, when WT plants were exposed to higher temperature treatments (42 and 45 °C), the transcript levels of SlMAPK3 significantly reduced after heat treatment (Fig. 1d and e, P < 0.05). These results indicated that SlMAPK3 might participate in heat response.
Fig. 1.
Expression analysis of SlMAPK3 under different temperature conditions in WT tomato plants. (a) at 25 °C, (b) at 30 °C, (c) at 38 °C, (d) at 42 °C, (e) at 45 °C. Data are represented as mean ± SD of three biological replicates. Statistical differences at each time point of treatment are labeled with different letters according to Duncan’s multiple range test at P < 0.05
Phenotype of slmapk3 mutants under heat stress
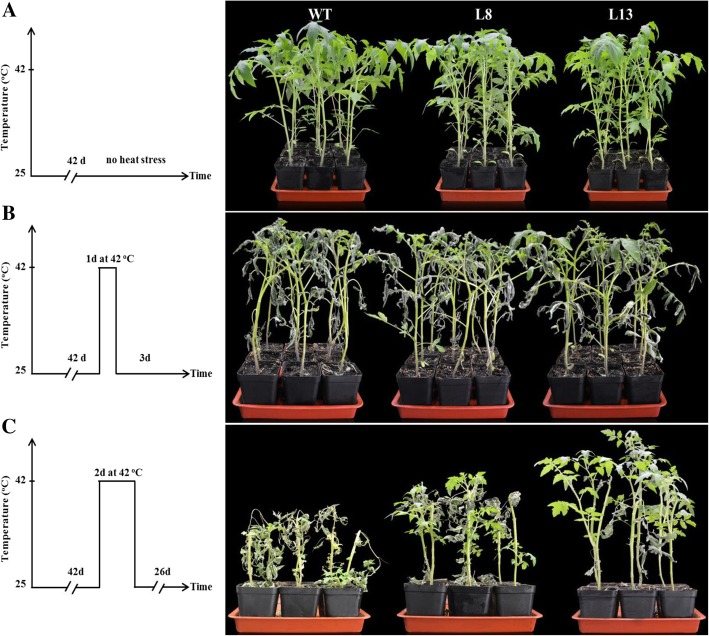
Before heat treatment, no significant differences between slmapk3 mutants and WT plants could be observed (Fig. 2a). However, after 1 day’s exposure to 42 °C, visible symptoms of leaves wilting and stem bending were aggravated in WT plants, compared with slmapk3 mutants which showed less severe wilting (Fig. 2b). A similar result could also be observed when heat stress was prolonged to 2 d, and the severest symptom was exhibited in WT plants (Fig. 2c). Meanwhile, the survival rate in slmapk3 mutants were 3.17 (in L8) and 4.17 (in L8) times higher than that in WT plants (Additional file 1: Figure S1). These results indicated that knockout of SlMAPK3 enhanced heat tolerance in tomato plants, suggesting that SlMAPK3 played a negative role in response to heat stress.
Fig. 2.
Phenotype of slmapk3 mutants and WT plants under heat stress. a Six-week-old tomato plants of slmapk3 mutants and WT under normal conditions. b Six-week-old tomato plants of slmapk3 mutants and WT were subjected to 42 °C for 1 d then placed them to 25 °C for 3 d. c Six-week-old tomato plants of slmapk3 mutants and WT were subjected to 42 °C for 2 d then placed them to 25 °C for 26 d
Effects of slmapk3 mutants on cell membrane damage, MDA content, and ion leakage under heat stress
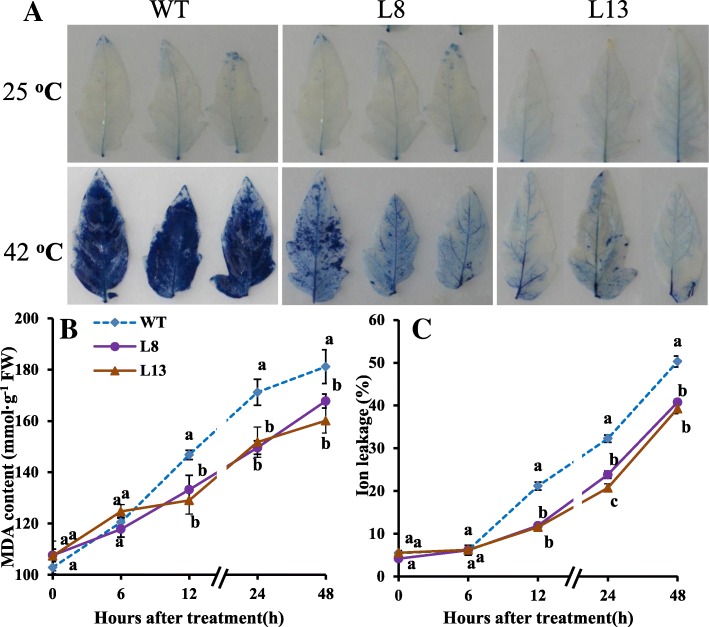
Cell membrane damage was analyzed by trypan blue staining. Under normal conditions, there was no significant difference between WT and slmapk3 mutants. However, after 42 °C treatment for 24 h, staining intensity in slmapk3 mutants was lower than that in WT (Fig. 3a). Ion leakage and MDA content are physiological indices of cell membrane damage. Under normal conditions, MDA content and ion leakage were not significantly different between WT and slmapk3 mutants (Fig. 3b and c, P < 0.05). In contrast, MDA content and ion leakage were significantly increased under heat stress, and MDA contents and ion leakage levels were remarkably higher in WT plants, in comparison with slmapk3 mutants (Fig. 3b and c, P < 0.05).
Fig. 3.
Effects of slmapk3 mutants on cell membrane damage under heat stress. a Trypan blue staining, b MDA content, c ion leakage level. For (a), the top panel represents leaves grown at 25 °C, and the bottom panel represents leaves treated with 42 °C for 24 h. Data are represented as mean ± SD of three biological replicates. Statistical differences at each time point of treatment are labeled with different letters according to Duncan’s multiple range test at P < 0.05
Effects of slmapk3 mutants on ROS accumulation under heat stress
The accumulations of H2O2 and O2•−, which are two major components of ROS, were detected by DAB and NBT staining. After heat treatment for 24 h, DAB and NBT staining results showed that slmapk3 mutants accumulated less H2O2 and O2•− than WT (Fig. 4a and b). This was consistent with the results of quantitative analysis, in which H2O2 and O2•− contents were 28.0, 7.1% (in L8), and 32.5, 9.4% (in L13) lower than those in WT plants at 24 h (Fig. 4c and d, P < 0.05).
Fig. 4.
Effects of slmapk3 mutants on ROS production under heat stress. a DAB staining, b NBT staining, c H2O2 content, d O2•− content, and (e) SlRBOH1 relative expression. For (a) and (b), the top panel represents leaves grown at 25 °C, and the bottom panel represents leaves treated with 42 °C for 24 h. Data are represented as mean ± SD of three biological replicates. Statistical differences at each time point of treatment are labeled with different letters according to Duncan’s multiple range test at P < 0.05
Under normal conditions, SlRBOH1 transcript levels in slmapk3 mutants were significantly lower than that in WT plants (Fig. 4e, P < 0.05). After 1 h of heat stress, SlRBOH1 transcript levels increased both in WT and in slmapk3 mutants, but transcript levels in L8 and L13 were 23.9 and 25.5% lower than that in WT plants (Fig. 4e, P < 0.05). Moreover, after 24 h of heat stress, SlRBOH1 transcript levels decreased dramatically, and no significant difference was observed between WT and slmapk3 mutants (Fig. 4e, P > 0.05). Taken together, these results indicated that knockout of SlMAPK3 reduced the overproduction of ROS under heat stress.
Effects of slmapk3 mutants on antioxidant enzymes under heat stress
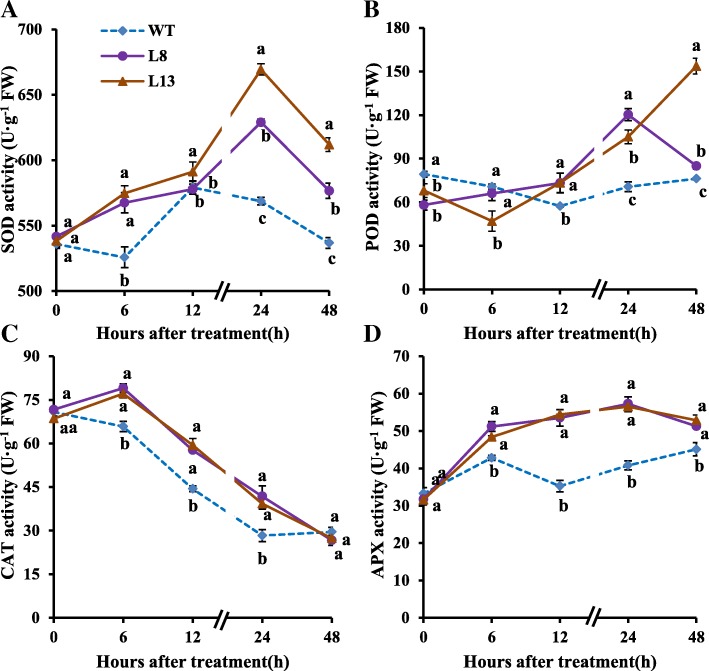
During the whole stress period, slmapk3 mutants showed significant higher SOD activities than those in WT plants except the twelfth hour (Fig. 5a, P < 0.05). POD activities in WT plants reduced gradually from hours 6 to 12 and then increased afterward. POD activities in slmapk3 mutants showed a fluctuating increase and were significantly higher than those in WT plants after 12 h heat treatment (Fig. 5b, P < 0.05). CAT activities reached a maximum of 79.0 U·g−1FW in L8 and 77.1 U·g−1FW in L13 on the sixth hour and then declined, but they were significantly higher than those in WT plants up to 24 h after heat treatment (Fig. 5c, P < 0.05). APX activities in slmapk3 mutants increased gradually after heat treatment for 24 h, the average CAT activities in slmapk3 mutants were 16.3, 53.1, 39.5 and 15.4% higher than those in WT plants at hours 6, 12, 24 and 48, respectively (Fig. 5d, P < 0.05).
Fig. 5.
Effects of slmapk3 mutants on antioxidant enzymes activities under heat stress. a SOD, b POD, c CAT, and d APX. Data are represented as mean ± SD of three biological replicates. Statistical differences at each time point of treatment are labeled with different letters according to Duncan’s multiple range test at P < 0.05
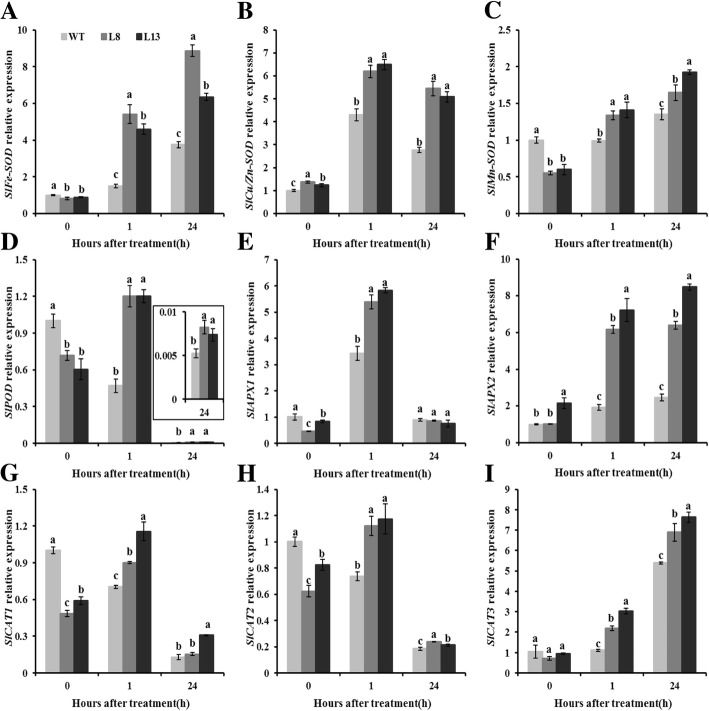
Relative expressions of genes involved in encoding SOD, POD, CAT, and APX were assayed at the transcriptional level using qRT-PCR. In comparison with WT plants, transcript levels of SlFe-SOD, SlMn-SOD, SlPOD, SlAPX1, SlCAT1, SlCAT2 and SlCAT3 in slmapk3 mutants were significantly lower under normal conditions (Fig. 6a, c, d, e, g, h and i, P < 0.05). After heat treatment, transcript levels of SlFe-SOD, SlMn-SOD, SlAPX2, and SlCAT3 were upregulated, and the levels in slmapk3 mutants were significantly higher than those in WT plants (Fig. 6a, c, f and i, P < 0.05). In addition, heat exposure enhanced the transcript levels of SlCu/Zn-SOD, SlPOD, SlAPX1, SlCAT1 and SlCAT2 at the time point of 1 h, and decreased those at 24 h in slmapk3 mutants (Fig. 6b, d, e, g and h, P < 0.05). The changing patterns of SlCu/Zn-SOD and SlAPX1 in WT plants were similar to the slmapk3 mutants under heat stress, while the changing patterns of SlPOD, SlCAT1 and SlCAT2 were different from the slmapk3 mutants, which decreased both at 1 h and at 24 h (Fig. 6b, d, e, g and h, P < 0.05). These results demonstrated that knockout of SlMAPK3 increased both activities and transcript levels of SOD, POD, CAT and APX under heat stress.
Fig. 6.
Effects of slmapk3 mutants on the transcript levels of key antioxidant enzymes genes under heat stress. a SlFe-SOD, b SlCu/Zn-SOD, c SlMn-SOD, d SlPOD, e SlAPX1, f SlAPX2, g SlCAT1, h SlCAT2, and i SlCAT3. Data are represented as mean ± SD of three biological replicates. Statistical differences at each time point of treatment are labeled with different letters according to Duncan’s multiple range test at P < 0.05
Effects of slmapk3 mutants on gene expressions of SlHSP70/90/100 and SlHSFA1a/2/3 under heat stress
Apart from antioxidant system, another key adaptive mechanism developed by plant species when subjected to heat stress is the accumulation of heat shock response-related genes, including HSPs and HSFs. Therefore, relative expression of SlHSP70, SlHSP90, SlHSP100 and SlHSFA1a, SlHSFA2, SlHSFA3 were analyzed. After 1 h of heat stress, the transcript levels of SlHSP70, SlHSP90, SlHSP100 and SlHSFA2 were rapidly induced, and significant difference were observed between WT and slmapk3 mutants. However, after 24 h of heat stress, transcript levels of these four genes decreased dramatically, but transcript levels in slmapk3 mutants were still higher than those in WT plants (Fig. 7a, b, c and e, P < 0.05). The relative expressions of SlHSFA1a and SlHSFA3 were significantly increased under heat stress, and slmapk3 mutants showed higher levels of these two genes after 1 h and 24 h of heat stress compared with WT plants (Fig. 7d and f, P < 0.05).
Fig. 7.
Effects of slmapk3 mutants on the transcript levels of key HSPs and HSFs genes under heat stress. a SlHSP70, b SlHSP90, c SlHSP100, d SlHSFA1a, e SlHSFA2, and f SlHSFA3. Data are represented as mean ± SD of three biological replicates. Statistical differences at each time point of treatment are labeled with different letters according to Duncan’s multiple range test at P < 0.05
Correlation analysis of heat stress related physiological indexes
As shown in Table 1, positive correlations were found between MDA content and ion leakage, between MDA content and H2O2 content, between MDA content and O2•− content, between ion leakage and H2O2 content, between ion leakage and O2•− content, between H2O2 content and O2•− content, between SOD activity and APX activity. In addition, negative correlations were found between MDA content and CAT activity, between ion leakage and CAT activity, between H2O2 content and CAT activity, between O2•− content and CAT activity (P < 0.01). The significant correlations among MDA content, ion leakage, H2O2 and O2•− contents could possibly be attributed to the fact that overproduction of ROS such as H2O2 and O2•− caused oxidative stress, which resulted in lipid peroxidation and disruption of membrane integrity under heat stress [24]. Besides, the close relationship among CAT activity and MDA content, ion leakage, H2O2 and O2•− contents (r > 0.72), indicated that CAT activity had significant negative correlations with the cell membrane damage and ROS accumulation under heat stress, which was consistent with a previous study that heat-induced decrease in CAT activity was strongly responsible for ROS detoxification in rice [25]. Furthermore, partial least square regression analysis (PLSR) results (Fig. 8) showed that MDA content was positively correlated with ion leakage, H2O2 and O2•− content, and negatively correlated with CAT activity. Path analysis (PA) results (Table 2) provided further information that the influence of ion leakage on MDA content was achieved via direct impact, whereas the influence of H2O2 content, O2•− content, POD activity and CAT activity on MDA content depended on other factors such as ion leakage.
Table 1.
Pearson’s correlations among MDA content, ion leakage, H2O2 content, O2•− content and antioxidant enzyme activities
| MDA content | Ion leakage | H2O2 content | O2•− content | SOD activity | POD activity | CAT activity | APX activity | |
|---|---|---|---|---|---|---|---|---|
| MDA content | 1.000 | 0.947b | 0.868b | 0.921b | 0.388 | 0.412 | −0.936b | 0.433 |
| Ion leakage | 1.000 | 0.883b | 0.846b | 0.245 | 0.492 | −0.932b | 0.310 | |
| H2O2 content | 1.000 | 0.768b | 0.071 | 0.188 | −0.722b | 0.339 | ||
| O2•− content | 1.000 | 0.279 | 0.383 | −0.873b | 0.373 | |||
| SOD activity | 1.000 | 0.604a | −0.421 | 0.722b | ||||
| POD activity | 1.000 | −0.575a | 0.513 | |||||
| CAT activity | 1.000 | −0.326 | ||||||
| APX activity | 1.000 |
aCorrelation is significant at the 0.05 level (2-tailed)
bCorrelation is significant at the 0.01 level (2-tailed)
Fig. 8.
Partial least square regression analysis for physiological indexes of tomato plants under heat stress. The MDA content was used as Y-variables and other indexes as the X-variables
Table 2.
Path coefficient analysis of characters on MDA content
| Characters | Direct Effect | Indirect Effect | Correlation coefficient with MDA content | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ion leakage | H2O2 content | O2•− content | SOD activity | POD activity | CAT activity | APX activity | Total | |||
| Ion leakage (X1) | 0.550 | – | 0.051 | 0.226 | 0.046 | −0.107 | 0.162 | 0.020 | 0.397 | 0.947 |
| H2O2 content (X2) | 0.057 | 0.486 | – | 0.206 | 0.013 | −0.041 | 0.125 | 0.022 | 0.811 | 0.868 |
| O2•− content (X3) | 0.268 | 0.465 | 0.044 | – | 0.052 | −0.083 | 0.152 | 0.024 | 0.654 | 0.921 |
| SOD activity (X4) | 0.186 | 0.135 | 0.004 | 0.075 | – | −0.131 | 0.073 | 0.046 | 0.202 | 0.388 |
| POD activity (X5) | −0.217 | 0.271 | 0.011 | 0.103 | 0.112 | – | 0.100 | 0.033 | 0.629 | 0.412 |
| CAT activity (X6) | −0.174 | −0.512 | −0.041 | − 0.234 | −0.078 | 0.124 | – | −0.021 | − 0.762 | −0.936 |
| APX activity (X7) | 0.064 | 0.170 | 0.019 | 0.100 | 0.134 | −0.111 | 0.057 | – | 0.370 | 0.433 |
Y = 0.642 + 0.906X1 + 7.024X2 + 14.760X3 + 0.119X4–0.200X5–0.224X6 + 0.173X7
R2 = 0.995
Discussion
MAPKs are serine-threonine protein kinases that are highly conserved in eukaryotes [26]. In tomato, SlMAPK3 has been studied extensively for its involvement in the responses to various stresses in plants, and silencing of SlMAPK3 differently influence plant tolerance to multiple environmental stresses [11]. Our previous studies demonstrated that knockout of SlMAPK3 in transgenic tomato plants resulted in reduced drought tolerance and decreased disease resistance to Botrytis cinerea in tomato plant, accompanied by lower antioxidant enzyme activity and higher H2O2 content [11, 14]. However, there is a lack of knowledge on the role and mechanisms of SlMAPK3 in response to heat stress. In the present study, we showed that SlMAPK3 relative expression was downregulated by high temperature treatments (42 °C, 45 °C) (Fig. 1d and e, P < 0.05), and knockout of SlMAPK3 in CRISPR/Cas9-mediated mutagenesis showed more tolerance to heat stress than WT plants (Fig. 2), which suggested that SlMAPK3 functioned as a negative regulator of heat response in tomato plants.
Generally, biological membranes are the first targets of diverse abiotic stresses, and loss of membranes integrity is a primary symptom of heat injury [27]. Previous studies documented that heat stress decreased membrane thermo-stability and increased the formation of membrane lipid peroxidation, as indicated by ion leakage and MDA content [28, 29]. Lower ion leakage level and MDA content could be observed in heat tolerant genotype, which have been successfully used as two important criteria for heat tolerant genotypes in tomato [30]. In this study, knockout of SlMAPK3 alleviated heat stress-induced damage to the membrane system (Fig. 3a). Moreover, elevations in both ion leakage and MDA content were significantly lower in slmapk3 mutants than in WT plants under heat stress (Fig. 3b and c, P < 0.05), which implied that knockout of SlMAPK3 maintained the relative integrity of cell membrane and reduced cell membrane damage caused by heat stress.
Heat stress always leads to the overproduction of ROS. Excessive ROS generation in plant tissues can directly cause oxidative damage, ultimately impairing the normal function of cells [28]. It has been reported that ROS levels in heat-sensitive rice increased more profoundly than that in heat-tolerant rice under the same heat conditions, suggesting that there is a direct correlation between ROS accumulation and plant tolerance to heat stress [31]. The NADPH oxidase is the major source of ROS under various abiotic and biotic stresses, which is encoded by RBOH genes [18]. The tomato SlRBOH1 has the highest transcript abundance within the SlRBOH family, which participates in the regulation of tolerance to heat stress [17, 32]. In our study, both H2O2 and O2•− contents increased under heat stress, while the contents in slmapk3 mutants were significantly lower than those in WT plants, and SlRBOH1 transcript levels in slmapk3 mutants were remarkably lower than that in WT plants after 1 h heat treatment (Fig. 4, P < 0.05). It’s indicated that knockout of SlMAPK3 suppressed ROS overproduction, which contributed to alleviate cell membrane damage caused by high temperature (Table 1, Fig. 3, P < 0.05).
Antioxidant enzymes, including SOD, POD, CAT, and APX are crucial in ROS detoxification, which are thought to be a part of heat-stress adaptation, and their strengths are positively correlated with the acquisition of thermotolerance in plants [33, 34]. Besides, the activation of antioxidant enzymes played a crucial role in MAPKs-mediated stress responses including heat stress response. A good example of this is RNAi-SlMAPK1 tomato plants, which showed higher heat tolerance than WT plants by increasing the antioxidant enzymes activities of SOD, POD, CAT, and APX [9]. In the present study, knockout of SlMAPK3 significantly enhanced activities of these four antioxidant enzymes under heat stress by upregulating relative expression of their corresponding genes (Figs. 5 and 6, P < 0.05), which helped to scavenge ROS and alleviate oxidative damage (Figs. 3 and 4, P < 0.05). These results indicated that antioxidant enzymes were involved in heat stress response mediated by SlMAPK3.
Currently, the role of SlMAPK3 in the regulation of heat-stress-related genes is still not entirely understood. HSFs and HSPs are known to play important roles in enhancing thermotolerance of plants. Larger HSPs, especially HSP70 and HSP90, were reported to act as molecular chaperone that participated in upregulation of several downstream genes associated with heat response in plants [35, 36]. Previous studies indicated that ClpB/Hsp100 proteins were critical in governing plant thermotolerance, the antisense lines which exhibited an extreme suppression of SlClpB/Hsp100 gene expression were hypersensitive to heat stress [37]. These studies supported our present data that slmapk3 mutants had higher transcript levels of SlHSP70, SlHSP90 and SlHSP100 than WT plants (Fig. 7a, b and c, P < 0.05), suggesting that slmapk3 mutants were more heat-resistant than WT plants. Moreover, three HSFs, namely HsfA1, HsfA2 and HsfB1, are critical components involved in mediating responsiveness of different heat stress-induced genes in tomato [22]. Thermotolerance was remarkably enhanced in SlHsfA1a-overexpressing lines, whereas the suppression lines exhibited heat-sensitive phenotypes [38]. Overexpression of AtHsfA2 showed enhanced tolerance to heat stress, indicating a correlation between HSFA2 expression level and heat tolerance in Arabidopsis thaliana [39]. In addition, ectopic overexpression of SlHsfA3 conferred increased thermotolerance in Arabidopsis thaliana [40]. In our study, relative expressions of SlHSFA1a, SlHSFA2 and SlHSFA3 were significantly higher in slmapk3 mutants than in WT plants under heat stress (Fig. 7d, e and f, P < 0.05). These results indicated that the increase in HSPs and HSFs genes relative expression might be associated with SlMAPK3-mediated heat stress response in tomato plants.
Conclusions
In conclusion, our current study demonstrated that knockout of SlMAPK3 enhanced heat tolerance in tomato plants. The decrease in MDA content and ion leakage implied that knockout of SlMAPK3 prevented cell membrane from oxidative damage caused by heat stress. In addition, knockout of SlMAPK3 reduced H2O2 and O2•− contents, downregulated SlRBOH1 relative expression, and increased both activities and transcript levels of SOD, POD, APX and CAT, suggesting that ROS production and scavenging were involved in SlMAPK3-mediated heat response (Fig. 9). Moreover, transcript levels of SlHSP70, SlHSP90, SlHSP100 and SlHSFA1a, SlHSFA2, SlHSFA3 were significantly higher in slmapk3 mutants than those in WT plants, indicated that SlHSFs and SlHSPs genes might be involved in SlMAPK3-mediated heat response (Fig. 9). These results revealed that, the enhanced heat tolerance in slmapk3 mutants could be associated with the suppression of ROS production and the activation of antioxidant enzymes, which led to lower ROS accumulation and alleviated oxidative damage under heat stress. Meanwhile, the transcript levels of SlHSFs and SlHSPs were also found to be modulated by SlMAPK3 silencing. Taken together, this study suggests a possible regulatory mechanism involving SlMAPK3-mediated heat stress response, and provides insights into the role that MAPK cascade plays against abiotic stress in tomato plants. Further studies will pay more attention to the specific relationships between HSFs/HSPs and SlMAPK3-mediated heat response.
Fig. 9.

A proposed model of the role of SlMAPK3 in tomato plants responses to heat stress involving ROS homeostasis. During heat stress, knockout of SlMAPK3 suppressed heat-induced upregulation of SlRBOH1 gene, and activated antioxidant enzymes, which alleviated ROS accumulation, in turn leading to a further mitigation in cell membrane damage in slmapk3 mutants. The involvement of SlHSFs/ SlHSPs genes expressions are also suggested by solid lines. Consequently, prolonged heat stress tolerance is induced
Methods
Plant materials and growth conditions
In this study, wild-type (WT) tomato plants (Solanum lycopersicum cv. Ailsa Craig) and T2 transgenic lines (L8 and L13) [14] were used. AC seeds were provided by Dr. Jim Giovannoni (Boyce Thompson Institute for Plant Research Ithaca, NY 14853, USA). All germinated tomato seeds were sown in plastic pots containing seedling substrate, soil, and vermiculite (2/1/1, by vol.), and grown in a greenhouse with a 16 h-light /8 h-dark photoperiod and 60–65% relative humidity, at a temperature of 25 °C.
For the heat stress tolerance assay, six-week-old plants were subjected to a 42/42 °C (day/night) illuminated chamber for two days. At each time point (0, 1, 3, 6, 12, 24 and 48 h) after treatment, five tomato plants were randomly selected and sampled from the same position. These samples were rapidly frozen in liquid nitrogen and stored at − 80 °C pending analysis. Three biological replicates were carried out in this experiment.
Analysis of expression patterns of SlMAPK3
Six-week-old WT tomato plants were exposed to different high temperature treatments (25, 30, 38, 42 and 45 °C) in an illuminated chamber. After each treatment, functional leaves from the same position were sampled at 0, 1, 3, 6, 12 and 24 h, and then immediately frozen in liquid nitrogen and stored at − 80 °C for RNA extraction. The transcript level of SlMAPK3 was measured by quantitative real-time PCR (qRT-PCR).
qRT-PCR analysis
In this study, an EasyPure Plant RNA Kit (Beijing Transgen Biotech Co. Ltd., Beijing, China) was used to extract total RNA from 0.15 g frozen leaf sample. The total RNA was quantified by using a NanoDrop 2000 Photometer spectrophotometer (Thermo Scientific, Waltham, MA, USA), and 2 μg of RNA was reverse transcribed to synthesized cDNA by the aid of the TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix Kit (Beijing Transgen Biotech Co. L td., Beijing, China).
The qRT-PCR was implemented using the TransStart Top Green qPCR SuperMix (Beijing Transgen Biotech Co.Ltd., Beijing, China), and the reaction mixture contains 5 μL of 2× SuperMix, 0.3 μL of both the forward and reverse specific primers (Additional file 2: Table S1), 1 μL of cDNA, and 3.4 μL of RNase-free water. The qRT-PCR was performed on a Bio-Rad CFX96 real-time PCR system (Bio-Rad, USA), and β-Actin was used as the reference gene. The expression levels of different genes were calculated using 2-ΔΔCT method.
Determination of MDA content and ion leakage
The lipid peroxidation and disruption of membrane integrity in cell membranes were estimated by measuring MDA content and ion leakage. MDA content was measured using the method as previously described by Ding et al. [41], and MDA content was expressed in mmol·g− 1 FW (fresh weight). Ion leakage was measured immediately from the leaf discs according to the method described by Zhao et al. [42], with some modifications.
Determination of H2O2 content and O2•− content
A H2O2 Detection Kit (A064, Jiancheng, Nanjing, China) and a superoxide radical anions (O2•−) Detection Kit (A052, Jiancheng, Nanjing, China) were used to assay the H2O2 and O2•− contents, and both H2O2 and O2•− contents were expressed as mmol·g− 1 FW.
Histochemical detection of cell damage
Twenty-four hours after heat treatment, leaves from WT and slmapk3 mutants were used for trypan blue staining analysis [43]. Detached leaves were soaked in 0.4% trypan blue solution at room temperature for 8 h. The photo was taken after decolorizing in boiling fixing liquid (lactic acid: glycerol: ethanol = 1:1:4).
Histochemical detection of ROS
Nitroblue tetrazolium (NBT) and 3,3′-diaminobenzidine (DAB) were used to detect the accumulation of O2•− and H2O2 as performed by Raina et al [44] Twenty-four hours after heat treatment, leaves from WT and slmapk3 mutants were soaked in NBT (1 mg·mL− 1) or DAB (1 mg·mL− 1) solutions at room temperature for 8 h. The photo was taken after decolorizing in boiling 95% (v/v) ethanol.
Determination of antioxidant enzyme activities
The activities of superoxide dismutase (SOD; EC 1.15.1.1), peroxidase (POD; EC 1.11.1.7), catalase (CAT; EC 1.11.1.6), and ascorbate peroxidase (APX; EC 1.11.1.11) were determined as previously described [45–48]. Frozen leaf sample (0.4 g, in powder form) was homogenized using an IKA Disperser in 5 mL of ice-cooled 100 mM potassium phosphate buffer (pH 7.0). The homogenate was centrifuged at 12000 g for 10 min at 4 °C, and then the supernatant was collected and used for antioxidant enzyme assays.
A SOD Detection Kit (A001, Jiancheng, Nanjing, China) was used to assay the SOD activity [45]. POD activity was assayed from the oxidation of guaiacol, and 1 unit of POD activity was defined as the 1 increase in absorbance at 470 nm per minute [46]. CAT activity was assayed from the consumption of H2O2, and 1 unit of CAT activity was defined as the 1 decrease in absorbance at 240 nm per minute [47]. APX activity was assayed by recording the absorbance of ascorbic acid at 290 nm, and 1 unit of CAT activity was defined as the 1 decrease in absorbance at 290 nm per minute [48]. All enzyme activities were calculated based on fresh weight, and were expressed as U·g− 1 FW.
Statistical analysis
All data were obtained from three independent replicates, and the data were expressed as the mean ± standard deviation (SD). All statistical analyses were performed with SPSS 20.0 (IBM Corp., Armonk, NY). The data were analyzed by one-way analysis of variance (ANOVA). Mean separations were performed by Duncan’s multiple range tests. Differences with P < 0.05 were considered to be significant. Pearson’s correlation analysis was performed to determine the correlations among heat stress related physiological indexes. The PLSR model was constructed using the Unscrambler software (CAMO AS., Norway), and PA model was constructed using the SPSS 20.0 (IBM Corp., Armonk, NY).
Additional files
Figure S1. Survival rate of tomato plants described in Fig. 2c. (DOCX 4305 kb)
Table S1. Sequences of specific primers used for qPCR analysis. (DOCX 31 kb)
Acknowledgments
We thank Prof. Yaoguang Liu (College of Life Sciences, South China Agricultural University) for gifting the CRISPR/Cas9 vector.
Abbreviations
- APX
Ascorbate peroxidase
- CAT
Catalase
- CRISPR
Clustered regularly interspaced short palindromic repeats
- DAB
DAB 3,3´diaminobenzidine
- FW
Fresh weight
- H2O2
Hydrogen peroxide
- HAMK
Heat-activated MAP kinase
- HSE
Heat stress elements
- HSFs
Heat stress transcription factors
- HSPs
Heat shock proteins
- MAPKKKs
MAPK kinase kinases
- MAPKKs
MAPK kinases
- MAPKs
Mitogen-activated protein kinases
- MDA
Malondialdehyde
- NBT
Nitroblue tetrazolium
- O2•−
superoxide radical anions
- PA
Path analysis
- PLSR
Partial least square regression analysis
- POD
Peroxidase
- qRT-PCR
quantitative real-time PCR
- RBOH
Respiratory burst oxidase homolog
- ROS
Reactive oxygen species
- SD
Standard deviation
- smHSPs
small HSPs
- SOD
Superoxide dismutase
- WT
Wild-type
Authors’ contributions
WY, and LS conceived and designed the experiments; LW provided slmapk3 mutants; WY, RL and SZ performed the experiments; WY, JS and LS analyzed the data. WY wrote the manuscript. RZ, JS and LS made manuscript revisions. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 31272215, 31371847, and 31571893). The funding agency had no role in the design of the study and collection, analysis, and interpretation of data, and in writing the manuscript.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the manuscript and its additional files, and the raw data is available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tubiello FN, Soussana JF, Howden SM. Crop and pasture response to climate change. Proc Natl Acad Sci U S A. 2007;104:19686–19690. doi: 10.1073/pnas.0701728104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iba K. Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance. Annu Rev Plant Biol. 2002;53:225–245. doi: 10.1146/annurev.arplant.53.100201.160729. [DOI] [PubMed] [Google Scholar]
- 3.Raja V, Majeed U, Kang H, Andrabi KI, John R. Abiotic stress: interplay between ROS, hormones and MAPKs. Environ Exp Bot. 2017;137:142–157. doi: 10.1016/j.envexpbot.2017.02.010. [DOI] [Google Scholar]
- 4.Kong F, Wang J, Cheng L, Liu S, Wu J, Peng Z, Lu G. Genome-wide analysis of the mitogen-activated protein kinase gene family in Solanum lycopersicum. Gene. 2012;499:108–120. doi: 10.1016/j.gene.2012.01.048. [DOI] [PubMed] [Google Scholar]
- 5.Nakagami H, Pitzschke A, Hirt H. Emerging MAP kinase pathways in plant stress signalling. Trends Plant Sci. 2005;10:339–346. doi: 10.1016/j.tplants.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Evrard A, Kumar M, Lecourieux D, Lucks J, von Koskull-Döring P, Hirt H. Regulation of the heat stress response in Arabidopsis by MPK6-targeted phosphorylation of the heat stress factor HsfA2. Peer J. 2013;1:e59. doi: 10.7717/peerj.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu L, Zu X, Zhang H, Wu L, Xi Z, Chen Y. Overexpression of ZmMAPK1 enhances drought and heat stress in transgenic Arabidopsis thaliana. Plant Mol Biol. 2015;88:429–443. doi: 10.1007/s11103-015-0333-y. [DOI] [PubMed] [Google Scholar]
- 8.Liu C, Wei C, Zhang M, Xu Y, Xiang Z, Zhao A. Mulberry MnMAPK1, a group C mitogen-activated protein kinase gene, endowed transgenic Arabidopsis with novel responses to various abiotic stresses. Plant Cell Tiss Org. 2017;131:151–162. doi: 10.1007/s11240-017-1272-x. [DOI] [Google Scholar]
- 9.Ding H, He J, Wu Y, Wu X, Ge C, Wang Y, Zhong S, Peiter E, Liang J, Xu W. The tomato mitogen-activated protein kinase SlMPK1 is as a negative regulator of the high temperature stress response. Physiol Plant. 2018:00067.2018. [DOI] [PMC free article] [PubMed]
- 10.Kandoth PK, Ranf S, Pancholi SS, Jayanty S, Walla MD, Miller W, Howe GA, Lincoln DE, Stratmann JW. Tomato MAPKs LeMPK1, LeMPK2, and LeMPK3 function in the systemin-mediated defense response against herbivorous insects. Proc Natl Acad Sci U S A. 2007;104:12205–12210. doi: 10.1073/pnas.0700344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang S, Wang L, Zhao R, Yu W, Li R, Li Y, Sheng J, Shen L. Knockout of SlMAPK3 reduced disease resistance to Botrytis cinerea in tomato plants. J Agr Food Chem. 2018;66:8949–8956. doi: 10.1021/acs.jafc.8b02191. [DOI] [PubMed] [Google Scholar]
- 12.Mayrose M, Bonshtien A, Sessa G. LeMPK3 is a mitogen-activated protein kinase with dual specificity induced during tomato defense and wounding responses. J Biol Chem. 2004;279:14819–14827. doi: 10.1074/jbc.M313388200. [DOI] [PubMed] [Google Scholar]
- 13.Yu L, Yan J, Yang Y, Zhu W. Overexpression of tomato mitogen-activated protein kinase SlMPK3 in tobacco increases tolerance to low temperature stress. Plant Cell Tiss Org. 2015;121:21–34. doi: 10.1007/s11240-014-0675-1. [DOI] [Google Scholar]
- 14.Wang L, Chen L, Li R, Zhao R, Yang M, Sheng J, Shen L. Reduced drought tolerance by CRISPR/Cas9-mediated SlMAPK3 mutagenesis in tomato plants. J Agr Food Chem. 2017;65:8674–8682. doi: 10.1021/acs.jafc.7b02745. [DOI] [PubMed] [Google Scholar]
- 15.Zang X, Geng X, Wang F, Liu Z, Zhang L, Zhao Y, Tian X, Ni Z, Yao Y, Xin M, Hu Z, Sun Q, Peng H. Overexpression of wheat ferritin gene TaFER-5B enhances tolerance to heat stress and other abiotic stresses associated with the ROS scavenging. BMC Plant Biol. 2017;17:14. doi: 10.1186/s12870-016-0958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song L, Xu X, Wang F, Wang Y, Xia X, Shi K, Zhou Y, Zhou J, Yu J. Brassinosteroids act as a positive regulator for resistance against root-knot nematode involving respiratory burst oxidase homolog-dependent activation of MAPKs in tomato. Plant Cell Environ. 2018;41:1113–1125. doi: 10.1111/pce.12952. [DOI] [PubMed] [Google Scholar]
- 17.Nie W, Wang M, Xia X, Zhou Y, Shi K, Chen Z, Yu JQ. Silencing of tomato RBOH1 and MPK2 abolishes brassinosteroid-induced H2O2 generation and stress tolerance. Plant Cell Environ. 2013;36:789–803. doi: 10.1111/pce.12014. [DOI] [PubMed] [Google Scholar]
- 18.Liu HB, Wang XD, Zhang YY, Dong JJ, Ma C, Chen WL. NADPH oxidase RBOHD contributes to autophagy and hypersensitive cell death during the plant defense response in Arabidopsis thaliana. Biol Plantarum. 2015;59:570–580. doi: 10.1007/s10535-015-0519-9. [DOI] [Google Scholar]
- 19.Wang L, Zhao R, Li R, Yu W, Yang M, Sheng J, Shen L. Enhanced drought tolerance in tomato plants by overexpression of SlMAPK1. Plant Cell Tiss Org. 2018;133:27–38. doi: 10.1007/s11240-017-1358-5. [DOI] [Google Scholar]
- 20.Young JC. Mechanisms of the Hsp70 chaperone system. Biochem Cell Biol. 2010;88:291–300. doi: 10.1139/O09-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hahn A, Bublak D, Schleiff E, Scharf K. Crosstalk between Hsp90 and Hsp70 chaperones and heat stress transcription factors in tomato. Plant Cell. 2012;23:741–755. doi: 10.1105/tpc.110.076018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baniwal SK, Bharti K, Chan KY, Fauth M, Ganguli A, Kotak S, et al. Heat stress response in plants: a complex game with chaperones and more than twenty heat stress transcription factors. J Bio Sci. 2004;29:471–487. doi: 10.1007/BF02712120. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed Mansour ASRD. Membrane-based activation of HSFs by heat shock in tobacco cells. Plant Stress. 2008;2:138–144. [Google Scholar]
- 24.Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Bioch. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Q, Zhou L, Liu J, Cao Z, Du X, Huang F, Pan G, Cheng F. Involvement of CAT in the detoxification of HT-induced ROS burst in rice anther and its relation to pollen fertility. Plant Cell Rep. 2018;37:741–757. doi: 10.1007/s00299-018-2264-y. [DOI] [PubMed] [Google Scholar]
- 26.Soares-Silva M, Diniz FF, Gomes GN, Bahia D. The mitogen-activated protein kinase (MAPK) pathway: role in immune evasion by Trypanosomatids. Front Microbiol. 2016;7:183. doi: 10.3389/fmicb.2016.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blum A, Ebercon A. Cell membrane stability as a measure of drought and heat tolerance in wheat1. Crop Sci. 1981;21:43. doi: 10.2135/cropsci1981.0011183X002100010013x. [DOI] [Google Scholar]
- 28.Hasanuzzaman M, Nahar K, Alam MM, Fujita M. Modulation of antioxidant machinery and the methylglyoxal detoxification system in selenium-supplemented brassica napus seedlings confers tolerance to high temperature stress. Biol Trace Elem Res. 2014;161:297–307. doi: 10.1007/s12011-014-0120-7. [DOI] [PubMed] [Google Scholar]
- 29.Foyer CH, Noctor G. Oxygen processing in photosynthesis: regulation and signalling. New Phytol. 2000;146:359–388. doi: 10.1046/j.1469-8137.2000.00667.x. [DOI] [Google Scholar]
- 30.Camejo D, Rodríguez P, Angeles Morales M. Miguel Dell Amico J, Torrecillas a, Alarcón JJ. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J Plant Physiol. 2005;162:281–289. doi: 10.1016/j.jplph.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Q, Zhou L, Liu J, Du X, Asad M, Huang F, Pan G, Cheng F. Relationship of ROS accumulation and superoxide dismutase isozymes in developing anther with floret fertility of rice under heat stress. Plant Physiol Bioch. 2018;122:90–101. doi: 10.1016/j.plaphy.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Yi C, Yao K, Cai S, Li H, Zhou J, Xia X, Shi K, Yu J, Foyer CH, Zhou Y. High atmospheric carbon dioxide-dependent alleviation of salt stress is linked to respiratory burst oxidase 1 (RBOH1)-dependent H2O2 production in tomato (Solanum lycopersicum) J Exp Bot. 2015;66:7391–7404. doi: 10.1093/jxb/erv435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tutar O, Marín-Guirao L, Ruiz JM, Procaccini G. Antioxidant response to heat stress in seagrasses. A gene expression study. Mar Environ Res. 2017;132:94–102. doi: 10.1016/j.marenvres.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 34.Esposito MP, Nakazato RK, Vaz Pedroso AN, Leite Lima ME, Figueiredo MA, Diniz AP, Kozovits AR, Domingos M. Oxidant-antioxidant balance and tolerance against oxidative stress in pioneer and non-pioneer tree species from the remaining Atlantic Forest. Sci Total Environ. 2018;625:382–393. doi: 10.1016/j.scitotenv.2017.12.255. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Li J, Liu B, Zhang L, Chen J, Lu M. Genome-wide analysis of the Populus Hsp90 gene family reveals differential expression patterns, localization, and heat stress responses. BMC Genomics. 2013;14:532. doi: 10.1186/1471-2164-14-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar RR, Goswami S, Gupta R, Verma P, Singh K, Singh JP, Kumar M, Sharma SK, Pathak H, Rai RD. The stress of suicide: temporal and spatial expression of putative heat shock protein 70 protect the cells from heat injury in wheat (Triticum aestivum) J Plant Growth Regul. 2016;35:65–82. doi: 10.1007/s00344-015-9508-7. [DOI] [Google Scholar]
- 37.Yang J, Sun Y, Sun A, Yi S, Qin J, Li M, Liu J. The involvement of chloroplast HSP100/ClpB in the acquired thermotolerance in tomato. Plant Mol Biol. 2006;62:385–395. doi: 10.1007/s11103-006-9027-9. [DOI] [PubMed] [Google Scholar]
- 38.Mishra SK, Tripp J, Winkelhaus S, Tschiersch B, Theres K, Nover L, Scharf KD. In the complex family of heat stress transcription factors, HSfA1 has a unique role as master regulator of thermotolerance in tomato. Genes Dev. 2002;16:1555–1567. doi: 10.1101/gad.228802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogawa D, Yamaguchi K, Nishiuchi T. High-level overexpression of the Arabidopsis HsfA2 gene confers not only increased themotolerance but also salt/osmotic stress tolerance and enhanced callus growth. J Exp Bot. 2007;58:3373–3383. doi: 10.1093/jxb/erm184. [DOI] [PubMed] [Google Scholar]
- 40.Li Z, Zhang L, Wang A, Xu X, Li J. Ectopic overexpression of SlHsfA3, a heat stress transcription factor from tomato, confers increased thermotolerance and salt hypersensitivity in germination in transgenic Arabidopsis. PLoS One. 2013;8:e54880. doi: 10.1371/journal.pone.0054880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding Z, Tian S, Zheng X, Zhou Z, Xu Y. Responses of reactive oxygen metabolism and quality in mango fruit to exogenous oxalic acid or salicylic acid under chilling temperature stress. Physiol Plant. 2007;130:112–121. doi: 10.1111/j.1399-3054.2007.00893.x. [DOI] [Google Scholar]
- 42.Zhao DY, Shen L, Fan B, Liu KL, Yu MM, Zheng Y, Ding Y, Sheng JP. Physiological and genetic properties of tomato fruits from 2 cultivars differing in chilling tolerance at cold storage. J Food Sci. 2009;74:C348–C352. doi: 10.1111/j.1750-3841.2009.01156.x. [DOI] [PubMed] [Google Scholar]
- 43.Ma X, Chen C, Yang M, Dong X, Lv W, Meng Q. Cold-regulated protein (SlCOR413IM1) confers chilling stress tolerance in tomato plants. Plant Physiol Bioch. 2018;124:29–39. doi: 10.1016/j.plaphy.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Ijaz R, Ejaz J, Gao S, Liu T, Imtiaz M, Ye Z, Wang T. Overexpression of annexin gene AnnSp2, enhances drought and salt tolerance through modulation of ABA synthesis and scavenging ROS in tomato. Sci Rep. 2017;7:12087. doi: 10.1038/s41598-017-11168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L, Zhao R, Zheng Y, Chen L, Li R, Ma J, Hong X, Ma P, Sheng J, Shen L. SlMAPK1/2/3 and antioxidant enzymes are associated with H2O2-induced chilling tolerance in tomato plants. J Agric Food Chem. 2017;65:6812–6820. doi: 10.1021/acs.jafc.7b01685. [DOI] [PubMed] [Google Scholar]
- 46.Doerge DR, Divi RL, Churchwell MI. Identification of the colored guaiacol oxidation product produced by peroxidases. Anal Biochem. 1997;250:10–17. doi: 10.1006/abio.1997.2191. [DOI] [PubMed] [Google Scholar]
- 47.Larrigaudière C, Vilaplana R, Soria Y, Recasens I. Oxidative behaviour of Blanquilla pears treated with 1-methylcyclopropene during cold storage. J Sci Food Agric. 2004;84:1871–1877. doi: 10.1002/jsfa.1850. [DOI] [Google Scholar]
- 48.Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Survival rate of tomato plants described in Fig. 2c. (DOCX 4305 kb)
Table S1. Sequences of specific primers used for qPCR analysis. (DOCX 31 kb)
Data Availability Statement
The datasets supporting the conclusions of this article are included within the manuscript and its additional files, and the raw data is available from the corresponding author on reasonable request.