Abstract
Background
Risk-based screening in women 40–49 years old has not been evaluated in routine screening mammography practice.
Purpose
To use a cross-sectional study design to compare the trade-offs of risk-based and age-based screening for women 45 years of age or older to determine short-term outcomes.
Materials and Methods
A retrospective cross-sectional study was performed by using a database of 20 539 prospectively interpreted consecutive digital screening mammograms in 10 280 average-risk women aged 40–49 years who were screened at an academic medical center between January 1, 2006, and December 31, 2013. Two hypothetical screening scenarios were compared: an age-based (≥45 years) scenario versus a risk-based (a 5-year risk of breast cancer greater than that of an average 50-year-old) scenario. Risk factors for risk-based screening included family history, race, age, prior breast biopsy, and breast density. Outcomes included breast cancers detected at mammography, false-positive mammograms, and benign biopsy findings. Short-term outcomes were compared by using the χ2 test.
Results
The screening population included 71 148 screening mammograms in 24 928 women with a mean age of 55.5 years ± 8.9 (standard deviation) (age range, 40–74 years). In women 40–49 years old, usual care included 50 screening-detected cancers, 1787 false-positive mammograms, and 384 benign biopsy results. The age-based (≥45 years) screening strategy revealed more cancers than did the risk-based strategy (34 [68%] vs 13 [26%] of 50; P < .001), while prompting more false-positive mammograms (899 [50.3%] vs 216 [12.1%] of 1787; P < .001) and benign biopsy results (175 [45.6%] vs 49 [12.8%] of 384; P < .001). The risk-based strategy demonstrated low levels of eligibility (few screenings) in the 40–44-year age group. Differences in outcomes in the 45–49-year age group explained the overall hypothetical screening strategy differences.
Conclusion
Risk-based screening for women 40–49 years old includes few women in the 40–44-year age range. Significant trade-offs in the 45–49-year age group explain the overall difference between hypothetical screening scenarios, both of which reduce the benefits as well as the harms of mammography for women 40–49 years old.
© RSNA, 2019
Online supplemental material is available for this article.
See also the editorial by Joe and Hayward in this issue.
Summary
Hypothetical screening scenarios show that for women 40–49 years old, initiating age-based mammography screening starting at age 45 reveals more cancers and results in more false-positive mammograms and benign biopsy results than risk-based screening; however, short-term outcome differences depended on age range: 40–44-year-olds in both scenarios had similar outcomes, which resembled those of not screening, while 45–49-year-olds showed significant differences for all outcomes.
Key Points
■ Age-based mammography screening beginning at age 45 detected more cancers than risk-based screening (34 vs 13 of 50; P < .001), while prompting more false-positive mammograms (899 vs 216 of 1787; P < .001) and benign biopsy results (175 vs 49 of 384; P < .001).
■ Both of the hypothetical screening scenarios of age-based (≥45 years) screening and risk-based screening decreased the benefits as well as the harms of screening women 40–49 years old.
■ Significant differences between hypothetical screening scenarios in terms of cancers detected, false-positive mammograms, and benign biopsy results were driven by outcome divergence in the 45–49-year age range because screening is comparably low in the 40–44-year age range.
Introduction
Breast cancer is the leading cause of death for women 35–49 years old (1,2). Mammography reduces mortality for women between the ages of 40 and 49 years, but the benefits-to-harms ratio described in the literature is less favorable compared with that in women in older age ranges (3–5). The United States Preventive Services Task Force recommends that women 40–49 years old forgo screening unless motivated by risk or values when weighing potential benefits and harms (5). The American Cancer Society (ACS) encourages the option to screen starting at age 40 years considering personal risk and recommends annual screening starting at age 45 (4).
Risk-based screening in women 40–49 years old aims to balance the benefits with harms: early cancer detection and mortality reduction with false-positive mammograms, benign biopsy results, overdiagnosis, and associated anxiety (6). However, risk-based protocols may eliminate the mortality benefits of screening (7). Risk-based screening policies promote the use of breast cancer risk to determine the age to start screening in women younger than 50 years (4,5) and to inform the screening interval in women in age groups starting at 50 years and older (8). In this study, we focus on the former.
Risk prediction models designed to personalize mammography screening include personal and familial risk factors (9–11), which have been validated in case-control data (12) but less frequently in screening populations (13). A substantial number of women diagnosed with breast cancer do not exhibit breast cancer risk factors (14).
Virtually all mammography screening trials use an age-based rather than a risk-based recruitment strategy, emphasizing the importance of studying the effectiveness of risk-based breast cancer screening strategies prior to implementation. Growing interest in risk-based screening creates an urgent mandate to determine effectiveness (4–6,15). The Women Informed to Screen Depending on Measures of Risk, or WISDOM, trial (NCT02620852) will examine the effectiveness of a personalized, risk-based approach to breast cancer screening (16), with the risk of a 50-year-old woman as a threshold. However, in light of the expense and long-term commitment of randomized trials, observational studies can also assess risk-based screening in diverse and real-world environments (17). We examined short-term outcomes, meaning the immediate sequelae of the screening examination, including benefits (namely, screening-detected cancer) and harms (namely, false-positive mammograms and benign biopsy results), and did not examine the long-term outcome of mortality. We hypothesized that risk-based screening in this age group will reduce both short-term benefits and harms compared with age-based (≥45 years) screening. Our purpose was to compare the trade-offs of risk-based and age-based screening for women 45 years of age or older.
Materials and Methods
The University of Wisconsin Health Sciences Institutional Review Board waived the need to obtain written informed consent for this Health Insurance Portability and Accountability Act–compliant database. Our study population was a subpopulation of a larger radiologic-epidemiologic database (details of which have been previously published [18]).
We derived the following two hypothetical cohorts: (a) a risk-based screening cohort of women 40–49 years old and (b) an age-based (≥45 years) screening cohort from a screening program that allows screening mammography from age 40 years.
Definitions and Measures
We used the Breast Cancer Surveillance Consortium (BCSC) Risk Calculator (9,10,16,19) to identify women who were eligible for risk-based screening. The BCSC model (https://tools.bcsc-scc.org/BC5yearRisk/intro.htm) uses variables to calculate 5- and 10-year risks of invasive breast cancer. These variables include age, family history of breast cancer in a first-degree relative, race/ethnicity, history of prior breast biopsy, and the American College of Radiology Breast Imaging Reporting and Data System, or BI-RADS, density category. Risk factors included in the BCSC Risk Calculator model were either clinically available or were routinely collected in our practice at the time of mammography.
Family history of breast cancer in a first-degree relative (mother, daughter, sister), race/ethnicity, and number of breast biopsies were all obtained from each woman’s self-report on a clinical intake form at the time of mammography. The breast biopsy variable in the BCSC calculator allows the following entries: none (no prior biopsy); prior biopsy, unknown diagnosis; nonproliferative; proliferative without atypia; proliferative with atypia; and lobular carcinoma in situ (19). Detailed breast pathologic findings at prior breast biopsy are difficult to collect by self-report; thus, we used what each woman could report: either “none (no prior biopsy)” or “prior benign biopsy, unknown diagnosis.”
We considered a mammogram to be a woman’s baseline (first) mammogram if no prior examinations were cited in the current mammography report and no prior screening mammography reports were available in our system (PenRad Mammography module [commercially available], version 6; PenRad Technologies, Buffalo, Minn). Like investigators in previously published studies, we categorized mammographic intervals on the basis of the date of the prior screening mammogram as annual (9–18 months), biennial (19–30 months), or longer than biennial (>30 months) (20,21). Breast density was derived from each woman’s prior mammography report and was categorized by using a scale on which a level of A indicated almost entirely fat; a level of B, scattered fibroglandular density; a level of C, heterogeneously dense; and a level of D, extremely dense. Breast density would not be available for risk calculation before a baseline mammogram. Therefore, for baseline studies, we considered this variable unknown. In addition, if the prior mammography report did not contain information on breast density (a rare circumstance) (Table 1), we also performed risk assessment using “unknown” for the breast density variable.
Table 1:
Distribution of Risk Factors Recorded for Screening Mammograms by Age
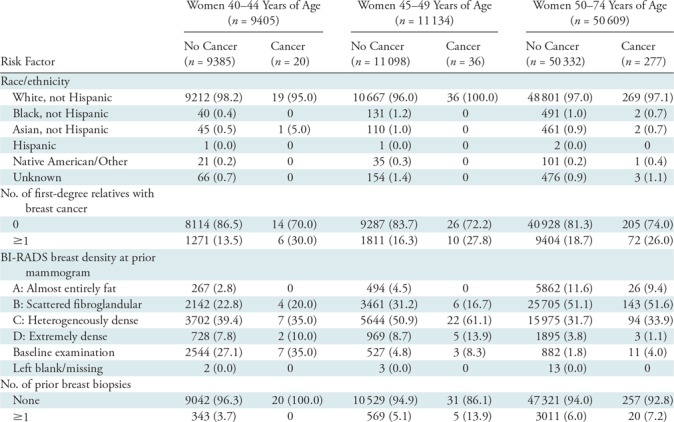
Note.—Data are numbers of women, with percentages in parentheses. BI-RADS = Breast Imaging Reporting and Data System.
Study Population
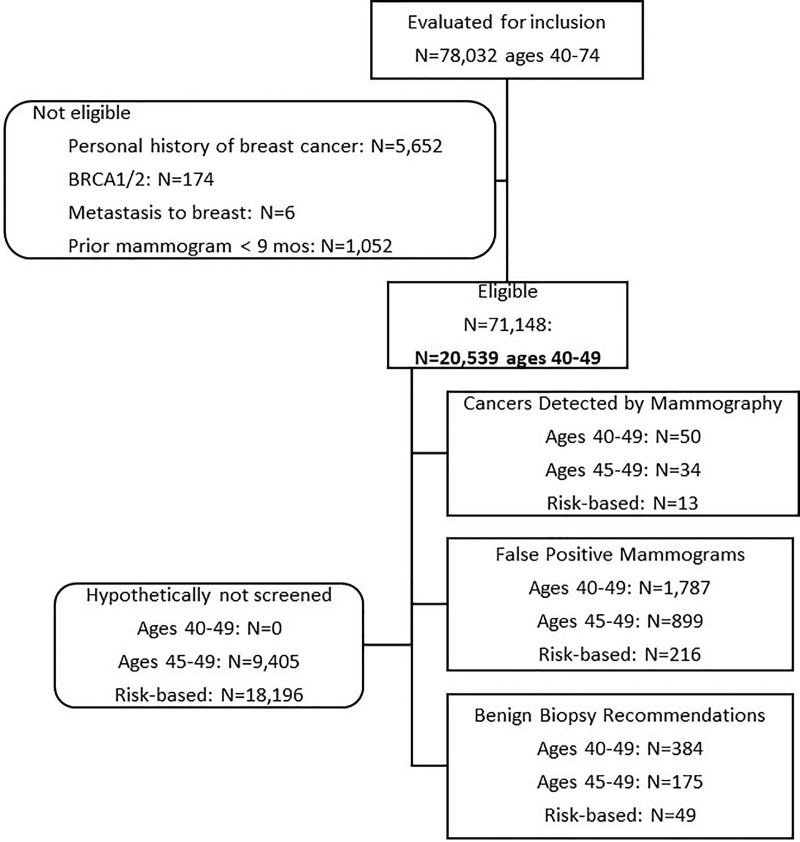
We included prospectively interpreted consecutive screening mammograms as recorded in PenRad that were acquired in average-risk women 40–49 years old at our academic medical center from January 1, 2006, to December 31, 2013. The appropriate population for risk-based screening is women at an average rather than high risk for breast cancer. In fact, high-risk women may be screened by using protocols that include supplemental modalities like MRI. Therefore, we excluded mammograms in high-risk women (ie, those with a personal history of breast cancer [n = 5652] or a known high-risk genetic mutation like BRCA [n = 174]). We also excluded screening mammograms in women who had undergone mammography within the previous 9 months to avoid misclassifying a diagnostic examination as a screening examination (20), and we excluded mammograms that resulted in a diagnosis of non-breast cancers metastatic to the breast (n = 6) (Fig 1).
Figure 1:
Flow diagram enumerates the mammograms included in this study.
First, we constructed an age-based (≥45 years) screening scenario similar to the ACS guidelines (4) by removing all women younger than 45 years of age. Second, we constructed a risk-based screening strategy by designating the Surveillance, Epidemiology, and End Results, or SEER, average race-specific risk of invasive breast cancer plus ductal carcinoma in situ (DCIS) for a 50-year-old woman as a threshold for screening eligibility. For example, the average SEER 5-year risk for white women is 1.23% (16) for invasive breast cancer and 0.35% for DCIS; therefore, the threshold for risk-based screening was the sum of these two risks: 1.58%.
Outcomes
We took a population perspective, viewing outcomes from the vantage point of the screening program as a whole. Breast cancer characteristics (invasive cancer vs DCIS, estrogen and progesterone receptor status, nodal status, and grade) were obtained by matching data with our Comprehensive Cancer Center cancer registry, which draws from the local health system and checks this population against the Wisconsin statewide cancer registry (22). If the registry recorded a diagnosis of invasive breast cancer or DCIS within 12 months after a mammogram, the mammogram, if positive, was a true-positive result, or, if negative, was a false-negative result. False-positive results included a false-positive screening mammogram (a recommendation for additional testing) or a benign biopsy result and no registry match within 12 months.
Our primary outcome variables were numbers of cancers detected at mammography, false-positive mammograms, and benign biopsy results. The secondary outcome was the proportion of DCIS diagnosed at screening. Although we did not do statistical comparisons to our screening practice starting at age 40 years, for each hypothetical screening scenario, we calculated the following: mammograms not obtained, mammographically detectable cancers not diagnosed, false-positive mammograms avoided, and benign biopsy results avoided. We also calculated the time from the actual screening detection to the time that the woman became eligible for the given hypothetical screening scenario (age based or risk based) to determine the potential time lapse for screening detection associated with each strategy.
The cancer detection rate and the recall rate were calculated on the basis of the Breast Imaging Reporting and Data System, or BI-RADS, 5th Edition (23). All physicians interpreting mammograms in the context of routine clinical care (n = 19, including E.S.B.) were certified under the Mammography Quality Standards Act and had 5–15 years of experience; four were breast imaging fellowship trained.
Statistical Analysis
We limited our analysis to predefined primary and secondary outcome variables for the two hypothetical screening strategies using the χ2 statistical test, defining a P value of less than .05 as indicating a statistically significant difference. We also estimated 95% confidence intervals (CIs) using generalized estimating equations, with an independence correlation structure to account for correlation among examinations for the same woman (24). We did not adjust for multiplicity of inferences in the study. We calculated the accuracy of the BCSC model in terms of discrimination and calibration to predict invasive cancer for comparison with prior literature (12,19). We calculated the discrimination by using areas under the receiver operating characteristic curve (AUCs) and 95% CIs, using logistic regression for the BCSC 5-year breast cancer risk estimate. We determined the calibration of the BCSC risk prediction model by calculating the ratio of the expected breast cancers to the observed breast cancers in the subset of mammograms obtained from 2006 to 2009, because 5-year outcomes were available in this group. Statistical computations were performed by using SAS, version 9.4 (SAS Institute, Cary, NC). To account for dependencies between women who had more than one mammogram, we performed a similar analysis with one randomly selected mammogram per individual woman. We included all baseline mammograms, which are associated with higher rates of false-positive results than subsequent mammograms (for which comparison to prior mammograms is available [25]) in a secondary analysis (Appendix E1 [online]).
Results
The mean age at screening mammography for our screening population, which included 71 148 screening mammograms in 24 928 women, was 55.5 years ± 8.9 (age range, 40–74 years) (Fig 1). The younger age group (40–49 years) was comparable to the overall screening population in terms of race and ethnicity and demonstrated the following expected differences: higher breast density, less family history of breast cancer, and fewer breast biopsies (Table 1). The 56 women aged 40–49 years given a diagnosis of breast cancer showed a higher proportion of known risk factors than did women without breast cancer in each age group. Fifty of the 56 cancers were screening detected—there were 33 invasive cancers (24 local, seven regional, and two unclassified) and 17 instances of DCIS.
Age-based versus Risk-based Screening Outcomes
In women 40–49 years old, age-based (≥45 years) screening (Table 2) detected more cancers than did risk-based screening (34 of 50; 68%; 95% CI: 55.1%, 80.9% vs 13 of 50; 26%; 95% CI: 13.8%, 38.2%) (P < .001), while prompting more false-positive mammograms (899 of 1787; 50.3%; 95% CI: 48.0%, 52.7% vs 216 of 1787; 12.1%; 95% CI: 10.6%, 13.6%) (P < .001) and benign biopsy results (175 of 384; 45.6%; 95% CI: 40.6%, 50.6% vs 49 of 384; 12.8%; 95% CI: 9.4%, 16.1%) (P < .001). The proportion of breast cancers diagnosed as DCIS at mammography in the risk-based strategy was greater than that in the age-based strategy (five of 13; 38.5%; 95% CI: 12.0%, 64.9% vs 10 of 34; 29.4%; 95% CI: 14.1%, 44.7%) (P = .05). We demonstrated similar results when we included only one randomly selected record per woman within the 40–49-year age range and all baseline mammograms were included in the hypothetical scenarios (Appendix E1 [online]).
Table 2:
Short-term Outcomes of Mammography in Women 40–49 Years of Age
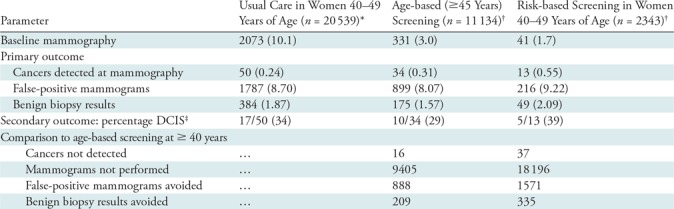
Note.—Unless otherwise specified, data are numbers of women, with percentages in parentheses. DCIS = ductal carcinoma in situ.
*Observed usual-care screening outcomes.
†Hypothetical screening strategies in women 40–49 years of age.
‡No. of instances of DCIS detected/total no. of cancers detected at mammography.
Age-based (≥45 years) screening included 6390 (62.2%; 95% CI: 61.2%, 63.1%) of the 10 280 women aged 40–49 years and 11 134 (54.2%; 95% CI: 53.5%, 54.9%) of the 20 539 mammograms, while risk-based screening included 1339 (13.0%; 95% CI: 12.4%, 13.7%) of the 10 280 women aged 40–49 years and 2343 (11.4%; 95% CI: 11.0%, 11.8%) of the 20 539 mammograms (Fig 2). The differences between hypothetical age-based (≥45 years) and risk-based screening included 21 of 50 detectable cancers (42.0%; 95% CI: 28.3%, 55.7%), 683 of 1787 false-positive mammograms (38.2%; 95% CI: 36.0%, 40.5%), and 126 of 384 benign biopsy results (32.8%; 95% CI: 28.1%, 37.5%). Of the 50 screening-detected cancers diagnosed in the 40–49-year age group, 13 (26.0%; 95% CI: 13.8%, 38.2%) were detected in women who had no risk factors.
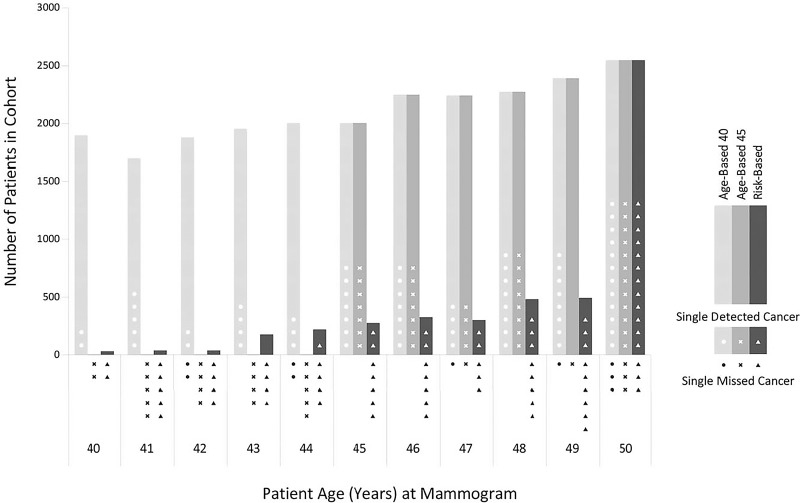
Figure 2:
Bar graph shows the numbers of mammograms in usual care (screening at ages 40–49 years) (light gray bar) and for the hypothetical screening strategies of age-based (≥45 years) screening (medium gray bar) and risk-based screening (dark gray bar). The shapes within or below the respective bars represent screening-detectable breast cancers either detected at mammography (white shapes) or not detected at mammography (black shapes) according to screening strategy.
In women 40–44 years old, 16 cancers (nine invasive, seven DCIS) were detected on 9405 mammograms in our screening practice starting at age 40 years. In the 40–44-year age group, by definition, age-based (≥45 years) screening included no mammograms, no recalls, no biopsies, and no cancers detected. Risk-based screening included 480 mammograms, 46 recalls, 12 biopsies, and a single cancer detected (a case of DCIS).
The majority of cancers in our study were estrogen receptor positive and lymph node negative, with cancer characteristics for each hypothetical screening scenario listed in Table 3. The difference in time-to-detection that would have occurred between cancer detection in our screening practice starting at age 40 years and aged-based program eligibility varied from 1 year to 5 years. This same difference for the risk-based program varied between 1 year to 10 years. The average hypothetical delay for risk-based screening (3.9 years) was longer than that for age-based (≥45 years) screening (2.4 years). The time difference between actual detection and eligibility for a given hypothetical scenario did not appear to differ according to tumor subtype (invasive vs DCIS), hormone receptor status, or nodal status (Table 3).
Table 3:
Cancer Characteristics and Time to Detection in Hypothetical Scenarios
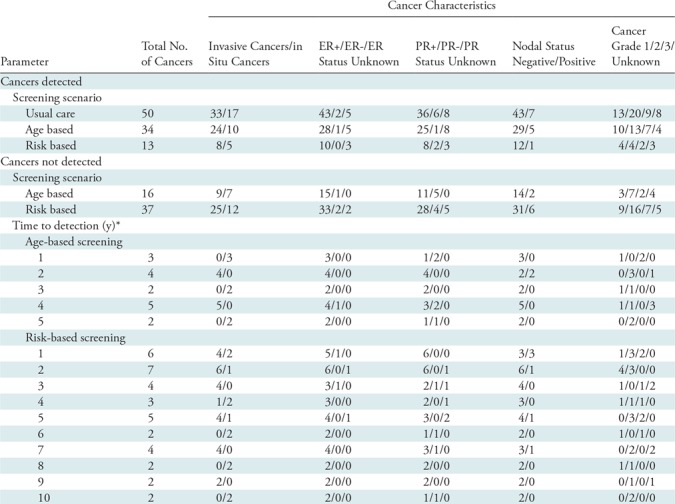
Note.—Data are numbers of cancers with a given characteristic. ER = estrogen receptor, PR = progesterone receptor.
*The time difference between actual detection in the screening program starting at age 40 years and the hypothetical scenario (age-based or risk-based) eligibility.
Risk Model Discrimination and Calibration
In screening mammograms in women aged 40–74 years, the BCSC model produced an AUC of 0.61 (95% CI: 0.58, 0.65) and a calibration expected/observed ratio of 1.06. In women aged 40–49 years, the BCSC model demonstrated an AUC of 0.678 (95% CI: 0.595, 0.761) and a calibration expected/observed ratio of 0.91.
Screening Practice and Performance
In women aged 40–74 years, 42 332 (59.5%; 95% CI: 59.1%, 59.9%) of 71 148 mammograms were annual, 16 729 (23.5%; 95% CI: 23.2%, 23.8%) were biennial, and 8113 (11.4%; 95% CI: 11.2%, 11.6%) were greater than biennial. In women aged 40–49 years, 9218 (44.9%; 95% CI: 44.2%, 45.6%) of 20 539 mammograms were annual, 5324 (25.9%; 95% CI: 25.3%, 26.5%) were biennial, and 2916 (14.2%; 95% CI: 13.7%, 14.7%) were greater than biennial. The majority of baseline mammograms were obtained in the 40–49-year age group (n = 3081) as compared with those in the 50–74 age group (n = 893). Performance metrics for all 71 148 mammograms showed a cancer detection rate of 4.19 per 1000 women and a recall rate of 8.19%.
Discussion
From a population perspective, our study showed that age-based (≥45 years) screening detected more cancers with more false-positive mammograms and benign biopsy results compared with a risk-based strategy. Because almost all women younger than 45 years old have a breast cancer risk lower than that of an average 50-year-old according to the Breast Cancer Surveillance Consortium (BCSC) model, risk-based screening in the 40–44-year age group generated fewer mammograms and detected only one out of 16 screening-detectable cancers, making the age-based (≥45 years) and the risk-based scenarios similar in this younger age range. Our study illustrates the short-term implications of changing practice from screening starting at age 40 years to either an American Cancer Society-like age-based (≥45 years) strategy or a WISDOM-like, risk-based strategy. Because we focused on short-term outcomes, we cannot know the long-term impact of undetected cancers. If a woman has a mammographically detectable cancer but is not eligible for screening, the impact of undetected cancers depends on the length of the diagnostic delay and tumor behavior, which tends to be more aggressive in younger women. A substantial delay would increase the likelihood that a cancer would be detected clinically, be larger, and be node positive.
A risk-based screening strategy depends on the accuracy of the risk model used and the threshold chosen. Performance for the BCSC model is available only for women 40–74 years of age (12,19). For women aged 40–74 years in our study, the BCSC model achieved an area under the receiver operating characteristic curve (AUC) of 0.614 for the task of discriminating whether a woman would develop breast cancer in 5 years—slightly lower than prior studies showing AUCs of 0.66 (95% CI: 0.61, 0.70) (12) and 0.664 (19). However, the AUC for our study population in the 40–49-year age group of 0.678 is comparable. For women aged 40–74 years in our study, the BCSC model calibration showed an expected/observed ratio of 1.06, similar to the ratio of 1.04 published previously (19). However, the expected/observed ratio for our study population in the 40–49-year age group of 0.91 is somewhat lower. We chose the risk of an average 50-year-old for invasive breast cancer plus ductal carcinoma in situ (DCIS) as our risk-based eligibility threshold. Although risk-based screening may provide the opportunity to align the decision to undergo mammography with women’s risk (and perhaps personal values and preferences if effective shared decision making is available), risk-based screening will be inherently more complicated to perform compared with age-based (≥45 years) screening (26–28), a topic that this study does not address. The decision to include DCIS may have resulted in the slightly higher proportion of DCIS in the risk-based scenario. When we included baseline mammograms in the hypothetical scenario results, conclusions do not change. Including baseline mammograms is clinically reasonable, reflecting a screening strategy that would enable breast density assessed on baseline mammograms (obtained in women older than 40 years, but otherwise without limitations of risk or age considerations), to influence the decision about future screening.
Our approach had some notable strengths. We showed that systematic risk factor collection in the clinic at the time of mammography is practical. Our method minimizes the problem of recall bias encountered in cross-sectional study designs. Importantly, our practice is well within performance benchmarks for screening mammography (23,29,30).
Our limitations included the lack of mortality follow-up, which precluded us from drawing conclusions on long-term outcomes. Potential biases of a retrospective, nonrandomized, observational study required us to draw conclusions with caution, although such investigations have been shown to provide accurate outcomes data (31,32). Reliance on patient self-report for previous breast biopsy information meant we could not collect detailed pathology data, which may have improved risk prediction performance; however, we demonstrated that the BCSC risk calculator performance is comparable to that in prior literature (12,19). In our primary analysis, hypothetical screening scenarios disproportionately excluded baseline mammograms in younger age groups, thereby potentially underestimating false-positive mammograms. However, subsequent analysis including all baseline examinations reinforced our initial results and conclusion. Our single-institution study had limited precision because of its modest sample size. Our study population had a high proportion of white women (the population of our screening mammography practice and catchment area), so results may not be generalizable to a more diverse group. We did not shed light on patient decision making or the practicality or cost of program implementation. We included only women reporting for screening rather than all women in the health system or region (ie, those that elect/do not elect to undergo screening).
In conclusion, we demonstrated that outcomes in an age-based (≥45 years) screening strategy versus a risk-based screening strategy in a high-quality screening practice differed significantly in the 45–49-year age group but not in the 40–44-year age group. The risk-based screening strategy exposed more women to delays in diagnosis prior to eligibility, and these delays were, on average, longer than those with age-based (≥45 years) screening. However, savings related to lower mammogram utilization and fewer false-positive mammograms and benign biopsy results accompany these missed detections. Understanding the projected differences in short-term outcomes between age-based and risk-based strategies—in the context of screening starting at 40 years of age—will enable researchers to predict outcomes that might occur in prospective studies. When choosing a screening strategy, physicians, patients, and policymakers will need to determine whether the trade-offs in both short-term and long-term outcomes are acceptable, while also weighing the practicality and complexity of each strategy.
APPENDIX
Acknowledgments
Acknowledgement
We thank Elizabeth A. Simcock, MS, for figure development and graphic design.
Supported by the National Institutes of Health (NIH) (K24CA194251 and U54AI117924), the Clinical and Translational Science Award program through the NIH National Center for Advancing Translational Sciences (UL1TR000427), the University of Wisconsin Carbone Cancer Center (P30CA014520), and the School of Medicine and Public Health University of Wisconsin-Madison Wisconsin Partnership Program.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the other funding sources listed above. Design and conduct of the study; data collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication was initiated by the study team and was not directly influenced by the funding sources listed above.
Disclosures of Conflicts of Interest: E.S.B. disclosed no relevant relationships. A.T. disclosed no relevant relationships. C.M.S. disclosed no relevant relationships. J.M.H. disclosed no relevant relationships. O.A. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for Biovector. Other relationships: disclosed no relevant relationships. J.R.C. disclosed no relevant relationships. E.M. disclosed no relevant relationships. S.B.S. disclosed no relevant relationships. L.G.W. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is the founder of and holds stock options in Elucent Medical; holds patents with Elucent Medical; institution has received support from Perimeter for a margin assessment clinical trial. Other relationships: disclosed no relevant relationships.
Abbreviations:
- ACS
- American Cancer Society
- AUC
- area under the receiver operating characteristic curve
- BCSC
- Breast Cancer Surveillance Consortium
- CI
- confidence interval
- DCIS
- ductal carcinoma in situ
References
- 1. What are the top causes of death by age and gender? http://visual.ons.gov.uk/what-are-the-top-causes-of-death-by-age-and-gender/. Accessed October 26, 2018 .
- 2. Leading Causes of Death in Females, 2014 (current listing) . https://www.cdc.gov/women/lcod/2014/all-females/index.htm. Accessed October 26, 2018 .
- 3. NHS Public Health Function Agreement 2015-16: Breast Screening Programme . London, England: : Department of Health; , 2014. . https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/383198/1516_No24_Breast_Screening_Programme_FINAL.pdf. Accessed October 26, 2018 . [Google Scholar]
- 4. Oeffinger KC, Fontham ET, Etzioni R, et al . Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society . JAMA 2015. ; 314 ( 15 ): 1599 – 1614 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Siu AL; U.S. Preventive Services Task Force . Screening for breast cancer: U.S. preventive services task force recommendation statement . Ann Intern Med 2016. ; 164 ( 4 ): 279 – 296 . [DOI] [PubMed] [Google Scholar]
- 6. Kerlikowske K, O’Kane ME, Esserman LJ. . Fifty years of age-based screening: time for a new risk-based screening approach . Evid Based Med 2014. ; 19 ( 5 ): 183 . [DOI] [PubMed] [Google Scholar]
- 7. Yen AM, Tsau HS, Fann JC, et al . Population-based breast cancer screening with risk-based and universal mammography screening compared with clinical breast examination: a propensity score analysis of 1 429 890 Taiwanese women . JAMA Oncol 2016. ; 2 ( 7 ): 915 – 921 . [DOI] [PubMed] [Google Scholar]
- 8. Trentham-Dietz A, Kerlikowske K, Stout NK, et al . Tailoring breast cancer screening intervals by breast density and risk for women aged 50 years or older: collaborative modeling of screening outcomes . Ann Intern Med 2016. ; 165 ( 10 ): 700 – 712 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Breast Cancer Surveillance Consortium . Breast Cancer Surveillance Consortium Risk Calculator . https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm. Published 2015. Accessed October 26, 2018 . [Google Scholar]
- 10. Tice JA, Cummings SR, Smith-Bindman R, Ichikawa L, Barlow WE, Kerlikowske K. . Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model . Ann Intern Med 2008. ; 148 ( 5 ): 337 – 347 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tyrer J, Duffy SW, Cuzick J. . A breast cancer prediction model incorporating familial and personal risk factors . Stat Med 2004. ; 23 ( 7 ): 1111 – 1130 . [DOI] [PubMed] [Google Scholar]
- 12. Vachon CM, Pankratz VS, Scott CG, et al . The contributions of breast density and common genetic variation to breast cancer risk . J Natl Cancer Inst 2015. ; 107 ( 5 ): 1 – 4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brentnall AR, Harkness EF, Astley SM, et al . Mammographic density adds accuracy to both the Tyrer-Cuzick and Gail breast cancer risk models in a prospective UK screening cohort . Breast Cancer Res 2015. ; 17 ( 1 ): 147 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Price ER, Keedy AW, Gidwaney R, Sickles EA, Joe BN. . The potential impact of risk-based screening mammography in women 40-49 years old . AJR Am J Roentgenol 2015. ; 205 ( 6 ): 1360 – 1364 . [DOI] [PubMed] [Google Scholar]
- 15. Onega T, Beaber EF, Sprague BL, et al . Breast cancer screening in an era of personalized regimens: a conceptual model and National Cancer Institute initiative for risk-based and preference-based approaches at a population level . Cancer 2014. ; 120 ( 19 ): 2955 – 2964 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shieh Y, Eklund M, Madlensky L, et al . Breast cancer screening in the precision medicine era: risk-based screening in a population-based trial . J Natl Cancer Inst 2017. ; 109 ( 5 ): djw290 . [DOI] [PubMed] [Google Scholar]
- 17. Nickson C, Mason KE, English DR, Kavanagh AM. . Mammographic screening and breast cancer mortality: a case-control study and meta-analysis . Cancer Epidemiol Biomarkers Prev 2012. ; 21 ( 9 ): 1479 – 1488 . [DOI] [PubMed] [Google Scholar]
- 18. Wu Y, Rubin DL, Woods RW, Elezaby M, Burnside ES. . Developing a comprehensive database management system for organization and evaluation of mammography datasets . Cancer Inform 2014. ; 13 ( Suppl 3 ): 53 – 62 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tice JA, Miglioretti DL, Li CS, Vachon CM, Gard CC, Kerlikowske K. . Breast density and benign breast disease: risk assessment to identify women at high risk of breast cancer . J Clin Oncol 2015. ; 33 ( 28 ): 3137 – 3143 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hubbard RA, Kerlikowske K, Flowers CI, Yankaskas BC, Zhu W, Miglioretti DL. . Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study . Ann Intern Med 2011. ; 155 ( 8 ): 481 – 492 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dittus K, Geller B, Weaver DL, et al . Impact of mammography screening interval on breast cancer diagnosis by menopausal status and BMI . J Gen Intern Med 2013. ; 28 ( 11 ): 1454 – 1462 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Foote M. . Wisconsin Cancer Reporting System: a population-based registry . WMJ 1999. ; 98 ( 4 ): 17 – 18 . [PubMed] [Google Scholar]
- 23. American College of Radiology . Breast Imaging Reporting and Data System (BI-RADS) . 4th ed. Reston, Va: : American College of Radiology; , 2003. . [Google Scholar]
- 24. Liang KY, Zeger SL. . Longitudinal data analysis using generalized linear models . Biometrika 1986. ; 73 ( 1 ): 13 – 22 . [Google Scholar]
- 25. Horsley RK, Kling JM, Vegunta S, Lorans R, Temkit H, Patel BK. . Baseline mammography: what is it and why is it important? a cross-sectional survey of women undergoing screening mammography . J Am Coll Radiol 2019. ; 16 ( 2 ): 164 – 169 . [DOI] [PubMed] [Google Scholar]
- 26. Collins IM, Steel E, Mann GB, et al . Assessing and managing breast cancer risk: clinicians’ current practice and future needs . Breast 2014. ; 23 ( 5 ): 644 – 650 . [DOI] [PubMed] [Google Scholar]
- 27. Schapira MM, Neuner J, Fletcher KE, Gilligan MA, Hayes E, Laud P. . The relationship of health numeracy to cancer screening . J Cancer Educ 2011. ; 26 ( 1 ): 103 – 110 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Evans DG, Donnelly LS, Harkness EF, et al . Breast cancer risk feedback to women in the UK NHS breast screening population . Br J Cancer 2016. ; 114 ( 9 ): 1045 – 1052 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carney PA, Sickles EA, Monsees BS, et al . Identifying minimally acceptable interpretive performance criteria for screening mammography . Radiology 2010. ; 255 ( 2 ): 354 – 361 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lehman CD, Arao RF, Sprague BL, et al . National performance benchmarks for modern screening digital mammography: update from the breast cancer surveillance consortium . Radiology 2017. ; 283 ( 1 ): 49 – 58 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Concato J, Shah N, Horwitz RI. . Randomized, controlled trials, observational studies, and the hierarchy of research designs . N Engl J Med 2000. ; 342 ( 25 ): 1887 – 1892 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Demissie K, Mills OF, Rhoads GG. . Empirical comparison of the results of randomized controlled trials and case-control studies in evaluating the effectiveness of screening mammography . J Clin Epidemiol 1998. ; 51 ( 2 ): 81 – 91 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.