Abstract
Background
The prevalence of dementia, which presents as cognitive decline in one or more cognitive domains affecting function, is increasing worldwide. Traditional cognitive screening tools for dementia have their limitations, with emphasis on memory and, to a lesser extent, on the cognitive domain of executive function. The use of virtual reality (VR) in screening for cognitive function in older persons is promising, but evidence for its use is sparse.
Objective
The primary aim was to examine the feasibility and acceptability of using VR to screen for cognitive impairment in older persons in a primary care setting. The secondary aim was to assess the module’s ability to discriminate between cognitively intact and cognitively impaired participants.
Methods
A comparative study was conducted at a public primary care clinic in Singapore, where persons aged 65-85 years were recruited based on a cut-off score of 26 on the Montreal Cognitive Assessment (MoCA) scale. They participated in a VR module for assessment of their learning and memory, perceptual-motor function, and executive function. Each participant was evaluated by the total performance score (range: 0-700) upon completion of the study. A questionnaire was also administered to assess their perception of and attitude toward VR.
Results
A total of 37 participants in Group 1 (cognitively intact; MoCA score≥26) and 23 participants in Group 2 (cognitively impaired; MoCA score<26) were assessed. The mean time to completion of the study was 19.1 (SD 3.6) minutes in Group 1 and 20.4 (3.4) minutes in Group 2. Mean feedback scores ranged from 3.80 to 4.48 (max=5) in favor of VR. The total performance score in Group 1 (552.0, SD 57.2) was higher than that in Group 2 (476.1, SD 61.9; P<.001) and exhibited a moderate positive correlation with scores from other cognitive screening tools: Abbreviated Mental Test (0.312), Mini-Mental State Examination (0.373), and MoCA (0.427). A receiver operating characteristic curve analysis for the relationship between the total performance score and the presence of cognitive impairment showed an area under curve of 0.821 (95% CI 0.714-0.928).
Conclusions
We demonstrated the feasibility of using a VR-based screening tool for cognitive function in older persons in primary care, who were largely in favor of this tool.
Keywords: virtual reality, feasibility studies, mental status and dementia tests, technology, video games, dementia, cognitive dysfunction
Introduction
Background and Rationale
Dementia is becoming more prevalent worldwide. About half of the dementia cases are in Asia, and the total number of cases worldwide is forecasted to increase to 63 million in 2030 [1]. In Singapore, one in ten people aged ≥60 years may have dementia [2]. Patients with dementia demonstrate significant cognitive decline in at least one or more cognitive domains, including complex attention, executive function, learning and memory, language, perceptual-motor function, and social cognition [3]. Mild cognitive impairment (MCI) represents a “middle ground” between normal ageing and dementia, and there has been growing interest in its timely diagnosis [4]. Although the Mini-Mental State Examination (MMSE) is the most widely applied test for dementia screening, the Montreal Cognitive Assessment (MoCA) is considered the best alternative for screening of MCI [5].
Besides the MMSE and MoCA, there are more than 40 other tests available for cognitive screening in health care settings [5]. A challenge with the application of commonly used paper-and-pencil or even digitalized screening tools is the limited cognitive domains that they assess. This is at the expense of other cognitive domains like executive function [6] and perceptual-motor function, which, when deficient, are associated with a high risk of progression to dementia [7]. Furthermore, the scoring in many of these screening tools is influenced by factors such as education level and cultural background [8]. Functional status scales used to assess the severity of dementia, such as the Barthel Index for Activities of Daily Living (ADL), also heavily depend on subjective observational measures. One plausible solution to overcome some of these limitations is the employment of virtual reality (VR) technology.
Virtual reality is a technology that provides interaction between a user and artificially generated environments. In recent years, with technological advancement, the use of VR has become more widespread. Beyond entertainment purposes, VR has also found purpose within certain fields of medicine [9] such as cognitive rehabilitation and training [10]. As a screening tool, VR has shown to have greater ecological validity [11], which reflects how well these tests predict real-world settings. This is in contrast with the contrived testing environment around the routine screening tests used today.
Several studies have attempted to investigate the use of VR to screen for cognitive impairment in older persons. Tong et al [12] used a tablet technology to screen for abnormal cognitive status in the emergency department, while Zygouris et al [13] developed a cognitive training app and compared its performance with established neuropsychological tests. Neither of these studies applied VR to cognitive screening in primary care, which is the most relevant setting for early detection of cognitive impairment [14].
Study Aims
The primary aim of this study was to examine the feasibility and acceptability of using VR to screen for cognitive impairment in older persons in a primary care setting. The VR platform used in this study is a new tool (referred to as the RE@CH assessment module or VR module) developed by the Institute of Technical Education (ITE), College West, Singapore. Feasibility is defined by the proportion of participants who successfully complete the RE@CH assessment module within a stipulated time. Acceptability is based on the feedback received at the end of the study with regard to acceptance, perception, and experience with VR.
The secondary aim was to assess the ability of the RE@ACH assessment module to discriminate between cognitively intact and cognitively impaired participants. Performance was assessed by first establishing a scoring algorithm on the module, followed by comparing the performance scores between the two groups. Validity was assessed by examining the correlation of the scores against other routine cognitive screening assessments including the Abbreviated Mental Test (AMT) [15], MMSE [16], and MoCA [17]. This was followed by receiver operating characteristic (ROC) curve analysis to determine a useful cut-off score.
Methods
Study Design
This study was conducted at SingHealth Polyclinics-Outram, a public primary care clinic located in the central region of Singapore and serving a multiethnic Asian population.
Most patients attend these polyclinics to visit their primary care physicians or family physicians for management of their noncommunicable diseases such as hypertension, dyslipidemia, and type 2 diabetes mellitus. Subspecialized clinics operate on selected days of the month at this study site, such as the GeRiAtric serviCE (GRACE) memory clinic, which screens patients for suspected cognitive impairment including MCI and dementia.
Participants and Eligibility
The participants were older persons registered at SingHealth Polyclinics-Outram, aged between 65 and 85 years. Sixty participants were targeted based on a small to medium standardized effect size [18] of the secondary aim (difference between mean performance scores and correlation coefficient), calculated using the upper confidence limit approach [19]. Of the 137 participants who were approached, 60 (43.8%) were recruited and enrolled after providing consent. All were recruited directly at the study site from March 2019 to April 2019 and were assessed by either the primary investigator (PI) or co-PI of the study project. Potential participants were patients waiting to see the doctor in the general clinic or GRACE memory clinic (Figure 1). They were either screened selectively at the waiting area or referred by physicians from those clinics.
Figure 1.
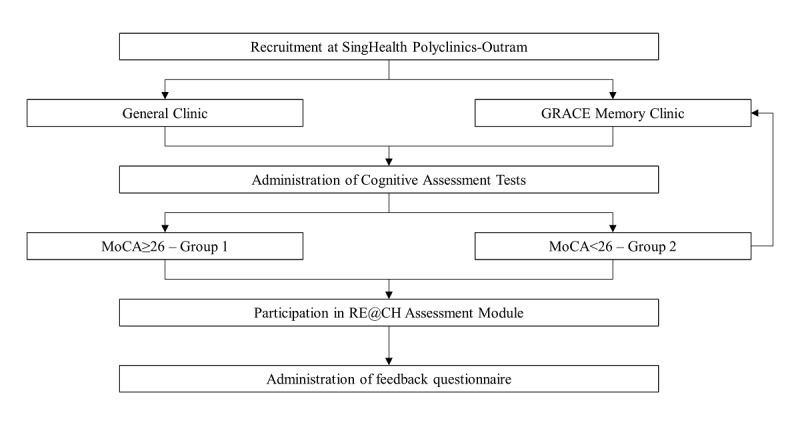
Study flow. GRACE: GeRiAtric serviCE; MoCA: Montreal Cognitive Assessment.
All participants were required to understand the procedure for using the RE@CH assessment module, perform the movements involved in the study, and have the mental capacity to provide written informed consent. Those with poor vision, inability to follow verbal commands, aphasia, impairment of kinetic abilities that could inaccurately affect their performance on the assessment module, and unwillingness or inability to comply with the study protocol were excluded. Those with severe functional impairment based on the Lawton instrumental ADL (iADL) scale [20] were also excluded.
Enrolled participants provided written informed consent before entering the study, and this included consent to access their electronic medical records. All participants had an acceptable mental capacity, and consent was obtained in the presence of a witness. The MoCA cognitive assessment screen was performed and participants were divided into two groups (Figure 1), each meeting specific eligibility criteria:
Group 1: Cognitively intact individuals, as defined by a MoCA score of ≥26. Those with pre-existing formal diagnosis of cognitive impairment of any degree or a history of cerebrovascular accident or neurological deficits were excluded.
Group 2: Cognitively impaired individuals, as defined by a MoCA score of <26.
RE@CH Assessment Module
The RE@CH assessment module (Figure 2) uses VR and motion sensor (Leap motion) technology to replicate activities encountered in daily living as 3D games (Figure 3). The virtual environment was projected on a 55-inch 2D screen that recreates an immersive experience for the user. Through these activities, several cognitive domains were assessed. These included learning and memory, perceptual-motor function, and executive function, which had the greatest emphasis.
Figure 2.
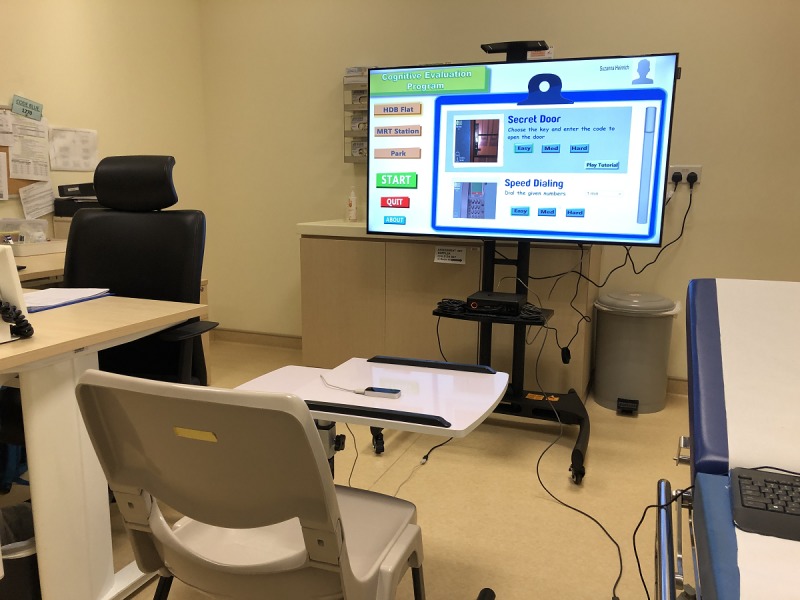
Setup of the RE@CH assessment module in the consultation room.
Figure 3.
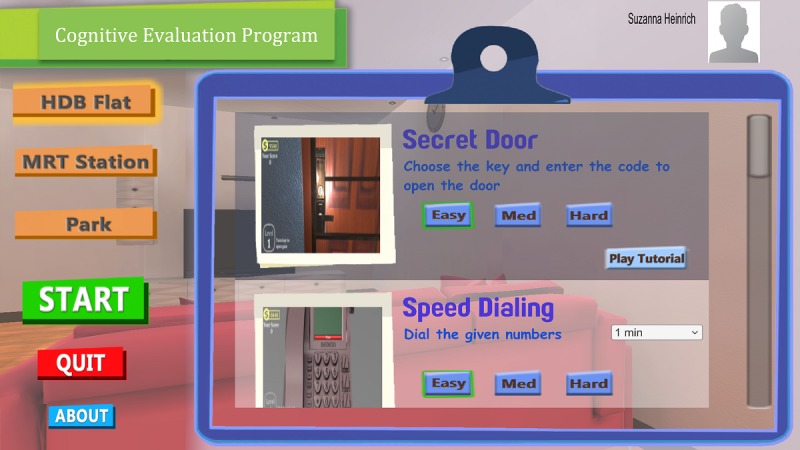
Main screen page of the RE@CH assessment module, showing two 3D games: Secret Door (opening a door using the right key) and Speed Dialling (making a phone call by recalling a predefined 8-digit number).
The study team designed a scoring algorithm (Table 1) to appraise the participant’s performance on the RE@CH assessment module. Seven relevant tasks were selected and the scored depending on the participants’ ability to complete the task correctly within the stipulated time, the number of attempts, and the proportion of tasks performed correctly.
Table 1.
Scoring algorithm on RE@CH assessment module.
| Cognitive domain, task, content | Score | Remarks | ||||||
| 0 | 25 | 50 | 75 | 100 | ||||
| Perceptual-Motor | ||||||||
| 1—Opening door with correct key and passcode number | No attempt | Unable to complete Step 1 | Complete Step 1 | Complete Step 1 and 2 | Complete Steps 1, 2, and 3 |
|
||
| Learning and Memory | ||||||||
| 2—Make a phone call, recalling the 8-digit number in predefined sequence | No attempt | Unable to complete on 3 attempts | Complete on 3 attempts | Complete on 2 attempts | Complete on 1 attempt |
|
||
| Executive Function | ||||||||
| 3—Identify items in each category from the newspaper: (1) famous people, (2) advertisement of groceries, and (3) 4-digit number on a lottery slip | No attempt | Fail to identify any category correctly | Identify 1 category correctly | Identify 2 categories correctly | Identify all 3 categories correctly |
|
||
| 4—Housekeeping: Sort things inside the room | No attempt | Sort 0 items correctly | Sort 1 item correctly | Sort 2 items correctly | Sort all 3 items correctly |
|
||
| 5—Dressing/grooming: Pick appropriate outfit for occasion | No attempt | Pick appropriate outfit for 0 of 3 tries | Pick appropriate outfit for 1 of 3 tries | Pick appropriate outfit for 2 of 3 tries | Pick appropriate outfit for all 3 tries |
|
||
| 6—Handling finances: Withdrawing cash from ATMa | No attempt | Unable to complete Step 1 | Complete Step 1 | Complete Step 1 and 2 | Complete Steps 1, 2, and 3 |
|
||
| 7—Handling finances: Shopping at provision shop | No attempt | Unable to complete Objective 1 with 0 correct item | Complete Objective 1 with 1 correct item | Complete Objective 1 with 2 or more correct items | Complete Objective 1 and 2 |
|
||
aATM: automated teller machine.
bPIN: personal identification number.
Participants had to complete various tasks via the module using appropriate hand gestures. Before the formal VR assessment, they were guided through one orientation task to familiarize themselves with the mechanics of the system. Their performance on the next seven key activities was scored manually using the scoring algorithm (Table 1) in the following order:
Opening a door using the correct key and passcode number
Making a phone call by recalling a predefined 8-digit number
Identifying: (a) Famous people, (b) Advertisement of groceries, and (c) 4-digit number on a lottery slip on a newspaper
Sorting household objects according to category
Picking an outfit appropriate for a specified occasion
Withdrawing cash from an automated teller machine
Shopping for groceries in a provision shop
Prior to each task, the study team guided the participant on the requirement of the task through a short tutorial.
Data Collection
First, AMT, MMSE, and MoCA cognitive screening tests were carried out in order and scored as part of the cognitive function assessment to determine eligibility and classification into either of the two groups.
Next, baseline characteristics were collected, including demographics, functional status (Barthel Index and Lawton iADL), clinical parameters, and past medical history. All data were obtained directly from the participants, except for their past medical history, which was from their electronic medical records.
Subsequently, the VR component of the study began once the PI or co-PI of the study started the briefing of the participant on the module and ended after the completion of the seventh activity on the RE@CH assessment module. The start and end time were recorded and used to calculate the participant’s time spent on the module, which included the time spent on the tutorial and explanation by the facilitator.
Finally, the participants completed an interviewer-administered questionnaire to assess their perception toward VR (Textbox 1). There were six questions, and the answers were rated on a Likert scale (1 - strongly disagree, 2 - disagree, 3 - neutral, 4 - agree, 5 - strongly agree). Total feedback scores were calculated by summing the scores from the six questions.
Sample questionnaire assessing subjects’ perception toward virtual reality experience, categorized by individual questions.
The VR software I have experienced is easy to use.
The amount of time I spent with the VR software is acceptable to me.
The use of VR software helps to make the experience in the clinic more interactive.
The use of VR technology to help diagnose medical condition appeals to me.
I would not mind seeing more new technologies being used by the doctor/medical staff during the consultation.
Overall, I enjoyed the VR experience in the clinic.
Outcome Measures
To achieve the primary aim of examining the feasibility and acceptability of the VR module, the following outcomes were measured: percentage of participants who successfully completed the RE@CH assessment module, time taken to complete the RE@CH assessment module, and scores from the feedback questionnaire.
To achieve the secondary aim of assessing the performance and validity of the RE@CH assessment module, the following outcomes were measured: performance scores on the RE@CH assessment module and scores on other cognitive assessment tests including AMT, MMSE, and MoCA. Additional statistical analysis was performed to derive the correlation between these outcome measures and to obtain the ROC curve.
All outcome measures were recorded in both study groups.
Statistical Analysis
Baseline characteristics were compared between the groups using a two-sample t test and the Fisher exact test for continuous and categorical variables, respectively. Summary statistics were calculated individually for the recruitment statistics and questionnaire-dependent feedback scores. Groups 1 and 2 were compared using the two-sample t test.
Performance scores were analyzed at each task level, given a total score, and compared between the two groups using the methods described above, as appropriate. Correlation between performance scores and other variables was assessed using Pearson correlation. A logistic regression was performed based on the total performance scores, followed by ROC analysis to assess its predictive capability to discriminate between cognitively intact and cognitively impaired individuals.
A P value<.05 was considered statistically significant (two-sided). Analyses were performed using SAS University Edition software (Version 9.4M6 of the SAS System for Windows; SAS Institute Inc, Cary, NC).
Results
Sample Description
Based on their MoCA scores, 37 participants with a score≥26 were placed in Group 1 (cognitively intact) and 23 participants with a score<26 were placed in Group 2 (cognitively impaired). Of those in Group 2, 10 were new cases pending referral to the GRACE memory clinic. Baseline characteristics of participants in both groups were compared (Table 2). There were no statistically significant differences in age and gender between the two groups. Mean cognitive assessment scores, based on AMT, MMSE and MoCA, were all higher in Group 1 than in Group 2.
Table 2.
Baseline characteristics by cognitive status.
| Characteristics | Cognitively intact (n=37) | Cognitively impaired (n=23) | P valuea | |
| Age (years), mean (SD) | 70.7 (3.6) | 73.2 (5.4) | .06 | |
| Gender, n (%) | .21 | |||
| Female | 26 (70.3) | 15 (65.2) | ||
| Male | 11 (29.7) | 8 (34.8) | ||
| Years of education, n (%) | <.001 | |||
| <6 | 4 (10.8) | 12 (52.2) | ||
| 6-10 | 23 (62.2) | 8 (34.8) | ||
| >10 | 10 (27.0) | 3 (13.0) | ||
| Functional status scores, mean (SD) | ||||
| ADLb | 98.5 (2.6) | 98.3 (3.9) | .78 | |
| iADLc | 22.8 (0.5) | 21.7 (1.4) | .002 | |
| Cognitive assessment scores, mean (SD) | ||||
| AMTd | 9.6 (0.6) | 8.3 (1.3) | <.001 | |
| MMSEe | 28.8 (1.0) | 25.9 (3.4) | <.001 | |
| MoCAf | 27.8 (1.2) | 22.2 (3.3) | <.001 | |
aIn categories where the mean values can be directly compared, the P values are given individually. In categories where the distribution across subcategories are compared, only P values for the main categories are given.
bADL: activities of daily living, maximum score of 100.
ciADL: instrumental activities of daily living, maximum score of 23.
dAMT: Abbreviated Mental Test, maximum score of 10.
eMMSE: Mini-Mental State Examination, maximum score of 30.
fMoCA: Montreal Cognitive Assessment, maximum score of 30.
Primary Aim: Feasibility and Acceptability
Recruitment Statistics and Time to Completion
All 60 (100%) enrolled participants successfully completed the study. The mean total time spent on the RE@CH assessment module was not significantly different between the two groups (Group 1: 19.1 [SD 3.6] minutes; Group 2: 20.4 [SD 3.4] minutes; P=.17; Table 3).
Table 3.
Total time spent on the RE@CH assessment module and feedback scores between study groups.
| Factor | Cognitively intact (n=37) | Cognitively impaired (n=23) | P value | Overalla (N=60), mean (SD) | ||||||||||
| Mean (SD) | Median | Mean (SD) | Median | |||||||||||
| Time spent on the virtual reality module | ||||||||||||||
| Time (minutes) | 19.1 (3.6) | 17 | 20.4 (3.4) | 18 | .17 | 19.6 (3.6) | ||||||||
| Feedback score from the questionnaire | ||||||||||||||
| Mean feedback score by questionb | ||||||||||||||
| Question 1 | 3.76 (1.01) | 3 | 3.87 (0.97) | 3 | .67 | 3.80 (0.99) | ||||||||
| Question 2 | 4.11 (0.77) | 4 | 4.13 (0.87) | 4 | .92 | 4.12 (0.80) | ||||||||
| Question 3 | 4.08 (0.76) | 4 | 4.17 (0.94) | 4 | .68 | 4.12 (0.83) | ||||||||
| Question 4 | 3.92 (1.04) | 3 | 4.26 (0.81) | 4 | .18 | 4.05 (0.96) | ||||||||
| Question 5 | 4.30 (0.81) | 4 | 4.26 (0.69) | 4 | .86 | 4.28 (0.76) | ||||||||
| Question 6 | 4.51 (0.56) | 4 | 4.43 (0.95) | 4 | .72 | 4.48 (0.72) | ||||||||
| Total feedback scorec | 24.7 (3.6) | 25 | 25.1 (3.9) | 26 | .62 | 24.8 (3.7) | ||||||||
aOverall mean values from both study groups.
bDescription of the individual questions can be found in Textbox 1.
cSum of the numerical scores (ranging from 1 to 5) given for each of the six questions.
Assessment of Perception Toward Virtual Reality
Results from the questionnaire were favorable toward the VR experience. Mean feedback scores for each question (over a scale of 1-5) ranged from 3.80 to 4.48 (Table 3). The largest proportion of responses to all statements was “Agree” or “Strongly agree.” The difference in total feedback scores between the study groups was not statistically significant (P=.62; Table 3).
Secondary Aim: Performance and Validity
Discriminating Performance Between Cognitively Intact and Cognitively Impaired Participants
In general, the mean scores for each task was higher in the cognitively intact compared to the cognitively impaired participants, except for task 1 (Table 4). These differences were statistically significant for tasks 2, 3 and 7, and the total performance score (sum of task 1 to task 7 scores) exhibited statistically significant differences. Results of the individual performance scores in the RE@CH assessment module are summarized in Table 4.
Table 4.
Performance scores on the RE@CH assessment module by cognitive status for individual tasks and total scores.
| Task | Cognitively intact (n=37), mean (SD) | Cognitively impaired (n=23), mean (SD) | P value |
| 1—Opening door | 96.6 (14.6) | 96.7 (11.4) | .97 |
| 2—Making phone call | 67.6 (33.3) | 41.3 (23.4) | .002 |
| 3—Reading newspaper and identifying specific components | 87.8 (20.9) | 65.2 (26.9) | <.001 |
| 4—Sorting items | 81.1 (19.0) | 71.7 (29.5) | .18 |
| 5—Choosing the right clothes | 66.9 (25.0) | 57.6 (23.2) | .16 |
| 6—Withdrawing money at ATMa | 53.4 (10.5) | 50.0 (0.0) | .06 |
| 7—Shopping at supermarket | 98.6 (5.7) | 93.5 (11.2) | .049 |
| Total performance scoreb | 552.0 (57.2) | 476.1 (61.9) | <.001 |
aATM: automated teller machine.
bSum of individual scores (ranging from 0 to 100) in each of the seven tasks.
Correlation of the RE@CH Assessment Module and Routine Cognitive Screening Assessment
The total performance score showed moderate positive correlation with scores from the other routine cognitive screening assessment tools, namely, AMT (0.312, P=.02), MMSE (0.373, P=.003), and MoCA (0.427, P<.001). In addition, the correlation of the total performance score with age was negative and poor (–0.291, P=.02). Table 5 presents the correlation matrix between the different variables.
Table 5.
Correlation matrix demonstrating the degree of association among ADL score, iADL score, AMT score, MMSE score, MoCA score, age, education, total feedback, and total performance scores.
| ADLa | iADLb | AMTc | MMSEd | MoCAe | Age | Education | Feedback score | Performance score | ||
| ADL | ||||||||||
| Pearson correlation coefficient | —f |
–0.01 | –0.04 | 0.30 | 0.12 | –0.40 | 0.07 | 0.20 | –0.03 | |
| P value | — | .94 | .79 | .02 | .35 | .002 | .61 | .13 | .85 | |
| iADL | ||||||||||
| Pearson correlation coefficient | –0.01 | — |
0.22 | 0.30 | 0.49 | –0.23 | 0.003 | –0.03 | 0.20 | |
| P value | .94 | — | .09 | .02 | <.001 | .07 | .98 | .79 | .13 | |
| AMT | ||||||||||
| Pearson correlation coefficient | –0.04 | 0.22 | — | 0.73 | 0.73 | –0.22 | 0.37 | 0.05 | 0.31 | |
| P value | .79 | .09 | — | <.001 | <.001 | .10 | .00 | .68 | .02 | |
| MMSE | ||||||||||
| Pearson correlation coefficient | 0.30 | 0.30 | 0.73 | — | 0.76 | –0.31 | 0.57 | 0.07 | 0.37 | |
| P value | .02 | .02 | <.001 | — | <.001 | .02 | <.001 | .62 | .00 | |
| MoCA | ||||||||||
| Pearson correlation coefficient | 0.12 | 0.49 | 0.73 | 0.76 | — | –0.23 | 0.40 | –0.07 | 0.43 | |
| P value | .35 | <.001 | <.001 | <.001 | — | .08 | .002 | .59 | <.001 | |
| Age | ||||||||||
| Pearson correlation coefficient | –0.40 | –0.23 | –0.22 | –0.31 | –0.23 | — | –0.13 | –0.07 | –0.29 | |
| P value | .002 | .07 | .10 | .02 | .08 | — | .33 | .59 | .02 | |
| Education | ||||||||||
| Pearson correlation coefficient | 0.07 | 0.003 | 0.37 | 0.57 | 0.40 | –0.13 | — | –0.09 | 0.24 | |
| P value | .61 | .98 | .003 | <.001 | .002 | .33 | — | .48 | .06 | |
| Feedback score | ||||||||||
| Pearson correlation coefficient | 0.20 | –0.03 | 0.05 | 0.07 | –0.07 | –0.07 | –0.09 | — | –0.02 | |
| P value | .13 | .79 | .68 | .62 | .59 | .59 | .48 | — | .85 | |
| Performance score | ||||||||||
| Pearson correlation coefficient | –0.03 | 0.20 | 0.31 | 0.37 | 0.43 | –0.29 | 0.24 | –0.02 | — | |
| P value | .85 | .13 | .02g | .003g | <.001g | .02g | .06 | .85 | — | |
aADL: activities of daily living.
biADL: instrumental activities of daily living.
cAMT: Abbreviated Mental Test.
dMMSE: Mini-Mental State Examination.
eMoCA: Montreal Cognitive Assessment.
fNot applicable.
gSignificance at P<.05.
Receiver Operating Characteristic Curve Analysis
ROC curve analysis (Figure 4) was conducted over the continuous total performance score to assess its capability to discriminate between cognitively intact (MoCA≥26) and cognitively impaired individuals (MoCA<26). The area under the curve (AUC) was found to be 0.821 (95% CI 0.714-0.928). An optimal statistical cutoff is achieved at a cut-off score of 500 (78.2% sensitivity, 75.7% specificity, 66.7% positive predictive value, and 84.8% negative predictive value; Figure 5).
Figure 4.
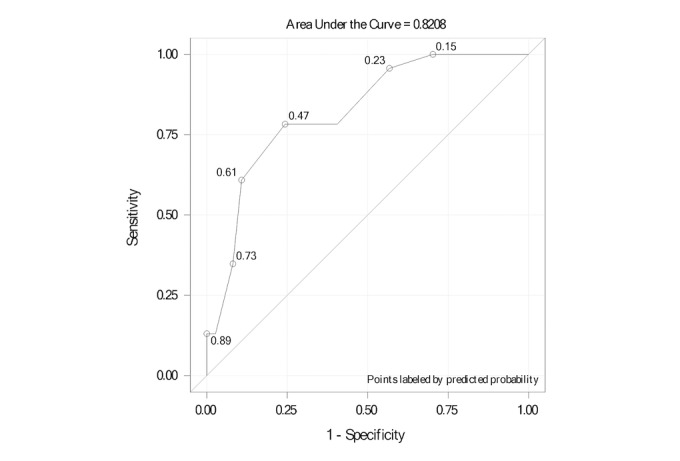
Receiver operating characteristic curve for the RE@CH assessment module’s ability to discriminate between the two groups through the total performance scores.
Figure 5.
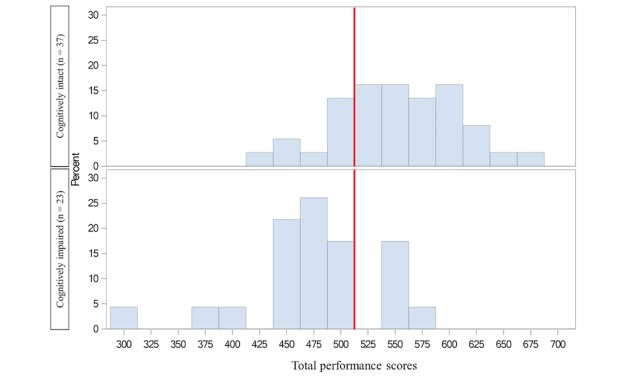
Distribution of the total performance scores of the RE@CH assessment module by group. The red line indicates the optimal cut-off score of 500.
Discussion
Primary Aim: Feasibility and Acceptability
Assessing Feasibility of the Virtual Reality Module in Primary Care
The primary aim of this study was to determine the feasibility and acceptability of VR to screen for cognitive impairment among older persons in primary care. We found the RE@CH assessment module feasible for this setting. All participants recruited successfully completed the study, regardless of their performance score. There were no withdrawals or any immediate adverse effects reported. The time to completion was comparable to the total time to completion of all three of the routine cognitive screening tools (AMT, MMSE, and MoCA) [5] used at SingHealth Polyclinics-Outram for memory-related complaints. Most of the participants agreed that the time spent on the VR was acceptable, as supported by a mean feedback score of 3.8 of 5 for Question 1 (Table 3).
Assessing Acceptance of Virtual Reality Among Participants
The RE@CH assessment module was well accepted by both participant groups, as evidenced by the positive feedback scores across all six questions. The response rate of 43.8% in this study suggests that almost one in two older persons are receptive to the use of novel technology for assessing their cognitive function. The scores for immersion in the VR experience (Table 3) were overall above average. This was supported by earlier studies that showed that older adults generally have a positive attitude toward the VR environment [21] and new technology [22]. A few felt that the VR software was not easy to use, but this could be because of unfamiliarity with VR technology.
Secondary Aim: Performance and Validity
Comparing Performance Between Cognitively Intact and Impaired Participants
The secondary aim of this study was to determine the performance and validity of the RE@CH assessment module in screening for cognitive impairment. We found that although the total performance scores were significantly higher in the cognitively intact group, it was attributed to a few individual tasks. Tasks 2, 3, and 7 were better at discriminating between the two groups. The remaining tasks could be affected by the technical limitations inherent in the VR module and the structure of the scoring algorithm. Tasks 4 and 5 were challenging to both groups, because the motion sensor was not precise enough to pick up all hand gestures. Tasks 1 and 6 were relatively easy, and all participants performed well. This might have led to underperformance or overperformance across the groups, but it is not likely to have differentiated between the two groups well.
Assessing Validity of the Virtual Reality Module Compared to Other Screening Assessments
The RE@CH assessment module generated valid total performance scores that had a moderate positive correlation with all the other three validated cognitive screening assessments. A high positive correlation was not expected, since the latter focused specifically on a few cognitive domains and the VR module could have identified cognitive deficits that other screening assessments were unable to detect. The ROC analysis indicated relatively strong prognostic classification capability [23] with an AUC of 0.821 when benchmarked against MoCA scores.
Clinical Implications
Our findings provide preliminary evidence that VR modalities, such as the RE@CH assessment module, can be used for cognitive screening among older person in primary care. We built upon the positive results from previous studies [11-13,24] and introduced another integral component of a VR setup—the Leap motion sensor—that detects hand gestures [25]. Instead of a single game, we introduced multiple short VR-based activities to assess the different cognitive domains.
Our findings show that participants could still perform most of the tasks within a time frame of about 20 minutes (mean 19.6 minutes). In addition, we showed that VR was not only feasible, but also relatively practical to execute within the premise of a primary care clinic. The screening can be executed prior to consultations with the primary care physician to optimize their clinic visits by at-risk patients. Implementation studies are needed in future research to assess its roll-out in clinical practice.
Limitations
This study had several limitations. First, there was a significant difference in the level of education between the two groups. More participants with MoCA scores≥26 had higher educational levels than those with lower MoCA scores, which could impact the outcomes of their performance. The disparity could be related to the recruitment process. Participants who were better educated and more likely to have been exposed to health technology and innovations were likely and willing to be enrolled into the study, even in the absence of cognitive symptoms. The sample size was small in this feasibility study. An adequately powered randomized controlled trial, stratified by education level, is planned to further assess the use of VR in cognitive assessment.
Second, the participants might not be representative of the entire target population at risk of cognitive impairment (older persons aged≥65 years) [26], since recruitment was carried out only at one location with multiple exclusion criteria. Furthermore, the study population was classified solely on the basis of the MoCA scores, which is a screening tool and not considered diagnostic of cognitive impairment. Participants with subjective cognitive impairment but normal MoCA scores could have been assigned erroneously to the cognitively intact group.
Lastly, the RE@CH assessment module was a prototype that was originally used for rehabilitation and then adapted for cognitive screening. It included tasks that might be challenging to complete because of the prototype content design, and not due to the participant’s cognitive impairment. A specially designed VR program that will be developed by us to assess cognitive function has been recently awarded funding by a local public information technology agency, which will address the limitations of the current prototype. The next prototype will eliminate tasks that do not differentiate between the two groups to both reduce redundancy and optimize the time spent to complete the cognitive assessment. The next study will include time motion measurements to evaluate accuracy, efficiency, and cost-effectiveness of our tool in comparison with the conventional screening tools such as MoCA.
Conclusions
The study successfully demonstrated the feasibility of a VR-based screening tool in primary care. The target population expressed a positive perception of and attitude toward VR and were open to the use of this type of technology for their cognitive assessment. The results of this feasibility study are invaluable in the design of a novel VR program and study protocol by validating its use as a comprehensive, multidomain cognitive function screening tool in the next phase of development.
Acknowledgments
We would like to extend our gratitude to our health care colleagues at SingHealth Polyclinics (Outram) who supported us in various ways, including screening and referring patients for the study. We also appreciate our collaborators at ITE College West, Mr Teh Tuan Ann and Dr Lim Soon Huat, for development of the RE@CH assessment module and their technical assistance. We are thankful to the SingHealth-Duke NUS Family Medicine Academic Clinical Programme (FM ACP) secretariat (Ms Winnie Seah, Winnie Ong, Eugene Quek) and SingHealth Polyclinics Department of Research Administration (Ms Patricia Kin, Caris Tan, and Usha Sankari) for their assistance in ethics board review and procurement of equipment. This study received funding from the FM ACP Seed Grant and the Duke-NUS MSF Grant.
Abbreviations
- ADL
activities of daily living
- AMT
Abbreviated Mental Test
- AUC
area under curve
- iADL
instrumental activities of daily living
- GRACE
GeRiAtric serviCE
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- MoCA
Montreal Cognitive Assessment
- PI
primary investigator
- ROC
receiver operating characteristic
- VR
virtual reality
Footnotes
Authors' Contributions: SILC, WTW, JHMQ, TO, and NCT designed the study protocol. SILC and WTW implemented the study and collected the data. JCA and SILC analyzed the data. SILC, JCA, NCT, RM, and TO reviewed and interpreted the results. SILC drafted the manuscript. All authors reviewed the draft and approved the final manuscript.
Conflicts of Interest: None declared.
References
- 1.Wimo A, Winblad B, Aguero-Torres H, von Strauss E. The magnitude of dementia occurrence in the world. Alzheimer Dis Assoc Disord. 2003;17(2):63–67. doi: 10.1097/00002093-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Subramaniam M, Chong SA, Vaingankar JA, Abdin E, Chua BY, Chua HC, Eng GK, Heng D, Hia SB, Huang W, Jeyagurunathana A, Kua J, Lee SP, Mahendran R, Magadi H, Malladi S, McCrone P, Pang S, Picco L, Sagayadevan V, Sambasivam R, Seng KH, Seow E, Shafie S, Shahwan S, Tan LL, Yap M, Zhang Y, Ng LL, Prince M. Prevalence of Dementia in People Aged 60 Years and Above: Results from the WiSE Study. J Alzheimers Dis. 2015;45(4):1127–1138. doi: 10.3233/JAD-142769. [DOI] [PubMed] [Google Scholar]
- 3.Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Washington, DC: American Psychiatric Association Publishing; 2013. [Google Scholar]
- 4.Dubois B, Padovani A, Scheltens P, Rossi A, Dell'Agnello G. Timely Diagnosis for Alzheimer's Disease: A Literature Review on Benefits and Challenges. J Alzheimers Dis. 2016;49(3):617–631. doi: 10.3233/JAD-150692. http://europepmc.org/abstract/MED/26484931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsoi KKF, Chan JYC, Hirai HW, Wong SYS, Kwok TCY. Cognitive Tests to Detect Dementia: A Systematic Review and Meta-analysis. JAMA Intern Med. 2015 Sep;175(9):1450–1458. doi: 10.1001/jamainternmed.2015.2152. [DOI] [PubMed] [Google Scholar]
- 6.Cullen B, O'Neill B, Evans JJ, Coen RF, Lawlor BA. A review of screening tests for cognitive impairment. J Neurol Neurosurg Psychiatry. 2007 Aug;78(8):790–799. doi: 10.1136/jnnp.2006.095414. http://europepmc.org/abstract/MED/17178826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, Belleville S, Brodaty H, Bennett D, Chertkow H, Cummings JL, de Leon M, Feldman H, Ganguli M, Hampel H, Scheltens P, Tierney MC, Whitehouse P, Winblad B, International Psychogeriatric Association Expert Conference on mild cognitive impairment Mild cognitive impairment. Lancet. 2006 Apr 15;367(9518):1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 8.Yokomizo JE, Simon SS, Bottino CMDC. Cognitive screening for dementia in primary care: a systematic review. Int Psychogeriatr. 2014 Nov;26(11):1783–1804. doi: 10.1017/S1041610214001082. [DOI] [PubMed] [Google Scholar]
- 9.Satava RM. Medical applications of virtual reality. J Med Syst. 1995 Jun;19(3):275–280. doi: 10.1007/BF02257178. [DOI] [PubMed] [Google Scholar]
- 10.Pensieri C, Pennacchini M. Overview: Virtual Reality in Medicine. JVWR. 2014 Jan 19;7(1):1–34. doi: 10.4101/jvwr.v7i1.6364. [DOI] [Google Scholar]
- 11.Tarnanas I, Schlee W, Tsolaki M, Müri René, Mosimann U, Nef T. Ecological validity of virtual reality daily living activities screening for early dementia: longitudinal study. JMIR Serious Games. 2013 Aug 06;1(1):e1. doi: 10.2196/games.2778. http://games.jmir.org/2013/1/e1/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tong T, Chignell M, Tierney MC, Lee J. A Serious Game for Clinical Assessment of Cognitive Status: Validation Study. JMIR Serious Games. 2016 May 27;4(1):e7. doi: 10.2196/games.5006. http://games.jmir.org/2016/1/e7/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zygouris S, Giakoumis D, Votis K, Doumpoulakis S, Ntovas K, Segkouli S, Karagiannidis C, Tzovaras D, Tsolaki M. Can a virtual reality cognitive training application fulfill a dual role? Using the virtual supermarket cognitive training application as a screening tool for mild cognitive impairment. J Alzheimers Dis. 2015;44(4):1333–1347. doi: 10.3233/JAD-141260. [DOI] [PubMed] [Google Scholar]
- 14.Olazarán Javier, Torrero P, Cruz I, Aparicio E, Sanz A, Mula N, Marzana G, Cabezón Dionisio, Begué Concepción. Mild cognitive impairment and dementia in primary care: the value of medical history. Fam Pract. 2011 Aug;28(4):385–392. doi: 10.1093/fampra/cmr005. [DOI] [PubMed] [Google Scholar]
- 15.Hodkinson HM. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing. 1972 Nov;1(4):233–238. doi: 10.1093/ageing/1.4.233. [DOI] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005 Apr;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 18.Coe R. It's the effect size, stupid: What effect size is and why it is important. Annual Conference of the British Educational Research Association; September 12-14, 2002; University of Exeter, England. 2002. [Google Scholar]
- 19.Sim J, Lewis M. The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. J Clin Epidemiol. 2012 Mar;65(3):301–308. doi: 10.1016/j.jclinepi.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Graf C. The Lawton instrumental activities of daily living scale. Am J Nurs. 2008 Apr;108(4):52–62. doi: 10.1097/01.NAJ.0000314810.46029.74. [DOI] [PubMed] [Google Scholar]
- 21.Torres ACS. Cognitive effects of video games on old people. International Journal on Disability and Human Development. 2011 Mar 09;10(1):55–58. doi: 10.1515/ijdhd.2011.003. [DOI] [Google Scholar]
- 22.Lee B, Chen Y, Hewitt L. Age differences in constraints encountered by seniors in their use of computers and the internet. Computers in Human Behavior. 2011 May;27(3):1231–1237. doi: 10.1016/j.chb.2011.01.003. [DOI] [Google Scholar]
- 23.Zhu W, Zeng N, Wang N. Sensitivity, specificity, accuracy, associated confidence interval and ROC analysis with practical SAS implementations. NESUG proceedings: Health Care and Life Sciences; NESUG 2010; November 14-17, 2010; Baltimore, Maryland. 2010. p. 6724. [Google Scholar]
- 24.Werner P, Rabinowitz S, Klinger E, Korczyn AD, Josman N. Use of the virtual action planning supermarket for the diagnosis of mild cognitive impairment: a preliminary study. Dement Geriatr Cogn Disord. 2009;27(4):301–309. doi: 10.1159/000204915. [DOI] [PubMed] [Google Scholar]
- 25.Martín-Gutiérrez J. Virtual Technologies Trends in Education. Eurasia J Math Sci Tech Ed. 2016 Dec 16;13(1):469–486. doi: 10.12973/eurasia.2017.00626a. [DOI] [Google Scholar]
- 26.Nagaendran K, Chen L H Christopher, Chong M S, Chua Esther Vanessa, Goh C K Shirley, Kua Joshua, Lee Theresa, Shiong Lim Wee, Marziyana A R, Ng C C David, Ng L L, Seow Dennis, Sitoh Y Y, Yap L K Philip, Yeo Donald, Yeo Y. Ministry of health clinical practice guidelines: dementia. Singapore Med J. 2013 May;54(5):293–299. doi: 10.11622/smedj.2013112. [DOI] [PubMed] [Google Scholar]


