Abstract
Purpose
AMD is the leading cause of blindness in the United States. The role of secondary inflammatory disease on AMD progression is largely unknown. Here we investigate the association between AMD and rheumatoid arthritis (RA), using MarketScan data for patients aged ≥65 years on Medicare.
Methods
Baseline data were extracted for subjects with at least two International Classification, Ninth Revision (ICD-9) diagnosis codes of RA and control subjects (no RA) and were matched at baseline by propensity score. Matched cohort data were extracted post-baseline time and examined up to 4.5 years of follow-up for ICD-9 diagnosis code AMD records. Multivariable regression models compared risk of an AMD diagnosis post-baseline for RA subjects and matched controls. Days until first AMD diagnosis between RA patients and controls was examined using survival analysis.
Results
Risk of new AMD diagnosis was elevated in RA patients (odds ratio [OR] 2.08; 95% confidence interval [CI] 1.98–2.18), with an increase in nonexudative AMD patients (P < 0.0001). Risk was elevated in female (n = 27,548) (OR 1.11; 95% CI 1.05–1.17) compared with male (n = 9704; P < 0.001) patients. The time to first AMD diagnosis was shorter for RA subjects than controls (P < 0.0001).
Conclusions
Our analysis provides support of association between RA diagnosis and increased nonexudative AMD diagnosis.
Keywords: age-related macular degeneration, dry AMD, wet AMD, MarketScan, rheumatoid arthritis
AMD is the leading cause of blindness among individuals older than 50 in the United States. Currently, an estimated 2 million Americans are believed to suffer from this disease.1 AMD is a disease affecting the macula, the area of retina needed for central vision, and consists of two types, wet (neovascular or exudative) AMD and dry (atrophic, nonexudative). Wet AMD is characterized by abnormal blood vessel growth or choroidal neovascularization, whereas dry AMD is characterized by retinal atrophy.2 Rheumatoid arthritis (RA) is also a disease that typically presents itself in mature individuals, with early onset between the ages of 40 and 60 years. Although this disease results in inflammation of the joints, it shares many similarities with AMD. Both AMD and RA are inflammatory diseases and share risk factors that include age, smoking, obesity, family history, and sex.3–6 In addition, both diseases are regulated in part by the complement pathway of the immune response (as reviewed in Refs. 7 and 8). The complement system can be initiated through three separate pathways: the classical, the lectin, or the alternative pathway. As with AMD, both the classical and the alternative pathway have been found to play a role in complement activation in RA.9 In AMD, single nucleotide polymorphisms for the complement genes, including Complement Factor H (CFH),10 Complement Factor B (CFB),11 Complement Factor I (CFI),12 and C313 have been identified. In addition, both C3 and C5 have been identified in drusen,14 and alterations in expression and localization of CD59 are observed in ocular tissues associated with AMD.15 Similarly, complement components C2, C3, C4, and C5 have been identified in rheumatoid synovial tissue.16 Increased mRNA expression of C3, CFB, factor D, CFH, CFI, S-protein, C5, C6, C7, C9, and CD59,17 as well as C3a and C5a receptor mRNA is found in synovial tissues in the presence of RA, as well.18 Several complement cleavage products also have been identified in synovial fluid, including Bb, SC5b-9, C3a, C3c, C5a, and C1-C1INH complexes.19–22 In addition to the complement pathway, both AMD and RA are regulated in part by similar inflammatory cytokines. One of these cytokines is TNF-α. TNF-α acts as a proinflammatory cytokine that is produced by macrophages and T cells.23 As a powerful proinflammatory cytokine, TNF-α has been targeted in RA24–28 as well as neovascular AMD therapies,29 and recent findings have supported interactions between TNF-α and the complement system.30,31 Biologics targeting TNF-α, such as infliximab, adalimumab, and etanercept, are currently approved treatments for RA by the US Food and Drug Administration. A case report of a neovascular AMD patient found systemic treatment with adalimumab to be a beneficial combination with anti-VEGF treatment.29 However, systemic infusion of infliximab (Remicade) demonstrated no benefit to neovascular AMD in a noncontrolled trial.32 These studies indicate the need for further examination on the role of TNF-α inhibitor biologics used to treat RA on AMD progression.
Although similarities between AMD and RA have been identified, much is still unknown about the pathogenesis for these diseases. Likewise, the effect of a preexisting inflammatory disease on AMD is yet to be determined. In a report published by McGeer and Sibley,33 RA patients were believed to be spared from unspecified AMD. These results were disputed in a recent study by Keenan and colleagues34 in which patients with osteoarthritis or RA were observed to have an increased risk for AMD among patients undergoing anti-VEGF treatment.
In this study, we provide further evidence of a correlation between RA and AMD through use of retrospective data from the MarketScan medical billing record database for Medicare patients. In addition, we examine the potential correlation between RA and the two types of AMD, wet and dry. As TNF-α inhibitors are currently used as therapeutic treatment for RA; we also investigate the effect of specific biologics on the risk of AMD.
Methods
Cohort Identification
The MarketScan Commercial Claims and Encounters Database (2010–2014) was analyzed to determine the odds of having AMD in the presence of RA. Subjects were identified within the Medicare population, and therefore were selected to be 64 years or older. Patients were chosen with at least two records of the ICD-9 (International Classification of Diseases, Ninth Revision) diagnosis code of 714.0 for RA, and insurance coverage for a minimum of 365 days was extracted for 2010. RA subjects were matched to subjects with no RA selected from the remaining population. Records for RA cases and controls were examined for 2010 to 2014 to identify AMD diagnoses using ICD-9 codes 362.5 (unspecified macular degeneration), 362.51 (dry AMD), and 362.52 (wet AMD). Subjects with a cancer diagnosis or a diagnosis of late effects of stroke were excluded from the groups to avoid early censoring due to death.
To measure the risk of AMD in RA subjects receiving TNF-α inhibitor monoclonal antibodies (MABs; infliximab, infliximab-dyyb, adalimubab, adalimumab-atto, certolizumab pegol, golimubab, entanercept, and etanercept-szzs), all pharmacy billing records were by therapeutic name and an MAB use indicator variable was constructed.
Baseline Patient Characteristics
Data from a baseline period of 6 months was used to construct measures of 24 chronic conditions treated in outpatient settings for all records using the Elixhouser method.35 Admission and comorbid conditions recorded in hospital discharge records were measured using the Charlson comorbidity score.36 To measure incident AMD cases during the follow-up study time, all subjects with a diagnosis code of AMD during the 6-month baseline period also were excluded. RA and control group subjects were matched using propensity scoring with a greedy algorithm. The propensity score model used 33 baseline variables to predict a member of the population being diagnosed with RA (see Supplementary Table S1 for baseline distribution of demographic and morbidity measure). The baseline matching conditions included age, sex, region, any hospital admission, Charlson comorbidity score from the hospital discharge records, and recorded outpatient diagnoses in the Elixhouser score (asthma; cardiac dysrhythmias; chronic obstructive pulmonary disease; conduction disorders of the heart; congestive heart failure; cystic fibrosis; diabetes with chronic complications; diabetes without chronic complications; diverticulosis and diverticulitis; epilepsy; heart valve disorders; hepatitis; HIV infection; hypertension; multiple sclerosis; otitis media; Parkinson's disease; peri-, endo-, and myocarditis; pulmonary heart disease; schizophrenia; senility; systemic lupus erythematosus; vertigo). Data for the matched cohorts were examined for up to 4.5 years following the 6-month baseline period (5 years total).
Survival Analysis
Survival analysis was used to show differences in time until AMD diagnosis among RA and control patients using the LIFETEST procedure. A period of 4.5 years (from July 1, 2010, to December 31, 2014) was used for this analysis, with the baseline period of the first 6 months excluded (January 1, 2010, to June 30, 2010) to rule out the possibility of measuring preexisting AMD diagnosis.
Statistical Analysis
Data on the matched RA and control population were analyzed using multivariable logistic and Cox regression. We compared the odds of having an AMD diagnosis during the follow-up time (controlling for days in study) and the proportional hazard rates of having an AMD diagnosis in the absence and presence of RA. The multivariable models controlled for any baseline differences in demographics, Charlson score, or Elixhouser diagnoses, which differed between groups after matching. All models included subject characteristics, and the logistic models included time in the study. Risk of AMD in RA patients receiving TNF-α inhibitor treatment was compared with unspecified AMD risk in the nontreatment group and multivariable logistic regression was applied. Survival curves were used to show the differences in days until the first AMD diagnosis between patients with RA and those without RA (control).
Results
Study Population
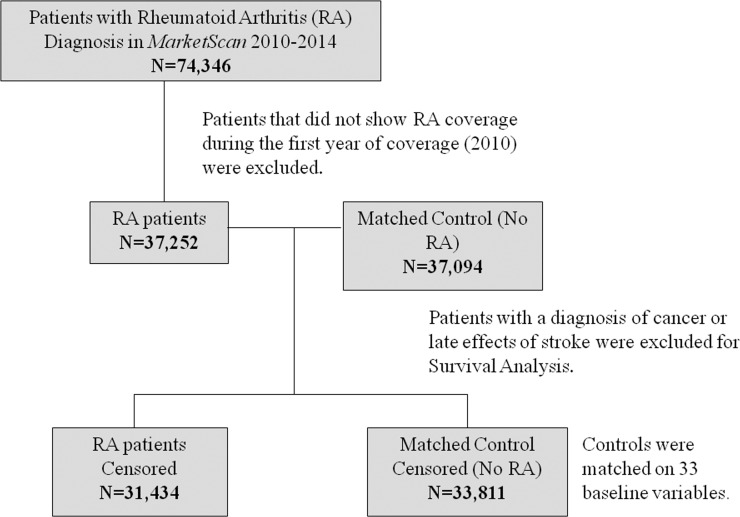
A total of 74,346 patients age 64 or older were identified with RA within the MarketScan database during the study period of January 1, 2010, to December 31, 2010. Patients who did not show RA coverage during the first year of coverage (2010) were excluded, leaving 37,252 patients identified with RA, and 37,094 patients were used to match for non-RA controls (Fig. 1). Mean age of patients within the control group was 73.70 (±7.343) years, and average age of patients with RA was 73.62 (±6.806) years. A higher percentage of female patients to male patients was observed in both RA patients (73.95% female) and the matched control (75.38% female) population (Table 1). Geographic regions of patients (northeast, north central, south, and west) were divided fairly evenly with the highest percentage of patients gathered from the north central region for both RA patients (30.44%) and control patients without RA (31.12%).
Figure 1.
Flow chart depicting cohort selection.
Table 1.
Demographic and Clinical Characteristics, by Cohort
|
RA Patients (n
= 37,252) |
Matched Control (No RA) Patients (n
= 37,094) |
|
| Sex, n (%) | ||
| Male | 9,704 (26.05) | 9,134 (24.62) |
| Female | 27,548 (73.95) | 27,960 (75.38) |
| Region, n (%) | ||
| Northeast | 7,425 (19.93) | 7,272 (19.60) |
| North central | 11,338 (30.44) | 11,543 (31.12) |
| South | 10,641 (28.56) | 10,633 (28.67) |
| West | 7,125 (19.13) | 6,958 (18.76) |
| Unknown | 723 (1.94) | 688 (1.85) |
| Patients with AMD, n (%) | ||
| Unspecified (including atrophic “dry”) | 5,294 (90.99) | 2,728 (83.09) |
| Exudative (wet) | 524 (9.01) | 555 (16.91) |
Association of AMD Diagnosis Among RA Patients
Unspecified AMD diagnosis was increased in the presence of RA (5818 patients) versus control with AMD (3283 patients). Analysis by specific AMD type indicated that a greater amount of dry AMD was observed in RA patients (5294; 90.99%) compared with control patients with no RA (2728; 83.09%). The number of wet AMD subjects was evenly matched between RA patients (524 patients; 16.91%) and patients with no RA (555 patients; 9.01%). Statistical analysis of these data revealed a 2.076 odds ratio (95% confidence interval 1.981–2.176) risk for AMD in RA patients with a significant increase in dry AMD patients (P < 0.0001). Risk of wet AMD was not significant, with an odds ratio of 0.978 (95% CI 0.610–1.348; P = 0.9129). The Cox model showed a hazard ratio of 1.928 (P ≤ 0.0001) for RA patients compared with controls in the fully adjusted model. The incidence of AMD also was observed more frequently in women with an odds ratio of 1.107 (95% CI 1.048–1.169; P ≤ 0.001; Table 2).
Table 2.
Risk of AMD Diagnosis in the Presence of RA
|
Odds Ratio (95% CI) |
P |
|
| Atropic (dry) AMD | 2.076 (1.981–2.176) | <0.0001 |
| Exudative (wet) AMD | 0.978 (0.610–1.348) | 0.9129 |
| Female versus male | 1.107 (1.059–1.169) | 0.0002 |
Survival Analysis
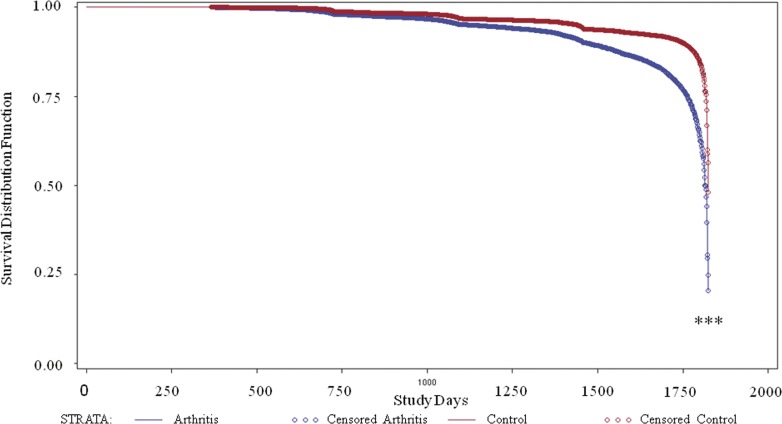
Because RA is a systemic inflammatory disease typically diagnosed before AMD, we decided to analyze whether or not the presence of RA would increase the likelihood of an AMD diagnosis. Of the 37,252 patients with RA, 5818 patients with a diagnosis of cancer or late effects of stroke were removed, with 31,434 remaining. Another 3288 patients were removed from the control group, leaving 33,885 patients. Here we observed a significant decrease in the number of days until AMD diagnosis in patients with RA (P < 0.0001; Fig. 2).
Figure 2.
Days to AMD diagnosis for RA patients versus control. Using the LIFETEST procedure, survival analysis of AMD diagnosis among RA (blue) and control (red) patients was determined over a 5-year period. The baseline period of 6 months was excluded to ensure RA diagnosis proceeded AMD diagnosis. ***P < 0.0001 of RA compared with control.
Effect of RA Treatment on AMD Diagnosis
TNF-α is found to be upregulated in RA,23 as well as play a role in the inflammatory response in the eye.37 However, although clinical outcomes for TNF-α inhibitors for the treatment of wet AMD have been variable,38–42 they have not yet been studied in the context of dry AMD. Therefore, we sought to examine the effect of TNF-α inhibitor MABs within the RA group. The risk of AMD diagnosis for RA patients receiving any of the TNF-α inhibitor monoclonal antibodies (infliximab, infliximab-dyyb, adalimubab, adalimumab-atto, certolizumab pegol, golimubab, entanercept, etanercept-szzs) were compared with the control population. Similar to the RA population as a whole, statistical analysis revealed a significant increase in unspecified AMD in RA patients receiving treatment (n = 9101; P < 0.0001), with a 2.097 odds ratio (95% CI 1.923–2.286) for unspecified AMD (including dry AMD). This increased risk is identical to that of the entire RA population, and therefore does not suggest a change in risk for AMD in the presence of MAB therapy. In addition, risk of wet AMD was not altered in RA patients receiving TNF-α inhibitor treatment (n = 1079; P = 0.9129; Table 3).
Table 3.
Risk of AMD Diagnosis in the Presence of RA Diagnosis and Undergoing TNF-α Inhibitor* Treatment
|
Odds Ratio (95% CI) |
P |
|
| Unspecified AMD (including dry)† | 2.097 (1.923–2.286) | <0.0001 |
| Exudative (wet) AMD | 0.978 (0.610–1.348) | 0.9129 |
TNF-α inhibitor monoclonal antibodies included infliximab, infliximab-dyyb, adalimubab, adalimumab-atto, certolizumab pegol, golimumab, entanercept, and etanercept-szzs.
When compared with unspecified AMD risk in the nontreated group, no significant difference was observed.
Discussion
Autoimmune diseases such as RA affect 1.3 million Americans alone, with numbers expected to dramatically increase by the year 2040.43,44 This inflammatory disease has no cure, and like many autoimmune diseases, is found to be diagnosed 2.4 to 4.1 times more often in female than male individuals.45,46 Although RA is a chronic inflammatory disease, affecting the joints, it shares many common risk factors, and overlap in immune response with AMD. As a systemic disease with early onset between 40 and 60 years of age, we question whether prolonged activation of commonly shared immune pathways, such as the complement cascade, would put patients at a greater risk of developing AMD, a disease typically diagnosed in individuals older than 60. Previous studies have shown conflicting results when analyzing the relationship between RA and AMD.33,47 In a previously published study by McGeer and Sibley,33 it was proposed that RA patients do not suffer from AMD due to their long-term use of anti-inflammatory drugs. However, in addition to using a small sample size, this study omitted important controls such as a prevalence survey of AMD, as well as potential bias in reporting of AMD cases.
Using the MarketScan database, we were able to use a much larger dataset to further examine the evidence of a role of a secondary inflammatory disease in AMD pathogenesis. Here we report that risk of dry AMD diagnosis is increased in patients with RA compared with a matched control group. Using ICD-9 codes, our results provide further analysis of specific AMD type, wet and dry, and its increased risk with RA diagnosis. As dry AMD makes up approximately 90% of all AMD cases, it is no surprise that both RA and control groups observed a respective 90.99% and 83.09% of the dry AMD cases as well. However, although the number of wet AMD patients detected among both RA and control groups remained similar (n = 524 RA group; n = 555 control), more dry AMD patients were detected in the RA group (n = 5294) compared with control (n = 2728). Using the MarketScan database, we also demonstrated that after matching the numbers of female patients for both RA (n = 27,548) and matched controls (n = 27,960), and correcting for sex, an increased risk for AMD was observed among female RA patients.
As use of anti-TNF-α biologics are commonly used in treatment of RA, we examined their potential role on AMD diagnosis. TNF-α is a powerful proinflammatory cytokine, therefore anti-TNF-α agents are used as treatments for RA,26–28 and are being investigated as potential therapies for the treatment of wet AMD.29,48 Interestingly, the complement system, which is activated in both RA and AMD, is also found to be modulated by TNF-α.30,31 In synovial fluids from RA patients, TNF-α levels are increased and correlate with the complement activation markers of C3a, SCSb-9, and Bb.49 Conversely, use of anti-TNF-α agents demonstrated a decrease of C1q-C4 complexes in RA patients,50 as well as a decrease in C3b and C4b.51 Due to the role of complement and TNF-α in both RA and AMD, we examined the effect of patients receiving the commonly prescribed anti-TNF-α inhibitors infliximab, adalimubab, certolizumab, golimumab, and entanercept on risk of AMD diagnosis. As anti-TNF-α agents are able to decrease complement levels, and reduce the number of inflammatory cells at site of inflammation, we might expect that a decreased risk of AMD diagnosis would be observed in RA patients receiving these biologics. However, we observed the same increased risk of AMD diagnosis for RA patients taking these biologics when compared with the entire RA population. One reason for this may be due to increased RA severity among patients prescribed TNF-α inhibitor treatments. Although not within the scope of this study, RA severity is likely to play a role in AMD risk. Another potential reason for a lack of observed difference among those prescribed MABs may be due to individual efficacy of anti-TNF-α biologics for specific disease. Although entanercept is effective for treating RA, it is not for the treatment of diseases such as Crohn's disease52 or uveitis.53,54 In addition, a recent study by Jin et al.55 found that patients with commercial insurance compared with Medicaid were 87% more likely to initiate biologic disease-modifying RA drugs. The lower percentage of Medicaid patients receiving biologics was further decreased in African American patients.55 As our study was conducted using MarketScan data from the Medicare population, the patients in our cohort also may be less likely to engage or maintain prolonged anti-TNF-α therapy sufficient to provide a therapeutic effect. In addition, to effectively treat RA, medications are frequently changed over the course of many years, and medication dosage may be increased in response to RA flare-ups. Although this study analyzed commonly prescribed TNF-α inhibitors, it did not take into account the use of nonsteroidal anti-inflammatory drugs, steroids, and various disease-modifying anti-rheumatic drugs that may have been taken in combination during or before our analysis. Age of RA onset is also not accounted for in this study, and data indicate that earlier onset of RA is linked to disease severity.56 Finally, anti-TNF-α agents might be effective only in wet AMD, a population too small among our RA patients to identify a potential treatment effect.
Last, in this study we demonstrated that RA diagnosis leads to a significantly earlier time of AMD diagnosis. This information may prove to be very important in RA patient care, as RA patients may need to be proactive in receiving regular eye examinations to monitor early signs of AMD.
Taken together, these data provide further evidence of a role for systemic inflammation in diseases such as AMD. RA patients were found to be at significant increased risk for dry AMD using the MarketScan database. Use of the MarketScan database allowed for the analysis of retrospective patient data from a large data set. However, the use of insurance claim databases, such as with Medicare, is not without its limitations. Use of insurance databases does not account for those suffering from disease without insurance coverage. The coding patterns of clinical professionals for diagnosis codes may be different among individuals, and disease diagnosis may vary or be altogether missed.57 In addition, clinical outcome and severity of disease are typically not reported through use of diagnosis codes. Use of the new ICD-10 codes, implemented in October 2015, should, however, provide information that is more detailed for future analysis. In the case of AMD, these updated codes can be used to identify different stages of AMD as well as the eye affected. As smoking is a major risk factor for both RA and AMD, we hope to complete future analysis of the associated risk of RA and AMD within the smoking population. Using either individual patient data or improved ICD-10 codes, these studies would incorporate smoking history including smoking dependence or smoker remission. For future analysis we also aim to include patient use of hydroxychloroquine, a treatment used for autoimmune conditions and capable of leading to retinal toxicity at higher dose concentrations. However, insurance claim databases at this time do not generally include dosage frequency, and inconsistencies in ICD-9 coding for hydroxychloroquine retinal toxicity have been observed.58 Despite the current limitations in health claim analysis, our analyses provide compelling data on the association between RA and dry AMD diagnosis. This finding suggests a potential need for proactive AMD screenings among RA patients. Following further investigation, nutritional supplementation used for the treatment of AMD, such as those provided by the Age-related Eye Disease Study, also may prove to be beneficial in preventive care for RA patients. Forthcoming analysis on the role of smoking on increased RA and AMD disease risk could also impact cessation counseling among dependent smokers. Last, further studies using individual patient data must be performed to determine the role, if any, that anti-TNF-α agents play in risk of AMD.
Supplementary Material
Acknowledgments
Supported by the National Institutes of Health (NIH) K12HD055885 Building Interdisciplinary Research Careers in Women's Health (BIRCWH) fellowship (GS). Funding for BR was provided in part by the NIH R01EY019320, the Department of Veterans Affairs RX000444, BX003050 (BR) and the South Carolina Smart State Endowment. Additional support was provided by the CEDAR Core funded by the Medical University of South Carolina Provost Office, and from resources provided by NIH/National Center for Advancing Translational Sciences South Carolina Clinical and Translational Research Institute Grant UL1 TR001450.
Disclosure: G. Schnabolk, None; B. Rohrer, None; K.N. Simpson, None
References
- 1.Friedman DS, O'Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 2.Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration--emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38:450–471. doi: 10.1080/07853890600946724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan JC, Thurlby DA, Shahid H, et al. Smoking and age related macular degeneration: the number of pack years of cigarette smoking is a major determinant of risk for both geographic atrophy and choroidal neovascularisation. Br J Ophthalmol. 2006;90:75–80. doi: 10.1136/bjo.2005.073643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104:7–21. doi: 10.1016/s0161-6420(97)30368-6. [DOI] [PubMed] [Google Scholar]
- 5.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deane KD, Demoruelle MK, Kelmenson LB, Kuhn KA, Norris JM, Holers VM. Genetic and environmental risk factors for rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2017;31:3–18. doi: 10.1016/j.berh.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballanti E, Perricone C, di Muzio G, et al. Role of the complement system in rheumatoid arthritis and psoriatic arthritis: relationship with anti-TNF inhibitors. Autoimmun Rev. 2011;10:617–623. doi: 10.1016/j.autrev.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 8.van Lookeren Campagne M, Strauss EC, Yaspan BL. Age-related macular degeneration: complement in action. Immunobiology. 2016;221:733–739. doi: 10.1016/j.imbio.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Ruddy S. Altered local metabolism of complement components in rheumatoid arthritis. Rheumatology. 1975;6:17–23. [PubMed] [Google Scholar]
- 10.Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 11.Gold B, Merriam JE, Zernant J, et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38:458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fagerness JA, Maller JB, Neale BM, Reynolds RC, Daly MJ, Seddon JM. Variation near complement factor I is associated with risk of advanced AMD. Eur J Hum Genet. 2009;17:100–104. doi: 10.1038/ejhg.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yates JR, Sepp T, Matharu BK, et al. Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med. 2007;357:553–561. doi: 10.1056/NEJMoa072618. [DOI] [PubMed] [Google Scholar]
- 14.McHarg S, Clark SJ, Day AJ, Bishop PN. Age-related macular degeneration and the role of the complement system. Mol Immunol. 2015;67:43–50. doi: 10.1016/j.molimm.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 15.Ebrahimi KB, Fijalkowski N, Cano M, Handa JT. Decreased membrane complement regulators in the retinal pigmented epithelium contributes to age-related macular degeneration. J Pathol. 2013;229:729–742. doi: 10.1002/path.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruddy S, Colten HR. Rheumatoid arthritis. Biosynthesis of complement proteins by synovial tissues. N Engl J Med. 1974;290:1284–1288. doi: 10.1056/NEJM197406062902304. [DOI] [PubMed] [Google Scholar]
- 17.Guc D, Gulati P, Lemercier C, Lappin D, Birnie GD, Whaley K. Expression of the components and regulatory proteins of the alternative complement pathway and the membrane attack complex in normal and diseased synovium. Rheumatol Int. 1993;13:139–146. doi: 10.1007/BF00301260. [DOI] [PubMed] [Google Scholar]
- 18.Neumann E, Barnum SR, Tarner IH, et al. Local production of complement proteins in rheumatoid arthritis synovium. Arthritis Rheum. 2002;46:934–945. doi: 10.1002/art.10183. [DOI] [PubMed] [Google Scholar]
- 19.Konttinen YT, Ceponis A, Meri S, et al. Complement in acute and chronic arthritides: assessment of C3c, C9, and protectin (CD59) in synovial membrane. Ann Rheum Dis. 1996;55:888–894. doi: 10.1136/ard.55.12.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doherty M, Richards N, Hornby J, Powell R. Relation between synovial fluid C3 degradation products and local joint inflammation in rheumatoid arthritis, osteoarthritis, and crystal associated arthropathy. Ann Rheum Dis. 1988;47:190–197. doi: 10.1136/ard.47.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jose PJ, Moss IK, Maini RN, Williams TJ. Measurement of the chemotactic complement fragment C5a in rheumatoid synovial fluids by radioimmunoassay: role of C5a in the acute inflammatory phase. Ann Rheum Dis. 1990;49:747–752. doi: 10.1136/ard.49.10.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oleesky DA, Daniels RH, Williams BD, Amos N, Morgan BP. Terminal complement complexes and C1/C1 inhibitor complexes in rheumatoid arthritis and other arthritic conditions. Clin Exp Immunol. 1991;84:250–255. [PMC free article] [PubMed] [Google Scholar]
- 23.McDermott MF. TNF. and TNFR biology in health and disease. Cell Mol Biol (Noisy-le-grand) 2001;47:619–635. [PubMed] [Google Scholar]
- 24.Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1–26. doi: 10.1002/art.39480. [DOI] [PubMed] [Google Scholar]
- 25.Weinblatt ME, Keystone EC, Furst DE, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48:35–45. doi: 10.1002/art.10697. [DOI] [PubMed] [Google Scholar]
- 26.Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999;354:1932–1939. doi: 10.1016/s0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- 27.Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594–1602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- 28.Moreland LW, Schiff MH, Baumgartner SW, et al. Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med. 1999;130:478–486. doi: 10.7326/0003-4819-130-6-199903160-00004. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-Vega B, Fernandez-Vega A, Rangel CM, et al. Blockade of tumor necrosis factor-alpha: a role for adalimumab in neovascular age-related macular degeneration refractory to anti-angiogenesis therapy? Case Rep Ophthalmol. 2016;7:154–162. doi: 10.1159/000445102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perlmutter DH, Dinarello CA, Punsal PI, Colten HR. Cachectin/tumor necrosis factor regulates hepatic acute-phase gene expression. J Clin Invest. 1986;78:1349–1354. doi: 10.1172/JCI112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.An E, Gordish-Dressman H, Hathout Y. Effect of TNF-alpha on human ARPE-19-secreted proteins. Mol Vis. 2008;14:2292–2303. [PMC free article] [PubMed] [Google Scholar]
- 32.van Hagen PM, Baarsma GS, van Bilsen CE, et al. A noncontrolled trial of anti-TNF-alpha chimeric monoclonal antibody (infliximab, Remicade®) in exudative age-related macular degeneration. Acta Ophthalmol. 2014;92:e691–e692. doi: 10.1111/aos.12471. [DOI] [PubMed] [Google Scholar]
- 33.McGeer PL, Sibley J. Sparing of age-related macular degeneration in rheumatoid arthritis. Neurobiol Aging. 2005;26:1199–1203. doi: 10.1016/j.neurobiolaging.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Keenan TD, Goldacre R. Goldacre MJ. Associations between age-related macular degeneration, osteoarthritis and rheumatoid arthritis: record linkage study. Retina. 2015;35:2613–2618. doi: 10.1097/IAE.0000000000000651. [DOI] [PubMed] [Google Scholar]
- 35.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 37.Mirshahi A, Hoehn R, Lorenz K, Kramann C, Baatz H. Anti-tumor necrosis factor alpha for retinal diseases: current knowledge and future concepts. J Ophthalmic Vis Res. 2012;7:39–44. [PMC free article] [PubMed] [Google Scholar]
- 38.Markomichelakis NN, Theodossiadis PG, Sfikakis PP. Regression of neovascular age-related macular degeneration following infliximab therapy. Am J Ophthalmol. 2005;139:537–540. doi: 10.1016/j.ajo.2004.09.058. [DOI] [PubMed] [Google Scholar]
- 39.Arias L, Caminal JM, Badia MB, Rubio MJ, Catala J, Pujol O. Intravitreal infliximab in patients with macular degeneration who are nonresponders to antivascular endothelial growth factor therapy. Retina. 2010;30:1601–1608. doi: 10.1097/IAE.0b013e3181e9f942. [DOI] [PubMed] [Google Scholar]
- 40.Wu L, Arevalo JF, Hernandez-Bogantes E, Regatieri CV, Roca JA, Farah ME. Intravitreal tumor necrosis factor-alpha inhibitors for neovascular age-related macular degeneration suboptimally responsive to antivascular endothelial growth factor agents: a pilot study from the Pan American Collaborative Retina Study Group. J Ocul Pharmacol Ther. 2013;29:366–371. doi: 10.1089/jop.2012.0203. [DOI] [PubMed] [Google Scholar]
- 41.Theodossiadis PG, Liarakos VS, Sfikakis PP, Vergados IA, Theodossiadis GP. Intravitreal administration of the anti-tumor necrosis factor agent infliximab for neovascular age-related macular degeneration. Am J Ophthalmol. 2009;147:825–830. doi: 10.1016/j.ajo.2008.12.004. 830.e821. [DOI] [PubMed] [Google Scholar]
- 42.Giganti M, Beer PM, Lemanski N, Hartman C, Schartman J, Falk N. Adverse events after intravitreal infliximab (Remicade) Retina. 2010;30:71–80. doi: 10.1097/IAE.0b013e3181bcef3b. [DOI] [PubMed] [Google Scholar]
- 43.Barbour KE, Helmick CG, Boring M, Brady TJ. Vital signs: prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation - United States, 2013–2015. MMWR Morb Mortal Wkly Rep. 2017;66:246–253. doi: 10.15585/mmwr.mm6609e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 45.Crowson CS, Matteson EL, Myasoedova E, et al. The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis Rheum. 2011;63:633–639. doi: 10.1002/art.30155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gleicher N, Barad DH. Gender as risk factor for autoimmune diseases. J Autoimm. 2007;28:1–6. doi: 10.1016/j.jaut.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 47.Keenan TD, Goldacre R, Goldacre MJ. Associations between age-related macular degeneration, osteoarthritis and rheumatoid arthritis: record linkage study. Retina. 2015;35:2613–2618. doi: 10.1097/IAE.0000000000000651. [DOI] [PubMed] [Google Scholar]
- 48.Wang H, Han X, Wittchen ES, Hartnett ME. TNF-alpha mediates choroidal neovascularization by upregulating VEGF expression in RPE through ROS-dependent beta-catenin activation. Mol Vis. 2016;22:116–128. [PMC free article] [PubMed] [Google Scholar]
- 49.Brodeur JP, Ruddy S, Schwartz LB, Moxley G. Synovial fluid levels of complement SC5b-9 and fragment Bb are elevated in patients with rheumatoid arthritis. Arthritis Rheum. 1991;34:1531–1537. doi: 10.1002/art.1780341209. [DOI] [PubMed] [Google Scholar]
- 50.Wouters D, Voskuyl AE, Molenaar ET, Dijkmans BA, Hack CE. Evaluation of classical complement pathway activation in rheumatoid arthritis: measurement of C1q-C4 complexes as novel activation products. Arthritis Rheum. 2006;54:1143–1150. doi: 10.1002/art.21729. [DOI] [PubMed] [Google Scholar]
- 51.Makrides SC. Therapeutic inhibition of the complement system. Pharmacol Rev. 1998;50:59–87. [PubMed] [Google Scholar]
- 52.Mitoma H, Horiuchi T, Tsukamoto H, et al. Mechanisms for cytotoxic effects of anti-tumor necrosis factor agents on transmembrane tumor necrosis factor alpha-expressing cells: comparison among infliximab, etanercept, and adalimumab. Arthritis Rheum. 2008;58:1248–1257. doi: 10.1002/art.23447. [DOI] [PubMed] [Google Scholar]
- 53.Foster CS, Tufail F, Waheed NK, et al. Efficacy of etanercept in preventing relapse of uveitis controlled by methotrexate. Arch Ophthalmol. 2003;121:437–440. doi: 10.1001/archopht.121.4.437. [DOI] [PubMed] [Google Scholar]
- 54.Smith JA, Thompson DJ, Whitcup SM, et al. A randomized, placebo-controlled, double-masked clinical trial of etanercept for the treatment of uveitis associated with juvenile idiopathic arthritis. Arthritis Rheum. 2005;53:18–23. doi: 10.1002/art.20904. [DOI] [PubMed] [Google Scholar]
- 55.Jin Y, Desai RJ, Liu J, Choi NK, Kim SC. Factors associated with initial or subsequent choice of biologic disease-modifying antirheumatic drugs for treatment of rheumatoid arthritis. Arthritis Res Ther. 2017;19:159. doi: 10.1186/s13075-017-1366-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Innala L, Berglin E, Moller B, et al. Age at onset determines severity and choice of treatment in early rheumatoid arthritis: a prospective study. Arthritis Res Ther. 2014;16:R94. doi: 10.1186/ar4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tyree PT, Lind BK, Lafferty WE. Challenges of using medical insurance claims data for utilization analysis. Am J Med Qual. 2006;21:269–275. doi: 10.1177/1062860606288774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chiu SY, Shaw JW, Luong TQ, Fong DS, Modjtahedi BS. Coding patterns used by ophthalmologists for hydroxychloroquine retinal toxicity. Clin Ophthal. 2018;12:2261–2265. doi: 10.2147/OPTH.S170789. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.