Human enterovirus 71 (EV71) is one of the causative agents of hand, foot, and mouth disease in children and is responsible for thousands of deaths in the past 20 years. Food azo dyes have been widely used since the nineteenth century; however, their biological effects on humans and microbes residing in humans are poorly understood. Here, we discovered that one of these dyes, brilliant black BN (E151), was particularly effective in inhibiting the infectivity of EV71 in both cell culture and mouse model studies. Mechanistic studies demonstrated that these sulfonated dyes mainly competed with EV71 attachment factors for viral binding to block viral attachment/entry to host cells. As no commercial antiviral drugs against EV71 are currently available, our findings open an avenue to exploit the development of permitted food dye E151 as a potential anti-EV71 agent.
KEYWORDS: AG129 mouse, antiviral agent, food azo dye, hand foot and mouth disease, human enterovirus, sulfonate, virus attachment factors, virus entry
ABSTRACT
Hand, foot, and mouth disease (HFMD), a highly contagious disease in children, is caused by human enteroviruses, including enterovirus 71 (EV71), coxsackievirus A16 (CVA16), and coxsackievirus A6 (CVA6). Although HFMD is usually mild and self-limiting, EV71 infection occasionally leads to fatal neurological disorders. Currently, no commercial antiviral drugs for HFMD treatment are available. Here, numerous sulfonated azo dyes, widely used as food additives, were identified as having potent antiviral activities against human enteroviruses. Among them, brilliant black BN (E151) was able to inhibit all EV71, CVA16, and CVA6 strains tested. In rhabdomyosarcoma cells, the 50% inhibitory concentrations of the dye E151 for various strains of EV71 ranged from 2.39 μM to 28.12 μM, whereas its 50% cytotoxic concentration was 1,870 μM. Food azo dyes, including E151, interacted with the vertex of the 5-fold axis of EV71 and prevented viral entry. Their efficacy in viral inhibition was regulated by amino acids at VP1-98, VP1-145, and/or VP1-246. Dye E151 not only prevented EV71 attachment but also eluted attached viruses in a concentration-dependent manner. Moreover, E151 inhibited the interaction between EV71 and its cellular uncoating factor cyclophilin A. In vivo studies demonstrated that E151 at a dose of 200 mg/kg of body weight/day given on the initial 4 days of challenge protected AG129 mice challenged with 10× the 50% lethal dose of wild-type EV71 isolates. Taken together, these data highlight E151 as a promising antiviral agent against EV71 infection.
IMPORTANCE Human enterovirus 71 (EV71) is one of the causative agents of hand, foot, and mouth disease in children and is responsible for thousands of deaths in the past 20 years. Food azo dyes have been widely used since the nineteenth century; however, their biological effects on humans and microbes residing in humans are poorly understood. Here, we discovered that one of these dyes, brilliant black BN (E151), was particularly effective in inhibiting the infectivity of EV71 in both cell culture and mouse model studies. Mechanistic studies demonstrated that these sulfonated dyes mainly competed with EV71 attachment factors for viral binding to block viral attachment/entry to host cells. As no commercial antiviral drugs against EV71 are currently available, our findings open an avenue to exploit the development of permitted food dye E151 as a potential anti-EV71 agent.
INTRODUCTION
Hand, foot and mouth disease (HFMD), a common and highly contagious disease in children, is caused by human enteroviruses, including enterovirus 71 (EV71), coxsackievirus A16 (CVA16), and coxsackievirus A6 (CVA6). The clinical features of HFMD include rashes on the hands and feet, buccal ulcerative lesions, fatigue, fever, and lymphadenopathy. The illness may last 7 to 10 days and is usually self-limiting; however, outbreaks result in substantial socioeconomic and public health burdens (1, 2). Moreover, infection with EV71 occasionally leads to fatal pulmonary edema or neurological disorders like encephalitis, aseptic meningitis, and acute flaccid paralysis (2, 3). Millions of cases and several thousand deaths have been reported during EV71 epidemics in the Asia-Pacific region since the 1990s (4–8). Although EV71 vaccines have been available since 2015 (9, 10), there are still no commercially available antiviral drugs to treat HFMD and its associated morbidities and risk of mortality.
Human enteroviruses, belonging to the genus Enterovirus in the family Picornaviridae, have a positive-sense, single-stranded-RNA genome encapsidated in a nonenveloped, icosahedral particle with 60 copies of each of the 4 viral structural proteins, VP1, VP2, VP3, and VP4. VP1 is immunodominant, and based on its nucleotide sequence, EV71 is divided into genogroups A, B, and C. Genogroups B and C are further divided into subgenogroups B1 to B5 and C1 to C5, respectively. VP1 is also involved in the cell entry of enteroviruses. Every VP1 pentamer on the surface of an EV71 virion forms a prominent star-shaped plateau at the 5-fold axis of symmetry, surrounded by a deep depression called the canyon region (11, 12). Scavenger receptor class B, member 2 (SCARB2), serves as a functional receptor for all EV71 strains and has been shown to bind the canyon region (13, 14). In contrast, sulfated P-selectin glycoprotein ligand-1 (PSGL-1) and glycosaminoglycans (GAGs) on the cell surface interact with the positively charged vertex of the 5-fold axis of viral capsid to facilitate EV71 attachment and infection (15–20). Similarly, sulfated/sulfonated heparin, suramin, and suramin derivatives have been shown to bind to the vertex of the 5-fold axis to block EV71 attachment to host cells (21–23). The cellular protein cyclophilin A has been determined to be an EV71-uncoating regulating factor through interacting with and modifying the conformation of the H-I loop of VP1 at the vertex of the 5-fold axis (24). Antibodies binding to the vertex of the 5-fold axis also prevent EV71 infection (25). Moreover, positively charged amino acids arginine and lysine around the canyon and the vertex of the 5-fold axis on the surface of EV71 are important for the production of viable viruses (26). Therefore, chemical and biological agents targeting positively charged amino acids around the canyon and the vertex of the 5-fold axis of EV71 could be promising candidates as therapeutics against EV71 infection.
Azo dyes are usually synthesized from petroleum and constitute up to 70% of all organic dyes in the world (27). They are characterized by the presence of an azo bond (-N=N-) in their structure, conjugated with two distinct or identical, mono- or polycyclic aromatic groups. They may contain only one azo moiety, but some have two (diazo) or more. The synthesis and production of azo dyes are easy, and it is estimated that more than 10,000 types of azo dyes have been synthesized and about 3,000 types are used globally in various fields (27, 28). Many azo dyes have one or more sulfonate groups linked to aromatic rings and exist as sodium salts. Sulfonation of azo dyes increases their solubility in water and, at the same time, decreases their cellular absorption and enhances their urinary excretion in humans and animals. Sulfonated azo dyes can interact with positively charged amino acids, such as lysine and arginine, and possess antimicrobial activities (29–33). Some azo dyes, called food azo dyes, have been authorized to be used as additives in the food and beverage industry since the nineteenth century. Common food azo dyes individually labeled with an E number can be found in the list of food additives from the European Food Safety Authority (EFSA) (53). However, the biological effects and medical potentials of food azo dyes are largely unknown.
We discovered that almost all sulfonated azo dyes in the EFSA list had inhibitory effects on the infectivity of EV71 in vitro. Brilliant black BN (E number E151) was demonstrated to inhibit EV71, CVA16, and CVA6 strains. E151 had the highest efficiency in blocking virus entry of all the dyes tested. Furthermore, dye E151 protected AG129 mice challenged by lethal doses of wild-type EV71. As E151 is safe and has been used for many years as a food additive, it is a promising antiviral agent to prevent and/or treat HFMD in children.
RESULTS
Inhibitory effects of food dyes on the propagation of EV71-GFP.
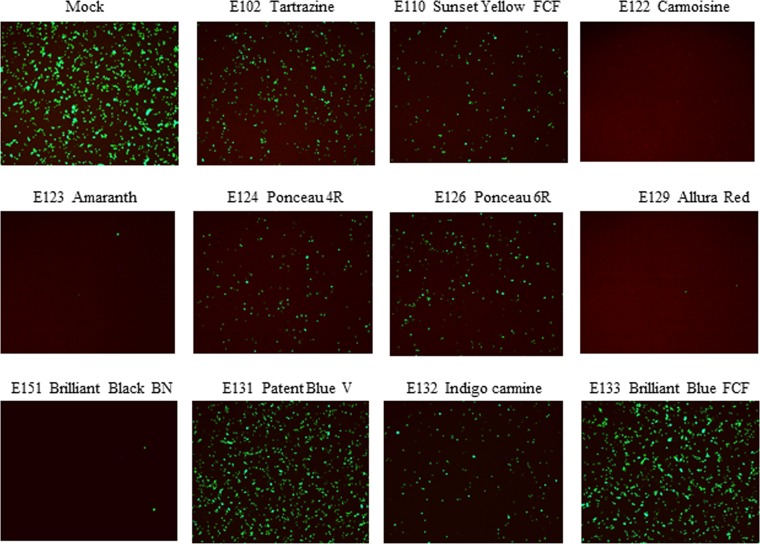
Eleven widely used food dyes were screened for their potency in inhibiting the propagation of EV71-GFP (producing green fluorescent protein [GFP] as a reporter) in rhabdomyosarcoma (RD) cells at a multiplicity of infection (MOI) of 1. By comparing the GFP signal in the presence of each dye to that in the mock controls at 24 h postinfection, all of the sulfonated azo dyes tartrazine (E102), sunset yellow FCF (E110), carmoisine (E122), amaranth (E123), ponceau 4R (E124), ponceau 6R (E126), allura red (E129), and brilliant black BN (E151) inhibited EV71-GFP infection at a concentration of 300 μM, as evidenced by the reduced GFP signals (Fig. 1). Among the other three sulfonated food dyes, patent blue V (E131), indigo carmine (E132), and brilliant blue FCF (E133), only E132 exhibited inhibition of EV71-GFP at the concentration of 300 μM. Overall, E122, E123, E129, and E151 were the four strongest inhibitors of EV71-GFP infection in vitro (Fig. 1).
FIG 1.
Effects of food dyes on infection of RD cells by EV71-GFP. EV71-GFP viruses were incubated with different dyes at 300 μM in cell culture medium at 37°C for 1 h and then inoculated into RD cells in 96-well plates at a multiplicity of infection (MOI) of 1. The GFP signals were observed and recorded at 24 h postinfection.
Antiviral activity of dyes E122, E123, E129, and E151 in vitro.
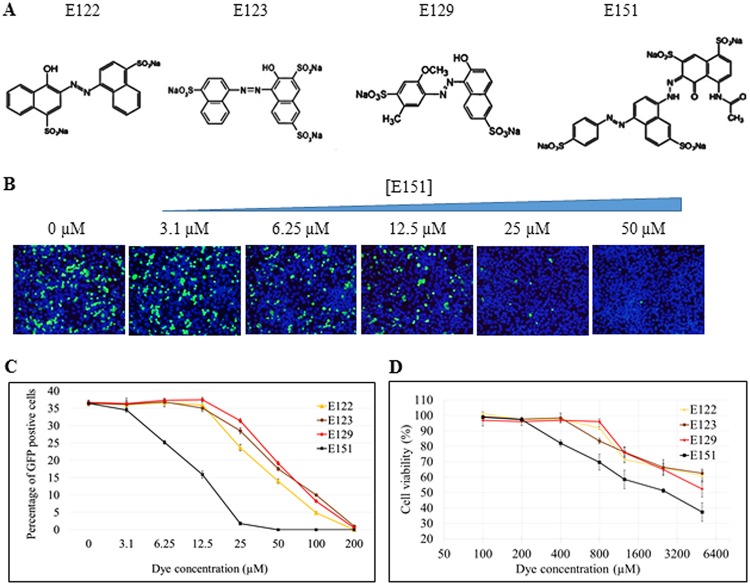
The four azo dyes E122, E123, E129, and E151, all of which contain sulfonated aromatic rings (Fig. 2A), were further tested in antiviral and cytotoxic studies. They exhibited dose-dependent inhibition of EV71-GFP in infected RD cells (Fig. 2B and C). Dye E151 was the most effective in reducing infection by EV71-GFP in RD cells. Based on the percentage of GFP-positive cells, the 50% inhibitory concentration (IC50) of E151 was determined to be 10.1 μM, while the IC50s of the other three dyes, E122, E123, and E129, were approximately 50 μM (Fig. 2C). In contrast, the 50% cellular cytotoxic concentration (CC50) of E151 in RD was 1,870 μM and the CC50s of the other three dyes were more than 5,000 μM as evaluated by an MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] assay (Fig. 2D).
FIG 2.
Antiviral and cytotoxic studies of dyes E122, E123, E129, and E151. (A) Chemical structures of the 4 anti-EV71 food azo dyes. All structures contain sulfonated aromatic rings. (B) Concentration-dependent inhibition of EV71-GFP infection by E151 in vitro. RD cells were infected by EV71-GFP at an MOI of 1 for 12 h in the presence of different concentrations of E151. After cell fixation, the nuclei of all cells (blue) were visualized by Hoechst 33258 staining and infected cells were identified by the GFP signal (green) under a fluorescence microscope. (C) Percentages of infected cells (GFP positive) after treatment with one of 4 food azo dyes at the indicated concentrations from 0 to 200 μM. The average value ± standard error of 3 random spots for each concentration is presented. (D) Cytotoxicity study of E122, E123, E129, and E151 in RD cells. The cells were cultured with different concentrations of each of the dyes in DMEM-10. After 3 days, the cell viability in the presence of each respective dye was measured by an MTT assay, and the results are presented as percentages of the result in the absence of the dye. The average values ± standard errors of triplicated experiments are shown.
By using a virus titer reduction assay, the inhibitory effects of the four azo dyes on the infectivity of wild-type human enteroviruses were investigated in RD cells. E151 at 100 μM significantly prevented cytopathic effect (CPE) induced by viral infection and reduced the titers of all 8 RD cell-adapted EV71 strains, belonging to different subgenogroups (Fig. 3A), and 4 clinical EV71 isolates (Fig. 3B). However, E122, E123, and E129 failed to reduce the titers of EV71-C1 and EV71-C4 at the concentration of 300 μM. In addition, E151 inhibited the infectivity of all 3 CVA16 and 1 CVA6 virus, while it did not show inhibitory effects on the infectivity of 1 CVA4 and 1 CVA10 virus (Fig. 3B). The inhibitory effect of E151 on the growth of 5 representative enteroviruses was also evaluated by titrating progeny viruses from RD cells infected at an MOI of 0.1 for 48 h in the presence of various concentrations of E151. The IC50s of E151 for EV71 ranged from 2.39 μM (for the highly sensitive EV71-B2) to 28.12 μM (for the slightly sensitive EV71-C1) (Fig. 3C).
FIG 3.
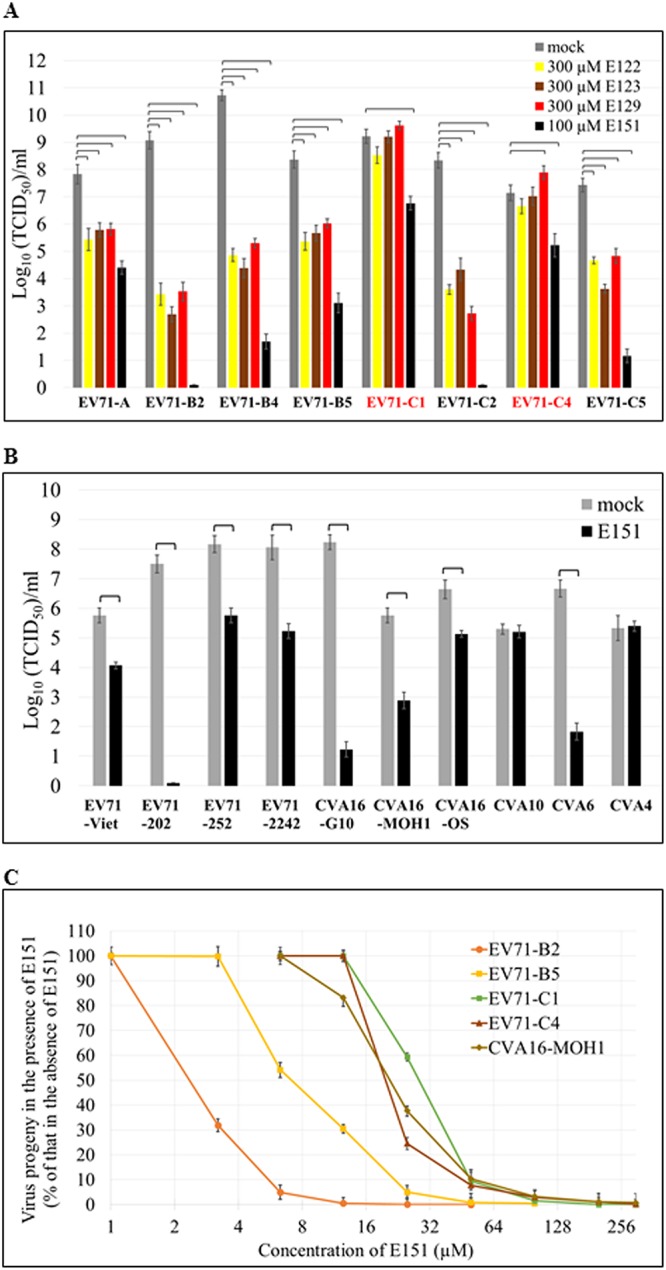
Inhibitory effects of azo dyes on the titers of human enteroviruses. (A and B) Titer reduction assays of 8 RD cell-adapted EV71 strains from different subgenogroups (A) and of 4 other clinical isolates of EV71 and 6 coxsackie A viruses (see Table 2) (B) in the presence of E122, E123, E129, or E151. Brackets indicate P values of <0.01 (one-way ANOVA with Dunnett’s posttest in panel A and two-tailed Student’s t test in panel B). (C) Inhibitory effect of E151 on the virus progeny yields of 5 enteroviruses. RD cells were infected with the virus at an MOI of 0.1 in the presence of various concentrations of E151 for 48 h. The titers of the respective virus progeny were determined in RD cells and are presented as percentages of the titer without E151 treatment. The average values ± standard errors of triplicated experiments are shown.
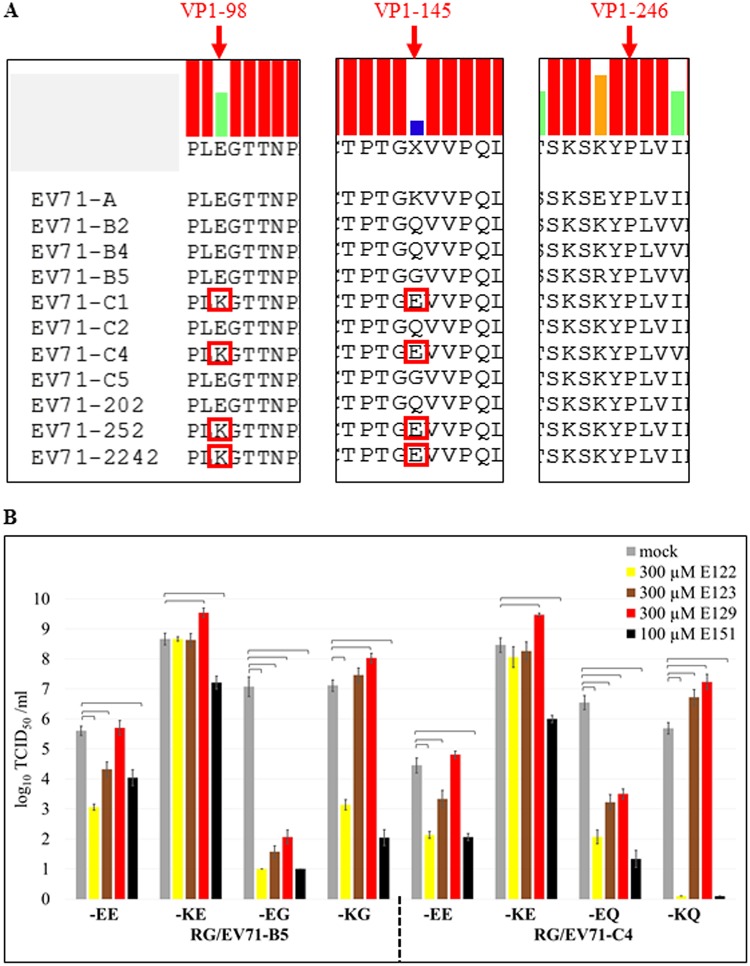
The inhibitory effects of the dye E151 on human enteroviruses varied. PSGL-1 binding EV71 strains with VP1-98E,145G/Q, such as EV71-B2, EV71-B4, EV71-B5, EV71-C2, EV71-C5, and EV71-202 (Fig. 4A), were highly sensitive to E151, with titer reductions of >105, while non-PSGL-1 binding strains with VP1-98K,145E, such as EV71-C1, EV71-C4, EV71-252, and EV71-2242 (Fig. 4A), were slightly sensitive to E151, with titer reductions of ∼102 in the presence of 100 μM E151 (Fig. 3A and B). In order to determine whether the amino acids at VP1-98 and/or VP1-145 affect the sensitivity of EV71 to E151 and the other 3 dyes, variants of EV71-B5 and EV71-C4 with different amino acid combinations at VP1-98 and VP1-145 were generated using a reverse genetics (RG) system (34). In the titer reduction assay, the EE variants (VP1-98E,145E) of RG/EV71-B5 and RG/EV71-C4 were slightly sensitive to E122, E123, and E151 but resistant to E129. The KE variants were only slightly sensitive to E151 and resistant to E122 and E123. The EG and EQ variants were highly sensitive to all 4 dyes, while the KG and KQ variants were highly sensitive only to E122 and E151. Surprisingly, E129 increased the titers of EV71 variants with VP1-98K in RD cells (Fig. 4B). The multiple-protein alignment of 6,585 full EV71 VP1 sequences released in GenBank until 2018 indicated that the EE variants were dominant, and their percentages ranged from 64.0% to 88.7%, while the percentages of KE variants ranged from 6.5% to 15.6% since 2008 (Table 1). The total percentage of EE and KE variants was 90.7% (82.4% + 8.3%) in all VP1 sequences. E151 inhibited both EE and KE variants, suggesting that this dye would be an effective antiviral against wild-type EV71 infections.
FIG 4.
Amino acids at VP1-98 and VP1-145 of EV71 modulated EV71’s sensitivity to the dyes. (A) Multiple protein alignment of EV71 VP1. EV71-B2, EV71-B4, EV71-B5, EV71-C4, and EV71-202, which were highly sensitive to all 4 of the dyes E122, E123, E129, and E151, had VP1-98E,145G/Q, while EV71-C1, EV71-C4, EV71-252, and EV71-2242, which were slightly sensitive or resistant to the 4 dyes, had VP1-98K,145E (highlighted by red boxes). (B) Effects of the 4 dyes on the infectivity of EV71 variants with different amino acid combinations at VP1-98 and VP1-145. The titer reduction assays of the EV71 variants were performed in RD cells in the absence or presence of each respective dye. The average values ± standard errors of triplicated experiments are shown. Brackets indicate P values of <0.01 (one-way ANOVA with Dunnett’s post-test).
TABLE 1.
Percentages of EV71 variants with different amino acid combinations at VP1-98 and VP1-145a
| EV71 variant | % of variants with indicated no. of sequences from indicated time period |
Total (n = 6,585) | |||||
|---|---|---|---|---|---|---|---|
| To 2007 (n = 607) | 2008–2009 (n = 497) | 2010–2011 (n = 1,481) | 2012–2013 (n = 1,314) | 2014–2015 (n = 1,388) | 2016–2017 (n = 1,298) | ||
| VP1-98E,145E | 64.0 | 68.5 | 81.9 | 88.7 | 88.3 | 84.3 | 82.4 |
| VP1-98K,145E | 10.7 | 15.6 | 6.5 | 6.5 | 6.7 | 9.8 | 8.3 |
| VP1-98E,145G/Q | 24.3 | 15.1 | 10.8 | 4.6 | 4.6 | 5.5 | 8.8 |
| VP1-98K,145G/Q | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Others | 1 | 0.8 | 0.8 | 0.2 | 0.4 | 0.4 | 0.5 |
A total of 6,585 EV71 VP1 protein sequences from the NCBI GenBank database were collected and aligned using DNASTAR MegAlign Pro 13.
Selection and characterization of dye E151-resistant EV71 mutants.
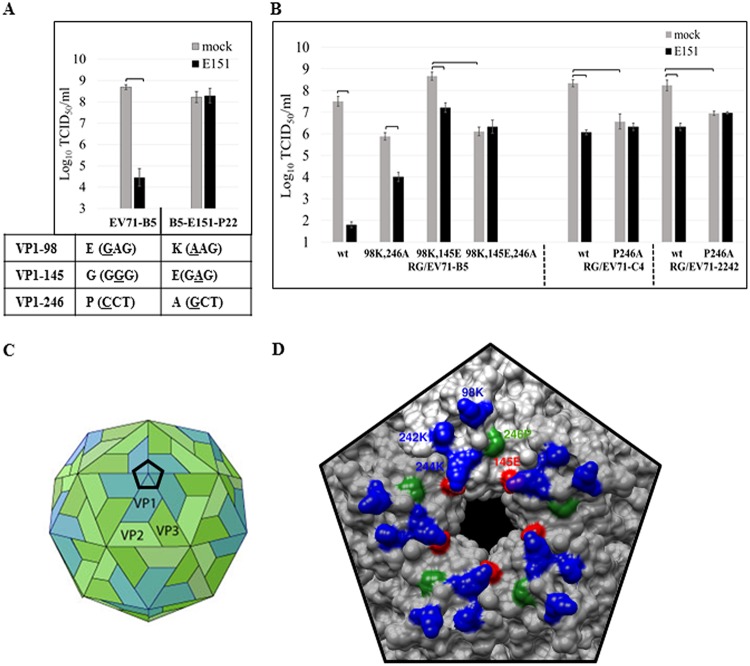
Dye E151-resistant EV71 mutants were selected by passaging viruses in RD cells in the presence of E151. The mutant EV71-B5, passaged 22 times and named B5-E151-P22 or EV71-B5res, was completely resistant to E151 at the concentration of 100 μM in RD cells (Fig. 5A). Sequencing of the genome of EV71-B5res revealed three amino acid mutations, E98K (a change of E to K at position 98), G145E, and P246A, in its VP1 protein compared to the sequence of its parental-wild type, EV71-B5 (Fig. 5A), implying that switching from a strain highly sensitive to E151 (EG variant) to a slightly sensitive strain (KE variant) was necessary for EV71-B5 to develop E151 resistance. The sequencing result also suggested that one additional mutation, P246A, was sufficient for KE strains of EV71 to become resistant to E151. Most importantly, VP1-246P is highly conserved (>99.9%) in EV71 (Fig. 4A), indicating that E151 has the potential to inhibit all circulating EV71 strains.
FIG 5.
Selection and characterization of E151-resistant EV71 mutants. (A) EV71-B5 was passaged in RD cells in the presence of E151. The 22nd-passage virus EV71-B5res (EV71-E151-P22) exhibited complete E151 resistance with 3 amino acid changes, E98K, G145E, and P246A, in VP1 compared to the sequence of the parental wild-type EV71-B5. Nucleotide mutations are underlined. (B) VP1-P246A conferred total E151 resistance on the EV71 KE (VP1-98K,145E) strains. P246A was introduced into EV71-B5, EV71-C4, and EV71-2242 strains by reverse genetics (RG). The titers of the 2nd-passage viruses were determined in RD cells in the absence or presence of 100 μM E151. The average values ± standard errors of triplicated experiments are shown. Brackets indicate P values of <0.01 (one-way ANOVA with Dunnett’s posttest). (C) Cartoon image of the icosahedral EV71 virion with VP1 (blue), VP2 (light green), and VP3 (green) on viral surface (adapted from ViralZone [https://viralzone.expasy.org/; Swiss Institute of Bioinformatics] under a Creative Commons CC BY-NC-ND 4.0 license). The regular black pentagon indicates the vertex of the 5-fold axis formed by a VP1 pentamer. (D) Three-dimensional surface of the vertex of an EV71 KE strain (PDB ID 3VBS). Amino acids at VP1-145 (red) and VP1-246 (green) are critical for E151 binding to prevent EV71 infection. VP1-246P is conserved, while VP1-145E is dominant. EV71 strains with VP1-145G/Q are highly sensitive to E151, whereas strains with VP1-145E have low sensitivity to E151. Positively charged VP1-98K, VP1-242K, and VP1-244K (blue) might also interact with E151 and other azo food dyes through electrostatic attraction.
According to the results of testing the EV71-B5 mutants in a titer reduction assay in the presence of 100 μM E151, mutation E98K did not significantly affect the sensitivity of EV71-B5 to E151 (Fig. 4B), but it compensated the growth defects caused by P246A, which did not induce CPE after 3 passages in RD cells (data not shown). Compared to the wild-type (wt) strain RG/EV71-B5-wt, which had a titer reduction of >105, the mutants with double mutation E98K G145E or E98K P246A became slightly sensitive to E151, with a titer reduction of ∼102. Only the triple mutation E98K G145E P246A conferred E151 resistance on EV71-B5 (Fig. 4B and 5B). Moreover, the single mutation P246A in EV71-C4 and EV71-2242 (KE strains) rendered them E151 resistant. However, the mutation P246A reduced the titers of the mutants about 10 to 100 times (Fig. 5B). Based on the crystal structure of an EV71 KE strain (PDB identification number [ID] 3VBS), VP1-98, VP1-145, and VP1-246 were located at the vertex of the 5-fold axis and proximal to conserved amino acids VP1-242K and VP1-244K (Fig. 5C and D).
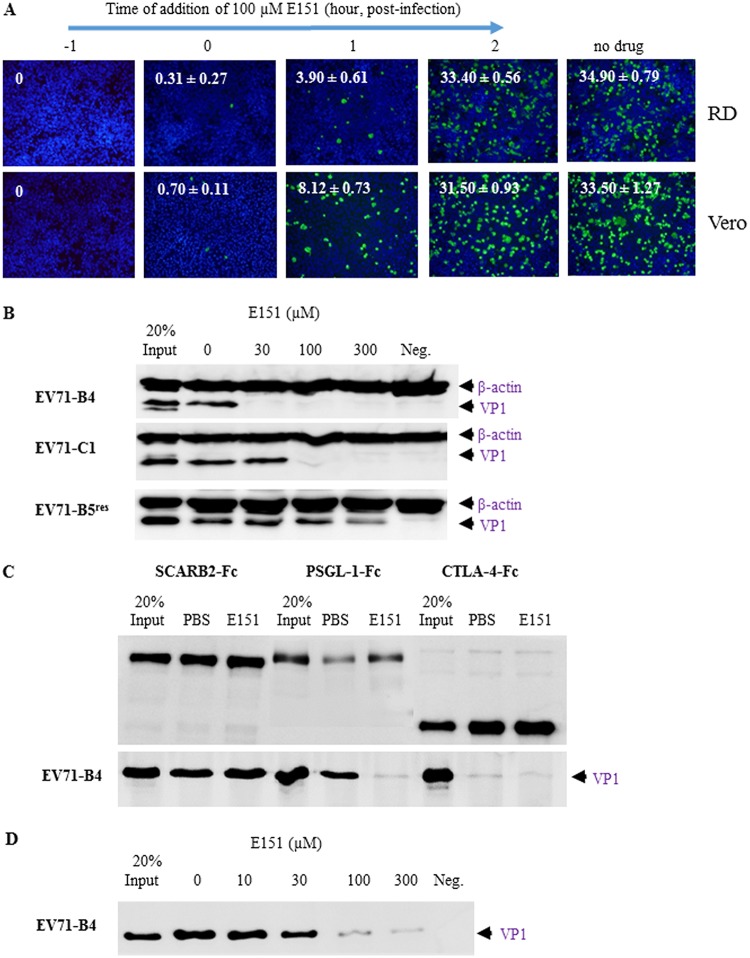
Inhibition of EV71 attachment by dye E151.
To investigate which steps of the EV71 life cycle were affected by E151, E151 was added to RD or Vero cells at an MOI of 1 at different time points during EV71-GFP infection, and infected cells were quantified at 12 h postinfection (Fig. 6A). The percentage of infected cells with positive GFP signals was significantly reduced when E151 was added at −1, 0 or 1 h but not at 2 h postinfection. Therefore, the inhibitory effects of E151 occurred at the early stages of EV71 infection (<2 h postinfection), which include viral attachment, internalization, and uncoating but not viral replication.
FIG 6.
Inhibition of EV71 attachment to cells or attachment factors by E151. (A) E151 affected the early stage (cell entry) of EV71-GFP infection. RD or Vero cells were infected by EV71-GFP at an MOI of 1. E151 was added at −1, 0, 1, or 2 h postinfection at a final concentration of 100 μM. At 12 h postinfection, the cells were fixed and stained with Hoechst 33258. The percentage of infected cells (green) at 3 random spots under each condition is presented as the average value ± standard error in the top left corner of a representative image. (B) E151 prevented the attachment of EV71-B4 (highly sensitive to E151) and EV71-C1 (slightly sensitive to E151) to RD cells in a concentration-dependent manner. Prechilled viruses and cells were incubated at 4°C for attachment, and then attached viruses and cells were analyzed by Western blotting. (C) E151 disrupted the binding of EV71-B4 to the human PSGL-1–Fc but not to the human SCARB2-Fc recombinant protein in a pulldown assay. Amounts of 3 μg SCARB2-Fc, 1 μg PSGL-1–Fc, and 3 μg CTLA-4–Fc (control) were bound to protein A/G-Sepharose and then incubated with purified EV71-B4. The precipitated proteins were analyzed by Western blotting. (D) E151 prevented the binding of EV71-B4 to heparin-Sepharose in a dose-dependent manner.
To further dissect the inhibitory mechanisms of the dye E151, assays of the attachment of EV71 to cells and attachment factors were performed in the absence or presence of E151. In a cell-virus binding assay, E151 prevented EV71 attachment to RD cells in a concentration-dependent manner. The attachment of EV71-B4 (highly sensitive to E151) and EV71-C1 (slightly sensitive to E151) to RD cells was significantly blocked by E151 at concentrations of 30 μM and 100 μM, respectively, based on the results of the Western blot analysis using a specific anti-VP1 monoclonal antibody, 1D9 (35). Although EV71-B5res was resistant to E151, E151 also partially blocked its attachment to RD cells at a very high concentration of 300 μM (Fig. 6B). However, E151 at 300 μM did not reduce the infectivity of EV71-B5res in RD cell culture (data not shown). In a pulldown assay, E151 blocked the binding of EV71-B4 to the PSGL-1–Fc recombinant protein as PSGL-1, similar to the interaction of E151 with the vertex of the viral 5-fold axis (17), while it did not prevent the interaction between EV71-B4 and human SCARB2-Fc recombinant protein, which is shown to bind the viral canyon region (Fig. 6C) (14). Furthermore, E151 prevented the binding of EV71-B4 to heparin in a concentration-dependent manner (Fig. 6D).
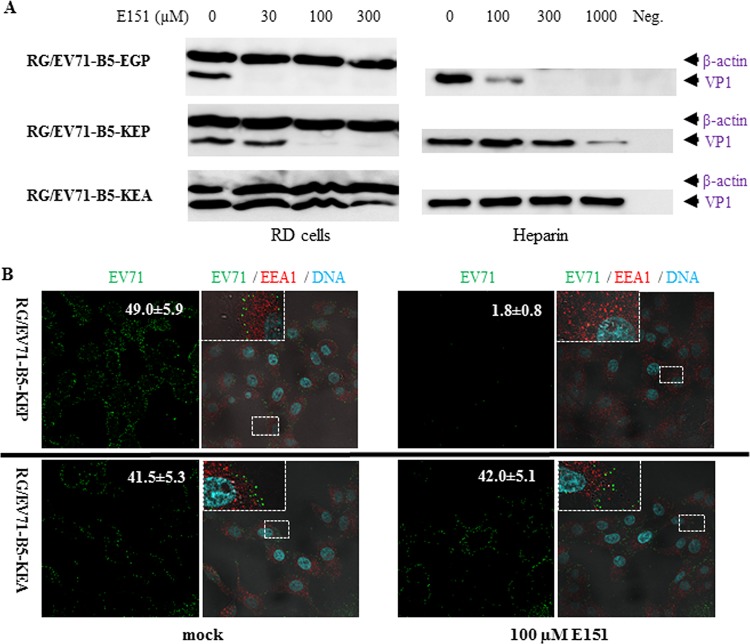
In order to evaluate whether amino acids at VP1-145 and VP1-246 determined the effects of the dye E151 on EV71 attachment, RG/EV71-B5-EGP (VP1-98E,145G,246P, highly sensitive to E151), RG/EV71-B5-KEP (VP1-98K,145E,246P, slightly sensitive to E151) and RG/EV71-B5-KEA (VP1-98K,145E,246A, resistant to E151) mutants were tested in the same cell-virus attachment assay. The results concurred with those of corresponding wild-type EV71 variants. Compared to RG/EV71-B5-EGP, whose attachment to RD cells and heparin was inhibited by E151 at concentrations of 30 μM and 100 μM, respectively, the attachment of RG/EV71-B5-KEP to both RD cells and heparin was only inhibited at higher concentrations of E151. As expected, P246A in RG/EV71-B5-KEA rendered attachment of the mutant unaffected by E151 (Fig. 7A). In an immunofluorescence confocal microscopy assay, attached EV71 virions on Vero cells were detected by anti-EV71-B4 antiserum after 1 h of incubation at 4°C. The attachment of RG/EV71-B5-KEP was strongly prevented by 100 μM E151, as the number of viral puncta (green) per cell was significantly reduced, from 49 ± 5.9 (average number ± standard error) in the absence of E151 to 1.8 ± 0.8 in the presence of E151, while the attachment of E151-resistant RG/EV71-B5-KEA was not significantly affected by E151 (Fig. 7B).
FIG 7.
Inhibitory effects of E151 on the attachment of EV71 KE strains abolished by VP1-P246A. (A) E151 prevented the attachment of RG/EV71-B5-EGP (VP1-98E,145G,246P, highly sensitive to E151) and RG/EV71-B5-KEP (VP1-98K,145E,246P, slightly sensitive to E151) but not RG/EV71-B5-KEA (VP1-98K,145E,246A, resistant to E151) to RD cells and heparin in a dose-dependent manner in Western blot assay. (B) E151 prevented the attachment of RG/EV71-B5-KEP but not RG/EV71-B5-KEA virions to Vero cells in immunofluorescence confocal microscopy assay. Virions were allowed to attach to prechilled Vero cells at 4°C for 1 h in the presence or absence of 100 μM E151. Attached virions (green), cellular early endosomes (red), and cellular DNA (cyan) were stained by anti-EV71 antiserum, anti-EEA1 antibody, and Hoechst 33258, respectively, after cell fixation. The numbers of EV71 puncta per cell (average value ± standard error) in a representative green EV71 channel image (×600 magnification) are presented in the top right corners. Enlarged views of the small boxed areas in the corresponding multiple EV71/EEA1/DNA channel images are in the top left corners.
Inhibition of EV71 infection at postattachment stage by dye E151.
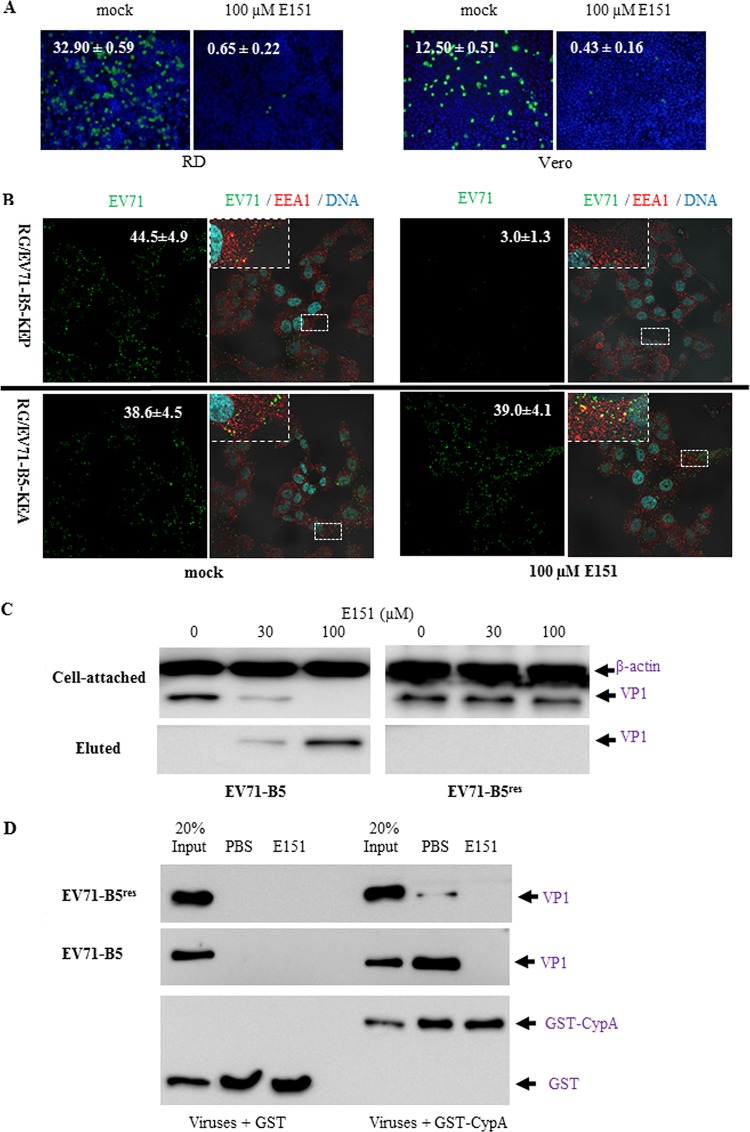
At the postattachment stage, E151 at 100 μM significantly prevented EV71-GFP infection and reduced the percentage of infected cells from 32.9 ± 0.59 to 0.65 ± 0.22 in RD cells and from 12.5 ± 0.51 to 0.43 ± 0.16 in Vero cells (Fig. 8A). The influence of E151 on the internalization of purified EV71 was further examined by immunofluorescence confocal microscopy assay. RG/EV71-B5-KEP and RG/EV71-B5-KEA were incubated with Vero cells at 4°C in the absence of E151 for attachment. After unattached viruses were washed away with cold phosphate-buffered saline (PBS), the attached viruses and cells in the absence or presence of 100 μM E151 were incubated at 37°C for 30 min to allow virus internalization. The cells were then fixed, and internalized EV71 virions and early endosome antigen 1 (EEA1) were observed. In the absence of E151 (mock treatment), the internalization of both RG/EV71-B5-KEP and RG/EV71-B5-KEA occurred, and some of the EV71 puncta (green) were colocalized with EEA1 (red), indicating that internalized virions fused with early endosomes (Fig. 8B). However, in the presence of E151, fewer RG/EV71-B5-KEP puncta per cell (3 ± 1.3) were detected compared to the number in the mock-treated cells (44.5 ± 4.9), while the number of RG/EV71-B5-KEA puncta per cell was not significantly different between mock-treated (38.6 ± 4.5) and E151-treated (39 ± 4.1) cells. As the degradation of internalized RG/EV71-B5-KEP virions is unlikely within 30 min of infection, further experiments were performed to determine if E151 detached cell bound virions. RD cells with attached E151-sensitive EV71-B5 or -resistant EV71-B5res viruses were resuspended in ice-cold PBS with 0, 30, or 100 μM E151. After incubation at 4°C for 15 min with gentle agitation, the cell pellets and supernatants were separated for EV71 antigen detection by Western blot analysis. The results indicated that the attached EV71-B5 but not EV71-B5res was eluted or released from the cells into the supernatants by E151 in a concentration-dependent manner (Fig. 8C).
FIG 8.
Inhibition of EV71 infection by E151 at the postattachment stage. (A) E151 inhibited EV71-GFP infection following attachment. Prechilled RD or Vero cells were incubated with ice-cold EV71-GFP at an MOI of 10 and attachment allowed at 4°C for 1 h. The unattached viruses were then washed away from cells using ice-cold PBS. Fresh DMEM-10 with or without 100 μM E151 was added, and the cells were transferred into a 37°C incubator. After 11 h, the cells were fixed and stained with Hoechst 33258. The percentage of infected cells (green) at 3 random spots under each condition is presented as the average value ± standard error in the top left corner of a representative image. (B) E151 detached cell-bound RG/EV71-B5-KEP but not RG/EV71-B5-KEA in the immunofluorescence confocal microscopy assay. Attached virions were allowed to internalize into the cells at 37°C for 30 min in the presence or absence of 100 μM E151. Virions (green), cellular endosomes (red), and cellular DNA (cyan) were stained by anti-EV71 antiserum, anti-EEA1 antibody, and Hoechst 33258, respectively, after cell fixation. The numbers of EV71 puncta per cell (average values ± standard errors) in representative green EV71 channel images (×600 magnification) are presented in the top right corners. Enlarged views of the small boxed areas in the corresponding multiple EV71/EEA1/DNA channel images are in the top left corners. (C) Attached EV71 virions were eluted from RD cells by E151 in a concentration-dependent manner. E151-sensitive EV71-B5 or -resistant EV71-B5res was incubated with RD cells at 4°C for 1 h for attachment, and unattached viruses were washed away with ice-cold PBS. The attached viruses-cells were divided into 3 portions and resuspended in ice-cold PBS with 0, 30, or 100 μM E151. After incubation at 4°C for 15 min with gentle agitation, the cell pellets and supernatants were separated for EV71 antigen detection using Western blotting. (D) EV71 binding to the uncoating factor cyclophilin A (CypA) was prevented by E151. In the GST pulldown assay, glutathione-Sepharose-bound GST or GST-CypA was incubated with purified EV71-B5 or EV71-E5res in the absence or presence of E151. The precipitated proteins were analyzed by Western blotting with the antibody 1D9 and anti-GST antiserum.
After viral internalization, E151 was further found to prevent the binding of EV71-B5 to an EV71-uncoating factor, cyclophilin A (CypA). In a glutathione S-transferase (GST) pulldown assay, EV71-B5 interacted with GST-CypA but not GST and failed to bind to GST-CypA in the presence of 100 μM E151 (Fig. 8D). The P246A mutation in EV71-B5res significantly weakened the interaction between the virus and GST-CypA, as reported previously (24), and this weak interaction was also inhibited by E151 (Fig. 8D).
Protection of EV71-challenged AG129 mice by dye E151.
The efficacy of E151 in preventing EV71 infection in vivo was assessed in 14-day-old AG129 mice, which were intraperitoneally challenged with 10 50% lethal doses (LD50) of highly E151-sensitive EV71-B4 (3 × 1010 50% tissue culture infective dose [TCID50]) or slightly E151-sensitive EV71-C1 (1.5 × 108 TCID50). The mice (8 pups per group) were then treated with 200 mg E151/kg of body weight/day or 200 μl PBS/day through intraperitoneal inoculation from the 14th to the 17th day (0 to 3 days postchallenge). The PBS-treated mice in control groups began to exhibit clinical symptoms, such as a hunched back and limb paralysis, from as early as day 6 postchallenge and succumbed to infection from day 8 postchallenge. Eventually they all died, with the survival percentage declining to 0. In the E151 treatment groups, some challenged mice only exhibited mild illness with clinical scores of less than 3 and subsequently recovered. All E151-treated mice were completely protected throughout the experiment (Fig. 9A and B).
FIG 9.
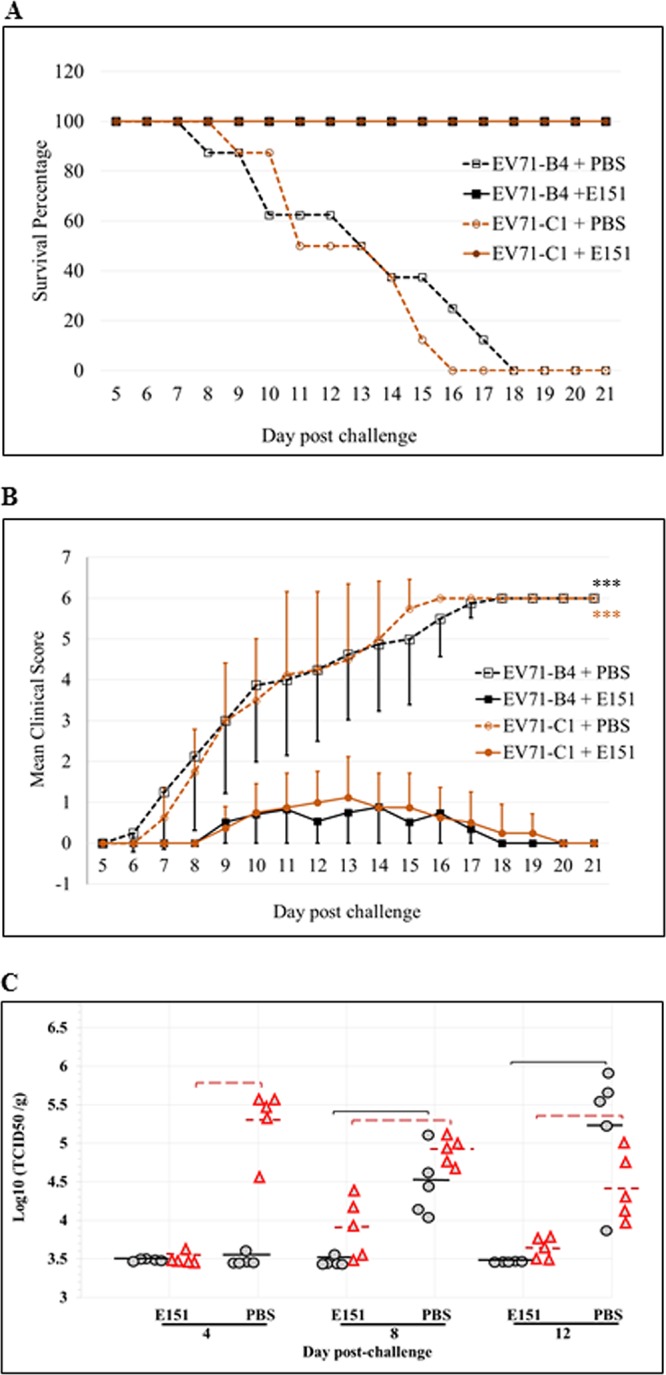
Protection of EV71-challenged AG129 mice by E151. Ten LD50 of EV71-B4 (highly sensitive to E151) or EV71-C1 (slightly sensitive to E151) were intraperitoneally inoculated into 14-day-old AG129 mice with 1 dose of E151 (200 mg/kg/day) or PBS, and then 1 dose of E151 was administered daily on the following 3 days (i.e. on the 15th to 17th day after birth of the mice). The daily percentage of survival (A) and mean clinical score (B) of the challenged mice with or without E151 treatment (n = 8) was recorded until 21 days after the challenge (***, P < 0.001). (C) Titers of progeny viruses in brains (black circles) and hind limb muscles (red triangles) from EV71-C1-infected mice that received PBS or E151 treatment. The mice (n = 5) were euthanized at 4, 8, or 12 days postchallenge, and titers of infectious viruses in the homogenized tissue samples were determined in RD cells. Mean values are shown as solid black lines (brain) or dashed red lines (hind limb muscle), and brackets indicate P values of <0.01 (Mann-Whitney test) for comparisons between PBS- and E151-treated mice.
To further evaluate the protective efficacy of E151, the EV71-C1-challenged mice treated with PBS or E151 were euthanized at 4, 8, or 12 days postchallenge (5 mice per group per time point), and titers of progeny virus in the brain and hind limb muscle tissues were determined (Fig. 9C). For the PBS-treated mice, viruses were detected in the muscle and brain from day 4 postchallenge. The viral titers in brains continually increased with time, while those in muscle tissue reached the highest levels at day 4 postchallenge and then declined, as previously reported (36). In contrast, significantly lower virus titers in both brain and muscle tissues were observed in the mice treated with E151. Although the viral titers in muscle of E151-treated mice increased after the cessation of E151 treatment from day 4 postchallenge, those in brains did not increase to the detectable level (103.5 TCID50/g).
DISCUSSION
The cell entry of EV71 begins with attachment to the molecules on the cell membrane and ends with the release of the viral genome into the cytoplasm (37). Multiple anti-EV71 drugs targeting EV71 entry have been reported (38, 39). Most food azo dyes contain sulfonated aromatic ring structures, like sulfonated naphthalene moieties, which are also found in other antiviral azo dyes and chemicals, such as Congo red, suramin, and the suramin derivative NF449. These sulfonated compounds can function as mimics of sulfated GAGs, which are attachment factors and/or receptors of many pathogens, including EV71 (19). The naphathalenetrisulfonic acid group in suramin has been reported to interact with EV71 virions in a saturation transfer difference nuclear magnetic resonance (STD-NMR) study (22), and the binding sites of NF449 on EV71 virions included amino acids VP1-98E and VP1-244K at the vertex of the 5-fold axis of viral capsid (23). Similarly, dye E151 interacted with virions to prevent viral attachment for the following reasons. First, E151 prevented the binding of EV71 virions to heparin-Sepharose (Fig. 6D). As both E151 and heparin are small molecules and negatively charged, E151 interacted with virions but not heparin to disrupt their binding. Second, E151 prevented the virions from interacting with PSGL-1 (Fig. 6C) and CypA (Fig. 8D), both of which have been reported to target the vertex of the 5-fold axis of EV71 (17, 24). Therefore, E151 is deduced to interact with EV71 at the vertex. Third, the results for E151-resistant mutants, which had mutations at the vertex (Fig. 5), also supported the idea that E151 interacted with EV71 at the vertex. Fourth, the vertex contains positively charged amino acids VP1-242K and VP1-244K (Fig. 5D) and possibly interacts with E151 through electrostatic attraction, although this has to be corroborated by further studies. However, the possibility that E151 interacted with other cellular components to adversely affect EV71 attachment/infection cannot be completely excluded.
Neither E151 nor NF449 affected the binding of EV71 to human SCARB2, a functional receptor interacting with the canyon region of EV71. These results suggest that human SCARB2 might not be necessary for EV71 attachment and coincide with studies where knockdown of SCARB2 in RD or Vero cells had no influence on the EV71 attachment (40). Interestingly, E151 prevented EV71 infection at the postattachment stage by eluting the attached virions from RD and Vero cells, implying that the EV71 attachment to the cells was reversible and that E151 competed with cellular attachment factors to bind to EV71 (Fig. 8C). Moreover, E151 disrupted the interaction between EV71 and the viral-uncoating factor CypA (Fig. 8D) but not the interaction with human SCARB2. However, the total effects of E151 on the uncoating and RNA release of EV71 need further evaluation. After cell entry, the ensuing replication of EV71 seemed not to be inhibited by E151 (Fig. 6A). Overall, E151 prevents the entry of EV71 in vitro.
The vertex of the 5-fold axis of EV71 plays an important role in not only viral entry but also viral pathogenicity. Molecular epidemiological studies have suggested that VP1-98K and VP1-145G/Q isolates are more frequently detected in cases with severe neurological disease in humans than are VP1-98E and VP1-145E isolates, respectively (41, 42). However, on the other hand, substitution of VP1-145G/Q for VP1-145E was found to be responsible for adaptation and/or virulence in both murine and primate models, either alone or in combination with other amino acids (43–46). VP1-K244E increased the virulence and neurotropism of EV71 in immunodeficient AG129 mice (47). Therefore, the vertex of the 5-fold axis of EV71 plays an important role in viral cell/tissue tropism and virulence in vivo. In this report, the dye E151, targeting to the vertex, effectively inhibited EV71 infection in a mouse model (36). The 14-day-old AG129 mice challenged by lethal doses of wild-type EV71 isolates were fully protected when E151 was intraperitoneally administered at 200 mg/kg/day for 4 continuous days at the beginning of the challenge. Compared to the results for the control groups treated with PBS, E151 treatment dramatically reduced EV71 titers in both muscle and brain tissues. The virus titers in muscle were noted to increase significantly at 8 days postchallenge, after cessation of E151 treatment, suggesting that an extended E151 treatment would produce better protection (Fig. 9C).
The dye E151 exhibited more inhibitory effects on infection of EV71 isolates with VP1-145G/Q than on the strain with VP1-145E (Fig. 3 and 4). Interestingly, the substitution of G/Q for E at VP1-145 also decreased the efficacy of suramin in preventing EV71 infection in vitro by 30 times (48), suggesting that negatively charged VP1-145E altered the electrostatic property of the vertex (Fig. 5D) and weakened the interaction between EV71 and E151/suramin. Besides this, VP1-246P was also involved in the interaction of E151 and EV71. The mutation VP1-P246A conferred E151 resistance on the EV71 strains with VP1-98K,145E but reduced the growth titers of the mutants, implying that the E151-resistant EV71 might be evolutionarily disadvantaged. Although the amino acids at VP1-98 and VP1-145 constantly switch from E to K and E to G/Q, respectively (49), VP1-246P is highly conserved in EV71 (Fig. 4A). Therefore, E151 could potentially inhibit all known circulating EV71 strains. Both of the mutations VP1-G/Q145E and VP1-P246A individually reduced the binding between E151 and EV71, and together, they abolished the binding totally (Fig. 4B). Considering that sulfonated groups of azo dyes, such as Congo red, interacted with positively charged amino acids (31), it is possible for E151 and other food azo dyes to interact with VP1-242K and VP1-244K, which are located near VP1-145 and VP1-246 on the virion (Fig. 5D), to affect the EV71 infection through electrostatic attraction. E151 also inhibited the infectivity of CVA6 and CVA16 in vitro; however, no protein sequence similarity was found among EV71, CVA6, and CVA16 at the vertexes of their 5-fold axes (data not shown). E151 might block the entry of or prevent the infection of CVA6 and CVA16 in different ways. Knowledge of the precise interaction between the dye E151 and these enteroviruses will contribute to the optimization of E151 for further antiviral drug development.
An acceptable daily intake (ADI) of 1 to 5 mg/kg/day in humans has been established for E151 in many countries, including Singapore (50). It will be interesting to evaluate whether E151 at the ADI level is potent enough to prevent EV71 infection of the digestive tract of children. However, growing concern about the safety of E151 could be a hurdle in using it to treat EV71 infection. First, absorption of E151 through the digestive tract is poor and parenteral administration is not allowed in humans at present. Oral administration may not allow E151 to efficiently inhibit EV71 infection in children with symptoms of HFMD. Second, it is controversial as to whether azo dyes, including E151, increase or cause intolerance, hyperactivity, and allergic reactions in children (50). Third, although no evidence indicates that E151 caused carcinogenic and genotoxic activities in several long-term studies using rats and mice, E151 was found to be genotoxic and to induce micronuclei in two cell lines (51). Finally, the food azo dyes tartrazine and carmoisine were reported to adversely affect biochemical markers in vital organs in rats even at low doses (52). Whether E151 alters biochemical markers in humans, especially children contracting illnesses, will affect its use as an antiviral agent. Therefore, extensive safety evaluation of E151 is required, especially when parenteral administration of E151 is considered.
Drug development for human infectious diseases faces obstacles due to the requirement for prolonged safety evaluation and huge experimental costs. Discovery of novel uses of chemicals with proven safety records and previous approved usage is an attractive approach. Food azo dyes, approved as food additives, are very stable at room temperature and easy to produce at a low cost (27, 33). In our study, the dye brilliant black BN, E number E151, was demonstrated to be able to inhibit the infectivity of EV71, CVA16, and CVA6, which are 3 major causative agents of HFMD (2). Although HFMD is self-limiting, the disease is associated with significant morbidities, such as severe pain, irritability, and reduced oral fluid and food intake, as well as a small proportion of mortality. Therefore, antiviral drugs, especially against EV71, are urgently needed to combat HFMD in two scenarios for the therapeutic treatment of children with HFMD: (i) to speed up recovery and reduce morbidity, and (ii) to reduce the infectivity of the shed virus, which will indirectly reduce virus transmissibility. Based on the fact that E151 effectively prevented EV71 infection in vitro and in vivo by blocking viral entry, our results highlight a novel function of the food dye E151 as a potential prophylactic/therapeutic antiviral agent against EV71 infection in humans. This potentiality should be investigated in future clinical studies, which will evaluate its efficacy in reducing the morbidity of children with HFMD and the transmissibility of the virus in the community, especially during an outbreak situation.
MATERIALS AND METHODS
Cells, viruses, and food azo dyes.
Human rhabdomyosarcoma (RD; ATCC number CCL-136) and monkey Vero (ATCC number CCL-81) cell lines were maintained in Dulbecco’s modified Eagles’ medium (DMEM) (Life Technologies) supplemented with 10% fetal bovine serum (FBS) (Biowest) and 1× antibiotic-antimycotic (Life Technologies) at 37°C in a 5% CO2 incubator. A total of 18 clinical isolates of human enteroviruses (Table 2) and one synthetic EV71-C4 generated by a reverse genetics system (34) were propagated in RD cells and then stored at −80°C for future experiments. The titration of viruses was determined in RD cells as 50% tissue culture infective dose (TCID50) using the Reed-and-Muench formula. The P1 gene of EV71 and CVA16 was amplified by using a OneStep reverse transcription-PCR (RT-PCR) kit (Qiagen) with the primers seqEV71-P1-F and seqEV71-P1-R, and then it was inserted into the pJET1.2 vector (Thermo Scientific) for sequencing (Table 3).
TABLE 2.
List of viruses used in this experimental study
| Strain name | Subgenogroup | Location | Yr | Major symptoms | GenBank accession no.a |
|---|---|---|---|---|---|
| EV71-A (BrCr) | A | USA | 1970 | Meningitis | U22521 |
| EV71-Viet | A | Vietnam | 2010 | HFMD | NA |
| EV71-B2 (7423) | B2 | USA | 1987 | Encephalitis | U22522 |
| EV71-B4 (#HFMD47) | B4 | Singapore | 2002 | Death | AF316321 |
| EV71-B5 (NUH0083) | B5 | Singapore | 2008 | HFMD | FJ461781 |
| EV71-C1 (Y90) | C1 | Japan | Unknown | Unknown | AB433864 |
| EV71-C2 (NUH0075) | C2 | Singapore | 2008 | HFMD | FJ172159 |
| EV71-202 | C2 | Japan | 2010 | HFMD | NA |
| EV71-252 | C2 | Japan | 2010 | HFMD | NA |
| EV71-2242 | C2 | Japan | 2010 | HFMD | NA |
| EV71-C4 (FY08) | C4 | China | 2008 | HFMD | EU703813 |
| EV71-C5 (3437) | C5 | Singapore | 2006 | HFMD | GU222654 |
| CVA16-G10 | A | South Africa | 1951 | HFMD | U05876 |
| CVA16-MOH1 | 2B2 | Japan | 2010 | HFMD | NA |
| CVA16-OS | 2B2 | Malaysia | Unknown | HFMD | NA |
| CVA10b | Singapore | 2008 | HFMD | NA | |
| CVA4b | Singapore | 2008 | HFMD | NA | |
| CVA6b | Singapore | 2008 | HFMD | NA |
NA, not applicable; the genomic sequences of viruses in our laboratory are different from ones published in GenBank due to mutations during viral propagation in vitro.
See reference 7.
TABLE 3.
List of primers used in this experimental study
| Primer name | Sequence (5′→3′) |
|---|---|
| GFP/EV71-B4-F | GCCATTACTACCCTTGGCTCACAGGTGTCTACTC |
| GFP/EV71-B4-R | CTTGGACTGGGCCATGTTTGATTGTATTGAGG |
| GFP-F | ATGGCCCAGTCCAAGCAC |
| GFP-R | AAGGGTAGTAATGGCGGGCAAGGCGGAGCCGGAG |
| GST-CypA-F | GGTTCCGCGTGGATCCGTCAACCCCACCGTGTTC |
| GST-CypA-R | GGGAATTCGGGGATCTTATTCGAGTTGTCCACAG |
| B5-VP1-E98K-F | CCCCCTAAAGGGTACCACCAATCC |
| B5-VP1-E98K-R | GTACCCTTTAGGGGGAGATCTATCTC |
| B5-VP1-G145E-F | ACTGGTGAGGTCGTTCCACAATTAC |
| B5-VP1-G145E-R | AACGACCTCACCAGTAGGAGTG |
| B5-VP1-P246A-F | CAAGTACGCTTTGGTTGTCAGAATATAC |
| B5-VP1-P246A-R | ACCAAAGCGTACTTGGACTTTGAAGAC |
| C4-VP1-E98K-F | CCCTCTTAAGGGCACAACTAACCC |
| C4-VP1-E98K-R | GTGCCCTTAAGAGGGAGATCTATCTC |
| C4-VP1-E145Q-F | CACCGGGCAAGTTGTCCCACAATTG |
| C4-VP1-E145Q-R | ACAACTTGCCCGGTGGGTGTG |
| C4-VP1-P246A-F | AAGTACGCTTTAGTGGTTAGGATTTAC |
| C4-VP1-P246A-R | CACTAAAGCGTACTTGGACTTGGAG |
| 2242-VP1-P246A-F | AAGTATGCACTGGTGATCAGGATTTAC |
| 2242-VP1-P246A-R | CACCAGTGCATACTTGGACTTCGAG |
| seqEV71-P1-F | CCTCCGGCCCCTGAATG |
| seqEV71-P1-R | GCGRGAGCTRTCTTCCCA |
| EV-Genotyping-F | CTTGTRATACCATGGATYAG |
| EV-Genotyping-R | GCGRGAGCTRTCTTCCCA |
| seqEV71-C-f1188 | GGAAGTTCCCGGATGTGTTAAC |
| seqEV71-C-r2909 | GTGGCACAAACATATATTGGAG |
| seqEV71-B-f | GTGGGAAAAGTCATCCAAG |
| seqEV71-B-r | CATCGTGTCTCAATCATACTC |
| seqCVA16-P1-f2 | GGAAATTCCCKGAYGTTTTGAC |
| seqCVA16-P1-r2 | GTGGAGTGATGGTTCAACAC |
All food dyes, i.e., tartrazine (03322-25MG), sunset yellow (68775-25MG), carmoisine (52245-25MG), amaranth (87612-25MG), ponceau 4R (18137-25MG), ponceau 6R (96365-25MG), allura red AC (38213-25MG and 458848-100G), brilliant black BN (11220-25MG and 211842-10G), patent blue V (74748-25MG), indigo carmine (73436-25MG), and brilliant blue FCF (80717-100MG), were purchased from Sigma-Aldrich. All the dyes were dissolved in sterile water at 10 mM and then diluted to desired concentrations with DMEM.
Antibodies and recombinant proteins.
Mouse anti-EV71 antisera were produced in AG129 pups immunized with purified EV71-B4 at a sublethal dose. Mouse anti-GST antisera were prepared in-house by immunizing BALB/c mice with purified GST. Mouse anti-EV71 monoclonal antibody 1D9 (35) was used to detect and quantify EV71 VP1 by Western blot analysis. Horseradish peroxidase (HRP)-conjugated mouse anti-β-actin monoclonal antibody (sc-47778) was purchased from Santa Cruz Biotechnology, and HRP-conjugated goat anti-mouse immunoglobin (P0260; Dako) and rabbit anti-human IgG (P021402-2; Dako) were from Agilent. Alexa Fluor 594 (AF 594)-conjugated rabbit anti-early endosome antigen 1 (EEA1) monoclonal antibody (ab206913) and AF488-conjugated goat anti-mouse IgG (A32723) were from Abcam and Life Technologies, respectively. EV71 attachment factors human SCARB2 and PSGL-1 fused to the Fc region of human IgG1 (SCARB2-Fc and PSGL-1–Fc) and CTLA-4–Fc (a negative-control recombinant Fc protein) were purchased from R&D Systems.
Expression and purification of GST-CypA.
The human cyclophilin A open reading frame (ORF) (accession number NM_203431) was amplified from total RNA of RD cells by using the Qiagen OneStep RT-PCR kit (Qiagen) with primers GST-CypA-F and GST-CypA-R (Table 3). It was then cloned into the BamHI-digested pGEX-4T-1 expression vector using the In-Fusion HD cloning kit (Clontech). The recombinant plasmids were transformed into Escherichia coli BL21(DE3) cells, and the correctness of the insert was confirmed by sequencing after plasmid purification. To express GST or GST-tagged cyclophilin A (GST-CypA), the transformed cells were cultured at 37°C in LB medium containing 100 mg/liter ampicillin. After the optical density at 600 nm (OD600) reached 0.8, protein expression was induced at 22°C by adding IPTG (isopropyl-β-d-thiogalactopyranoside) at a final concentration of 0.2 mM. After 5 h of induction, the cells were harvested, and soluble GST and GST-CypA were purified. The purified proteins were dissolved in PBS, and their respective concentrations were measured at Å280.
Generation of EV71-GFP reporter virus.
The genome of EV71-B4 with a green fluorescent protein (GFP) gene was constructed as described previously (13), with modifications. The infectious plasmid pJET-hPolI-B4 (34) was linearized by PCR using primers GFP/EV71-B4-F and GFP/EV71-B4-R (Table 3) so that the 5′ untranslated region (UTR) and VP4 were separated. The GFP gene from the pLVX-IRES-ZsGreen1 vector (Clontech) with an EV71 protease 2A recognition sequence AITTL at the 3′ end was amplified with primers GFP-F and GFP-R (Table 3). All PCR was performed using Q5 high-fidelity 2× master mix (New England Biolabs), and PCR products were extracted using the QIAquick gel extraction kit (Qiagen) after DNA gel electrophoresis. The purified GFP-AITTL gene and linearized pJET-hPolI-B4 plasmid were ligated together by using the In-Fusion HD cloning kit (Clontech), forming the recombinant plasmid pJET-hPolI-B4/GFP. The infectious EV71-GFP reporter virus was generated by direct transfection of the plasmid pJET-hPolI-B4/GFP into RD cells using Lipofectamine 2000 (Thermo Fisher Scientific) and then further propagated in RD cells for 2 passages.
Cytotoxicity assay of food azo dyes.
An MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] assay kit (TOX-1; Sigma) was used to determine the cytotoxicity of azo dyes in cell culture. Amounts of 104 RD cells in 100 μl DMEM with 10% FBS (DMEM-10) were seeded into 96-well plates and incubated for 6 h at 37°C. The medium was then replaced by fresh DMEM-10 containing different concentrations of azo dyes. After 3 days of incubation, the medium was removed and extra azo dyes were washed away with PBS. An amount of 100 μl of MTT at the concentration of 0.5 mg/ml in DMEM-10 was added to each well, and the plates further incubated for 3 h at 37°C. Then, 100 μl of MTT solubilization solution was added, and the plates were gently swirled at room temperature for 15 min. The absorbance of each well at a wavelength of 570 nm was measured by using a Tecan Sunrise microplate reader. The cytotoxicity of azo dyes was estimated by comparing the absorbances of the azo dye-treated cells with that of mock-treated cells.
Antiviral activity of food azo dyes against EV71-GFP in vitro.
For screening of anti-EV71 drugs, RD cells were seeded in 96-well plates at a density of 104 cells per well and incubated overnight in an incubator. EV71-GFP was diluted to 104 TCID50/100 μl in DMEM-10 containing an azo dye at a concentration of 300 μM and incubated for 1 h at 37°C. The treated viruses were inoculated into RD cells at an MOI of 1, and the plates were transferred into the incubator. At 24 h postinfection, the GFP signals in each well were observed and recorded under a UV light microscope (Olympus).
In the assay of azo dye concentration-dependent inhibition of EV71 infection, 104 TCID50 of EV71-GFP was suspended in 100 μl DMEM-10 with dyes 2-fold serially diluted in a range from 200 μM to 3.1 μM and incubated for 1 h at 37°C. The treated viruses were inoculated into RD cells in 96-well plates at a multiplicity of infection (MOI) of 1. In the assay of time-dependent inhibition of EV71-GFP infection, RD or Vero cells in 96-well plates were infected by EV71-GFP at an MOI of 1. E151 was added into cell culture medium at a final concentration of 100 μM at −1, 0, 1, and 2 h postinfection. In assays of inhibition of EV71-GFP infection at the postattachment stage, RD or Vero cells in 96-well plates were cooled for 10 min in a 4°C chiller and incubated with 105 TCID50 of EV71-GFP at 4°C for viral attachment. The unattached viruses were washed away with ice-cold PBS twice 1 h later, and fresh DMEM-10 containing 0 or 100 μM E151 was added. After 12 h of incubation at 37°C, all cells were fixed with 4% paraformaldehyde (PFA) and stained with Hoechst 33258 (1 μg/ml). Images were captured using a UV light microscope (Olympus) and merged using Adobe Photoshop software. The percentage of GFP-positive cells in 3 random images of each sample was calculated.
Viral titer reduction assay.
The sensitivity of enteroviruses to food azo dyes was determined in a cell culture assay that measured the degree of protection by the dyes of RD cell monolayers from undergoing cytopathic effect (CPE) caused by viral infection. RD cells were seeded into 96-well plates at 104 cells in 100 μl DMEM with 1% FBS (DMEM-1) per well and incubated at 37°C for 6 h. Amounts of 100 μl of 10-fold serial dilutions of viruses in DMEM-1 containing azo dyes were incubated for 1 h at room temperature and transferred into the plates. After 4 days, positive infected wells were counted as showing clear CPE on cell monolayers. TCID50 values were determined by the Reed-and-Muensch method.
Determination of IC50 of E151 on virus infection.
EV71 and CVA16 viruses were separately diluted to a concentration of 104 TCID50/0.5 ml and treated with different concentrations of E151 in DMEM-10 for 1 h at 37°C. RD cells were seeded into 24-well plates at a density of 105 cells per well and incubated for 6 h at 37°C, and then the medium was replaced by 0.5 ml of EV71 diluents so that the cells were infected at an MOI of 0.1. After 48 h of infection at 37°C, the infected cells were frozen-thawed 3 times and titers of viruses in supernatants were determined. Infections of each virus at each concentration of E151 were done in triplicate. Two independent experiments were performed.
Selection of E151-resistant EV71s.
Highly E151-sensitive EV71-B4, EV71-B5, and EV71-C5 strains were passaged in RD cells in the presence of 10 μM E151 10 times and then passaged in the presence of 100 μM E151 another 12 times, while slightly E151-sensitive EV71-2242, EV71-C1, and EV71-C4 were directly passaged in RD cells in the presence of 100 μM E151 12 times. E151-resistant EV71-B5res was selected for further study. Briefly, the RNAs were extracted from supernatants of infected cells with RNeasy kits (Qiagen), and the P1 genes were amplified for sequencing.
Generation of EV71 mutants by reverse genetics.
The genomic RNA of EV71-B5 and EV71-C4 was first amplified by RT-PCR and then processed using a human RNA polymerase I promoter reverse genetics (RG) system as described previously (34), and the infectious plasmids were named pJET-EV71-B5 and pJET-EV71-C4, respectively. The mutations at VP1-98 and VP1-145 were introduced into pJET-B5 by site-directed mutagenesis with corresponding primers (Table 3) according to the In-Fusion protocol (Clontech). Similarly, the mutation VP1-P246A was also introduced into the infectious plasmids pJET-EV71-B5, pJET-EV71-C4, and pJET-EV71-2242. The infectious mutants were generated by transfection of their corresponding infectious plasmids into RD cells. Briefly, 1 μg plasmid DNA was mixed with 2.5 μl Lipofectamine 2000 (Life Technologies) in Opti-MEM (Life Technologies) and then transfected into cell monolayers in 6-well plates. All viruses generated were further propagated in RD cells for 2 passages, and the sequence of the VP1 gene from the 2nd-passage viruses was confirmed. All subsequent cell infection experiments used the 2nd-passage RG viruses.
Binding inhibition assay of EV71 and cells.
RD cells were detached using PBS with 5 mM EDTA and washed with DMEM-1 twice. Cells were then resuspended in DMEM-1 and chilled on ice for 15 min. Amounts of 108 TCID50 of purified EV71-B4 (highly sensitive to E151), EV71-C1 (slightly sensitive to E151), EV71-B5res (resistant to E151), or RG viruses were mixed with 2 × 106 RD cells in 500 μl of DMEM-1 containing E151 at a final concentration of 0, 30, 100, or 300 μM. After incubation at 4°C for 1 h with gentle agitation, cells were washed with ice-cold PBS 3 times and the bound viruses were analyzed and detected by Western blotting.
For the assay of E151’s elution of attached EV71 from RD cells, 3 × 108 TCID50 of E151-sensitive EV71-B5 or E151-resistant EV71-B5res was incubated with 6 × 106 prechilled RD cells at 4°C for 1 h for attachment. Unattached viruses were then washed away with ice-cold PBS. The attached viruses and cells were divided into 3 aliquots and resuspended in ice-cold PBS with 0, 30, or 100 μM E151. After incubation at 4°C for 15 min with gentle agitation, the cell pellets and supernatants were separated for EV71 antigen detection as described above.
Binding inhibition assay of EV71 and viral attachment/uncoating factors.
All Sepharose beads were first treated with 3% bovine serum albumin (BSA) in PBS with gentle agitation at 4°C for at least 3 h to block nonspecific interaction with EV71 particles. Amounts of 40 μl of BSA-pretreated protein A/G-Sepharose beads (Santa Cruz) were directly mixed with 3 μg of SCARB2-Fc, 1 μg of PSGL-1–Fc, or 3 μg of CTLA-4–Fc in 250 μl of PBS+ (pH 7.4, 0.1% BSA and 0.2% Igepal CA-630). Simultaneously, 108 TCID50 of purified EV71-B4 was diluted in 250 μl of PBS+ or PBS+ containing 600 μM E151. After incubation at 4°C for 30 min, the viruses were added and incubated with beads and recombinant proteins for another 2 h at 4°C with gentle agitation. Amounts of 20 μl of BSA-treated heparin-Sepharose (Abcam) were incubated with 108 TCID50 of purified EV71 in 500 μl of PBS+ containing E151 at a concentration of 0, 10, 30, 100, 300, or 1,000 μM at 4°C for 2 h with gentle agitation. BSA-treated glutathione-Sepharose beads (Thermo Fisher) containing 30 μg of GST or GST-cyclophilin A were incubated with 108 TCID50 of purified EV71-B5 or EV71-B5res particles in 500 μl of PBS+ or PBS+ containing 300 μM E151 at 4°C with gentle agitation. After incubation, the beads were washed 3 times with ice-cold PBS containing 0.01% Tween 20 and the bound proteins and viruses were analyzed by Western blotting.
Western blot analysis.
The samples were dissolved in SDS loading buffer. After being heated at 100°C for 5 to 10 min, the proteins in the samples were separated by 12% SDS–PAGE gel and transferred onto 0.2-μm nitrocellulose membrane (Bio-Rad). The membrane was first blocked using 5% nonfat milk in PBS with 0.05% Tween 20 (PBST). For detection of EV71 VP1 protein, the membranes were blotted with the monoclonal antibody 1D9 (supernatant from hybridoma culture), followed by HRP-conjugated goat anti-mouse IgG (1:2,000). For detection of GST-tagged proteins, the membranes were blotted with mouse anti-GST antiserum (1:5,000), followed by HRP-conjugated goat anti-mouse IgG (1:2,000). For detection of human Fc recombinant proteins, the membranes were blotted with HRP-conjugated rabbit anti-human IgG (1:5,000). For detection of cellular beta-actin, the membranes were blotted with HRP-conjugated mouse anti-human beta-actin monoclonal antibody (1:8,000). The blotting buffer was PBST with 3% nonfat milk, and blotting time was usually 2 h at room temperature or overnight at 4°C. The membranes were washed 3 times for 5 to 10 min using PBST after each blotting. After the membranes were incubated with Clarity Western ECL substrate (Bio-Rad), the chemiluminescent signals were detected and recorded by a Gel-Imager ChemiDoc Touch (Bio-Rad) and analyzed using the Image Lab software.
Immunofluorescence confocal microscopy assay.
Vero cells (5 × 104) in 300 μl DMEM-10 were seeded onto μ-Slide 8-well glass bottom slides (ibidi), and 24 h later, the slides were prechilled at 4°C for 15 min and the medium was removed. For viral attachment, purified EV71 viruses were diluted in ice-cold DMEM-10 with or without 100 μM E151 and then added to the cells at an MOI of 100. After 1 h of incubation at 4°C, the cells were washed with ice-cold DMEM 3 times to remove the unattached viruses and then fixed with 4% PFA in PBS for 15 min. For viral internalization, the cells with attached viruses were topped up with 300 μl of fresh DMEM-10 with or without 100 μM E151 and further incubated at 37°C in an incubator for 15 min to 1 h, followed by fixation. The fixed cells were permeabilized with 0.5% Triton X-100 in PBS for 10 min and blocked overnight with PBS containing 5% BSA at 4°C. After being washed with PBST, the cells were incubated at 4°C with mouse anti-EV71 antiserum in PBS+ (1:1,000) for at least 3 h with gentle agitation. After washing with PBST, the cells were incubated with AR594-conjugated rabbit anti-EEA1 monoclonal antibody (1:1,000) and AR448-conjugated anti-mouse IgG (1:1,000) overnight. The cells were then stained with Hoechst 33258 in PBS (0.1 μg/ml) for 10 min. After washing with PBST another 3 times, the cells in PBS were observed under an FV3000 confocal laser scanning microscope (Olympus).
Ethics statement.
AG129 mice from B&K Universal (United Kingdom) were housed in individual ventilated cages inside an animal biosafety level 2 (ABSL2) laboratory for animal care and use. All animal experiments were carried out in accordance with the Guides for Animal Experiments of the National Institute of Infectious Diseases (NIID), and experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the Temasek Life Sciences Laboratory Ltd, Singapore (IACUC project approval no. TLL-16-023: Influence of food colorings on the infection of human enterovirus 71 in vivo).
EV71 infection in AG129 mice.
Eight 14-day-old AG129 mouse neonates in each group were challenged with 10 LD50 of EV71-B4 (3 × 1010 TCID50) or EV71-C1 (1.5 × 108 TCID50) in 0.2 ml of PBS via the intraperitoneal (i.p.) route. From 0 to 3 days postchallenge, the mice in the E151 protection group were administered 1 dose daily of E151 in PBS at 200 mg/kg through the i.p. route, while only PBS was used in the mock treatment group. The mice were monitored at a frequency of 24 h for clinical illness until 21 days postchallenge. Clinical illness was scored as follows: 0, normalcy; 1, ruffled hair and hunchbacked appearance; 2, lethargy with limb weakness; 3, paralysis in one limb; 4, paralysis in two limbs but with ability to move and ingest; 5, immobility or unconsciousness; 6, death. As mice with a clinical score of 5 usually die in 1 day, they were euthanized with carbon dioxide to reduce their suffering and their death days were accounted on the next day. At the end of the experiments, all mice were euthanized with carbon dioxide. To study viral propagation in the EV71-C1-challenged mice, the muscles of hind limbs and the brains (5 pups per group) were harvested, weighed, and stored at −80°C after euthanasia with carbon dioxide at 4, 8, and 12 days postchallenge. The samples were homogenized at 500 mg/ml in DMEM with 10% FBS and 5× antibiotic-antimycotic by using a TissueLyser LT homogenizer (Qiagen). The homogenates were frozen-thawed twice and then kept at 4°C for 1 h. The titers of virus progeny in the supernatants of clarified homogenates (10,000 × g for 10 min at 4°C) were determined in RD cells.
Statistics.
All quantification of viral titer and binding assays was performed in duplicates or triplicates. Their values were compared using Student’s t test, analysis of variance (ANOVA), or the nonparametric Mann-Whitney test in Excel (Microsoft) and/or GraphPad Prism version 8.0.1 (GraphPad software, USA). Kaplan-Meier survival curves and mean clinical score curves were analyzed by GraphPad Prism using the log-rank test and the Wilcoxon test, respectively. Two-tailed P values of <0.05 were considered statistically significant.
ACKNOWLEDGMENTS
K.-B.C. and T.M. conceived the study. K.-B.C., S.-M.W., and T.M. designed the experiments. T.M. performed the experiments and collected the data. Q.J. assisted with cell culture work. K.-B.C., S.-M.W., and T.M. analyzed the data. T.M. wrote the manuscript. K.-B.C. and S.-M.W. revised the manuscript.
We thank Daiwen Yang (Department of Biological Sciences, NUS), Justin Chu, and Vincent Chow (Department of Microbiology and Immunology, NUS) for their kind and constructive suggestions. We are grateful to Danielle Anderson (Duke-NUS Medical School) for her contribution in proofreading the manuscript.
This research was fully supported by core funding of Temasek Life Sciences Laboratory, Limited.
The funder has filed a patent application on food azo dyes, including brilliant black BN, for prophylactic/therapeutic agents against hand, foot, and mouth disease (Singapore patent application no. 10201809449R).
The funder had no role in the design and execution of the experiments or the analysis or interpretation of data.
REFERENCES
- 1.Chumakov M, Voroshilova M, Shindarov L, Lavrova I, Gracheva L, Koroleva G, Vasilenko S, Brodvarova I, Nikolova M, Gyurova S, Gacheva M, Mitov G, Ninov N, Tsylka E, Robinson I, Frolova M, Bashkirtsev V, Martiyanova L, Rodin V. 1979. Enterovirus 71 isolated from cases of epidemic poliomyelitis-like disease in Bulgaria. Arch Virol 60:329–340. doi: 10.1007/BF01317504. [DOI] [PubMed] [Google Scholar]
- 2.Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, Ooi MH. 2010. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis 10:778–790. doi: 10.1016/S1473-3099(10)70194-8. [DOI] [PubMed] [Google Scholar]
- 3.Chang LY, Lin TY, Hsu KH, Huang YC, Lin KL, Hsueh C, Shih SR, Ning HC, Hwang MS, Wang HS, Lee CY. 1999. Clinical features and risk factors of pulmonary oedema after enterovirus-71-related hand, foot, and mouth disease. Lancet 354:1682–1686. doi: 10.1016/S0140-6736(99)04434-7. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu H, Utama A, Yoshii K, Yoshida H, Yoneyama T, Sinniah M, Yusof MA, Okuno Y, Okabe N, Shih SR, Chen HY, Wang GR, Kao CL, Chang KS, Miyamura T, Hagiwara A. 1999. Enterovirus 71 from fatal and nonfatal cases of hand, foot and mouth disease epidemics in Malaysia, Japan and Taiwan in 1997-1998. Jpn J Infect Dis 52:12–15. [PubMed] [Google Scholar]
- 5.McMinn P, Stratov I, Nagarajan L, Davis S. 2001. Neurological manifestations of enterovirus 71 infection in children during an outbreak of hand, foot, and mouth disease in Western Australia. Clin Infect Dis 32:236–242. doi: 10.1086/318454. [DOI] [PubMed] [Google Scholar]
- 6.Wu JM, Wang JN, Tsai YC, Liu CC, Huang CC, Chen YJ, Yeh TF. 2002. Cardiopulmonary manifestations of fulminant enterovirus 71 infection. Pediatrics 109:E26. doi: 10.1542/peds.109.2.e26. [DOI] [PubMed] [Google Scholar]
- 7.Wu Y, Yeo A, Phoon MC, Tan EL, Poh CL, Quak SH, Chow VT. 2010. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int J Infect Dis 14:e1076–e1081. doi: 10.1016/j.ijid.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Yang F, Ren L, Xiong Z, Li J, Xiao Y, Zhao R, He Y, Bu G, Zhou S, Wang J, Qi J. 2009. Enterovirus 71 outbreak in the People's Republic of China in 2008. J Clin Microbiol 47:2351–2352. doi: 10.1128/JCM.00563-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu FC, Meng FY, Li JX, Li XL, Mao QY, Tao H, Zhang YT, Yao X, Chu K, Chen QH, Hu YM, Wu X, Liu P, Zhu LY, Gao F, Jin H, Chen YJ, Dong YY, Liang YC, Shi NM, Ge HM, Liu L, Chen SG, Ai X, Zhang ZY, Ji YG, Luo FJ, Chen XQ, Zhang Y, Zhu LW, Liang ZL, Shen XL. 2013. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 381:2024–2032. doi: 10.1016/S0140-6736(13)61049-1. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Y, Li JX, Jin PF, Wang YX, Zhu FC. 2016. Enterovirus 71: a whole virion inactivated enterovirus 71 vaccine. Expert Rev Vaccines 15:803–813. doi: 10.1080/14760584.2016.1191357. [DOI] [PubMed] [Google Scholar]
- 11.Plevka P, Perera R, Cardosa J, Kuhn RJ, Rossmann MG. 2012. Crystal structure of human enterovirus 71. Science 336:1274. doi: 10.1126/science.1218713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Peng W, Ren J, Hu Z, Xu J, Lou Z, Li X, Yin W, Shen X, Porta C, Walter TS, Evans G, Axford D, Owen R, Rowlands DJ, Wang J, Stuart DI, Fry EE, Rao Z. 2012. A sensor-adaptor mechanism for enterovirus uncoating from structures of EV71. Nat Struct Mol Biol 19:424–429. doi: 10.1038/nsmb.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamayoshi S, Yamashita Y, Li J, Hanagata N, Minowa T, Takemura T, Koike S. 2009. Scavenger receptor B2 is a cellular receptor for enterovirus 71. Nat Med 15:798–801. doi: 10.1038/nm.1992. [DOI] [PubMed] [Google Scholar]
- 14.Dang M, Wang X, Wang Q, Wang Y, Lin J, Sun Y, Li X, Zhang L, Lou Z, Wang J, Rao Z. 2014. Molecular mechanism of SCARB2-mediated attachment and uncoating of EV71. Protein Cell 5:692–703. doi: 10.1007/s13238-014-0087-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishimura Y, Shimojima M, Tano Y, Miyamura T, Wakita T, Shimizu H. 2009. Human P-selectin glycoprotein ligand-1 is a functional receptor for enterovirus 71. Nat Med 15:794. doi: 10.1038/nm.1961. [DOI] [PubMed] [Google Scholar]
- 16.Nishimura Y, Wakita T, Shimizu H. 2010. Tyrosine sulfation of the amino terminus of PSGL-1 is critical for enterovirus 71 infection. PLoS Pathog 6:e1001174. doi: 10.1371/journal.ppat.1001174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishimura Y, Lee H, Hafenstein S, Kataoka C, Wakita T, Bergelson JM, Shimizu H. 2013. Enterovirus 71 binding to PSGL-1 on leukocytes: VP1-145 acts as a molecular switch to control receptor interaction. PLoS Pathog 9:e1003511. doi: 10.1371/journal.ppat.1003511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pourianfar HR, Poh CL, Fecondo J, Grollo L. 2012. In vitro evaluation of the antiviral activity of heparan sulfate mimetic compounds against enterovirus 71. Virus Res 169:22–29. doi: 10.1016/j.virusres.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 19.Tan CW, Poh CL, Sam IC, Chan YF. 2013. Enterovirus 71 uses cell surface heparan sulfate glycosaminoglycan as an attachment receptor. J Virol 87:611–620. doi: 10.1128/JVI.02226-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan CW, Sam IC, Lee VS, Wong HV, Chan YF. 2017. VP1 residues around the five-fold axis of enterovirus A71 mediate heparan sulfate interaction. Virology 501:79–87. doi: 10.1016/j.virol.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Qing J, Sun Y, Rao Z. 2014. Suramin inhibits EV71 infection. Antiviral Res 103:1–6. doi: 10.1016/j.antiviral.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Ren P, Zou G, Bailly B, Xu S, Zeng M, Chen X, Shen L, Zhang Y, Guillon P, Arenzana-Seisdedos F, Buchy P, Li J, von Itzstein M, Li Q, Altmeyer R. 2014. The approved pediatric drug suramin identified as a clinical candidate for the treatment of EV71 infection—suramin inhibits EV71 infection in vitro and in vivo. Emerg Microbes Infect 3:e62. doi: 10.1038/emi.2014.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishimura Y, McLaughlin NP, Pan J, Goldstein S, Hafenstein S, Shimizu H, Winkler JD, Bergelson JM. 2015. The suramin derivative NF449 interacts with the 5-fold vertex of the enterovirus A71 capsid to prevent virus attachment to PSGL-1 and heparan sulfate. PLoS Pathog 11:e1005184. doi: 10.1371/journal.ppat.1005184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qing J, Wang Y, Sun Y, Huang J, Yan W, Wang J, Su D, Ni C, Li J, Rao Z, Liu L, Lou Z. 2014. Cyclophilin A associates with enterovirus-71 virus capsid and plays an essential role in viral infection as an uncoating regulator. PLoS Pathog 10:e1004422. doi: 10.1371/journal.ppat.1004422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee H, Cifuente JO, Ashley RE, Conway JF, Makhov AM, Tano Y, Shimizu H, Nishimura Y, Hafenstein S. 2013. A strain-specific epitope of enterovirus 71 identified by cryo-electron microscopy of the complex with Fab from neutralizing antibody. J Virol 87:11363–11370. doi: 10.1128/JVI.01926-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan S, Li G, Wang Y, Gao Q, Wang Y, Cui R, Altmeyer R, Zou G. 2016. Identification of positively charged residues in enterovirus 71 capsid protein VP1 essential for production of infectious particles. J Virol 90:741–752. doi: 10.1128/JVI.02482-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zollinger H. 2003. Color chemistry: synthesis, properties, and applications of organic dyes and pigments, 3rd ed Wiley-VCH GmbH & Co. KGaA, Weinheim, Germany. [Google Scholar]
- 28.Robinson T, McMullan G, Marchant R, Nigam P. 2001. Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour Technol 77:247–255. doi: 10.1016/S0960-8524(00)00080-8. [DOI] [PubMed] [Google Scholar]
- 29.Baba M, Schols D, Mohan P, De Clercq E, Shigeta S. 1993. Inhibition of HIV-1-induced cytopathogenicity, syncytium formation, and virus-cell binding by naphthalenedisulfonic acids through interaction with the viral envelope gp120 glycoprotein. Antivir Chem Chemother 4:229–234. doi: 10.1177/095632029300400405. [DOI] [Google Scholar]
- 30.Ojala WH, Ojala CR, Gleason WB. 1995. The X-ray crystal structure of the sulfonated azo dye Congo red, a non-peptidic inhibitor of HIV-1 protease which also binds to reverse transcriptase and amyloid proteins. Antivir Chem Chemother 6:25–33. doi: 10.1177/095632029500600104. [DOI] [Google Scholar]
- 31.Ojala WH, Sudbeck EA, Lu LK, Richardson TI, Lovrien RE, Gleason WB. 1996. Complexes of lysine, histidine, and arginine with sulfonated azo dyes: model systems for understanding the biomolecular recognition of glycosaminoglycans by proteins. J Am Chem Soc 118:2131–2142. doi: 10.1021/ja951121f. [DOI] [Google Scholar]
- 32.Ono M, Wada Y, Wu Y, Nemori R, Jinbo Y, Wang H, Lo KM, Yamaguchi N, Brunkhorst B, Otomo H, Wesolowski J, Way JC, Itoh I, Gillies S, Chen LB. 1997. FP-21399 blocks HIV envelope protein-mediated membrane fusion and concentrates in lymph nodes. Nat Biotechnol 15:343–348. doi: 10.1038/nbt0497-343. [DOI] [PubMed] [Google Scholar]
- 33.Weglarz TE, Gorecki L. 2012. Azo dyes—biological activity and synthetic strategy. Chemik 66:1303–1307. [Google Scholar]
- 34.Meng T, Kiener TK, Kwang J. 2012. RNA polymerase I-driven reverse genetics system for enterovirus 71 and its implications for vaccine production. Virol J 9:238. doi: 10.1186/1743-422X-9-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang ML, Kiener TK, Lim XF, Kwang J. 2012. Identification and characterization of a monoclonal antibody recognizing the linear epitope RVADVI on VP1 protein of enterovirus 71. J Med Virol 84:1620–1627. doi: 10.1002/jmv.23372. [DOI] [PubMed] [Google Scholar]
- 36.Khong WX, Yan B, Yeo H, Tan EL, Lee JJ, Ng JK, Chow VT, Alonso S. 2012. A non-mouse-adapted enterovirus 71 (EV71) strain exhibits neurotropism, causing neurological manifestations in a novel mouse model of EV71 infection. J Virol 86:2121–2131. doi: 10.1128/JVI.06103-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dimitrov DS. 2004. Virus entry: molecular mechanisms and biomedical applications. Nat Rev Microbiol 2:109–122. doi: 10.1038/nrmicro817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shang L, Xu M, Yin Z. 2013. Antiviral drug discovery for the treatment of enterovirus 71 infections. Antiviral Res 97:183–194. doi: 10.1016/j.antiviral.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Tan CW, Lai JK, Sam IC, Chan YF. 2014. Recent developments in antiviral agents against enterovirus 71 infection. J Biomed Sci 21:14. doi: 10.1186/1423-0127-21-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ku Z, Ye X, Shi J, Wang X, Liu Q, Huang Z. 2015. Single neutralizing monoclonal antibodies targeting the VP1 GH loop of enterovirus 71 inhibit both virus attachment and internalization during viral entry. J Virol 89:12084–12095. doi: 10.1128/JVI.02189-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y, Fu C, Wu S, Chen X, Shi Y, Zhou B, Zhang L, Zhang F, Wang Z, Zhang Y, Fan C, Han S, Yin J, Peng B, Liu W, He X. 2014. A novel finding for enterovirus virulence from the capsid protein VP1 of EV71 circulating in mainland China. Virus Genes 48:260–272. doi: 10.1007/s11262-014-1035-2. [DOI] [PubMed] [Google Scholar]
- 42.Zhang B, Wu X, Huang K, Li L, Zheng L, Wan C, He ML, Zhao W. 2014. The variations of VP1 protein might be associated with nervous system symptoms caused by enterovirus 71 infection. BMC Infect Dis 14:243. doi: 10.1186/1471-2334-14-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang SW, Wang YF, Yu CK, Su IJ, Wang JR. 2012. Mutations in VP2 and VP1 capsid proteins increase infectivity and mouse lethality of enterovirus 71 by virus binding and RNA accumulation enhancement. Virology 422:132–143. doi: 10.1016/j.virol.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 44.Kataoka C, Suzuki T, Kotani O, Iwata-Yoshikawa N, Nagata N, Ami Y, Wakita T, Nishimura Y, Shimizu H. 2015. The role of VP1 amino acid residue 145 of enterovirus 71 in viral fitness and pathogenesis in a cynomolgus monkey model. PLoS Pathog 11:e1005033. doi: 10.1371/journal.ppat.1005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kobayashi K, Sudaka Y, Takashino A, Imura A, Fujii K, Koike S. 2018. Amino acid variation at VP1-145 of enterovirus 71 determines attachment receptor usage and neurovirulence in human scavenger receptor B2 transgenic mice. J Virol 92:e00681-18. doi: 10.1128/JVI.00681-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujii K, Sudaka Y, Takashino A, Kobayashi K, Kataoka C, Suzuki T, Iwata-Yoshikawa N, Kotani O, Ami Y, Shimizu H, Nagata N, Mizuta K, Matsuzaki Y, Koike S. 2018. VP1 amino acid residue 145 of enterovirus 71 is a key residue for its receptor attachment and resistance to neutralizing antibody during cynomolgus monkey infection. J Virol 92:e00682-18. doi: 10.1128/JVI.00682-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caine EA, Moncla LH, Ronderos MD, Friedrich TC, Osorio JE. 2016. A single mutation in the VP1 of enterovirus 71 is responsible for increased virulence and neurotropism in adult interferon-deficient mice. J Virol 90:8592–8604. doi: 10.1128/JVI.01370-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ren P, Zheng Y, Wang W, Hong L, Delpeyroux F, Arenzana-Seisdedos F, Altmeyer R. 2017. Suramin interacts with the positively charged region surrounding the 5-fold axis of the EV-A71 capsid and inhibits multiple enterovirus A. Sci Rep 7:42902. doi: 10.1038/srep42902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tee KK, Lam TT, Chan YF, Bible JM, Kamarulzaman A, Tong CY, Takebe Y, Pybus OG. 2010. Evolutionary genetics of human enterovirus 71: origin, population dynamics, natural selection, and seasonal periodicity of the VP1 gene. J Virol 84:3339–3350. doi: 10.1128/JVI.01019-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). 2010. Scientific Opinion on the re-evaluation of Brilliant Black BN (E 151) as a food additive on request from the European Commission. EFSA J 8:1540. doi: 10.2903/j.efsa.2010.1540. [DOI] [Google Scholar]
- 51.Macioszek VK, Kononowicz AK. 2004. The evaluation of the genotoxicity of two commonly used food colors: quinoline yellow (E 104) and brilliant black BN (E 151). Cell Mol Biol Lett 9:107–122. [PubMed] [Google Scholar]
- 52.Amin KA, Abdel Hameid H 2nd, Abd Elsttar AH. 2010. Effect of food azo dyes tartrazine and carmoisine on biochemical parameters related to renal, hepatic function and oxidative stress biomarkers in young male rats. Food Chem Toxicol 48:2994–2999. doi: 10.1016/j.fct.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 53.EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). 2010. Scientific opinion on the appropriateness of the food azo-colours Tartrazine (E 102), Sunset Yellow FCF (E 110), Carmoisine (E 122), Amaranth (E 123), Ponceau 4R (E 124), Allura Red AC (E 129), Brilliant Black BN (E 151), Brown FK (E 154), Brown HT (E 155) and Litholrubine BK (E 180) for inclusion in the list of food ingredients set up in Annex IIIa of Directive 2000/13/EC. EFSA J 8:1778. doi: 10.2903/j.efsa.2010.1778. [DOI] [Google Scholar]