Herpesviruses infect nearly all humans and persist quiescently for the life of the host. These viruses intermittently activate into the lytic phase to produce infectious virus, thereby causing disease. To ensure that lytic activation is not prematurely terminated, expression of the virally encoded lytic switch protein needs to be sustained. In studying Epstein-Barr virus, one of the most prevalent human herpesviruses that also causes cancer, we have discovered that a viral kinase activated by the viral lytic switch protein partners with a cellular kinase to deactivate a silencer of the lytic switch protein, thereby providing a positive feedback loop to ensure successful completion of the viral productive phase. Our findings highlight key nodes of interaction between the host and virus that could be exploited to treat lytic phase-associated diseases by terminating the lytic phase or kill cancer cells harboring herpesviruses by accelerating the completion of the lytic cascade.
KEYWORDS: epigenetics, human herpesviruses, lytic activation, viral protein kinase
ABSTRACT
Herpesviruses are ubiquitous, and infection by some, like Epstein-Barr virus (EBV), is nearly universal. To persist, EBV must periodically switch from a latent to a replicative/lytic phase. This productive phase is responsible for most herpesvirus-associated diseases. EBV encodes a latency-to-lytic switch protein which, upon activation, sets off a vectorially constrained cascade of gene expression that results in production of infectious virus. While triggering expression of the switch protein ZEBRA is essential to lytic cycle entry, sustaining its expression is equally important to avoid premature termination of the lytic cascade. We report that the viral protein kinase (vPK), encoded by a gene that is kinetically downstream of the lytic switch, sustains expression of ZEBRA, amplifies the lytic cascade, increasing virus production, and, importantly, prevents the abortive lytic cycle. We find that vPK, through a noncanonical site phosphorylation, activates the cellular phosphatidylinositol 3-kinase-related kinase ATM to cause phosphorylation of the heterochromatin enforcer KAP1/TRIM28 even in the absence of EBV genomes or other EBV proteins. Phosphorylation of KAP1 renders it unable to restrain ZEBRA, thereby further derepressing and sustaining its expression to culminate in virus production. This partnership with a host kinase and a transcriptional corepressor enables retrograde regulation by vPK of ZEBRA, an observation that is counter to the unidirectional regulation of gene expression reminiscent of most DNA viruses.
IMPORTANCE Herpesviruses infect nearly all humans and persist quiescently for the life of the host. These viruses intermittently activate into the lytic phase to produce infectious virus, thereby causing disease. To ensure that lytic activation is not prematurely terminated, expression of the virally encoded lytic switch protein needs to be sustained. In studying Epstein-Barr virus, one of the most prevalent human herpesviruses that also causes cancer, we have discovered that a viral kinase activated by the viral lytic switch protein partners with a cellular kinase to deactivate a silencer of the lytic switch protein, thereby providing a positive feedback loop to ensure successful completion of the viral productive phase. Our findings highlight key nodes of interaction between the host and virus that could be exploited to treat lytic phase-associated diseases by terminating the lytic phase or kill cancer cells harboring herpesviruses by accelerating the completion of the lytic cascade.
INTRODUCTION
Gene expression during the replicative phase of many DNA viruses, including papillomaviruses, polyomaviruses, adenovirus, and herpesviruses, follows a well-ordered temporal cascade (1–4). In herpesviruses, it begins with expression of immediate-early genes (IE) followed by early (E) genes and then late (L) genes (4). Vectorial regulation might be one way to ensure this chronological order of gene expression from viral genomes. Indeed, after a lytic trigger turns on a limited set of IE genes, these genes then transcriptionally activate a larger set of E genes. Products of most E genes form the replication machinery that replicates the viral genome. Viral genome replication is followed by expression of late genes that are structural components or regulate packaging and egress of the virion (5, 6). These functional characteristics of each kinetic class of replicative genes ensure tightly ordered expression of viral genes, resulting in infectious viral particles.
A second property, inherent to DNA viruses that activate from latency, primarily herpesviruses, is that physiologic and environmental triggers prompt the switch from latency to the replicative or lytic phase. These are generally considered transient, mild, and self-resolving (7–11); however, how these fleeting triggers drive the entire viral lytic cascade to completion is not well understood. Specifically, how is IE gene expression sustained despite the transient nature of lytic triggers? In contrast to most herpesviruses that infect humans, Epstein-Barr virus (EBV) provides a tractable biological system in which this and other questions pertaining to the latency-to-lytic switch can be readily examined. EBV transforms B lymphocytes in vitro to establish continuously proliferating latently infected cell lines. Similarly, latently infected B lymphocyte-derived EBV tumors can be explanted from patients into continuously growing cell lines. EBV in both types of infected cells is generally tightly latent but can be provoked to enter into the lytic phase through application of chemical triggers or ligation of immune molecules. However, other than changes in cell differentiation and metabolic states, the identities of in vivo physiologic triggers for EBV lytic activation are unclear; thus, while it is possible that other host mechanisms sustain the initial trigger, these have not been characterized and therefore cannot be tested. Another possibility is that the lytic cycle, once initiated, itself sustains the trigger. For example, retrograde feedback from E and L lytic genes may maintain or even amplify the expression of IE genes. We therefore asked if any E and L genes enhance the expression of BZLF1, the main IE gene of EBV.
EBV is one of two cancer-causing human herpesviruses. While natural infection results in life-long latency in B lymphocytes, EBV is a WHO class I carcinogen that is causally linked to cancers of B cells, epithelial cells, T cells, and natural killer cells (12–16). Episodic but well-regulated transition of EBV to the lytic phase in B cells results in production of infectious particles that are essential for persistence in the general population and tumorigenesis in vulnerable hosts. Transition from latency to the lytic state is mediated by two EBV-encoded IE genes: the critical IE BZLF1 gene, which produces ZEBRA (ZTA), and the BRLF1 gene, which produces RTA. ZEBRA expression precedes that of RTA in some Burkitt lymphoma-derived cell lines (e.g., Akata), while in other Burkitt-lymphoma backgrounds (e.g., Raji) and EBV-transformed B cell lines (i.e., lymphoblastoid cell lines [LCL]), only ZEBRA is able to disrupt viral latency (17–19). While ZEBRA and RTA contribute to the process of viral DNA replication, ZEBRA is able to bind the lytic origin to regulate replication of the viral genome (20). Importantly, both IE gene products activate their own and each other’s promoters as well as those of E and some L genes, thereby ensuring tight directional control of the complex activities that result in production of virions.
To address whether retrograde regulation enhances and thereby sustains the lytic switch signal, i.e., ZEBRA, we screened an EBV library comprised of E and L genes/open reading frames (ORFs) and report that the viral protein kinase (vPK), a product of the early lytic gene BGLF4, transcriptionally enhances expression of ZEBRA. We find that vPK directly activates the cellular phosphatidylinositol 3-kinase (PI3K)-related kinase ATM through phosphorylation at a noncanonical serine to ultimately phosphorylate KAP1/TRIM28, the universal repressor for the KRAB-ZFP superfamily of transcriptional repressors. Phosphorylation of KAP1 disrupts its ability to repress BZLF1, thereby enhancing and sustaining ZEBRA expression to not only avoid abortive lytic activation but also produce more progeny virions. These experiments, performed in the natural context of EBV latency, i.e., in B lymphocytes, also demonstrate an exception to the well-accepted rule of anterograde regulation of viral gene expression that is characteristic of most DNA viruses.
RESULTS
A library screen identifies the EBV early lytic gene BGLF4-encoded viral protein kinase as a candidate that enhances expression of the viral latency-to-lytic switch protein ZEBRA.
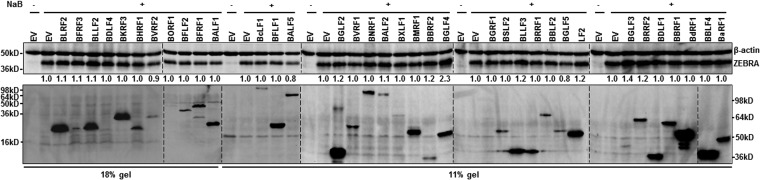
B lymphocytes represent the natural latency reservoir for EBV. To test if early or late lytic gene products are able to enhance expression of the latency-to-lytic switch protein ZEBRA in latently infected B cells, we examined HH514-16 endemic Burkitt lymphoma (eBL) cells that are tightly latent but can be induced into the lytic phase using exogenous triggers. We introduced fifty cloned EBV open reading frames (ORFs) encoding either E or L lytic gene products from a previously described library (21) and examined expression using an anti-FLAG antibody (Ab). As shown in Fig. 1, we were able to detect substantial levels of expression from thirty-six EBV ORFs. Compared to empty vector-transfected cells, introduction of vPK encoded by early lytic gene BGLF4 in cells exposed to the lytic cycle-inducing agent sodium butyrate (NaB) resulted in the greatest increase (2.3-fold) in ZEBRA protein (Fig. 1). Earlier studies had shown that two other EBV lytic proteins were able to enhance the levels of IE gene products. BRRF1 and BGLF2, genes that encode Na protein and a tegument protein, respectively, were shown to enhance the levels of IE gene products (19, 22, 23). While both Na and BGLF2 proteins were expressed at substantial levels in our screen, they resulted in minimal enhancement of ZEBRA levels.
FIG 1.
Screening of early and late lytic EBV gene products for candidates that increase expression of latency-to-lytic switch protein ZEBRA. HH514-16 Burkitt lymphoma (BL) cells were transfected with empty vector (EV) or plasmids expressing the indicated EBV early or late lytic genes, exposed to NaB after 24 h, harvested after another 24 h, and immunoblotted using anti-ZEBRA antibody or anti-FLAG antibody to detect FLAG-tagged EBV proteins. Numbers represent relative amounts of ZEBRA protein after normalizing to β-actin. Markers on the left correspond to proteins separated on 18% gels; those on the right correspond to 11% gels.
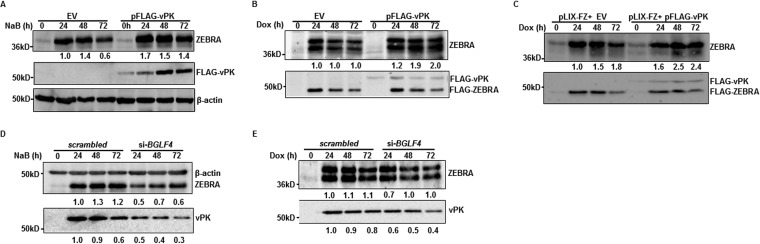
We validated vPK’s ability to enhance the level of ZEBRA by (i) harvesting cells at different times after exposure to a lytic trigger, (ii) examining the effect of exogenous vPK in HH514-16-derived CLIX-FZ cells that allow us to emulate physiologic lytic triggers by inducing expression of ZEBRA from a stably integrated doxycycline-inducible BZLF1 ORF (24), and (iii) testing LCL, i.e., non-eBL-derived B cell lines. Introduction of vPK resulted in increased levels of ZEBRA at all examined time points, in all 3 cell lines, and regardless of how the lytic cycle was triggered (Fig. 2A to C). In contrast, compared to scrambled short interfering RNA (siRNA)-transfected cells, knockdown of vPK with previously validated siRNA that specifically targets BGLF4 transcripts (25) resulted in blunted induction of ZEBRA protein (Fig. 2D and E); the lack of blunting at later times in CLIX-FZ cells (Fig. 2E) is likely due to autoinduction of endogenous ZEBRA by doxycycline-activated ZEBRA. Thus, EBV vPK, encoded by an early lytic gene, regulates the levels of the latency-to-lytic switch protein ZEBRA.
FIG 2.
EBV vPK regulates expression of ZEBRA in BLs and LCLs. (A and B) HH514-16 (A) and CLIX-FZ (B) BL cells were transfected with empty vector (EV) or pFLAG-vPK, treated with NaB (A) or doxycycline (B) after 24 h, harvested at the indicated times posttreatment, and immunoblotted with antibodies against ZEBRA, FLAG, and β-actin. Numbers represent relative amounts of ZEBRA after normalizing to β-actin (A) or the ratio between endogenous ZEBRA (lower-molecular-mass band) and doxycycline-induced FLAG-ZEBRA (higher-molecular-mass band) (B). (C) LCL was transfected with empty vector plus pLIX-FZ (encoding doxycycline-inducible BZLF1) or pFLAG-vPK and pLIX-FZ, exposed to doxycycline after 24 h, and harvested at indicated times posttreatment for immunoblotting with antibodies against ZEBRA or FLAG. Numbers reflect the ratio between endogenous ZEBRA and doxycycline-inducible FLAG-ZEBRA. (D and E) HH514-16 (D) and CLIX-FZ (E) cells were transfected with scrambled siRNA or si-BGLF4, treated with NaB (D) or doxycycline (E) after 8 h, and harvested at the indicated times after treatment for immunoblotting with antibodies against ZEBRA, β-actin, and FLAG. Top rows of numbers indicate relative amounts of ZEBRA protein normalized to β-actin (D) or the ratio between endogenous ZEBRA (lower-molecular-mass band) and doxycycline-induced FLAG-ZEBRA (higher-molecular-mass band) (E). Bottom rows of numbers represent relative amounts of vPK after normalization to β-actin (D) or FLAG-ZEBRA (E). All experiments were performed at least twice.
vPK regulates transcription of BZLF1.
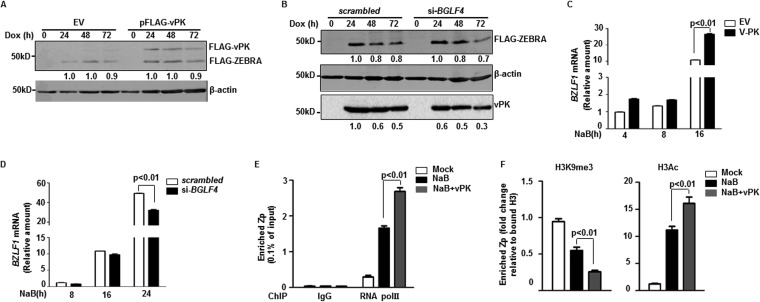
Manipulation of vPK levels showed corresponding changes in the levels of ZEBRA protein expressed specifically from viral genomes in eBL and LCL, as indicated by altered ratios of endogenous to doxycycline-induced ZEBRA shown in Fig. 2B and E and the increase in lower-molecular-mass ZEBRA shown in Fig. 2C. However, the levels of ZEBRA expressed from integrated BZLF1 detected by anti-FLAG antibody remained unchanged following manipulation of vPK (Fig. 3A and B). These results indicate that instead of affecting translation or stability of ZEBRA, vPK alters transcription of BZLF1 encoded by viral genomes. Indeed, introduction of vPK resulted in increased BZLF1 message while knockdown of vPK suppressed BZLF1 message (Fig. 3C and D). Simultaneously, overexpression of vPK during lytic induction increased the occupancy of RNA polymerase II at the BZLF1 promoter compared to that of cells that did not overexpress vPK (Fig. 3E). Following exposure to a lytic trigger, a parallel chromatin immunoprecipitation (ChIP) experiment also examining the BZLF1 promoter in vPK-overexpressing cells (versus cells with unaltered vPK expression) showed increased enrichment of acetylation marks alongside decreased trimethylated lysine 9 on histone 3 (H3) (Fig. 3F). These H3 modifications in cells expressing higher levels of vPK are consistent with further loss of repression at the BZLF1 promoter. Thus, EBV vPK prompts transition of the epigenetic status at the BZLF1 promoter from repression to activation, causing enhanced transcription of BZLF1.
FIG 3.
vPK epigenetically regulates to enhance transcription of BZLF1. (A and B) CLIX-FZ BL cells were transfected with empty vector (EV) or pFLAG-vPK (A) or with scrambled siRNA or si-BGLF4 (B), treated with doxycycline after 24 h (A) or 8 h (B), and harvested at different times after treatment for immunoblotting with the indicated antibodies. Numbers indicate the relative amounts of FLAG-ZEBRA and vPK after normalizing to β-actin. (C and D) HH514-16 BL cells were transfected with empty vector (EV) or pFLAG-vPK and treated with NaB after 24 h (C) or transfected with scrambled siRNA or si-BGLF4 and treated with NaB after 8 h (D). Cells were harvested at indicated times after treatment and examined by qRT-PCR using primers directed against BZLF1 transcript and using the ΔΔCT method. (E and F) CLIX-G4 eBL cells were left untreated or were treated with NaB or NaB plus doxycycline (to induce expression of vPK) and harvested after 24 h for chromatin immunoprecipitation with control antibody (IgG) or antibody against RNA polymerase II (E), histone 3 (F), H3K9me3 (F), or H3Ac (F). Relative occupancy of BZLF1 promoter (Zp) by RNA polymerase II (E) and modified histone 3 (F) was determined via qPCR using primers targeting the BZLF1 promoter. All experiments were performed between 2 and 3 times. Error bars in panels C to F represent 3 technical replicates from 2 experiments.
vPK epigenetically regulates BZLF1 through phosphorylation of KAP1.
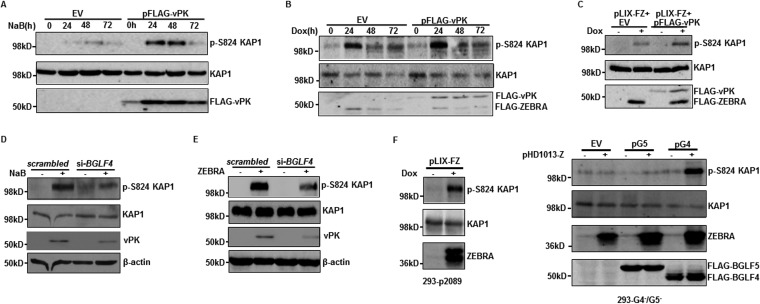
In addressing how vPK removes heterochromatin marks at the BZLF1 promoter, we turned our attention to the cellular corepressor KAP1/TRIM28, known to enforce heterochromatin through H3K9Me3 modification. Our previous work had demonstrated that (i) KAP1 silences BZLF1 (and other lytic genes) through recruitment by members of the KRAB-ZFP family of transcriptional repressors (26) and (ii) phosphorylation at serine 824 of KAP1 is essential for disassociation between KAP1 and heterochromatin-inducing factors, resulting in derepression of lytic genes and disruption of EBV latency (24). vPK-mediated loss of heterochromatin marks on the BZLF1 promoter prompted us to examine if vPK also caused phosphorylation of KAP1. As shown in Fig. 4A to C, introduction of vPK resulted in increased phosphorylation at S824 of KAP1 in eBL cells and LCL. In contrast, knockdown of vPK via BGLF4 siRNA resulted in impaired phosphorylation of KAP1 again in both types of lytically activated cells (Fig. 4D and E). Notably, in 293-p2089 cells, which maintain a bacmid harboring the EBV genome, induction of the lytic cycle via ZEBRA resulted in robust phosphorylation of KAP1, as was observed in BLs and LCLs; however, in 293 cells lacking EBV BGLF4 and BGLF5, ZEBRA-mediated lytic cycle induction was only able to cause phosphorylation of KAP1 when BGLF4 was simultaneously introduced (Fig. 4F). These results indicate that vPK directly or indirectly causes phosphorylation of KAP1 at serine 824.
FIG 4.
vPK causes phosphorylation of KAP1 at serine 824 during viral lytic activation. (A and B) HH514-16 (A) and CLIX-FZ (B) cells transfected with empty vector (EV) or pFLAG-vPK were treated with NaB (A) or doxycycline (B) 24 h later and harvested at the indicated times posttreatment. Cell lysates were analyzed by immunoblotting with antibodies against p-S824 KAP1, KAP1, and FLAG. (C) LCL was transfected with pLIX-FZ (encoding doxycycline-inducible BZLF1) plus empty vector (EV) or pLIX-FZ plus pFLAG-vPK, treated with doxycycline 24 h later, and harvested after another 24 h for immunoblotting with antibodies to p-S824 KAP1, KAP1, and FLAG. (D) HH514-16 cells were transfected with scrambled siRNA or si-BGLF4, treated with NaB after 8 h, and harvested after another 24 h. (E) LCL transfected with scrambled siRNA plus empty vector, scrambled siRNA plus ZEBRA-expressing plasmid (pHD1013-Z), si-BGLF4 plus empty vector, or si-BGLF4 plus pHD1013-Z were harvested 24 h later. Cell lysates from harvested HH514-16 cells and LCL were immunoblotted with the indicated antibodies. (F) 293-p2089 and 293-G4−/G5− cells were transfected with the indicated plasmid combinations (pLIX-FZ, pHD1013-Z, EV, pG5, and pG4) and harvested 24 h later for immunoblotting with the indicated antibodies. All experiments were performed at least 3 times.
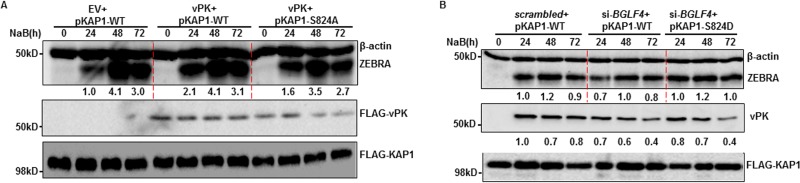
To determine if vPK-mediated induction of p-S824 KAP1 resulted in ZEBRA expression, we introduced wild-type (WT) KAP1 or an S824A (phospho-dead) mutant KAP1 along with FLAG-vPK into lytically induced HH514-16 cells. Cells expressing S824A KAP1 demonstrated blunted increases in ZEBRA levels compared to those expressing wild-type KAP1 (Fig. 5A). On the other hand, introduction of an S824D (phospho-mimetic) mutant of KAP1 alongside knockdown of vPK rescued ZEBRA levels compared to those of lytically induced cells expressing wild-type KAP1 alongside vPK knockdown (Fig. 5B). Taken together, these results demonstrate that vPK causes phosphorylation of the heterochromatin protein KAP1 to derepress BZLF1, resulting in increased expression of ZEBRA.
FIG 5.
vPK increases ZEBRA expression through induction of serine 824-phosphorylated KAP1. HH514-16 cells were transfected with combinations of the indicated plasmids (A) or siRNA plus plasmids (B). Transfected cells were treated with NaB 24 h later and harvested at different time points posttreatment for immunoblotting with the indicated antibodies. Numbers reflect relative amounts of ZEBRA and vPK after normalization to β-actin. All experiments were performed at least twice.
vPK induces phosphorylation of KAP1 independent of other EBV proteins and the viral genome.
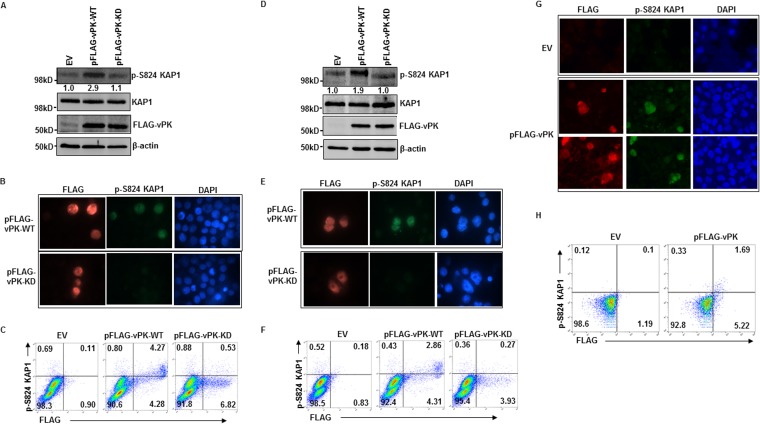
Because vPK has been previously reported to interact with a few lytic and latent proteins (27–31), we investigated whether vPK requires other EBV proteins to induce phosphorylation of KAP1. When transduced into eBL cells and LCL in the absence of exogenous lytic triggers, wild-type vPK successfully resulted in KAP1 phosphorylation in nearly every cell that expressed vPK (Fig. 6A to F). Since low-level activation of the viral lytic cascade due to endogenous lytic triggers could not be excluded, we also introduced wild-type vPK into BJAB cells, an EBV-negative B cell line. The ability of vPK to also cause phosphorylation of KAP1 in BJAB cells indicated that viral proteins, lytic or latent, were not required for vPK-mediated KAP1 phosphorylation. In fact, the viral genome also was not required for vPK to cause KAP1 phosphorylation (Fig. 6G and H). However, the kinase function of vPK was necessary for KAP1 phosphorylation, as demonstrated by suppression of phospho-S824 (p-S824) KAP1 following introduction of a kinase-dead mutant of vPK (Fig. 6A to F). Thus, while vPK requires its kinase function, other viral proteins and the viral genome are dispensable for KAP1 phosphorylation.
FIG 6.
vPK induction of KAP1 phosphorylation is independent of other EBV components. HH514-16 BL cells (A to C), LCL (D to F), and EBV-negative BJAB cells (G and H) were transfected with empty vector (EV), pFLAG-vPK-WT, or pFLAG-vPK-KD (A to F only) and harvested 24 h later for immunoblotting (A and D), immunofluorescence assay (B, E, and G), and flow cytometry (C, F, and H) with the indicated antibodies. Numbers in panels A and D indicate ratios of pKAP1 to total KAP1.
vPK activates ATM to phosphorylate KAP1, resulting in increased ZEBRA expression.
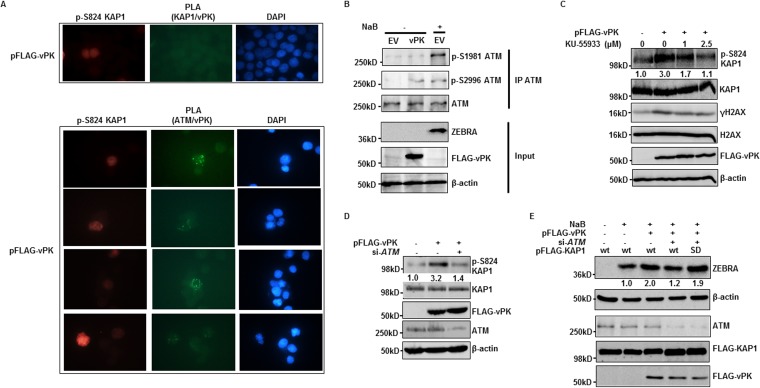
Experiments shown in Fig. 6 suggested that vPK either directly phosphorylates KAP1 or activates a cellular kinase to phosphorylate KAP1. To address if vPK is able to directly bind KAP1, a prerequisite for phosphorylating KAP1, we performed proximity ligation assay (PLA) that detects in situ direct interactions between two proteins that are less than 40 nm apart (32, 33). Despite phosphorylation of KAP1 following introduction of pFLAG-vPK in eBL cells, we were unable to demonstrate a direct interaction between FLAG-vPK and KAP1 (Fig. 7A). However, in parallel PLA experiments, we observed interactions between FLAG-vPK and the cellular kinase ATM specifically in phospho-KAP1-positive cells (Fig. 7A). Immunoprecipitation experiments showed that while lytic cycle activation resulted in phosphorylation of ATM at S2996 and S1981, introduction of vPK resulted in ATM phosphorylation exclusively at S2996 (Fig. 7B). Thus, vPK interacts with ATM to phosphorylate it preferentially at S2996 but not at S1981, a residue canonically phosphorylated after DNA damage.
FIG 7.
vPK interacts with and activates ATM to induce KAP1 phosphorylation. (A) HH514-16 BL cells were transfected with pFLAG-vPK and harvested 24 h later for proximity ligation assay (PLA) to examine direct interactions between FLAG-vPK protein and KAP1 or ATM. A subsequent immunofluorescence assay with anti-p-S824 KAP1 was performed to indicate pFLAG-vPK-transfected cells. p-S824 KAP1, phospho-S824 KAP1. (B) HH514-16 BL cells were transfected with empty vector or FLAG-vPK; 24 h later, empty vector-transfected cells were left untreated or were induced with NaB. Cells were harvested after another 24 h and subjected to immunoprecipitation with ATM antibody and immunoblotting with the indicated antibodies. (C and D) HH514-16 BL cells were transfected with empty vector or pFLAG-vPK and simultaneously treated with the indicated concentrations of KU-55933 (C) or cotransfected with si-ATM (D). Cells were harvested 24 h later and immunoblotted with the indicated antibodies. Numbers in panels C and D indicate ratios of pKAP1 to total KAP1. (E) HH514-16 BL cells were transfected with the indicated combinations of nucleotides and either exposed to NaB 24 h later or left untreated. After another 24 h, cells were harvested and immunoblotted with the indicated antibodies. Numbers represent relative amounts of ZEBRA protein after normalizing to β-actin. All experiments were performed between 2 and 3 times.
Others had previously reported on the role of ATM in the EBV lytic cycle (34–36), and we had shown that ATM directly bound to and phosphorylated KAP1 at S824 in lytic cells (24). We therefore asked if vPK induces phosphorylation of KAP1 via ATM. As shown in Fig. 7C, the ability of transfected vPK to cause KAP1 phosphorylation was progressively impaired with increasing concentrations of KU55933, an ATM inhibitor. As expected, KU55933 suppressed the levels of γH2AX, a known ATM substrate, without affecting total KAP1 levels. In parallel experiments, knockdown of ATM via siRNA also impaired vPK’s ability to cause phosphorylation of KAP1, again without affecting total KAP1 levels (Fig. 7D). Furthermore, in the presence of an exogenous lytic trigger, transfected vPK was unable to augment ZEBRA levels when ATM was knocked down. However, vPK-mediated ZEBRA amplification was rescued by a phosphomimetic (S-D) mutant of KAP1 despite impairment of ATM (Fig. 7E). These experiments established a linear but retrograde relationship between vPK, ATM, p-S824-KAP1, and ZEBRA, resulting in amplification of the viral latency-to-lytic switch.
vPK-mediated retrograde enhancement of ZEBRA amplifies the entire lytic cascade.
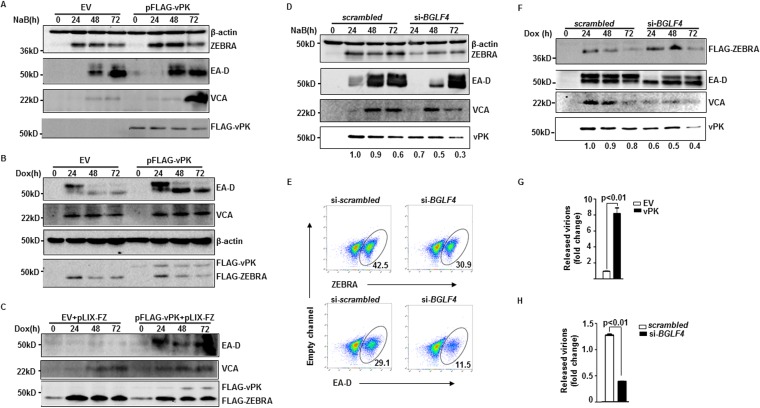
To ascertain if feedback amplification of ZEBRA by vPK is a self-limited property or has far-reaching effects on the rest of the lytic cycle, we examined markers of later stages of the lytic phase following manipulation of vPK levels in cells exposed to lytic triggers. As shown in Fig. 8A to C, introduction of vPK in the presence of a lytic trigger resulted in increased levels of EBV early lytic protein EA-D and late lytic protein VCA in both eBL and LCL; simultaneously, significantly more virus particles were released from cells expressing higher levels of vPK (Fig. 8G). In contrast, compared to levels for scrambled siRNA-transfected eBL cells, cells exposed to siRNA targeting BGLF4 expressed lower levels of EA-D and VCA and released significantly fewer virions (Fig. 8D, F, and H). The effect on L gene expression is consistent with our earlier observations that vPK and KAP1 regulate L genes (24–26). Notably, overexpression and particularly knockdown of vPK also coordinately altered the levels of the higher-molecular-mass, i.e., phosphorylated, form of EA-D (Fig. 8A, B, D, and F); this is consistent with EA-D being a substrate of vPK (28). Importantly, at the single-cell level shown in Fig. 8E, knockdown of vPK resulted in 27.3% reduction in ZEBRA-positive cells but a far greater, i.e., 60.5%, reduction in EA-D-positive cells, demonstrating that optimal expression of vPK is essential not just to sustain ZEBRA expression but to prevent the lytic cycle from prematurely terminating. Thus, by boosting the levels of EBV latency-to-lytic switch protein ZEBRA, vPK intensifies the entire lytic cascade, increases production of progeny virions, and, most importantly, prevents the abortive lytic cycle.
FIG 8.
vPK amplifies the lytic cascade by retrograde enhancement of the latency-to-lytic switch protein ZEBRA, thereby preventing abortive lytic activation. (A and B) HH514-16 (A) and CLIX-FZ (B) BL cells were transfected with empty vector (EV) or pFLAG-vPK, treated with NaB (A) or doxycycline (Dox) (B) 24 h later, and harvested at the indicated times posttreatment for immunoblotting with the indicated antibodies. (C) The lymphoblastoid cell line (LCL) was transfected with EV plus pLIX-FZ (encoding doxycycline-inducible BZLF1) or pFLAG-vPK plus pLIX-FZ and treated with doxycycline 24 h later. Cell lysates were harvested at different times posttreatment for immunoblotting using the indicated antibodies. (D and E) HH514-16 BL cells were transfected with scrambled siRNA or si-BGLF4, treated with NaB 8 h later, and harvested at the indicated time points for immunoblotting (D) or at 24 h posttreatment for flow cytometry (E) with the indicated antibodies. Numbers in panel D indicate relative amounts of vPK after normalization to β-actin, and numbers in panel E indicate percent ZEBRA- or EA-D-positive cells. (F) CLIX-FZ BL cells transfected with scrambled siRNA or si-BGLF4 were treated with doxycyline 8 h after transfection and harvested at the indicated time points for immunoblotting. Numbers indicate relative amounts of vPK after normalization to FLAG-ZEBRA. (G) HH514-16 BL cells were transfected with EV or pFLAG-vPK and treated with NaB after 24 h. (H) Cells were transfected with scrambled siRNA or si-BGLF4 and treated with NaB after 8 h. Released virus particles were collected from culture supernatants and quantified via qPCR 72 h after treatment with NaB. All experiments were performed at least twice. Error bars in panels G and H represent 3 technical replicates from 2 experiments.
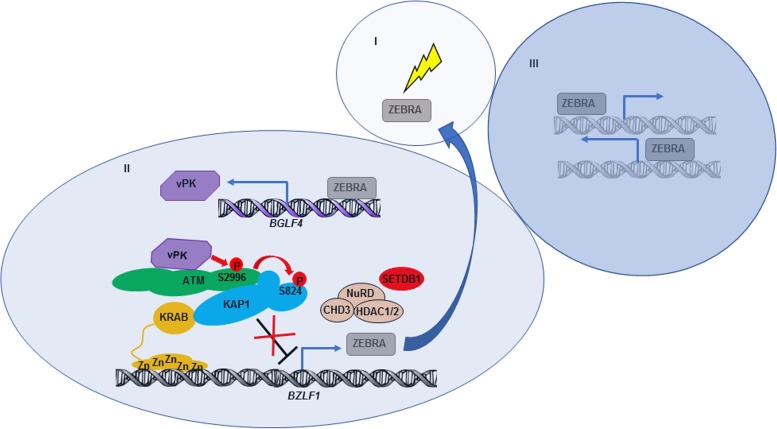
Taken together, our findings support a model in which disruption of EBV latency is followed by a lytic/replicative phase which can be divided into three stages, depicted in Fig. 9. In stage I, lytic triggers cause expression of a limited amount of the lytic switch protein ZEBRA. In stage II, ZEBRA transactivates early lytic gene BGLF4 and drives the expression of vPK. vPK, via cellular kinase ATM, induces phosphorylation of KAP1 at serine 824, which results in epigenetic derepression of BZLF1 and, thus, further enhancement of ZEBRA levels. In stage III, augmented expression of ZEBRA resulting from this retrograde feedback from vPK contributes to sustaining and amplifying the entire lytic cascade.
FIG 9.
Model illustrating the mechanism by which vPK, the product of early lytic gene BGLF4, epigenetically derepresses immediate-early lytic switch gene BZLF1 to sustain and amplify the EBV lytic cascade through phosphorylation of KAP1. In stage I, a limited amount of latency-to-lytic switch protein ZEBRA is induced by exogenous stimuli. In stage II, ZEBRA transcriptionally activates the EBV early lytic gene BGLF4, resulting in expression of vPK. vPK binds and activates ATM to induce ATM-mediated phosphorylation of KAP1 at S824, which results in derepression of BZLF1. In stage III, the resulting positive feedback on ZEBRA protein sustains and amplifies the EBV lytic cascade.
DISCUSSION
The amount of ZEBRA in an infected cell is a bottleneck for determining whether EBV remains latent or switches into the lytic phase. Activity of the BZLF1 promoter is determined by a balance of positive and negative transcription factors that epigenetically mediate activating and silencing marks on histones. Examples of positive transcription factors include CREB, ATF, AP-1, EBP, ZBP1, and MEF2, while ZEB1, ZEB2, KRAB-ZFPs, KAP1, and STAT3 are negative regulators (5, 24, 26, 37–39). If EBV switches into the lytic phase, the switch, i.e., ZEBRA, must remain on for successful production of infectious particles. However, the mechanisms that prevent the abortive lytic cycle are not clear. One strategy is through activation of the BZLF1 promoter by ZEBRA itself (40, 41). Another, that we describe here, is through vPK-mediated regulation of BZLF1. ZEBRA expression is impaired, and the lytic cascade is also prematurely terminated when vPK expression alone is experimentally blunted. This retrograde regulation of BZLF1 by an early gene product is counter to the generally accepted theme of anterograde regulation of the three kinetic classes of lytic genes and is orchestrated through a partnership between vPK, the viral protein kinase, and cellular DNA repair factors ATM (also a protein kinase) and KAP1 (a heterochromatin modifier). This partnership also functionally links two kinases to gene regulation via histone remodeling.
The viral protein kinase appears to be a common node during disruption of latency for both oncogenic human herpesviruses EBV and Kaposi’s sarcoma-associated herpesvirus (KSHV) (42). In both instances, vPK causes phosphorylation of KAP1; however, whether KSHV vPK also functions through ATM to phosphorylate KAP1 has not been tested. While vPK from EBV, KSHV, cytomegalovirus (CMV), and herpes simplex virus 1 (HSV1), representatives of all 3 herpesvirus subfamilies, has been shown to cause phosphorylation of ATM, whether activated ATM phosphorylates KAP1 in KSHV-, CMV-, and HSV1-infected cells is not known (43). Whether vPK directly phosphorylates ATM or functions through an intermediary kinase in herpesvirus-infected cells is also unclear. Our findings indicate that EBV vPK indeed directly interacts with and activates ATM, as demonstrated by phosphorylation of H2AX, and that this activation of ATM results in disruption of EBV latency. As a guardian of the cellular genome, ATM is activated by cellular DNA damage, resulting in intensive posttranslational modifications, including phosphorylation and acetylation. ATM then phosphorylates a series of substrates, including KAP1, Chk2, p53, AKT, and BRCA proteins (44). However, the relationships between posttranslational modifications on ATM, substrate specificity, and functional consequences are not entirely clear. In the context of EBV lytic infection, we find that vPK-mediated phosphorylation of ATM at serine 2996, a little-studied site, is responsible for phosphorylation of KAP1, resulting in amplification of the lytic cascade. Curiously, an earlier study of another EBV+ BL line had reported that overexpression of vPK resulted in phosphorylation of ATM preferentially at S2996 (45).
KAP1, a heterochromatin-inducing universal transcriptional corepressor, plays a key role in facilitating repair of damaged DNA on silenced chromatin (46). In response to double-strand breaks in heterochromatin, activated ATM causes phosphorylation of KAP1. Phospho-KAP1 disassociates from heterochromatin-inducing factors, allowing for relaxation of damaged chromatin, thereby providing access to DNA repair proteins. Whether double-strand break-induced active ATM directly phosphorylates KAP1 is not known. However, we have previously demonstrated that ATM activated by lytic triggers directly interacts with and phosphorylates KAP1 only in lytic EBV-infected cells (24).
Similar to the host genome, episomal herpesvirus genomes are also silenced by KAP1. This silencing maintains viral latency; at the same time, herpesviruses exploit this very mechanism involving KAP1 to disrupt latency. Indeed, phosphorylation of KAP1 at the same serine residue that also regulates DNA repair is key to disruption of 3 human herpesviruses: EBV, KSHV, and CMV (24, 42, 47).
Theoretically, any component of the vPK-ATM-KAP1 functional complex may be interfered with or activated by alterations in physiologic states or medical interventions to regulate the outcome of the EBV lytic cycle. For example, kinases, DNA lesions, or chemicals that activate ATM, such as chloroquine, may turn on or enhance the replicative phase (24, 34, 48). Similarly, interventions such as chemotherapeutic agents that cause DNA damage, resulting in KAP1 phosphorylation, may simultaneously spark and enhance the herpesvirus lytic cycle as a bystander effect. Indeed, even in the presence of a strong lytic trigger, such as NaB or inducible ZEBRA, the lytic cycle does not function at maximum capacity. There is room for further amplification of the lytic cascade, as we have demonstrated following overexpression of vPK in NaB-treated and doxycycline-treated cells.
The lytic cycle is often abortive largely due to robust EBV-directed immune surveillance, but weak lytic triggers may also contribute. Feedback amplification of ZEBRA, i.e., the switch, by an early protein may ensure that the lytic cycle goes to completion in at least some cells. Deletion of vPK from an EBV bacmid in 293 cells negatively impacted virion production through vPK’s effect on a set of lytic genes, although its retrograde effect on BZLF1 in this artificial non-B cell-related latency context was not examined (49). Notably, the effect of this retrograde amplification is likely to be more dramatic in immunocompromised subjects who lack EBV-directed T cells. Indeed, the effect of this vPK-ATM-KAP1-ZEBRA partnership may contribute to the often extraordinary EBV loads seen in posttransplant patients.
Components of the vPK-ATM-KAP1-ZEBRA partnership may be exploited therapeutically to enhance or impede the lytic cycle. Enhancement of the lytic cascade would result in direct cell death as well indirect cell killing caused by lytic protein-mediated activation of the immune response. Such oncolytic therapy would benefit patients with cancers such as EBV-positive Burkitt lymphomas and gastric or nasopharyngeal cell carcinomas. On the other hand, impedance to the lytic cascade to abort the lytic cycle would benefit patients with EBV-related diseases such as infectious mononucleosis, immunocompromise-associated EBV lymphomas, chronic active EBV infection, and oral hairy leukoplakia, and possibly even diseases caused by other human herpesviruses.
MATERIALS AND METHODS
Cell culture and chemical treatment.
EBV-positive endemic Burkitt lymphoma (eBL) cell line HH514-16 (a gift from George Miller, Yale University) and EBV-negative B lymphoma cell line BJAB (a gift from Janet Hearing, Stony Brook University) were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (Gibco) and 1% penicillin-streptomycin (Gibco). 293-p2089 and 293-G4−/G5− cells were derived from human embryonic kidney 293 cells stably transfected with bacmids harboring wild-type (p2089) or BGLF4/BGLF5-null EBV B95-8 genomes and maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, and 50 μg/ml of hygromycin B (Invitrogen). The CLIX-FZ (HH514-16 cells transfected with pLIX_402-FZ) cell line harboring a DNA cassette for inducible expression of ZEBRA protein was generated as described before (24). The CLIX-G4 (for clone-HH514-16 transfected with pLIX_402-G4) cell line expressing inducible vPK was generated from puromycin (P8833; Sigma-Aldrich)-selected HH514-16 cells transfected with pLIX_402-G4. LCL were generated using B95-8 EBV and maintained as described before (24, 50, 51); early-passage cells were used in experiments. Cells were treated with sodium butyrate (NaB; 3 mM; 303410; Sigma-Aldrich), doxycycline (5 μg/ml; D9891; Sigma-Aldrich), and KU-55933 (SML1109; Sigma-Aldrich) at the indicated concentrations.
Plasmids, siRNAs, and transfection.
The previously described EBV ORF library was a gift from Edward Gershburg (Southern Illinois University) (21). Plasmids pFLAG-vPK, pFLAG-vPK-KD, pFLAG-KAP1-wt, pFLAG-KAP1-S824A, FLAG-KAP1-S824D, pLIX-FZ, and pHD1013-Z were described before (24, 25, 52). Plasmid pLIX_402-G4 was constructed by inserting the BGLF4 coding sequence amplified from a cDNA library of HH514-16 cells with forward primer ATACAGCTAGCGCCACCATGGATGTGAATATGGCTGC and reverse primer ATACAACCGGTTCCACGTCGGCCATCTGGACC into pLIX_402 (a gift from David Root [Addgene plasmid number 41394]) at NheI and AgeI sites. siRNAs targeting human ATM transcripts and scrambled siRNA were purchased from Santa Cruz Biotechnology (sc-29761). A previously validated siRNA targeting BGLF4 transcript (25) was synthesized by Dharmacon.
HH514-16 cells, CLIX-FZ cells, and LCL (1 × 106) were transfected with 20 μg of plasmid or 200 pmol of siRNA in Ingenio solution (MIR50117; Mirus) using an Amaxa Nucleofector II (program A-024), except in Fig. 5 and 7D, where 30 μg total plasmid (Fig. 5A), 20 μg plasmid with 150 pmol of siRNA (Fig. 5B), or 30 μg plasmid plus 150 pmol of siRNA (Fig. 7D) were transfected.
Antibodies.
Antibodies included mouse anti-FLAG Ab (F3165; Sigma), rabbit anti-BGLF4 (vPK) Ab (AP8057b; Abgent), rabbit anti-KAP1 Ab (A300-274A; Bethyl Laboratories), goat anti-KAP1 Ab (A303-838A; Bethyl Laboratories), rabbit anti-ATM Ab (A300-299A; Bethyl Laboratories), goat anti-ATM Ab (A300-136A; Bethyl Laboratories), rabbit anti-p-S1981-ATM Ab (ab81292; Abcam), rabbit anti-p-S2996-ATM Ab as described before (53), mouse anti-β-actin Ab (AC-15; Sigma), rabbit anti-p-S824-KAP1 Ab (A300-767A; Bethyl Laboratories), mouse anti-EA-D Ab (MAB8186; EMD), rabbit anti-phospho-histone H2A.X Ab (9718S; Cell Signaling Technology), rabbit anti-histone H2A.X Ab (7631S; Cell Signaling Technology), rabbit anti-histone 3 Ab (AB1791; Abcam), rabbit anti-acetyl-histone 3 Ab (9649S; Cell Signaling Technology), rabbit anti-histone 3 (trimethyl K9) Ab (AB8898; Abcam), mouse anti-RNA polymerase II CTD Ab (AB817; Abcam), human polyclonal serum against EBV small viral capsid antigen (VCA), as previously described (6), mouse anti-ZEBRA Ab (a gift from Paul Farrell), horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (H+L) (AP308P; EMD Millipore), HRP-conjugated goat anti-rabbit IgG (H+L) (AP307P; EMD Millipore), HRP-conjugated protein A (101023; Thermo Fisher), fluorescein isothiocyanate (FITC)-conjugated donkey anti-rabbit IgG (31568; Thermo Fisher), phycoerythrin (PE)-conjugated goat anti-mouse IgG (sc-3738; Santa Cruz), PE-conjugated donkey anti-rabbit IgG (sc-3745; Santa Cruz), FITC-conjugated goat anti-mouse IgG (F0257; Sigma), and Alexa Fluor 647-conjugated goat anti-rabbit IgG (A-21245; Thermo Fisher).
Immunoblotting, immunofluorescence, and flow cytometry.
Cell lysates were harvested and analyzed via immunoblotting with the indicated antibodies as previously described (54). Densitometry was performed with Quantity One analysis software (Bio-Rad Laboratories).
Cells were fixed, incubated with the indicated antibodies, and subjected to immunofluorescence assays or flow cytometry as previously described (24).
PLA.
Proximity ligation assays testing interactions between KAP1 and FLAG-vPK protein as well as ATM and FLAG-vPK protein were performed in pFLAG-vPK-transfected HH514-16 cells according to the manufacturer’s instructions using goat anti-KAP1 Ab, goat anti-ATM Ab, mouse anti-FLAG Ab, Duolink In Situ PLA probe anti-goat MINUS (DUO92006; Sigma-Aldrich), anti-mouse PLUS (DUO92001; Sigma-Aldrich), Duolink In Situ detection reagent green (DUO92014; Sigma-Aldrich), and wash buffers (DUO82049; Sigma-Aldrich).
Quantitative PCR (qPCR) to quantitate transcript levels and EBV load.
Quantitative reverse transcriptase PCR (qRT-PCR) was performed as previously described and analyzed using the ΔΔCT method (where CT is threshold cycle) (54). Primer sequences included the following: GTAACCCGTTGAACCCCATT (forward) and CCATCCAATCGGTAGTAGCG (reverse) for 18S rRNA and TTCCACAGCCTGCACCAGTG (forward) and GGCAGAAGCCACCTCACGGT (reverse) for BZLF1.
Viral genomic DNA was prepared from released EBV particles that had been treated with DNase. The EBV BALF5 gene was amplified using qPCR with forward primer CGTCTCATTCCCAAGTGTTTC and reverse primer GCCCTTTCCATCCTCGTC as previously described (24).
ChIP-qPCR.
Chromatin immunoprecipitation (ChIP) was conducted using a SimpleChIP enzymatic chromatin IP kit (9003; Cell Signaling Technology) by following the manufacturer’s instructions, with minor modifications. Briefly, treated CLIX-G4 BL cells were cross-linked in RPMI with 1% formaldehyde for 10 min at room temperature and quenched with glycine. Cross-linked cells were washed with phosphate-buffered saline and resuspended in buffer A (7006; Cell Signaling Technology) for 10 min at 4°C. Nuclei were pelleted and resuspended in buffer B (7007; Cell Signaling Technology), treated with 0.5 μl of micrococcal nuclease (10011; Cell Signaling Technology) per IP sample, and incubated at 37°C for 30 min before stopping the digestion with EDTA. Digested nuclei were pelleted, resuspended in ChIP buffer (7008; Cell Signaling Technology), and homogenized on ice to disrupt nuclear membrane. Lysates were clarified by centrifugation at 9,400 × g for 10 min at 4°C; 2% of the supernatant was set aside as the input, and the remaining supernatant was divided equally, mixed with individual antibody as indicated plus protein G magnetic beads, and incubated overnight at 4°C. Beads were pelleted and washed with low- and high-salt solutions sequentially. Precipitated DNA was eluted from beads using elution buffer (7009; Cell Signaling Technology). DNA from elutes and input samples were recovered by treating with proteinase K (1002; Cell Signaling Technology) overnight at 65°C and purified using spin columns.
Purified DNA samples were subjected to qPCR amplification of the BZLF1 promoter with forward primer TCTTGTAACGGCATAGGG and reverse primer AGCCACTGGTCTCATCCT. RNA polymerase II occupancy at the BZLF1 promoter was determined by the ΔCT value generated from CT (ChIP sample) minus CT (input sample) and represented as a percentage of the input (Fig. 3E). The abundance of methylated or acetylated histone 3 at the BZLF1 promoter was measured using the ΔΔCT method with anti-histone 3 antibody-precipitated samples as an internal control (Fig. 3F).
Statistical analysis.
P values were calculated by comparing the means from two groups of interest using unpaired Student's t test.
ACKNOWLEDGMENTS
We thank Edward Gershburg at Southern Illinois University for providing the EBV ORF library, Janet Hearing at Stony Brook University for the BJAB cell line, Kum Kum Khanna at QIMR Berghofer Medical Research Institute, Australia, for providing the pFLAG-CMV2-KAP1 construct, and Eric Burton for technical support.
A.E.-G. was supported by RSG 16-201-01 from the American Cancer Society, and S.B.-M. was supported by NIH R01 AI113134, NIH R41 AI115834, funds from the University of Florida, and the Children’s Miracle Network. We have no conflicts of interest to declare.
REFERENCES
- 1.Zheng ZM, Baker CC. 2006. Papillomavirus genome structure, expression, and post-transcriptional regulation. Front Biosci 11:2286–2302. doi: 10.2741/1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berk AJ. 2005. Recent lessons in gene expression, cell cycle control, and cell biology from adenovirus. Oncogene 24:7673–7685. doi: 10.1038/sj.onc.1209040. [DOI] [PubMed] [Google Scholar]
- 3.White MK, Safak M, Khalili K. 2009. Regulation of gene expression in primate polyomaviruses. J Virol 83:10846–10856. doi: 10.1128/JVI.00542-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grinde B. 2013. Herpesviruses: latency and reactivation–viral strategies and host response. J Oral Microbiol 5:10.3402/jom.v5i0.22766. doi: 10.3402/jom.v5i0.22766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murata T, Tsurumi T. 2014. Switching of EBV cycles between latent and lytic states. Rev Med Virol 24:142–153. doi: 10.1002/rmv.1780. [DOI] [PubMed] [Google Scholar]
- 6.Bhaduri-McIntosh S, Miller G. 2006. Cells lytically infected with Epstein-Barr virus are detected and separable by immunoglobulins from EBV-seropositive individuals. J Virol Methods 137:103–114. doi: 10.1016/j.jviromet.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Sawtell NM, Thompson RL. 1992. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J Virol 66:2150–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook CH, Zhang Y, McGuinness BJ, Lahm MC, Sedmak DD, Ferguson RM. 2002. Intra-abdominal bacterial infection reactivates latent pulmonary cytomegalovirus in immunocompetent mice. J Infect Dis 185:1395–1400. doi: 10.1086/340508. [DOI] [PubMed] [Google Scholar]
- 9.Fietze E, Prosch S, Reinke P, Stein J, Docke WD, Staffa G, Loning S, Devaux S, Emmrich F, von Baehr R. 1994. Cytomegalovirus infection in transplant recipients. The role of tumor necrosis factor. Transplantation 58:675–680. doi: 10.1097/00007890-199409000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Bauer G, Hofler P, Simon M. 1982. Epstein-Barr virus induction by a serum factor. Characterization of the purified factor and the mechanism of its activation. J Biol Chem 257:11411–11415. [PubMed] [Google Scholar]
- 11.di Renzo L, Altiok A, Klein G, Klein E. 1994. Endogenous TGF-beta contributes to the induction of the EBV lytic cycle in two Burkitt lymphoma cell lines. Int J Cancer 57:914–919. doi: 10.1002/ijc.2910570623. [DOI] [PubMed] [Google Scholar]
- 12.Thorley-Lawson DA, Allday MJ. 2008. The curious case of the tumour virus: 50 years of Burkitt's lymphoma. Nat Rev Microbiol 6:913–924. doi: 10.1038/nrmicro2015. [DOI] [PubMed] [Google Scholar]
- 13.Jones JF, Shurin S, Abramowsky C, Tubbs RR, Sciotto CG, Wahl R, Sands J, Gottman D, Katz BZ, Sklar J. 1988. T-cell lymphomas containing Epstein-Barr viral DNA in patients with chronic Epstein-Barr virus infections. N Engl J Med 318:733–741. doi: 10.1056/NEJM198803243181203. [DOI] [PubMed] [Google Scholar]
- 14.Weiss LM, Movahed LA, Warnke RA, Sklar J. 1989. Detection of Epstein-Barr viral genomes in Reed-Sternberg cells of Hodgkin's disease. N Engl J Med 320:502–506. doi: 10.1056/NEJM198902233200806. [DOI] [PubMed] [Google Scholar]
- 15.Henle G, Henle W. 1976. Epstein-Barr virus-specific IgA serum antibodies as an outstanding feature of nasopharyngeal carcinoma. Int J Cancer 17:1–7. doi: 10.1002/ijc.2910170102. [DOI] [PubMed] [Google Scholar]
- 16.Chan CK, Mueller N, Evans A, Harris NL, Comstock GW, Jellum E, Magnus K, Orentreich N, Polk BF, Vogelman J. 1991. Epstein-Barr virus antibody patterns preceding the diagnosis of nasopharyngeal carcinoma. Cancer Causes Control 2:125–131. doi: 10.1007/BF00053132. [DOI] [PubMed] [Google Scholar]
- 17.Ragoczy T, Miller G. 1999. Role of the epstein-barr virus RTA protein in activation of distinct classes of viral lytic cycle genes. J Virol 73:9858–9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zalani S, Holley-Guthrie E, Kenney S. 1996. Epstein-Barr viral latency is disrupted by the immediate-early BRLF1 protein through a cell-specific mechanism. Proc Natl Acad Sci U S A 93:9194–9199. doi: 10.1073/pnas.93.17.9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong GK, Delecluse HJ, Gruffat H, Morrison TE, Feng WH, Sergeant A, Kenney SC. 2004. The BRRF1 early gene of Epstein-Barr virus encodes a transcription factor that enhances induction of lytic infection by BRLF1. J Virol 78:4983–4992. doi: 10.1128/jvi.78.10.4983-4992.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammerschmidt W, Sugden B. 1988. Identification and characterization of oriLyt, a lytic origin of DNA replication of Epstein-Barr virus. Cell 55:427–433. doi: 10.1016/0092-8674(88)90028-1. [DOI] [PubMed] [Google Scholar]
- 21.Goswami R, Shair KHY, Gershburg E. 2017. Molecular diversity of IgG responses to Epstein-Barr virus proteins in asymptomatic Epstein-Barr virus carriers. J Gen Virol 98:2343–2350. doi: 10.1099/jgv.0.000891. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Cohen JI. 2016. Epstein-Barr virus (EBV) tegument protein BGLF2 promotes EBV reactivation through activation of the p38 mitogen-activated protein kinase. J Virol 90:1129–1138. doi: 10.1128/JVI.01410-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagemeier SR, Barlow EA, Kleman AA, Kenney SC. 2011. The Epstein-Barr virus BRRF1 protein, Na, induces lytic infection in a TRAF2- and p53-dependent manner. J Virol 85:4318–4329. doi: 10.1128/JVI.01856-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Burton EM, Bhaduri-McIntosh S. 2017. Chloroquine triggers Epstein-Barr virus replication through phosphorylation of KAP1/TRIM28 in Burkitt lymphoma cells. PLoS Pathog 13:e1006249. doi: 10.1371/journal.ppat.1006249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Guindy A, Lopez-Giraldez F, Delecluse HJ, McKenzie J, Miller G. 2014. A locus encompassing the Epstein-Barr virus bglf4 kinase regulates expression of genes encoding viral structural proteins. PLoS Pathog 10:e1004307. doi: 10.1371/journal.ppat.1004307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Burton EM, Koganti S, Zhi J, Doyle F, Tenenbaum SA, Horn B, Bhaduri-McIntosh S. 2018. KRAB-ZFP repressors enforce quiescence of oncogenic human herpesviruses. J Virol 92:e00298-18. doi: 10.1128/JVI.00298-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asai R, Kato A, Kato K, Kanamori-Koyama M, Sugimoto K, Sairenji T, Nishiyama Y, Kawaguchi Y. 2006. Epstein-Barr virus protein kinase BGLF4 is a virion tegument protein that dissociates from virions in a phosphorylation-dependent process and phosphorylates the viral immediate-early protein BZLF1. J Virol 80:5125–5134. doi: 10.1128/JVI.02674-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang JT, Yang PW, Lee CP, Han CH, Tsai CH, Chen MR. 2005. Detection of Epstein-Barr virus BGLF4 protein kinase in virus replication compartments and virus particles. J Gen Virol 86:3215–3225. doi: 10.1099/vir.0.81313-0. [DOI] [PubMed] [Google Scholar]
- 29.Yue W, Gershburg E, Pagano JS. 2005. Hyperphosphorylation of EBNA2 by Epstein-Barr virus protein kinase suppresses transactivation of the LMP1 promoter. J Virol 79:5880–5885. doi: 10.1128/JVI.79.9.5880-5885.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kato K, Yokoyama A, Tohya Y, Akashi H, Nishiyama Y, Kawaguchi Y. 2003. Identification of protein kinases responsible for phosphorylation of Epstein-Barr virus nuclear antigen leader protein at serine-35, which regulates its coactivator function. J Gen Virol 84:3381–3392. doi: 10.1099/vir.0.19454-0. [DOI] [PubMed] [Google Scholar]
- 31.Chen MR, Chang SJ, Huang H, Chen JY. 2000. A protein kinase activity associated with Epstein-Barr virus BGLF4 phosphorylates the viral early antigen EA-D in vitro. J Virol 74:3093–3104. doi: 10.1128/jvi.74.7.3093-3104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fredriksson S, Gullberg M, Jarvius J, Olsson C, Pietras K, Gustafsdottir SM, Ostman A, Landegren U. 2002. Protein detection using proximity-dependent DNA ligation assays. Nat Biotechnol 20:473–477. doi: 10.1038/nbt0502-473. [DOI] [PubMed] [Google Scholar]
- 33.Leuchowius KJ, Weibrecht I, Soderberg O. 2011. In situ proximity ligation assay for microscopy and flow cytometry. Curr Protoc Cytom 9:Unit 9.36. doi: 10.1002/0471142956.cy0936s56. [DOI] [PubMed] [Google Scholar]
- 34.Hagemeier SR, Barlow EA, Meng Q, Kenney SC. 2012. The cellular ataxia telangiectasia-mutated kinase promotes Epstein-Barr virus lytic reactivation in response to multiple different types of lytic reactivation-inducing stimuli. J Virol 86:13360–13370. doi: 10.1128/JVI.01850-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang'ondu R, Teal S, Park R, Heston L, Delecluse H, Miller G. 2015. DNA damage signaling is induced in the absence of Epstein-Barr virus (EBV) lytic DNA replication and in response to expression of ZEBRA. PLoS One 10:e0126088. doi: 10.1371/journal.pone.0126088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kudoh A, Fujita M, Zhang L, Shirata N, Daikoku T, Sugaya Y, Isomura H, Nishiyama Y, Tsurumi T. 2005. Epstein-Barr virus lytic replication elicits ATM checkpoint signal transduction while providing an S-phase-like cellular environment. J Biol Chem 280:8156–8163. doi: 10.1074/jbc.M411405200. [DOI] [PubMed] [Google Scholar]
- 37.Daigle D, Megyola C, El-Guindy A, Gradoville L, Tuck D, Miller G, Bhaduri-McIntosh S. 2010. Upregulation of STAT3 marks Burkitt lymphoma cells refractory to Epstein-Barr virus lytic cycle induction by HDAC inhibitors. J Virol 84:993–1004. doi: 10.1128/JVI.01745-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koganti S, Clark C, Zhi J, Li X, Chen EI, Chakrabortty S, Hill ER, Bhaduri-McIntosh S. 2015. Cellular STAT3 functions via PCBP2 to restrain Epstein-Barr Virus lytic activation in B lymphocytes. J Virol 89:5002–5011. doi: 10.1128/JVI.00121-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill ER, Koganti S, Zhi J, Megyola C, Freeman AF, Palendira U, Tangye SG, Farrell PJ, Bhaduri-McIntosh S. 2013. Signal transducer and activator of transcription 3 limits Epstein-Barr virus lytic activation in B lymphocytes. J Virol 87:11438–11446. doi: 10.1128/JVI.01762-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Binne UK, Amon W, Farrell PJ. 2002. Promoter sequences required for reactivation of Epstein-Barr virus from latency. J Virol 76:10282–10289. doi: 10.1128/jvi.76.20.10282-10289.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flemington E, Speck SH. 1990. Autoregulation of Epstein-Barr virus putative lytic switch gene BZLF1. J Virol 64:1227–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang PC, Fitzgerald LD, Van Geelen A, Izumiya Y, Ellison TJ, Wang DH, Ann DK, Luciw PA, Kung HJ. 2009. Kruppel-associated box domain-associated protein-1 as a latency regulator for Kaposi's sarcoma-associated herpesvirus and its modulation by the viral protein kinase. Cancer Res 69:5681–5689. doi: 10.1158/0008-5472.CAN-08-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li R, Zhu J, Xie Z, Liao G, Liu J, Chen MR, Hu S, Woodard C, Lin J, Taverna SD, Desai P, Ambinder RF, Hayward GS, Qian J, Zhu H, Hayward SD. 2011. Conserved herpesvirus kinases target the DNA damage response pathway and TIP60 histone acetyltransferase to promote virus replication. Cell Host Microbe 10:390–400. doi: 10.1016/j.chom.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shiloh Y, Ziv Y. 2013. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol 14:197–210. doi: 10.1038/nrm3546. [DOI] [PubMed] [Google Scholar]
- 45.Li R, Liao G, Nirujogi RS, Pinto SM, Shaw PG, Huang TC, Wan J, Qian J, Gowda H, Wu X, Lv DW, Zhang K, Manda SS, Pandey A, Hayward SD. 2015. Phosphoproteomic profiling reveals Epstein-Barr virus protein kinase integration of DNA damage response and mitotic signaling. PLoS Pathog 11:e1005346. doi: 10.1371/journal.ppat.1005346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ziv Y, Bielopolski D, Galanty Y, Lukas C, Taya Y, Schultz DC, Lukas J, Bekker-Jensen S, Bartek J, Shiloh Y. 2006. Chromatin relaxation in response to DNA double-strand breaks is modulated by a novel ATM- and KAP-1 dependent pathway. Nat Cell Biol 8:870–876. doi: 10.1038/ncb1446. [DOI] [PubMed] [Google Scholar]
- 47.Rauwel B, Jang SM, Cassano M, Kapopoulou A, Barde I, Trono D. 2015. Release of human cytomegalovirus from latency by a KAP1/TRIM28 phosphorylation switch. Elife 4:e06068. doi: 10.7554/eLife.06068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bakkenist CJ, Kastan MB. 2003. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 49.Feederle R, Mehl-Lautscham AM, Bannert H, Delecluse HJ. 2009. The Epstein-Barr virus protein kinase BGLF4 and the exonuclease BGLF5 have opposite effects on the regulation of viral protein production. J Virol 83:10877–10891. doi: 10.1128/JVI.00525-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hui-Yuen J, Koganti S, Bhaduri-McIntosh S. 2014. Human B cell immortalization for monoclonal antibody production. Methods Mol Biol 1131:183–189. doi: 10.1007/978-1-62703-992-5_11. [DOI] [PubMed] [Google Scholar]
- 51.Hui-Yuen J, McAllister S, Koganti S, Hill E, Bhaduri-McIntosh S. 2011. Establishment of Epstein-Barr virus growth-transformed lymphoblastoid cell lines. J Vis Exp 57:3321. doi: 10.3791/3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bolderson E, Savage KI, Mahen R, Pisupati V, Graham ME, Richard DJ, Robinson PJ, Venkitaraman AR, Khanna KK. 2012. Kruppel-associated Box (KRAB)-associated co-repressor (KAP-1) Ser-473 phosphorylation regulates heterochromatin protein 1beta (HP1-beta) mobilization and DNA repair in heterochromatin. J Biol Chem 287:28122–28131. doi: 10.1074/jbc.M112.368381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kozlov SV, Graham ME, Jakob B, Tobias F, Kijas AW, Tanuji M, Chen P, Robinson PJ, Taucher-Scholz G, Suzuki K, So S, Chen D, Lavin MF. 2011. Autophosphorylation and ATM activation: additional sites add to the complexity. J Biol Chem 286:9107–9119. doi: 10.1074/jbc.M110.204065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.King CA, Li X, Barbachano-Guerrero A, Bhaduri-McIntosh S. 2015. STAT3 regulates lytic activation of Kaposi's sarcoma-associated herpesvirus. J Virol 89:11347–11355. doi: 10.1128/JVI.02008-15. [DOI] [PMC free article] [PubMed] [Google Scholar]