DNA from viruses has been “horizontally transferred” to mammalian genomes during evolution, but the impact of this process on mammalian biology remains poorly understood. The findings of our study indicate that a novel gene has evolved in guinea pigs through fusion of host and virus genes.
KEYWORDS: parvoviral EVE
ABSTRACT
Sequences derived from parvoviruses (family Parvoviridae) are relatively common in animal genomes, but the functional significance of these endogenous parvoviral element (EPV) sequences remains unclear. In this study, we used a combination of in silico and molecular biological approaches to investigate a fusion gene carried by guinea pigs (genus Cavia) that is partially derived from an EPV. This gene, named enRep-M9l, encodes a predicted polypeptide gene product comprising a partial myosin9-like (M9l) gene fused to a 3′ truncated, EPV-encoded replicase. We examined the genomic and phylogenetic characteristics of the EPV locus (enRep) that encodes the viral portions of enRep-M9l, revealing that it derives from an ancient dependoparvovirus (genus Dependoparvovirus) that was incorporated into the guinea pig germ line between approximately 22 and 35 million years ago (MYA). Despite these ancient origins, the regions of the enRep locus that are expressed in the enRep-M9l gene are conserved across multiple species in the family Caviidae (guinea pigs and cavies), consistent with a potential function at the amino acid level. Using molecular biological approaches, we further demonstrated that (i) enRep-M9l mRNA is broadly transcribed in guinea pig cells, (ii) the cloned enRep-M9l transcript can express a protein of the expected size in guinea pig cells in vitro, and (iii) the expressed protein localizes to the cytosol. Our findings demonstrate that, consistent with a functional role, the enRep-M9l fusion gene is evolutionarily conserved, broadly transcribed, and capable of expressing protein.
IMPORTANCE DNA from viruses has been “horizontally transferred” to mammalian genomes during evolution, but the impact of this process on mammalian biology remains poorly understood. The findings of our study indicate that a novel gene has evolved in guinea pigs through fusion of host and virus genes.
INTRODUCTION
Animal genomes contain numerous DNA sequences derived from viruses. These endogenous viral elements (EVEs) are thought to arise when infection of germ line cells (i.e., gametes or early embryonic cells) leads to viral sequences becoming integrated into the chromosomal DNA of the ancestral organism such that they are subsequently transferred from parent to offspring via inheritance as novel genes (1). Most EVEs derive from viruses that circulated in ancestral species millions of years ago. Most of the EVE sequences found in mammalian genomes are derived from retroviruses (family Retroviridae). Typically, tens of thousands of these endogenous retrovirus (ERV) sequences are present (e.g., ∼8% of the human genome is estimated to be comprised of ERVs [2]), compared to a relatively small number (e.g., ∼1 to ∼20) of EVE sequences derived from nonretroviral virus families (1). This likely reflects that retroviruses integrate into genomic DNA as an obligate step in their replication whereas integration is thought to occur only anomalously, via nonhomologous recombination (DNA viruses) or retrotransposition of virus-encoded RNA (DNA and RNA viruses), for nonretroviral viruses (3–5).
EVEs preserve information about viruses that circulated millions of years ago and can therefore provide unique insight into the long-term evolutionary interactions between viruses and cells (6). Furthermore, genomic and experimental research has revealed that during eukaryotic evolution, some of these “horizontally transferred” viral sequences have been coopted or exapted so that they now perform physiologically relevant functions. In mammals, EVEs have been shown to have roles related to cell function, embryonic development, and antiviral immunity (7–12). While most examples involve ERVs, potentially coopted/exapted EVEs derived from nonretroviral viruses have also been identified and investigated (12). For example, an endogenous bornavirus-like nucleoprotein (EBLN) element in the thirteen-lined ground squirrel (Ictidomys tridecemlineatus) encodes an intact nucleoprotein that colocalizes in the nucleus with viral factories and inhibits in vitro replication of Borna disease virus (BDV) (11). In humans, an EBLN gene (EBLN-1) is expressed in testis and brain (13). Although this gene contains an intact open reading frame (ORF), it is proposed to function in gene regulation as a noncoding RNA (ncRNA) (12, 13).
Among the nonretroviral EVEs that have been documented in mammals, parvoviruses (family Parvoviridae) are well represented (1, 14–16). Parvoviruses are small, nonenveloped viruses of icosahedral symmetry. They have a linear, single-stranded DNA (ssDNA) genome 4 to 6.3 kb in length (17) and encode at least two major open reading frames (ORFs) that are expressed to produce nonstructural (NS or Rep) proteins and structural (VP or capsid) proteins. Parvovirus replication occurs in the nucleus and can occasionally lead to viral genomes becoming integrated into host chromosomes via nonhomologous recombination, possibly facilitated by single-stranded breaks created by the nickase function of Rep (18). Accordingly, incorporation of parvovirus DNA into the germ line DNA might be expected to occur at a certain frequency as a natural consequence of the biology of these viruses. However, the EPVs generated in such events are rapidly eliminated from the gene pool via genetic drift unless they are selected for some reason (as is the case for any new allele). Thus, the presence of numerous independently acquired, fixed EPV insertions in animal genomes is unexpected and suggests that selective pressures may have occasionally favored retention of EPV genes in animal genomes during their evolution. Intriguingly, several EPV loci contain open reading frames (ORFs) capable of expressing complete or almost complete proteins (19, 20), and recent studies have shown that Rep-encoding EPV loci in rodents and elephants exhibit a similar, distinctive pattern of tissue-specific expression—being specifically transcribed in the liver—despite having been acquired in entirely independent germ line incorporation events (20).
In this study, we used a combination of in silico and molecular biological approaches to investigate enRep-M9l—a guinea pig fusion gene that is derived partially from an EPV and partially from a host gene.
(This article was submitted to an online preprint archive [21].)
RESULTS
Guinea pigs express enRep-M9l—a fusion gene partly derived from EPV sequences.
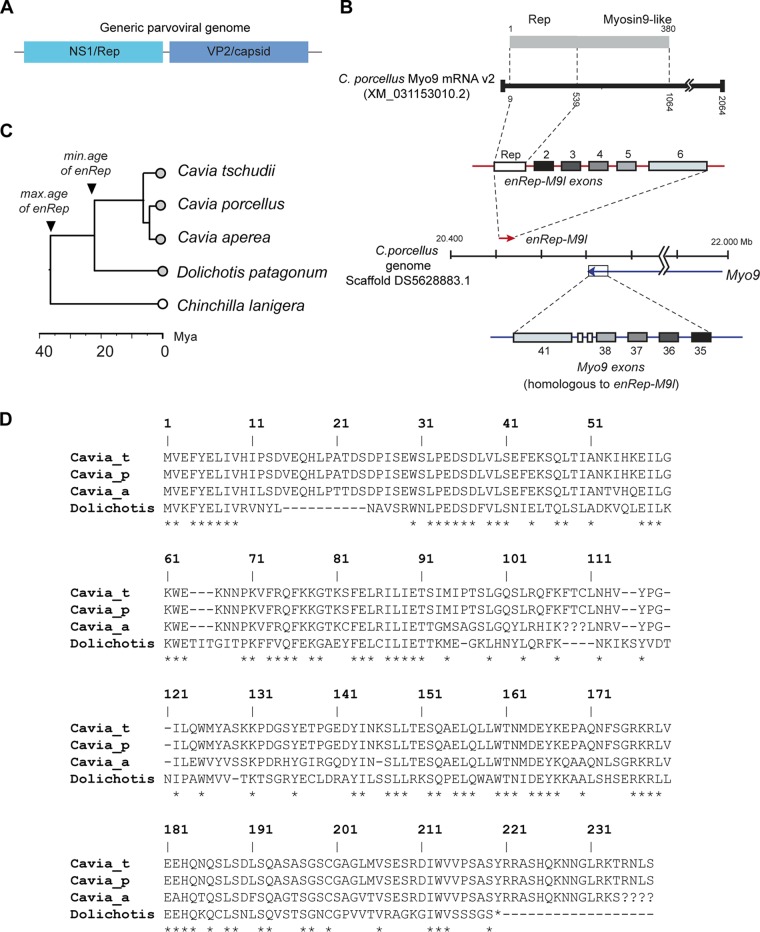
A predicted fusion gene containing EPV-derived sequences was identified in the guinea pig (Cavia porcellus) via similarity search-based screening of the reference RNA sequence (RefSeq RNA) database (22). We examined this predicted gene product (named myosin-9 variant [v2] in GenBank) using comparative approaches. This revealed it to be comprised of a 3′ truncated parvovirus rep gene fused to a region homologous to myosin-9 (Myo9). It also includes a long 3′ untranslated region (3′UTR) that is most likely of cellular origin and a very short 5′UTR of only 6 nucleotides (Fig. 1B). The Myo9-like regions of the predicted gene product are homologous to exons 35, 36, 37, 38, and 41 of Myo-9 but are in fact derived from a distinct Myo9-like gene (see Fig. 1B). This gene—which we named enRep-M9l—is encoded on the same chromosome as Myo9 but is located ∼200 Mb away on the opposite strand.
FIG 1.
Genomic and phylogenetic characteristics of the enRep-M9l gene. (A) Schematic diagram showing the structure of a generic parvovirus genome. (B) Diagram showing structure of the enRep-M9l locus. The upper part of the diagram shows the predicted enRep-M9l mRNA (black line) as represented in GenBank (accession no. XM_013153010.2) and the putative fusion protein containing Rep and Myosin9-like domains (gray bar). The middle section of the diagram shows the location of the enRep-M9l (red arrow) locus relative to the Myo9 locus (blue arrow) in the C. porcellus genome (scaffold DS5628883.1), with arrows indicating the direction of transcription. Above the line representing the scaffold, the exon structure of the enRep-M9l gene is shown (red line with overlaid boxes). The six exons (numbered 1 to 6 in the figure) of the enRep-M9l gene are color-coded to indicate their correspondence to a subset of the exons (i.e., exons 35 to 38 and 41) encoded by the Myo9 gene, as shown beneath the line representing the genomic scaffold (blue line with overlaid boxes). (C) A time-scaled phylogenetic tree showing the estimated divergence times of four extant species in the genus Cavia, relative to an outgroup taxon, the chinchilla (Chinchilla lanigera). Circles with gray fill indicate taxa in which enRep is present. Empty circles indicate taxa in which enRep is absent. (D) Multiple alignment of virtually translated enRep ORFs. Question marks indicate unresolved sequence. Asterisks indicate residues that are 100% conserved across all four taxa.
Similarity search-based screening of the rodent whole-genome sequence (WGS) assemblies revealed that the viral portions of enRep-M9l are carried by an EPV locus specific to the cavies (family Caviidae), which we named enRep. Cavies are rodents native to South America, and the group includes guinea pigs, wild cavies, and maras. The presence of the enRep insertion at an orthologous locus in three guinea pig species, as well as in the more distantly related Patagonian mara (Dolichotis patagonum), demonstrates that it was incorporated into the germ line prior to divergence of these species an estimated ∼22 million years ago (MYA) (confidence interval [CI], 14.8 to 27.6 MYA) (23–25). Meanwhile, the absence of enRep in more distantly related rodent species, such as the chinchilla (Chinchilla lanigera), indicates that germ line integration probably occurred less than 35 MYA (Fig. 1C).
Inspection of the enRep locus revealed that only a truncated rep gene is present, with no other parvovirus genes detectable nearby and no detectable target site duplication (TSD) or poly(A) tails (indicative of insertion via retrotransposition) being present. The start codon of the enRep-M9l gene corresponds to the canonical start codon of rep as encoded by dependoparvoviruses. Most of the enRep locus is highly degraded by mutation—reflecting a long presence in the guinea pig germ line and relaxed evolutionary constraints on sequence change. However, the coding region of the Rep ORF that is incorporated into the enRep-M9l gene contains no premature stop codons or frameshifting mutations. Although the coding sequence is preserved, it shows as much as 15% divergence between species at the amino acid level. The sequence in the Patagonian mara was much more divergent (48% similarity to the C. porcellus sequence at the amino acid level). Intriguingly, the enRep sequence in the mara also contains an ORF encoding the N-terminal portions of Rep that are incorporated into the enRep-M9l gene of C. porcellus, despite this region containing multiple indels relative to the guinea pig sequences. However, the predicted ORF is slightly shorter, being 220 amino acids (aa) in length, as opposed to 239 aa in guinea pigs (Fig. 1D).
Investigation of the enRep locus in silico revealed that it is located within 0.4 kb of the Myo9-like exons that are incorporated into the enRep-M9l gene. We next used PCR to amplify (i) the regions of enRep that are incorporated into the enRep-M9l gene, (ii) their immediate genomic context, and (iii) the full-length gene from genomic DNA (Fig. 2A). The resulting amplicons were sequenced, confirming that the locus is structured as in the published WGS assembly of the C. porcellus genome. Since computer program-based predictions of genes, transcripts and splicing sites need to be experimentally validated, we also generated and sequenced cDNA to confirm that in vivo expression and splicing of the enRep-M9l gene occurs as predicted by in silico approaches.
FIG 2.
Parvovirus-derived myosin fusion is expressed in guinea pig. (A) The presence of a parvovirus-derived rep gene in the genome of guinea pig was verified by PCR from genomic DNA. F1/R1 primers were used to amplify regions of the rep gene carried by an EPV (enRep). F2/R2 primers were used to amplified rep in its immediate genomic context (enRepGC). F1/R3 primers were used to amplified rep and Myo9-like exons (enRep-M9l). All amplicons were verified by sequencing. The locations of the primers on the gene are shown as follows: rep hexon in white, Myo9-like exons in different shades of gray, and introns as lines. pb, base pairs. (B) The expression of enRep-M9l transcript was detected in several guinea pig tissues. Total RNA was isolated, and cDNA was generated using oligo(dT) and used for PCR to detect enRep (enRep), enRep-M9l up to the exon 2/3 junction (enRep-M9l), or the full-length enRep-M9l coding sequence (FLenRep-M9l). C. porcellus GAPDH (CpGAPDH) mRNA was used as loading control. The locations of the primers on the enRep-M9l coding sequence are shown under the blots. Exon 5, in which alternative splicing occurs, is indicated by a red asterisk. A DNA migration ladder is indicated on the left-hand side. (C) The enRep-M9l coding sequence confirmed by sequencing of cDNA is shown. The start of each exon is indicated by a bold capital letter. The sequence missing in the alternatively spliced version is shown in red, and the sequence of enRep is underlined. (D) Alignment of the long (enRep-M9lL) and short (enRep-M9lS) proteins encoded by the cloned sequences. The Rep domain is framed in red.
The enRep-M9l gene is broadly transcribed in vivo.
We investigated in vivo transcription of enRep-M9l using reverse transcription-PCR (RT-PCR). RNA was isolated from several guinea pig (C. porcellus) tissues and used to generate cDNA. The absence of genomic DNA in cDNA preparations was confirmed prior to performing assays (data not shown). We first demonstrated that cDNA encoding the enRep section of the enRep-M9l mRNA was present in all tissues analyzed (Fig. 2B). Next, we verified that these sequences were expressed as part of a transcript encoding a fusion protein comprised of enRep sequences fused to Myo9-like exons as predicted. PCR using a forward primer at the end of enRep and a reverse primer aligning at the junction of exons 2 and 3 of Myo9-like (Fig. 2B) generated amplicons of the expected size in all samples analyzed, confirming that enRep-M9l was expressed as a fused transcript.
The presence of the full-length coding sequence was confirmed by PCR in all analyzed tissues (Fig. 2B), including ovary, liver, lung, kidney, spleen, heart, and brain. Those last-named amplicons were sequenced, demonstrating that there were at least two alternatively spliced forms of enRep-M9l. The isoforms differed at the junction of enRep-M9l exons 5 and 6, with the shorter isoform lacking a region spanning 10 codons of exon 5 (Fig. 2C and D). In some tissue samples (e.g., spleen), both isoforms were found to occur. We cannot be certain that the entire predicted transcript sequence as represented in the GenBank was expressed since, despite several attempts, we failed to obtain the untranslated regions of the mature enRep-M9l transcript. Nevertheless, our analysis does confirm that the entire coding sequence was expressed.
The enRep-M9l protein exhibits cytosolic localization in vivo.
To verify that the ORFs in the enRep-M9l transcript can be translated into a protein, we cloned and expressed the two isoforms of enRep-M9l in vitro. We cloned enRep-M9l long (380 amino acids) and short (370 amino acids) coding sequences from spleen-derived cDNA into eukaryotic expression vectors to express an N- or C-terminal Flag-tagged protein. Cells were transfected with the 3×Flag-enRep-M9lL (long) or 3×Flag-enRep-M9lS (short), enRep-M9l-FlagL or enRep-M9l-FlagS, or control plasmids; a Flag-tagged protein was observed when either construct was used (Fig. 3A), and there were no notable differences in mobility between the isoforms. This shows that it is possible to obtain a protein from the coding sequence present at the enRep-M9l transcripts.
FIG 3.
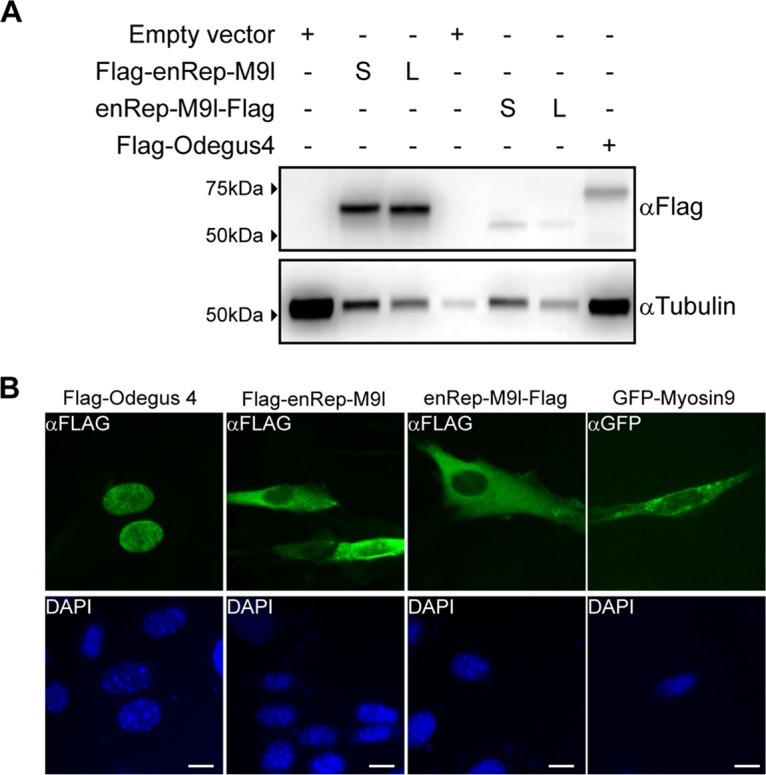
enRep-M9l encodes a protein with cytoplasmic localization. The expression of enRep-M9l isoforms was analyzed by Western blotting and immunofluorescence. (A) NIH 3T3 cells were transfected with plasmids encoding Flag-enRep-M9lL, Flag-enRep-M9lS, enRep-M9l-FlagL, enRep-M9l-FlagS, or Flag-Odegus4 or with empty vector. At 48 h later, cells were lysed, and Western blot assays were performed using anti-Flag or anti-tubulin antibodies as a loading control. The migration of the molecular marker is shown on the left-hand side, and the antibody used for each blot is indicated on the right-hand side. S, short; L, long. (B) NIH 3T3 cells seeded over coverslips were transfected with plasmids encoding Flag-enRep-M9lS, enRep-M9l-FlagS, Flag-Odegus4, or mouse GFP-Myosin9. At 24 h later, cells were fixed, and immunofluorescence was performed using anti-Flag or anti-GFP antibodies. DAPI was used to stain the nucleus. Bar, 10 μm.
Western blot assays showed a migration difference only between the Flag-enRep-M9l and enRep-M9l-Flag proteins, which was due to the addition of the three Flag epitopes when the protein was tagged at the amino terminal. The expected size of both proteins (including the epitopes) was <50 kDa, but we observed a migration above the 50-kDa level. It is known that parvoviral replicases are regulated by phosphorylation (26), and several programs showed putative posttranslational modification in both short enRep-M9l and long enRep-M9l. Thus, the lower level of migration observed for enRep-M9l might reflect posttranslational modification of the protein.
To further investigate the enRep-M9l protein, we asked where in the cell it might be located. Unlike adeno-associated virus (AAV) Rep and other EPVs containing intact ORFs encoding complete or nearly complete Rep proteins (e.g., the Odegus4 element in the genome of the degu [Octodon degus]) (19), the enRep-M9l sequence does not contain predicted nuclear localization signals. We cloned and expressed the Odegus4 element, demonstrating that this transcript can express a protein (Fig. 3A). We compared the localization of Odegus4 to that of enRep-M9lS expressed from both constructs and included green fluorescent protein-Myosin9 (GFP-Myosin9) from mouse in the analysis (Fig. 3B). As expected, the protein expressed by Odegus4 was mainly located in the nucleus, whereas GFP-Myosin9 had cytoplasmic localization; both enRep-M9l proteins had cytoplasmic localization, and no difference was observed between the isoforms (data not shown).
DISCUSSION
In this study, we investigated the evolution and molecular properties of a guinea pig fusion gene, namely, enRep-M9l. We showed that the viral portions of enRep-M9l derive from an EPV (named enRep) that was incorporated into the genome of caviomorph rodents between 22 and 35 MYA, while the host portions derive from five exons of a myosin 9-like (M9l) gene. Furthermore, while most of this EPV sequence is degraded, the portions included in the enRep-M9l gene are intact in multiple species of guinea pig (genus Cavia), consistent with selection to maintain a function at the amino acid level. Using PCR, we confirmed that enRep-M9l was expressed in vivo (Fig. 2B) and showed that in some tissues, two splicing forms were present (Fig. 2C).
The enRep-M9l protein contains two major domains, the N-terminal Rep_N domain (amino acids 4 to 176) and the C-terminal myosin tail domain (amino acids 58 to 325). The Rep_N domain includes the helicase domain of Rep78, while the C-terminal myosin tail domain includes a Rad50 and SMC subdomains. It was not possible to identify a nuclear localization signal (NLS) for the enRep section of the protein, and consistent with this, we observed cytosolic localization for the enRep-M9l protein regardless of whether it was tagged at the N terminus or the C terminus. We compared the localization of enRep-M9l with that of another EPV-encoded Rep protein, Odegus4, encoded by a dependoparvovirus-derived EPV in the degu genome. We previously reported that this EPV is specifically transcribed in degu liver (19). The predicted gene product contains at least two predicted NLSs, as it is expected for Rep proteins (which must locate to the nucleus to allow replication of parvoviral DNA). We demonstrated that the Odegus4 protein indeed exhibited nuclear localization. Thus, if both EPV-derived and partially EPV-derived gene products have functional roles, they are likely to be entirely different. We hypothesize that during the adaptation of enRep to its ancestral host, the NLS was lost as part of the adaptation process, allowing it to be coopted for a still unknown cellular role at the cytoplasm. The observation that enRep-M9l was expressed in all analyzed tissues (ovary, liver, lung, kidney, spleen, heart, and brain) may indicate that this fusion has an important functional role in guinea pig. The possible functions of the enRep-M9l protein remain a matter of speculation, but since the N-terminal region of the parvovirus Rep protein incorporated into this gene normally binds viral DNA during replication, it may have a function related to binding nucleic acids in the cytoplasm.
The selective forces underlying the fixation of EPVs (and EVEs derived from other kinds of virus) remain poorly understood. Nonetheless, it is now clear that sequences acquired from viruses can be “repurposed” by animal genomes in a variety of ways (1, 8, 12, 27). Our findings indicate that in guinea pigs, a novel gene may have arisen through fusion of EPV and host genes. We demonstrate that the enRep-M9l gene possesses molecular characteristics that are at least consistent with a functional role as a coding gene, being conserved, broadly transcribed, and capable of expressing protein. These findings provide indications that acquisition of genes from parvoviruses has shaped the evolution of mammals and establish a platform for future experiments designed to determine whether enRep-M9l plays a biologically relevant role in guinea pig physiology.
MATERIALS AND METHODS
Genome screening in silico.
Whole-genome sequence (WGS) data were obtained from the National Center for Biotechnology Information (NCBI) genome resource. WGS data of 60 rodent species were screened for parvovirus-like sequences using the database-integrated genome-screening (DIGS) tool (28). Parvovirus protein sequences were used as “probes” in similarity-search-based screens of WGS data. Sequences that produced statistically significant matches to probes were extracted and classified by BLAST-based comparison into a set of virus reference genomes. All the sequence data are available on https://github.com/giffordlabcvr/Parvoviridae-enRep.
Nucleic acid extractions and PCR.
Two female individuals of 300 g were euthanized by 1-cloro-2,2,2-trifluoroethyl difluoromethyl ether overdose according to a protocol approved by the Bioethical Committee of Facultad de Ciencias de la Vida, Universidad Andres Bello (Acta 010/2016). Genomic DNA was obtained from liver tissue, and total RNA was extracted from ovary, lungs, liver, kidney, spleen, heart, and brain using an RNeasy minikit (Qiagen). cDNA was synthesized using 500 ng of RNA, oligo(dT), and a SuperScript III first-strand synthesis system (Thermo Fisher).
To verify the presence of enRep in the genomic DNA, PCR was performed using primers F1 (5′-ATGGTGGAATTTTATGAGCTG-3′) and R1 (5′-CATAAGCCCTGCACCACAACTG-3′) with 75 ng of gDNA, GoTaq Flexi G2 (Promega), and 2.0 mM MgCl2 with a program of 95°C for 3 min; 35 cycles of 95°C for 30 s, 53.3°C for 30 s, and 72°C for 35 s; and a final extension at 72°C for 5 min. To confirm that enRep was in the correct genomic context, we used primers aligning 165 bp upstream (F2; 5′-AACCTGAGTTGTCATTCAGG-3′) and 55 bp downstream (R2; 5′-TGATGCCCTTCTGTATGAGG-3′) of enRep with the same conditions as before except for the use of 35 cycles of 95°C for 30 s, 53.3°C for 30 s, and 72°C for 45 s. To verify the full enRep-M9l exons-introns in the genome, we performed PCR using PfuUltra II (Agilent) with primers F1 and R3 (5′-GAGGTGGGCAGTCATCCATC-3′), which align in the last Myo9-like exon, and a program of 92°C for 2 min; 35 cycles of 92°C for 20 s, 60°C for 30 s, and 72°C for 1 min 50 s; and a final extension at 72°C for 3 min. All the amplicons were cloned in pGEM-T Easy (Promega) and sequenced.
To discard genomic DNA contamination in the cDNA preparations, primers F3 (5´-TGGATCCTATGAGACTCCTGGT-3´) and R2 were used for a PCR with 1 μl of cDNA with GoTaq Flexi G2 (Promega) and 2.0 mM MgCl2 and a program of 95°C for 3 min; 35 cycles of 95°C for 30 s, 57°C for 30 s, and 72°C for 45 s; and a final extension of 72°C for 5 min. To amplify enRep from cDNA, the same primers and conditions described for processing of enRep with genomic DNA were used, except that 40 PCR cycles were performed. To verify the presence of the enRep-M9l transcript, a forward primer aligning at the 3´ end of enRep (F4; 5´-ATGGTGGAATTTTATGAGGTG-3´) and a reverse primer aligning at the exon-exon junction of enRep-M9l exons 2 and 3 (R4; 5´-CATAAGCCCTGCAGCTGAATC-3´) were used under the same conditions as before except for the use of 40 cycles of 95°C for 30 s, 58°C for 30 s, and 72°C for 20 s. For GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (GenBank accession no. NM_001172951), primers Frw (5´-GAATCACGAGAAGTACGACA-3´) and Rev (5´-GTATTTGGCCGGTTTCTCC-3´) were used under the same conditions except for the use of 40 cycles of 95°C for 30 s, 57.3°C for 30 s, and 72°C for 25 s. The full-length enRep-M9l coding sequence was amplified from the cDNA using PFU II Ultra and primers Frw (5′-ATATCTCGAGCTATTCAGCAGGCTTGGCCTCGG-3′) and Rev (5′-ATATGAATTCAATGGTGGAATTTTATGAGCTG-3′) and 1 μl of cDNA and a program of 92°C for 2 min; 40 cycles of 92°C for 20 s, 60°C for 30 s, and 72°C for 50 s; and a final extension at 72°C for 3 min. The amplicons were cloned in pGEM-T Easy and sequenced.
Cloning and plasmids.
pGEM-T Easy-enRep-M9l containing the amplicons from spleen were digested with EcoRI and XhoI and cloned into pcDNA3×Flag to generate pcDNA3×Flag-enRep-M9lL and pcDNA3×Flag-enRep-M9lS, which encode 3×Flag-enRep-M9l isoforms. The coding sequences were also amplified from pGEM-T Easy-enRep-M9l using the primers Frw (5′-ATATGAATTCGCCACCATGGTGGAATTTTATG-3′) and Rev (5′-ATATCTCGAGTTCAGCAGGCTTGGCCTCGG-3′) using the same PCR conditions as described above. The PCR products were digested with EcoRI and XhoI and cloned into pCMVtag4C to generate pCMVTag4C-enRep-M9lL and pCMVTag4C-enRep-M9lS, which encode enRep-M9l-Flag isoforms. The Odegus4 coding sequence was amplified using primers Frw (5´-ATGGAATTCATGGTGCAGTTTTATGAGC-3´) and Rev (5´-ATCTCGAGCTAGAGGGCGCACTTTTTCC-3´) from degu genomic DNA. The PCR product was digested with BamHI and XhoI and cloned into pcDNA3×Flag to generate pcDNA3×Flag-Odegus4, which encodes 3×Flag-Odegus4. Plasmid MyosinIIA-GFP (29), which encodes mouse GFP-Myosin9, was a gift from Matthew Krummel (Addgene plasmid catalog no. 38297).
Western blotting and immunofluorescence.
NIH 3T3 cells were seeded in 12-well plates and transfected with 500 ng of pcDNA3×Flag-enRep-M9lL, pcDNA3×Flag-enRep-M9lS, pCMVtag4C-enRep-M9lL, pCMVtag4C-enRep-M9S, pcDNA3×Flag-Odegus4, or empty vector. At 48 h later, the cells were lysed in Reporter lysis buffer (Promega). Samples were then boiled in 5× sodium dodecyl sulfate (SDS) loading buffer, and the proteins were resolved by 10% acrylamide SDS-PAGE. After transfer to nitrocellulose membranes, the blots were probed with mouse anti-Flag (clone M2; Sigma) or mouse anti-α-tubulin (clone DM1A; Sigma). Secondary antibodies conjugated to horseradish peroxidase (HRP) and the enhanced chemiluminescence (ECL) reagents were used for development.
For immunofluorescence assays, 2.5 × 104 NIH 3T3 cells were seeded in a 12-mm-diameter coverslip and transfected with 500 ng of pcDNA3×Flag-enRep-M9lS, pCMVtag4C-enRep-M9lS, pcDNA3×Flag-Odegus4, or pEGFP-Myosin9. At 48 h later, the coverslips were fixed with 3.7% formaldehyde–phosphate-buffered saline. After permeabilization, the covers were incubated with mouse anti-Flag M2 (1:100) or mouse anti-GFP B2 (Santa Cruz Biotechnology) (1:100). For secondary antibodies, anti-mouse Alexa Fluor 488 (1:500) was used. Samples were mounted in Prolong Diamond Fade with 4′,6-diamidino-2-phenylindole (DAPI), and fluorescent images were acquired at ×40 magnification with a DFC3000G Leica charge-coupled-device (CCD) camera mounted on a Leica DMIL microscope running Leica LASX software. Digital images were processed with Photoshop CS3 (Adobe).
ACKNOWLEDGMENTS
This work was supported by Fondo Nacional de Desarrollo Científico y Tecnológico grant FONDECYT1180705 to G.A. I.V.-H. was supported by AAP2018-1 from Universidad Andres Bello. R.J.G. was funded by the Medical Research Council of the United Kingdom (MC_UU_12014/12).
REFERENCES
- 1.Katzourakis A, Gifford RJ. 2010. Endogenous viral elements in animal genomes. PLoS Genet 6:e1001191. doi: 10.1371/journal.pgen.1001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Parseval N, Heidmann T. 2005. Human endogenous retroviruses: from infectious elements to human genes. Cytogenet Genome Res 110:318–332. doi: 10.1159/000084964. [DOI] [PubMed] [Google Scholar]
- 3.Holmes EC. 2011. The evolution of endogenous viral elements. Cell Host Microbe 10:368–377. doi: 10.1016/j.chom.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horie M, Honda T, Suzuki Y, Kobayashi Y, Daito T, Oshida T, Ikuta K, Jern P, Gojobori T, Coffin JM, Tomonaga K. 2010. Endogenous non-retroviral RNA virus elements in mammalian genomes. Nature 463:84–87. doi: 10.1038/nature08695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimizu A, Nakatani Y, Nakamura T, Jinno-Oue A, Ishikawa O, Boeke JD, Takeuchi Y, Hoshino H. 2014. Characterisation of cytoplasmic DNA complementary to non-retroviral RNA viruses in human cells. Sci Rep 4:5074. doi: 10.1038/srep05074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feschotte C, Gilbert C. 2012. Endogenous viruses: insights into viral evolution and impact on host biology. Nat Rev Genet 13:283–296. doi: 10.1038/nrg3199. [DOI] [PubMed] [Google Scholar]
- 7.Dewannieux M, Heidmann T. 2013. Endogenous retroviruses: acquisition, amplification and taming of genome invaders. Curr Opin Virol 3:646–656. doi: 10.1016/j.coviro.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Frank JA, Feschotte C. 2017. Co-option of endogenous viral sequences for host cell function. Curr Opin Virol 25:81–89. doi: 10.1016/j.coviro.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gautam P, Yu T, Loh YH. 2017. Regulation of ERVs in pluripotent stem cells and reprogramming. Curr Opin Genet Dev 46:194–201. doi: 10.1016/j.gde.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Horie M, Tomonaga K. 6 April 2018, posting date Paleovirology of bornaviruses: what can be learned from molecular fossils of bornaviruses. Virus Res doi: 10.1016/j.virusres.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Fujino K, Horie M, Honda T, Merriman DK, Tomonaga K. 2014. Inhibition of Borna disease virus replication by an endogenous bornavirus-like element in the ground squirrel genome. Proc Natl Acad Sci U S A 111:13175–13180. doi: 10.1073/pnas.1407046111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horie M. 2017. The biological significance of bornavirus-derived genes in mammals. Curr Opin Virol 25:1–6. doi: 10.1016/j.coviro.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Sofuku K, Parrish NF, Honda T, Tomonaga K. 2015. Transcription profiling demonstrates epigenetic control of non-retroviral RNA virus-derived elements in the human genome. Cell Rep 12:1548–1554. doi: 10.1016/j.celrep.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Belyi VA, Levine AJ, Skalka AM. 2010. Sequences from ancestral single-stranded DNA viruses in vertebrate genomes: the parvoviridae and circoviridae are more than 40 to 50 million years old. J Virol 84:12458–12462. doi: 10.1128/JVI.01789-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Fu Y, Xie J, Cheng J, Ghabrial SA, Li G, Peng Y, Yi X, Jiang D. 2011. Widespread endogenization of densoviruses and parvoviruses in animal and human genomes. J Virol 85:9863–9876. doi: 10.1128/JVI.00828-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pénzes JJ, de Souza WM, Agbandje-McKenna M, Gifford RJ. 2019. An ancient lineage of highly divergent parvoviruses infects both vertebrate and invertebrate hosts. bioRxiv doi: 10.1101/571109:571109. [DOI] [PMC free article] [PubMed]
- 17.Cotmore SF, Agbandje-McKenna M, Chiorini JA, Mukha DV, Pintel DJ, Qiu J, Soderlund-Venermo M, Tattersall P, Tijssen P, Gatherer D, Davison AJ. 2014. The family Parvoviridae. Arch Virol 159:1239–1247. doi: 10.1007/s00705-013-1914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berns KI. 1990. Parvovirus replication. Microbiol Rev 54:316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arriagada G, Gifford RJ. 2014. Parvovirus-derived endogenous viral elements in two South American rodent genomes. J Virol 88:12158–12162. doi: 10.1128/JVI.01173-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi Y, Shimazu T, Murata K, Itou T, Suzuki Y. 2019. An endogenous adeno-associated virus element in elephants. Virus Res 262:10–14. doi: 10.1016/j.virusres.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Valencia-Herrera I, Cena-Ahumada E, Faunes F, Ibarra-Karmy R, Gifford RJ, Arriagada G. 2019. Molecular properties and evolutionary origins of a parvovirus-derived myosin fusion gene in guinea pigs. bioRxiv 10.1101/572735. [DOI] [PMC free article] [PubMed]
- 22.O'Leary NA, Wright MW, Brister JR, Ciufo S, Haddad D, McVeigh R, Rajput B, Robbertse B, Smith-White B, Ako-Adjei D, Astashyn A, Badretdin A, Bao Y, Blinkova O, Brover V, Chetvernin V, Choi J, Cox E, Ermolaeva O, Farrell CM, Goldfarb T, Gupta T, Haft D, Hatcher E, Hlavina W, Joardar VS, Kodali VK, Li W, Maglott D, Masterson P, McGarvey KM, Murphy MR, O'Neill K, Pujar S, Rangwala SH, Rausch D, Riddick LD, Schoch C, Shkeda A, Storz SS, Sun H, Thibaud-Nissen F, Tolstoy I, Tully RE, Vatsan AR, Wallin C, Webb D, Wu W, Landrum MJ, Kimchi A, et al. 2016. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res 44:D733–D745. doi: 10.1093/nar/gkv1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunnum JL, Salazar-Bravo J. 2010. Molecular systematics, taxonomy and biogeography of the genus Cavia (Rodentia: Caviidae). J Zool Syst Evol Res 48:376–388. doi: 10.1111/j.1439-0469.2009.00561.x. [DOI] [Google Scholar]
- 24.Opazo JC. 2005. A molecular timescale for caviomorph rodents (Mammalia, Hystricognathi). Mol Phylogenet Evol 37:932–937. doi: 10.1016/j.ympev.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Fabre PH, Hautier L, Dimitrov D, Douzery EJ. 2012. A glimpse on the pattern of rodent diversification: a phylogenetic approach. BMC Evol Biol 12:88. doi: 10.1186/1471-2148-12-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nuesch JP, Lachmann S, Corbau R, Rommelaere J. 2003. Regulation of minute virus of mice NS1 replicative functions by atypical PKClambda in vivo. J Virol 77:433–442. doi: 10.1128/jvi.77.1.433-442.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horie M, Tomonaga K. 2011. Non-retroviral fossils in vertebrate genomes. Viruses 3:1836–1848. doi: 10.3390/v3101836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu H, Dennis T, Hughes J, Gifford RJ. 2018. Database-integrated genome screening (DIGS): exploring genomes heuristically using sequence similarity search tools and a relational database. bioRxiv doi: 10.1101/246835:246835. [DOI]
- 29.Jacobelli J, Bennett FC, Pandurangi P, Tooley AJ, Krummel MF. 2009. Myosin-IIA and ICAM-1 regulate the interchange between two distinct modes of T cell migration. J Immunol 182:2041–2050. doi: 10.4049/jimmunol.0803267. [DOI] [PubMed] [Google Scholar]