Graphical abstract
Keywords: Organophosphate pesticides, Pyrethroids, Total diet study, LC-MS/MS, GC-MS/MS
Highlights
-
•
Pesticides were detected in 46% of representative samples of foods prepared as consumed.
-
•
Organophosphate pesticides and pyrethroids represent 83% of occurrences.
-
•
Chlorpyrifos was detected in 21% of samples.
-
•
Highest pesticide concentration (chlorpyrifos) detected in smoked fish from Mali.
Abstract
In the framework of the first regional Total Diet Study in Sub-Saharan Africa, 3696 foodstuffs, commonly consumed in Benin, Cameroon, Mali and Nigeria were purchased, prepared as consumed and pooled into 308 composite samples. Those core foods were tested for up to 470 pesticides residues by liquid and gas chromatography coupled with tandem mass spectrometry.
39 pesticides were detected with 294 total occurrences, including 47.3% organophosphate pesticides and 35.7% pyrethroids. More specifically, 6 substances represented 75.5% of all 3 organophosphates and 3 pyrethroids: chlorpyrifos (22.4%) cypermethrin (18.0%) dichlorvos (13.6%), lambda cyhalothrin (8.2%), permethrin (7.5%) and profenofos (5.8%).
One pesticide or more was detected in 45.8% of samples.
Strikingly, several pesticides were quantified in 2 composite samples of smoked fish from Mali: chlorpyrifos (5236–18 084 μg/kg), profenofos (30–182 μg/kg), cypermethrin (22–250 μg/kg), cyfluthrin (16–117 μg/kg), lambda cyhalothrin (9–17 μg/kg) and permethrin (3–6 μg/kg).
1. Introduction
The “pesticide” terminology describes a variety of substances, including among others insecticides, herbicides, fungicides and growth regulators (WHO, 2010). Loss of agricultural commodities, curtailed by pesticide utilization, needs to be balanced with negative health effects. Acute toxicity, accidentally (Pouokam, Lemnyuy-Album, Ndikontar, & Sidatt, 2017) or through self-poisoning mainly, can lead to nausea, cough, skin and eye irritation and respiratory failure, tachycardia, ulceration of the lips, tongue, pharynx and larynx and an estimated 300,000 deaths per year (Eddleston, 2015, Pearson et al., 2017). In this study, we aimed to generate mean contamination data, adequately reflecting the chronic exposure to pesticides from a typical diet, in specific areas. Consequences of chronic exposure to pesticides may include metabolic disorders, such as diabetes (Montgomery et al., 2008, Starling et al., 2014), reproductive disruption (Merviel et al., 2017, Ueker et al., 2016), genotoxicity (Eastmond & Balakrishnan, 2010) carcinogenesis (Engel et al., 2017, Uyemura et al., 2017), neurological (Muñoz-Quezada et al., 2016, Schmidt et al., 2017, Shelton et al., 2014, Wagner-Schuman et al., 2015) and sensory disorders (Sturza et al., 2016).
One way of assessing the dietary exposure of populations to food chemicals, such as pesticide residues, is the Total Diet Study (TDS) approach (EFSA, 2011). Two specific aspects characterize a TDS: (1) the representativeness of the sampling and (2) the preparation of the samples “as consumed”.
WHO and FAO endorse the TDS methodology (EFSA, 2011) as a pertinent public health risk assessment tool.
A regional Sub-Saharan Africa TDS (SSA-TDS), which generated the data presented in this paper, was implemented by FAO in Benin, Cameroon, Mali and Nigeria between 2014 and 2018, together with the four national food safety authorities, in close collaboration with Centre Pasteur of Cameroon (CPC) and WHO (FAO, 2014, Ingenbleek et al., 2019, Ingenbleek et al., 2019). The purpose of this project was to assess the contamination levels of the food of 8 African population groups. The dietary exposure of those population groups will then be compared with existing health-based guidance values or end points. The study methodology including the derivation of food consumption data from national household budget surveys was described elsewhere (Ingenbleek et al., 2017).
In this paper, we are presenting the occurrence of pesticides in composite samples, each resulting from the pooling of 12 subsamples. These composites are representative of the food consumption habits of 3 population groups living in coastal areas (Duala, the Littoral of Benin and Lagos) and 5 population groups in non-coastal areas (Bamako, the Borgou region of Benin, Kano, the North of Cameroon and Sikasso). Although our analytical plan included organochlorine pesticides, we will not discuss their occurrence in this paper, but in another article (in preparation) with other persistent organic pollutants (POPs).
2. Experimental
2.1. Sample selection and preparation of foods as consumed
Food consumption data were derived from household budget surveys validated by National Statistics Authorities, from Benin, Cameroon, Mali and Nigeria and gathering a total of 72,979 households. Core foods of each study centre were selected on the basis of the relative importance of mean food consumption by weight (Ingenbleek et al., 2017), so as to cover at least 90% of the mean total diet in grams per adult male equivalent per day (g/AME/d).
Each core food was sampled through available representation criteria, such as market share or the origins of the food, using 12 subsamples of equal size, prepared as consumed and pooled into composites fit for laboratory tests. Subsamples were collected and prepared individually according to recipe books (Gautier and Mallet, 2006, Madubike, 2013, Nya-Njike, 1998, Vinakpon-Gbaguidi, 2003), using inert kitchen utensils. These references are considered as representative of the diet of the study populations and were therefore selected by the national competent authorities. These recipe books allow the identification of the processes used in the preparation of the foods, especially cooking time and temperature. The actual recipes were however not prepared, as each composite sample only contained one food type or ingredient. The inedible parts were removed at the preparation stage, as a typical consumer would do. In order to avoid contamination, distilled water was used to prepare food as consumed, instead of tap water, which is also part of the sampling. The quantity of water added during the cooking process of each of the 12 subsamples was measured by weighing the food at each stage of the preparation process.
Two seasons were captured for 5 main food groups, which cover staple food and most of the mean total diet by weight (cereals, tubers, legumes, vegetables and fruits):
-
•
the rainy season in October-November 2017;
-
•
the dry season or harmattan in February 2018.
Other food groups were collected during the rainy season only (nuts and seeds, dairy, oils, beverages and miscellaneous).
Tap water was also tested for pesticide residues, with 16 composite samples of 12 representative subsamples by study centre for both the rainy and the dry season.
Samples were frozen and shipped by air in coolers with dry ice within a timeframe never exceeding 24 h from the kitchen laboratory (Benin, Cameroon, Mali and Nigeria) to the testing laboratory (France).
2.2. Chemicals and reagents
Acetone (pestinorm) and petroleum ether (40–60 °C) were obtained from VWR, acetonitrile (for LCMS grade), ethyl ether (pestipur), heptane (≥99%), hexane (pestipur), methanol (for HPLC grade), toluene (for analysis ISO ACS reag) and anhydrous sodium sulphate from Carlo Erba, dichloromethane (pestipur) from Biosolve, n-dodecane (For synthesis) from Merck and ammonium acetate from Fisher Chemical (France). Salts mixture 1 (4 g of anhydrous magnesium sulphate, 1 g of NaCl, 1 g ± 0.1 g of trisodium citrate dihydrate and 0.5 g of disodiumhydrogencitrates hydrate), mixture 2 (150 mg of Primary Secondary Amines (PSA) and 900 mg of anhydrous magnesium sulfate), mixture 3 (150 mg of PSA, 150 mg C18, 900 mg of anhydrous magnesium sulfate) and mixture 4 (150 mg PSA, 45 mg graphitized black carbon, 900 mg magnesium anhydrous sulfate) were purchased from Macherey Nagel (Chromabond Mix I, III, VI et V). Analytical standards (purity from 81.2% to 99.5%) of pesticides and metabolites were purchased from Dr. Ehrenstorfer GmbH (Germany) and Sigma-Aldrich. PTFE filter (Chromofil – 0.45 μm, for ethephon) was from Macherey Nagel, SPE C18 cartridge (12 ml/2 g; Sep-Pak®) from Waters (France) and Florisil cartridge (6 ml/1 g; Chromabond®) from Macherey Nagel.
Stock solutions of individual pesticide standards (1 mg/ml) were prepared by weighing in 20 ml of toluene, methanol, or acetonitrile according to their solubility and stability. Intermediate stock standard mixtures of pesticide were prepared in toluene (for GC mixture) and in methanol or acetonitrile (for LC mixture) and were stored at −20 °C. Working standard solutions were prepared by dilutions of the intermediate stock standard solutions in ethanol and stored at 4 °C.
2.3. Analytical methods
Three analytical methods were used in this study.
2.3.1 Multiresidue method QuEChERS for food of plant origin
The method used (Quick Easy Cheap Effective Rugged Safe, QuEChERS) was based on the standard method NF EN 15662 (AFNOR, 2009). 470 pesticides were analysed, including 122 compounds on GC and 348 on LC in this method.
The sample was blended to obtain a homogeneous sample, and 5 g (±1%) for water-poor products or 10 g (±1%) for water-rich products were weighed in a 50 ml screw-cap centrifuge tube and extracted with 10 ml of acetonitrile containing 100 μl of the internal standards (IS: HCH gamma D6, Chlorpyrifos-methyl D6, Diazinon D10 and PCB 170 for GC and Bentazone D6, Carbofuran D3, Isoproturon D6, Terbuthylazine D5, Propiconazole D5 for LC) at 2.5 μg/ml. After salts (mixture 1) addition, the mixture was shaken intensively and centrifuged for 20 min at 3000 rpm and kept at −15 °C for phase separation. 6 ml of the organic phase was taken for the clean-up with different bulk sorbents (the mixture 2, 3 or 4) depending on the type of matrix and MgSO4 anhydrous to remove the residual water. Extracts were shaken by vortex and centrifuged for 15 min at 3000 rpm and kept at −15 °C. 1 ml of supernatant was transferred into a vial for LC-MS/MS analysis. To get better specificity and sensitivity for LC-MS/MS analysis, 348 pesticides were divided into two groups according to their properties. So, 2 injections were made on the LC-MS/MS: one for methanol mobile phases (331 compounds) and another for acetonitrile mobile phases (17 compounds shown in Section 2.4.3). Another 1 ml of supernatant was transferred into a vial in the presence of 25 μl of n-dodecane and evaporated to dryness at 35 °C under nitrogen steam. The residue was dissolved in 225 μl of a solvent mixture (heptane/acetone; 90/10; v/v). The final extract was then transferred into the conical vial for GC–MS/MS analysis after ultrasonic shock.
2.3.1. The ANSES POPs 10 and POPs 11 methods for food of animal origin
The POPs 10 method (ANSES, PBM Pest LSA-INS-0165, Version 04, 2015) for analysis of organochlorines and the POPs 11 method (ANSES, PBM Pest LSA-INS-0166, Version 05, 2015) for analysis of organophosphorus are official methods recognized by the Ministry of Agriculture for the official control of pesticides in animal commodities as part of surveillance plans. In this study, 70 of the pesticides were analysed with these methods.
2.3.1.1. Extraction for the ANSES POPs 10 and POPs 11 methods
First, the fat in sample was extracted, and its percentage (MG) calculated and then the pesticides were analysed in the extracted fat.
The sample was blended to obtain a homogeneous sample, and 10 g mixed with 25 g of sodium sulphate and 25 g of Fontainebleau sand in a mortar to obtain a dry and brittle product. The extraction column was sealed with glass wool and a 2-cm thick layer of anhydrous sodium sulphate, and the mixture obtained previously was then poured. The column was then eluted with 3 × 50 ml of solvent mixture (hexane/acetone; 2/1; v/v), and the mortar thoroughly rinsed. The eluate was collected in a zymark tube surmounted by a funnel containing anhydrous sodium sulphate, and the funnel rinsed with 10 ml of the solvent mixture, which was collected into the same tube. The solvent mixture was then evaporated under nitrogen to about 5 ml at 35 °C, the fat extract transferred to a tared tube, and the zymark tube rinsed with 3 × 1 ml of hexane, which was collected into the tared tube. The fat extract was evaporated under nitrogen at about 35 °C, and the amount of fat weighed at constant weight.
2.3.1.2. Cryogenic extraction of pesticides for the POPs 10 and POPs 11 methods
A 0.5 g aliquot of the fat obtained was taken in a tube of 12 ml. 100 μl of the IS-1 (HCH gamma D6, Chlorpyriphos methyl D6 and Cypermethrin D6 at 0.2 μg/ml) for POPs 10 or the IS-2 (Diazinon D10 and Propiconazole D5 at 2.5 μg/kg) for POPs 11 and then 3 ml of solvent mixture (acetonitrile/dichloromethane; 75/25; v/v) were added. The tube was vortexed, and centrifuged for 20 min at 3000 rpm and kept at −15 °C. The supernatant was then transferred to another tared tube. A second cryogenic centrifugation was made with the same solvent mixture. Both extracts were combined and evaporated to dryness under a stream of nitrogen (set at 35 °C).
2.3.1.3. Purification on SPE columns for the POPs 10 and POPs 11 methods
Two types of cartridges (C18 and Florisil) were used for POPs 10 and only one (C18) for POPs 11.
2.3.1.3.1. Purification on a cartridge C18 (POPs 10)
The cartridge was conditioned with 5 ml of petroleum ether, 5 ml of acetone and then 2 × 5 ml of methanol. The cryogenic extract was dissolved into 1 ml of acetonitrile and deposited on the C18 cartridge, and the cartridge eluted with 10 ml of acetonitrile by rinsing the sample tube. The eluate was evaporated to dryness under a stream of nitrogen (set at 35 °C) in the presence of 100 μl of n-dodecane. 900 μl of hexane was added and shaken for 1 min, by vortex, then ultrasonically for 5 min, for the next purification step on the Florisil cartridge.
2.3.1.3.2. Purification on a cartridge of Florisil (POPs 10)
The cartridge was conditioned with 10 ml of hexane. The extract obtained after C18 was deposited on the Florisil cartridge. The cartridge was eluted by rinsing the sample tube with 2 ml then 10 ml of solvent mixture (petroleum ether/ethyl ether; 98/2; v/v), then 12 ml of another solvent mixture (petroleum ether/ethyl ether; 85/15, v/v). The eluate was evaporated to dryness under a stream of nitrogen (set at 35 °C) in the presence of 100 μl of n-dodecane. 900 μl of hexane was added and shaken for 1 min, by vortex, then sonicated for 5 min, and transferred into an injection vial suitable for GC.
2.3.1.3.3. Purification on a cartridge C18 (POPs 11)
The cartridge was conditioned with 5 ml of methanol, 5 ml of acetone and then 5 ml of solvent mixture (acetonitrile/dichloromethane; 95/5; v/v). The cryogenic extract was dissolved into 1 ml of the solvent mixture and deposited on the C18 cartridge, and the cartridge eluted with 10 ml of methanol by rinsing the sample tube. The eluate was evaporated to dryness under a stream of nitrogen (set at 35 °C), and the residue dissolved in 1 ml of ethanol. 500 μl of the solution was taken into a vial of 1 ml containing 50 μl of n-dodecane, and evaporated to dryness under a stream of nitrogen (set at 35 °C). The residue was then dissolved with 450 μl of hexane for GC-MS/MS analysis. Another half of the ethanol solution was evaporated to dryness under a stream of nitrogen (set at 35 °C), and the residue dissolved with 500 μl of solvent mixture (methanol/H2O; 50/50; v/v) for LC-MS/MS analysis.
2.4. GC-MS/MS and LC-MS/MS measurements
2.4.1. GC-MS/MS for multiresidue QuEChERS
A trace GC Ultra (Thermo Scientific) equipped with a split/splitless injector and a Quantum XLS Triple Quadrupole was used. A CP SIL 8CB column (Agilent) of 60 m × 0.25 mm ID × 0.25 μm was used for chromatographic separation, with helium (99.999%) as carrier gas (1.5 ml/min.). The mass spectrometer was operated in electron impact (EI) positive ionization mode, and data were acquired using selected reaction monitoring (SRM). Argon (1.5 mTorr) was used as collision gas, and the source temperature was set at 250 °C. The injection volume was 1 μl in splitless mode.
2.4.2. GC-MS/MS for POPs 10 and POPs 11
A trace GC Ultra (Thermo Scientific) equipped with a programmed temperature vaporizer (PTV) injector and a Quantum XLS Triple Quadrupole was used. A CP SIL 8CB column (Agilent) of 60 m × 0.25 mm ID × 0.25 μm was used for chromatographic separation, with helium (99.999%) as carrier gas (1.5 ml/min). The mass spectrometer was operated in electron impact (EI) positive ionization mode, and data were acquired using selected reaction monitoring (SRM). Argon (1.5 mTorr) was used as the collision gas, and the source temperature was set at 280 °C. The injection volume was 2 μl in PTV mode.
2.4.3. LC-MS/MS for multiresidue QuEChERS
A Shimadzu LC (NEXERA X2) system coupled to a triple-quadrupole mass spectrometer was used (8060, Shimadzu, Kyoto, Japan). Chromatographic separation was carried out at 40 °C using an Accucore C18 150 mm × 2.1 mm × 2.6 μm column (Thermo Electron, France) with a pre-column (Accucore C18 2.1 mm × 2.6 μm). The methanol mobile phases were methanol + 2 mM ammonium acetate + 0.002% formic acid + 5 ml H2O and H2O + 2 mM ammonium acetate + 0.002% formic acid. The acetonitrile mobile phases were acetonitrile + 0.05% formic acid and H2O + 0.05% formic acid. Injection volume was 5 μl, and the flow at 0.3 ml/min. ESI (electrospray ionization) was operated in a positive/negative polarity switching mode and data were acquired in multiple reaction monitoring mode (MRM). Interface voltage was at 1500 V, interface temperature, at 350 °C and CID Gas at 230 kPa.
2.4.4. LC-MS/MS for organophosphorus (POPs 11)
A LC (Dionex Ultimate 3000) system coupled to a triple-quadrupole mass spectrometer was used (TSQ Quantiva, Thermo Scientific, France). Chromatographic separation was carried out at 40 °C using an Accucore C18 150 mm × 2.1 mm × 2.6 μm column (Thermo Electron, France) with a pre-column (Accucore C18 2.1 mm × 2.6 μm). The mobile phases were methanol + 0.5 mM ammonium acetate and H2O + 0.5 mM ammonium acetate. Injection volume was 5 μl, and the flow at 0.3 ml/min. ESI was operated in a positive/negative polarity switching mode and data were acquired in MRM. The mass spectrometer parameters were: spray voltage of positive ion of 4000 V and negative of 2500 V; sheath gas of 40 (arbitrage); aux gas of 15 (arb); ion transfer tube temperature of 300 °C and vaporizer temperature of 300 °C.
2.5. Analytical performances
In this study, all applied methods of analysis have been accredited by COFRAC (Comité Français d'Accréditation, France). The QuEChERS method has been used since 2010, the POPs 10 method since 2011, the POPs 11 method since 2014.
The means recoveries of these methods were between 70% and 120% and their precision (RSD, Relative Standard Deviation %) was less than 20% for at least the accredited analytes. Therefore, it meets the requirements of the EU international standards (SANTE 31181, 2017).
The limits of quantification (LQ) for the QuEChERS method were different depending on the compounds, and were between 1 μg/kg (for 10 priority compounds: chlorpyrifos, cypermethrin, dichlorvos, dimethoate, endosulfan, lambda cyhalothrin, omethoate, permethrin, profenofos and trichlorfon) and 10 μg/kg (for others). The LQ for POPs 10 and 11, depending on the compounds and matrices considered, was between 1 and 8 μg/kg.
2.6. Internal quality controls
The quality of the analysis is ensured by analyzing internal reference material as well as blank control for each analytical series. The accuracy of the method is ensured by regular participation in inter laboratory tests such as EUPT (EU-Proficiency Tests), BIPEA (Interprofessional Bureau of Analytical Studies) and FAPAS (Proficiency testing from Fera).
In each batch of analyses, matrix-matched calibration with at least 4 levels was performed by using an internal standard method for quantification to reduce systematic errors and matrix effects; the deviations of the measured signal from their values estimated with linear regression (residuals) was controlled (±20%); a reagent blank and a matrix blank were checked for contamination (<LD) and a checkpoint (QC) supplemented close to LQ was conducted for performance verification (individual recovery from 60% to 140% and means one from 70% to 120%).
3. Results
3.1. Occurrence of pesticides
No pesticide residue was detected > LD in any of the 16 tap water composite samples.
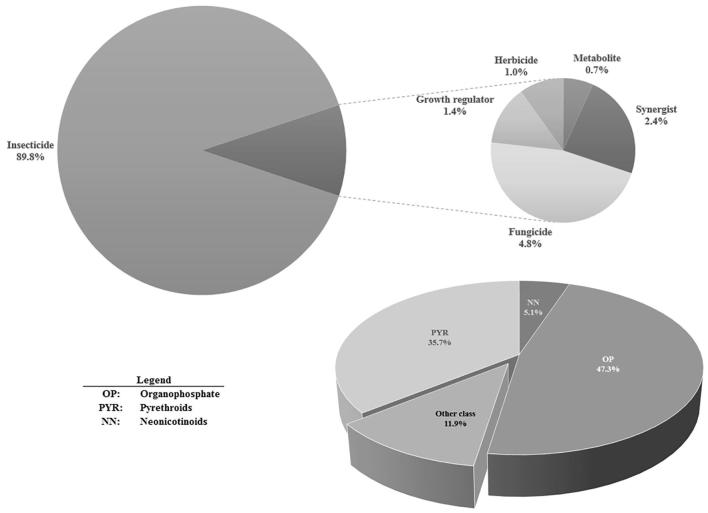
The list and proportion of detected pesticide residues with concentration > LD is presented in Table 1. Fig. 1 shows that 89.8% of pesticide occurrences concern insecticides and 4.8% are fungicides.
Table 1.
Occurrence of detected pesticides.
| Pesticide | Chemical class | Main use | Occurrences (n) |
|---|---|---|---|
| Chlorpyrifos | OP | INS | 66 |
| Cypermethrin | PYR | INS | 53 |
| Dichlorvos | OP | INS | 40 |
| Lambda Cyhalothrin | PYR | INS | 24 |
| Permetrhin | PYR | INS | 22 |
| Profenofos | OP | INS | 17 |
| Acetamiprid | NN | INS | 11 |
| Piperonyl butoxide | Other class | SYN | 7 |
| Pirimiphos methyl | OP | INS | 6 |
| Chlormequat | Other class | GR | 4 |
| Imidacloprid | NN | INS | 4 |
| Deltamethrin | PYR | INS | 3 |
| Dimethoate | OP | INS | 3 |
| Indoxacarb | Other class | INS | 3 |
| Omethoate | OP | INS | 3 |
| Chlorantraniliprole | Other class | INS | 2 |
| Cyfluthrin | PYR | INS | 2 |
| Fenpropimorph | Other class | FUN | 2 |
| Imazalil | Other class | FUN | 2 |
| 2,4-D | Other class | HER | 1 |
| 3,5-Dichloroaniline | Other class | ME | 1 |
| Acrinathrin | PYR | INS | 1 |
| Atrazine | Other class | HER | 1 |
| Azoxystrobin | Other class | FUN | 1 |
| Boscalid | Other class | FUN | 1 |
| Carbendazim | Other class | FUN | 1 |
| Chlorpropham | Other class | HER | 1 |
| Chlorpyrifos methyl | OP | INS | 1 |
| Malathion | OP | INS | 1 |
| Metalaxyl | Other class | FUN | 1 |
| Orthophenylphenol | Other class | FUN | 1 |
| Phthalimide | Other class | ME | 1 |
| Propamocarb | Other class | FUN | 1 |
| Propiconazole | Other class | FUN | 1 |
| Pyrimethanil | Other class | FUN | 1 |
| Thiabendazole | Other class | FUN | 1 |
| Triazophos | OP | INS | 1 |
| Trichlorfon | OP | INS | 1 |
| Tricyclazole | Other class | FUN | 1 |
| TOTAL | 294 | ||
Legend: FUN, Fungicide; GR, Growth regulator; INS, Insecticide; HER, Herbicide; ME, Metabolite; NN, Neonicotinoid; OP, Organophosphate; PYR, Pyrethroid; SYN, Synergist.
Fig. 1.
Proportions of pesticide occurrences by main use and by chemical class.
Organophoshates (47.3% of detected compounds) and pyrethroids (35.7%) represented the majority of occurrences, while neonicotinoids (acetamiprid and imidacloprid) represented 5.1% of all occurrences.
Other pesticides (21 analytes) represented 35 incidences or 11.9% of total occurrence.
3.2. Co-occurrence of pesticides
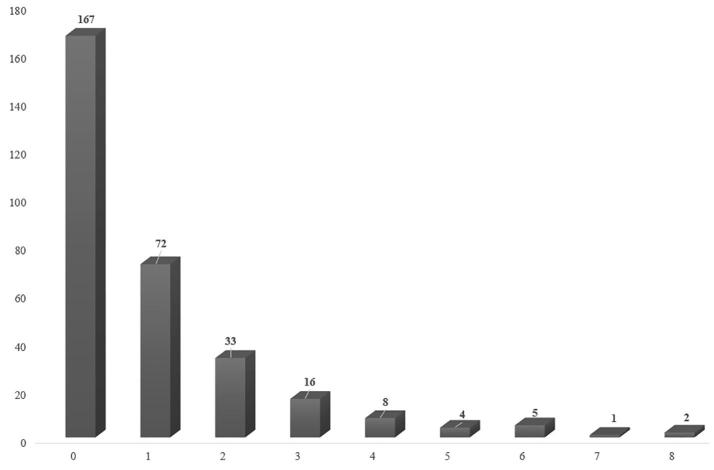
Fig. 2 shows the co-occurrence of pesticide in the SSA-TDS composite samples. Of 308 composite food samples, no pesticide residue was detected in 167 samples (54.2%). Whereas one pesticide only was detected in 72 samples (23.4%), 69 samples contained more than one and up to 8 pesticides (22.4%) and 36 samples contained 3 pesticides or more (11.7%).
Fig. 2.
Number of co-occurrences in SSA-TDS composite samples.
3.3. Contamination by most prevalent pesticides
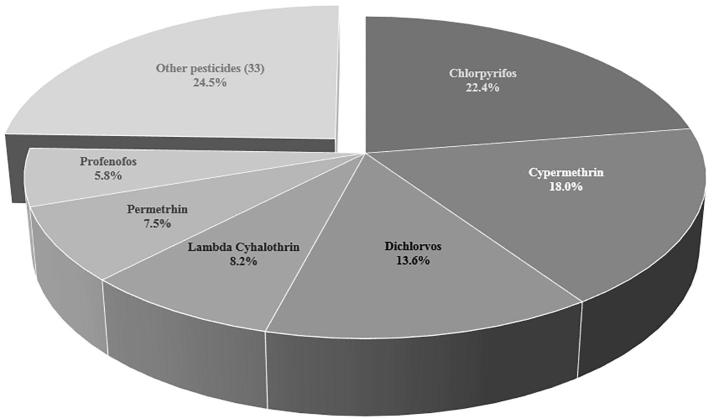
The sum of 6 most frequently detected substances chlorpyrifos (22.4%), cypermethrin (18.0%), dichlorvos (13.6%), permethrin (7.5%), lambda cyhalothrin (7.5%) and profenofos (5.8%) covered 75.5% of 294 occurrences (Fig. 3). Like 88.1% of pesticide occurrences in SSA-TDS samples these 6 analytes are either organophosphates, or pyrethroids.
Fig. 3.
Proportion of occurences of 6 most frequently detected pesticides in SSA-TDS composite samples.
Table 2 and Table 3 present the occurrence of these 6 pesticides, by food group and by study centre respectively.
Table 2.
detection rate (>LD), mean and maximum concentration (µg/kg wet weight) of most frequently detected pesticides by food group.
| ORGANOPHOSPHATES |
PYRETHROIDS |
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chlorpyrifos |
Dichlorvos |
Profenofos |
Cypermethrin |
Lambda cyhalothrin |
Permethrin |
|||||||||||||||||||||
| Samples (n) | >LD (%) | Concentration |
>LD (%) | Concentration |
>LD (%) | Concentration |
>LD (%) | Concentration |
>LD (%) | Concentration |
>LD (%) | Concentration |
||||||||||||||
| FOOD GROUP | Mean |
Max |
Mean |
Max |
Mean |
Max |
Mean |
Max |
Mean |
Max |
Mean |
Max |
||||||||||||||
| LB | UB | Matrix | LB | UB | Matrix | LB | UB | Matrix | LB | UB | Matrix | LB | UB | Matrix | LB | UB | Matrix | |||||||||
| CEREALS | 58 | 10 | 1.4 | 1.6 | 56.3 | 21 | 1.1 | 1.3 | 13.5 | 0 | 0.0 | 0.3 | ND | 7 | 1.6 | 1.9 | 65.1 | 3 | 0.0 | 0.3 | 1.378 | 0 | 0.0 | 0.3 | ND | |
| Rice | Bread | – | Pasta | Bread | – | |||||||||||||||||||||
| TUBERS | 50 | 12 | 0.4 | 0.6 | 7.9 | 20 | 2.0 | 2.2 | 18.6 | 0 | 0.0 | 0.3 | ND | 8 | 0.2 | 0.5 | 5.0 | 0 | 0.0 | 0.3 | ND | 6 | 1.2 | 1.5 | 49.1 | |
| Cassava dry | Cassava dry | – | Cassava dry | – | Cassava dry | |||||||||||||||||||||
| LEGUMES | 28 | 32 | 1.1 | 1.3 | 10.8 | 32 | 5.1 | 5.4 | 61.0 | 11 | 0.0 | 0.4 | NQ | 11 | 0.9 | 1.1 | 13.8 | 7 | 0.7 | 1.0 | 12.1 | 25 | 20.2 | 20.5 | 475.4 | |
| Peanut | Beans | Beans | Peas | Beans | Beans | |||||||||||||||||||||
| VEGETABLES | 56 | 27 | 3.6 | 3.8 | 91.2 | 7 | 0.5 | 0.8 | 10.0 | 13 | 10.2 | 10.6 | 555.0 | 34 | 16.8 | 17.0 | 257.8 | 29 | 1.9 | 2.2 | 41.1 | 4 | 0.1 | 0.4 | 6.4 | |
| Tomato | Tomato | Leafy vegetables | Tomato | Leafy vegetables | Leafy vegetables | |||||||||||||||||||||
| FRUITS | 36 | 8 | 0.0 | 0.3 | NQ | 11 | 0.3 | 0.6 | 5.6 | 6 | 0.1 | 0.4 | 3.2 | 19 | 0.7 | 0.9 | 8.9 | 3 | 0.0 | 0.3 | NQ | 3 | 0.0 | 0.3 | NQ | |
| Citrus | Citrus | Plantain | Citrus | Watermelon | Citrus | |||||||||||||||||||||
| NUTS/SEEDS | 4 | 75 | 2.5 | 2.6 | 5.2 | 0 | 0.0 | 0.3 | ND | 0 | 0.0 | 0.3 | ND | 25 | 0.4 | 0.6 | 1.6 | 0 | 0.0 | 0.3 | ND | 25 | 2.7 | 2.9 | 10.7 | |
| Sesame | – | – | Sesame | – | Sesame | |||||||||||||||||||||
| MEAT | 7 | 71 | 0.4 | 0.6 | 1.1 | 0 | 0.0 | 0.3 | ND | 14 | 0.0 | 0.3 | NQ | 57 | 2.3 | 2.5 | 10.0 | 0 | 0.0 | 0.3 | ND | 29 | 0.1 | 0.3 | NQ | |
| Beef | – | Beef | Beef | – | Beef | |||||||||||||||||||||
| EGGS | 4 | 75 | 0.2 | 0.3 | NQ | 0 | 0.0 | 0.3 | ND | 25 | 0.1 | 0.3 | NQ | 25 | 0.3 | 0.5 | 1.4 | 0 | 0.0 | 0.3 | ND | 0 | 0.0 | 0.3 | ND | |
| Hen eggs | – | Hen egg | Hen egg | – | – | |||||||||||||||||||||
| FISH | 9 | 67 | 2,591 | 2,591 | 18,084 | 0 | 0.0 | 0.3 | ND | 22 | 23.5 | 23.8 | 181.5 | 44 | 30.4 | 30.7 | 250.0 | 33 | 3.4 | 3.6 | 16.8 | 33 | 2.6 | 2.8 | 14.7 | |
| Smoked fish | – | Smoked fish | Smoked fish | Smoked fish | Smoked fish | |||||||||||||||||||||
| DAIRY | 7 | 14 | 0.0 | 0.3 | NQ | 0 | 0.0 | 0.3 | ND | 0 | 0.0 | 0.3 | ND | 43 | 0.5 | 0.7 | 2.8 | 0 | 0.0 | 0.3 | ND | 0 | 0.0 | 0.3 | ND | |
| Milk powder | – | – | Milk powder | – | – | |||||||||||||||||||||
| OIL/FAT | 11 | 45 | 8.9 | 9.2 | 94.7 | 0 | 0.0 | 0.3 | ND | 0 | 0.0 | 0.3 | ND | 0 | 0.0 | 0.3 | ND | 0 | 0.0 | 0.3 | ND | 9 | 1.6 | 1.8 | 17.3 | |
| Peanut oil | – | – | – | – | Palm oil | |||||||||||||||||||||
| BEVERAGES | 20 | 0 | 0.0 | 0.3 | ND | 5 | 0.2 | 0.5 | 3.0 | 0 | 0.0 | 0.3 | ND | 0 | 0.0 | 0.3 | ND | 0 | 0.0 | 0.3 | ND | 0 | 0.0 | 0.3 | ND | |
| – | Beer | – | – | – | – | |||||||||||||||||||||
| MISCELLANEOUS | 18 | 22 | 0.2 | 0.5 | 2.1 | 0 | 0.0 | 0.3 | ND | 6 | 0.4 | 0.6 | 6.5 | 17 | 0.5 | 0.8 | 4.0 | 0 | 0.0 | 0.3 | ND | 11 | 0.2 | 0.4 | 2.5 | |
| Chili pepper | – | Chili pepper | Chili pepper | – | Chili pepper | |||||||||||||||||||||
| TOTAL | (n) | 308 | 66 | 40 | 17 | 53 | 24 | 22 | ||||||||||||||||||
| (%) | 21.4 | 13.0 | 5.5 | 17.2 | 7.8 | 7.1 | ||||||||||||||||||||
Legend: ND: Non detected; NQ: Non quantified.
Table 3.
detection rate (>LD), mean and maximum concentration (µg/kg wet weight) of most frequently detected pesticides by study centre.
|
ORGANOPHOSPHATES |
PYRETHROIDS |
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Chlorpyrifos |
Dichlorvos |
Profenofos |
Cypermethrin |
Lambda cyhalothrin |
Permethrin |
|||||||||||||||||||||
| Samples (n) | >LD (%) |
Concentration |
>LD (%) |
Concentration |
>LD (%) |
Concentration |
>LD (%) |
Concentration |
>LD (%) |
Concentration |
>LD (%) |
Concentration |
||||||||||||||
| Centre |
Mean |
Max |
Mean |
Max |
Mean |
Max |
Mean |
Max |
Mean |
Max |
Mean |
Max |
||||||||||||||
| LB | UB | Matrix | LB | UB | Matrix | LB | UB | Matrix | LB | UB | Matrix | LB | UB | Matrix | LB | UB | Matrix | |||||||||
| BENIN | Littoral | 33 | 12 | 1.3 | 1.6 | 56.3 | 3 | 0.0 | 0.3 | 1.0 | 0 | 0.0 | 0.3 | 0.3 | 15 | 1.6 | 1.8 | 65.1 | 3 | 0.0 | 0.3 | 1.1 | 0 | 0.0 | 0.3 | 0.3 |
| Rice | Cassava dry | – | Pasta | Maize | – | |||||||||||||||||||||
| Borgou | 28 | 7 | 0.0 | 0.3 | 1.0 | 0 | 0.0 | 0.3 | 0.3 | 4 | 0.0 | 0.3 | 1.0 | 11 | 0.3 | 0.5 | 5.6 | 4 | 0.2 | 0.5 | 8.0 | 11 | 2.3 | 2.5 | 67.4 | |
| Smoked fish | – | Beef | Beans | Beans | Beans | |||||||||||||||||||||
| CAMEROON | Duala | 50 | 24 | 2.5 | 2.7 | 91.2 | 8 | 0.6 | 0.8 | 18.3 | 4 | 0.0 | 0.3 | 1.0 | 12 | 11.6 | 11.8 | 257.8 | 6 | 0.7 | 1.0 | 28.4 | 8 | 0.3 | 0.6 | 12.3 |
| Tomato | Peanuts | Onion & garlic | Tomato | Tomato | Beans | |||||||||||||||||||||
| Garoua | 28 | 25 | 1.1 | 1.5 | 33.0 | 7 | 0.9 | 1.2 | 0.0 | 4 | 0.0 | 0.3 | 1.0 | 21 | 4.1 | 4.4 | 126.3 | 18 | 1.6 | 1.8 | 41.1 | 7 | 14.0 | 14.3 | 474.4 | |
| Leafy vegetables | Peanuts | Tomato | Leafy vegetables | Leafy vegetables | Beans | |||||||||||||||||||||
| MALI | Bamako | 54 | 17 | 548.2 | 548.5 | 18,084 | 0 | 0.0 | 0.3 | 0.3 | 9 | 14.0 | 14.3 | 555.0 | 7 | 7.8 | 8.1 | 250.0 | 13 | 0.4 | 0.6 | 8.6 | 9 | 1.0 | 1.3 | 49.1 |
| Smoked fish | – | Leafy vegetables | Smoked fish | Smoked fish | Cassava dry | |||||||||||||||||||||
| Sikasso | 32 | 16 | 276.1 | 276.4 | 5,236 | 0 | 0.0 | 0.3 | 0.3 | 16 | 2.0 | 2.3 | 30.0 | 9 | 2.1 | 2.4 | 34.2 | 9 | 0.9 | 1.2 | 16.8 | 9 | 0.3 | 0.6 | 3.3 | |
| Smoked fish | – | Smoked fish | Leafy vegetables | Smoked fish | Smoked fish | |||||||||||||||||||||
| NIGERIA | Lagos | 50 | 36 | 1.0 | 1.2 | 28.7 | 56 | 3.2 | 3.4 | 18.6 | 6 | 0.2 | 0.5 | 19.0 | 34 | 1.4 | 1.6 | 13.8 | 6 | 0.2 | 0.4 | 5.8 | 6 | 0.6 | 0.9 | 17.3 |
| Other vegetables | Cassava fresh | Chili pepper | Peas | Other vegetables | Palm oil | |||||||||||||||||||||
| Kano | 33 | 27 | 4.8 | 5.0 | 94.7 | 15 | 1.7 | 2.0 | 61.0 | 0 | 0.0 | 0.3 | 0.3 | 27 | 1.2 | 1.4 | 10.0 | 3 | 0.3 | 0.6 | 12.1 | 6 | 0.5 | 0.8 | 10.8 | |
| Peanut oil | Beans | – | Beef | Beans | Other nuts/seeds | |||||||||||||||||||||
| TOTAL | (n) | 308 | 66 | 40 | 17 | 53 | 24 | 22 | ||||||||||||||||||
| (%) | 21.4 | 13.0 | 5.5 | 17.2 | 7.8 | 7.1 | ||||||||||||||||||||
So as to take into account the measurement uncertainty due to censored data (non-detected analytes) the mean concentrations presented are lower bound and upper bound values (LB-UB), which means that we use as concentration:
-
•
Zero (LB) and the limit of detection (UB) for non-occurrence;
-
•
The limit of detection (LB) and limit of quantification (UB) for detected but non quantified data.
3.3.1. Chlorpyrifos
Out of 66 chlorpyrifos occurrences, 37 exceeded LQ (1 μg/kg). The highest chlorpyrifos concentrations (Table 3) were quantified in two smoked fish samples composite samples from Mali (Bamako: 18 084 mg/kg and Sikasso: 5236 μg/kg). Those high concentrations alone considerably lift the mean chlorpyrifos concentration in fish up, which thereby becomes the highest concentration of all food groups (Table 2). Ten samples contained chlorpyrifos levels above 10 μg/kg, including one composite of peanut oil from Kano: 95 μg/kg, two composites of tomato from Duala: 26 and 91 μg/kg, rice from Cotonou (Benin): 56 μg/kg, leafy vegetables from Garoua: 33 μg/kg, vegetables from Lagos: 29 μg/kg, bread from Duala: with 14 μg/kg and peanuts from Bamako: with 11 μg/kg.
3.3.2. Dichlorvos
Of 40 dichlorvos occurrences, 38 exceeded LQ (1 μg/kg) and 11 composites comprised concentrations above 10 μg/kg. Dichlorvos was often detected in legumes (32% > LD), cereals (21% > LD), and tubers (20% > LD), as shown in Table 2. Dichlorvos was most frequently detected in Nigerian foods (Lagos 56% and Kano 15%), followed by Cameroon (Duala: 8% and Garoua 7%). Consistently, the maximum dichlorvos concentration (61 μg/kg) was quantified in a bean sample from Kano (Nigeria). Dichlorvos was only detected once in Benin (cassava: 1.0 μg/kg) and was not detected at all in Malian foods (Table 2).
3.3.3. Profenofos
Profenofos was more frequently detected in foods from Mali (Bamako: 9% and Sikasso 16%) than in other study centres. Concentrations in excess of 10 μg/kg were only quantified in Mali in two composites of leafy vegetables (16–555 μg/kg) and two composites smoked fish (30–182 μg/kg) collected in two distinct centres. Profenofos was also quantified (>LQ) in Bamako, in tomato (2 μg/kg) and plantain (3 μg/kg). Apart from samples collected in Mali, only one composite sample from Lagos exceeded the analytical limit of quantification (chili pepper: 7 μg/kg).
3.3.4. Cypermetrin
The highest cypermethrin concentrations were quantified in two tomato composite samples from Duala (240–258 μg/kg) and in smoked fish from Bamako (250 μg/kg). Cypermethrin was quantified in leafy vegetables collected during the dry season in Cameroon (Duala: 235 μg/kg; Garoua: 126 μg/kg) but was not detected (LD = 0.3 μg/kg) in leafy vegetables collected during the wet season. The same observation applies to leafy vegetables in Mali, which contained cypermethrin during the dry season (2–34 μg/kg) but was not detected during the rainy season. Similarly, okro collected contained cypermethrin in Kano (4 μg/kg) and Garoua (3 μg/kg) during the dry season, but concentrations did not exceed LD in samples collected during the wet season.
3.3.5. Lambda cyhalothrin
Lambda cyhalothrin was detected 24 times including in 16 vegetable samples (29% > LD) and in smoked fish from 3 different study centres (Garoua: 5 μg/kg; Bamako: 9 μg/kg; Sikasso: 17 μg/kg). 8/12 tomato composites also contained lambda cyhalothrin (ND-28 μg/kg), as well as 3/8 of leafy vegetables (ND-41 μg/kg), in which the maximum concentration was quantified.
We also quantified lambda cyhalothrin in beans from Borgou (8 μg/kg) and from Kano (12 μg/kg), bread from Bamako (1 μg/kg) and maize from the littoral of Benin (1 μg/kg).
3.3.6. Permethrin
Several bean composites samples enclosed permethrin concentrations (7/16 or 44%) unlike other legumes (peanuts and peas: 0%), which results in a permethrin detection rate of 25% in legumes (Table 2). Beans from Cameroon (Garoua: 475 μg/kg), and Benin (Borgou: 67 μg/kg) contained the highest permethrin content. Permethrin was also quantified in beans from Bamako: 2 μg/kg; Duala: 12 μg/kg; Lagos: 2 μg/kg; Sikasso: 2 μg/kg. Permethrin concentrations in excess of 10 μg/kg were quantified in cassava dry from Bamako (49 μg/kg), palm oil from Lagos (17 μg/kg), sesame seeds from Kano (11 μg/kg) and smoked fish from Garoua (15 μg/kg). Smoked fish from Mali also contained permethrin (Bamako: 6 μg/kg; Sikasso: 3 μg/kg). Vegetables from Duala also enclosed permethrin (leafy vegetables: 6 μg/kg; onion and garlic: LB: 0.3; UB: 1.0 μg/kg), as well as pepper from Lagos (3 μg/kg). Traces of permethrin (LB: 0.3; UB: 1.0 μg/kg) were detected in two beef composites from Bamako and Borgou, in broth from Kano and citrus (oranges) from Bamako.
3.3.7. Seasonal variation
In order to compare the occurrences of the 6 most prevalent pesticides in food groups, which were both collected (1) during the wet season and (2) during the dry season (cereals, tubers, legumes, vegetables and fruits) we completed a t test. Both the total number of occurrence (p < 0.05) and the number of occurrences below the limit of quantification (LQ = 0.001 mg/kg), which we arbitrarily qualify of “traces”, enabled identification of a seasonal pattern (p < 0.01). Pesticides were more frequently detected in samples collected during the wet season. However, no particular detection or concentration pattern was determined with regard to quantified samples (>LQ).
3.4. Contamination by other pesticides
Acetamiprid was detected in 11 composites, with higher concentrations in leafy vegetables (94–241 μg/kg) from Garoua. Two bean composites (19–23 μg/kg) from Garoua, one composite of leafy vegetables from Sikasso (31 μg/kg) and two composites of tomato (Duala: 14 μg/kg; Lagos: 15 μg/kg) also exceeded 10 μg/kg.
Of 4 imidacloprid occurrences, only 1 composite (maize from Bamako: 35 μg/kg) contained concentration above 10 μg/kg.
Pirimiphos methyl was detected in wheat bread and pasta from Bamako, Duala and Cotonou (3–181 μg/kg).
Piperonyl butoxide, a synergist was quantified in the same bread samples collected in Bamako, Duala (35–45 μg/kg), and in pasta from Cotonou (17 μg/kg). One millet (Bamako composite) also contained LB: 3; UB: 10 μg/kg of piperonyl butoxide.
Interestingly, growth regulator chlormequat was also detected in bread from Bamako, Duala and Lagos (3–12 μg/kg).
Omethoate was quantified in Duala (tomato: 135 μg/kg), Lagos (peas: 8 μg/kg; other vegetables: 5 μg/kg). Dimethoate concentrations were also quantified (Duala tomato: 31 μg/kg; Lagos peas: 15 μg/kg; and Lagos other vegetables 46 μg/kg).
4. Discussion
Neither the food preparation process (Huan et al., 2015, Mahugija et al., 2017), nor the dilution factor due to the pooling of 12 subsamples decreased initial pesticide concentration of raw agricultural commodities to a level that could not be detected in 46% of our food samples. The occurrence of 294 incidences of 39 pesticides scattered in 141/308 samples was possible thanks to the combination of (1) a sensitive analytical method (LD: 0.3; LQ: 1.0 μg/kg) for 10 priority analytes and (2) the collection of 12 different subsamples of equal weight by composite. Low limit of detection also ensured low uncertainty of measurement due to limited contribution of censored data to the conservative upper bound scenario. Had the sensitivity of the method been lower, for example with LD = 5 μg/kg, like in a previous total diet study by Gimou, Charrondiere, Leblanc, and Pouillot (2008), and assuming comparable recoveries, we would have obtained 121 incidences, including 85 incidences of chlorpyrifos, dichlorvos, profenofos, cypermethrin, lambda cyhalothrin and permethrin (70%).
In a recent study, (Lehmann, Turrero, Kolia, Konaté, & de Alencastro, 2017) was looking for 31 residues, with higher analytical limits than in the SSA-TDS, through the collection of raw agricultural commodities in Burkina Faso. This team detected 16 pesticides in vegetables and drinking water and concluded that vegetables they collected were 36% above Codex MRLs.
In this present study, it is impossible to conclude with regard to the conformity of pooled subsamples of food prepared as consumed against MRLs, which apply to commodities. It is however obvious that at least one of the 12 pooled subsamples contained pesticide, up to 12 times the amount quantified in the composite.
In the absence of Codex MRL, a food sample is non-conforming, regardless of the concentration.
In order to illustrate this, although chlorpyrifos is approved by the international standard, Codex Alimentarius (2018), did not publish a chlorpyrifos maximum residue limit (MRL) for commodities such as fish, tomato, peanut or peanut oil, from which these foods were prepared. Therefore, 15 composites all contained at least one subsample, which did not conform to Codex standard applicable to chlorpyrifos in commodities, from which they were prepared.
We would also like to emphasize that, of 39 detected pesticides, 11 (including 3 of the 6 most frequently detected residues in our analytical plan) are not currently approved by the EU (atrazine, carbendazim, cyfluthrin, dichlorvos, omethoate, permethrin, profenofos, triazofos, trichlorfon, tricyclazole). The European Commission retains more stringent regulation with regard to MRLs compared with Codex (European Commission, 2018).
Chlorpyrifos, only detected in fruits in the second French TDS, and in the NZ TDS (FSANZ, 2002, Nougadère et al., 2012), was detected in all food groups in the SSA-TDS, with the exception of beverages. The absence of concentrations > LD in beverages phenomenon may be due to degradation of residues in drinks (Radford, Panuwet, Hunter, Barr, & Ryan, 2018). In a study on market foods in Shaanxi Province, China (Wang, Wang, Zhang, Wang, & Guo, 2013) the detection rate of chlorpyrifos on individual raw agricultural commodities (1.75%) was over 12 fold lower than in SSA-TDS pooled samples (21.4%). We noted that (Wang et al., 2013) only detected chlorpyrifos in 5 Shaanxi Province raw vegetable samples (mean: 35 μg/kg; max: 129 μg/kg).
Strikingly, both the maximum chlorpyrifos concentration of all SSA-TDS composites (18 084 μg/kg) and the highest mean concentration (2591 μg/kg) applied to food group fish, due to the contribution of smoked fish from Mali. The identification of the origin of the presence of chlorpyrifos at such high levels in smoked fish (environmental contamination or catch/post catch bad practice) requires further investigations with regard to handling (capture techniques, processing, storage), and to the environment. Food group “oil and fat” contained, to a lesser amount chlorpyrifos concentration (9 μg/kg), mainly due to the contribution of one peanut oil sample from Kano (95 μg/kg).
Dichlorvos was mainly detected in Nigeria (33 of 40 occurrences) and was notably prevalent in legumes with 32% samples with concentration > LD (mean: 5; max: 61 μg/kg). In the second French TDS, dichlorvos was only detected in one fruit sample and was below LQ (10 μg/kg). In Shaanxi Province (Wang et al., 2013), dichlorvos was detected in 2 samples (mean: 1.6 µg/kg; max: 2.3 µg/kg), which is considerably lower than in composite samples from the SSA-TDS. Codex standard did not publish maximum residue limit (MRL) for dichlorvos in legumes or vegetables but was detected 16 times in food composites prepared from those commodities.
Profenofos was not tested in the French and NZ TDS and was not detected in Shaanxi either. Of all profenofos occurrences obtained in this present study, 58% occurred in Mali (10/17), including 4 quantified concentrations in leafy vegetables (16–555 µg/kg) and smoked fish (30–182 µg/kg). By comparison, Lehmann et al. detected profenofos in raw sorrel (median: 619 µg/kg; max: 2999 µg/kg) in Burkina Faso. There is no Codex MRL for profenofos in leafy vegetables, legumes, onion, plantain or fish, and yet profenofos was detected in foods prepared from these commodities with 14 occurrences.
Cypermethrin was detected in 34% of SSA-TDS vegetable samples (with concentrations mean: 17 µg/kg; max: 258 µg/kg). The tomato composite from Duala exceeded Codex MRL of 0.2 mg/kg. By contrast, cypermethrin was not detected > LD, neither in the second French TDS nor in the NZ TDS (FSANZ, 2002) but was detected (mean: 124 µg/kg; max: 631 µg/kg) in raw individual samples of sorrel from Burkina Faso.
Lambda cyhalothrin was detected in 29% of SSA TDS vegetable samples (mean: 2 µg/kg; max: 41 µg/kg) and only 2% > LD in vegetables from the second French TDS (mean: 12; max: 200 µg/kg). The difference in detection rate may be explained by different analytical limits of detection, as well as by different contamination patterns.
Permethrin was not detected in a Chinese survey (Wang et al., 2013), it was only detected (<LQ) in one vegetable sample from the French TDS (Nougadère et al., 2012) and was quantified in several vegetable samples, prepared as consumed from the New Zealand TDS (mean: 10 µg/kg; max: 140 µg/kg). In the SSA-TDS, permethrin was detected in 25% of legume samples (44% of bean composites), up to 475 µg/kg in beans, two of which exceeded the Code MRL for soy bean of 50 µg/kg (as no specific Codex MRL applicable to beans is recorded).
From a more general perspective, our survey of pesticide concentrations in African foods prepared as consumed, included the characterization of seasonal patterns, although the fact that traces of pesticide not in excess of 1 µg/kg are more frequently detected during the wet season is unlikely to matter with regard to consumer safety.
The completion of dietary exposure assessment and comparison of households’ actual intakes with acceptable daily intakes (manuscript in preparation) will be useful to include pesticide residues in the list of food safety priority concerns. We expect that the issue of chlorpyrifos found with exceptionally high levels in smoked fish in Mali foreshadows extensive health problems, which need to be tackled soonest.
5. Conclusion
To the best of our knowledge, a multi-centre total diet study is unprecedented in Africa. This systematic approach, covering 13 food groups and up to 470 analytes reveals the presence pesticides residues in foods as consumed, rather than in raw agricultural commodities. The concentrations we obtained therefore reflect a typical diet of 7291 households.
In order to conclude with regard to the occurrence of pesticides in 8 study centres:
-
•
The preparation of foods as consumed (e.g. washing and cooking) and the pooling of 12 samples (dilution effect) enabled characterization of significant and realistic contamination levels of food.
-
•
The TDS approach applied to this present study enabled capturing of seasonal variation of detection (frequent presence of pesticide traces detected in the rainy season).
-
•
The TDS methodology enabled identification of geographic patterns (dichlorvos frequently detected in Nigeria, high concentrations of chlorpyrifos in smoked fish from Mali).
-
•
6 most prevalent pesticides generated 75.5% of occurrences, which justifies scrutiny and examination of these analytes as top priority in terms of pesticide risk assessment.
Our next step is the completion of dietary exposure assessment of study populations to 6 most prevalent pesticides, taking into consideration food consumption data, at household level, for the populations of our 8 study centres. Although the exposure will be of great help to provide guidance to risk managers from Benin, Cameroon, Mali and Nigeria in setting the priority agenda, we would like to address our preliminary recommendations to risk managers and their technical and financial partners:
-
1.
Strengthen laboratory capacity to monitor most prevalent pesticides in commodities
-
2.
Implement a monitoring and surveillance plan with regard to concentrations of at least 6 of the most frequently detected pesticides
-
3.
Review practices in the smoked fish value chain in Mali
-
4.
Sensitize smoked fish value chain operators to the health consequences of high exposure to pesticides and to good practices
Additional recommendations will be available, once dietary exposure and risk characterization completed.
6. Disclaimer
The views expressed in this publication are those of the authors and do not necessarily reflect the views and policies of the Food and Agriculture Organization of the United Nations and the World Health Organization.
Declaration of Competing Interest
The authors declare that there is no conflict of interest.
Acknowledgments
Acknowledgements
We would like to remember the late Marie Madeleine Gimou, the initiator of this study.
The project was funded under grant STDF/PG/303 and the authors are thankful to Kenza le Mentec and Marlynne Hopper of the Standard and Trade Development Facility (STDF), the donor institution.
Many thanks also to FAO staff (Renata Clarke, Markus Lipp, Caroline Merten, Blaise Ouattara, Jean Kamanzi, Sekou Hebie and Alex Nyarko) who supported the total diet study at various stages of its submission and its implementation. The CPC management, as well as the various heads of national coordinating institutions of the other participating countries ABSSA, Benin; ANSSA, Mali and NAFDAC, Nigeria contributed to the success of this project. The scientific committee members, who provide guidance and validation of the methodology with their valuable experience of implementing total diet studies, are Katie Egan, Peter Fürst, Thierry.
Guérin, Adam Probert, Siswanto Siswanto and Christina Tlustos. We are extremely grateful for their support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2019.100034.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- AFNOR, NF EN 15662 January 2009 (2009). Aliments d’origine végétale - Méthode polyvalente de détermination des résidus des pesticides par CG-SM et SL/SM/SM avec extraction/partition avec de l’acétonitrile et nettoyage par SPE dispersés – Méthode QuEChERS.
- ANSES (Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail) PBM Pest LSA-INS-0165, Version 04, 2015 (2015). Dosage des pesticides organochlorés et des pyréthrinoïdes dans les denrées alimentaires d'origine animale par CG-DCE et/ou CG-SM/SM.
- ANSES PBM Pest LSA-INS-0166, Version 05 (2015). Dosage des pesticides polaires et apolaires dans les denrées alimentaires d'origine animale par CLSM/SM et CG-SM/SM.
- Codex Alimentarius (2018). Pesticide DATABASE. Available <http://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/pestres/pesticides/en/> (Accessed 26 June 2018).
- Eastmond D.A., Balakrishnan S. Genotoxicity of pesticides. In: Krieger R., editor. Hayes' Handbook of Pesticide Toxicology. 3rd ed. Academic Press; 2010. pp. 357–380. ISBN 9780123743671. [Google Scholar]
- Eddleston M. Pesticides. Medicine. 2015;44(3):193–196. ISSN 1357-3039. [Google Scholar]
- EFSA (European Food Safety Agency) (2011). Towards a harmonised Total Diet Study approach: a guidance document. EFSA Journal 9(11), 2450. European Food Safety Authority, Food and Agriculture Organization of the United Nations, World Health Organization.
- Engel L.S., Werder E., Satagopan J., Blair A., Hoppin J.A., Koutros…Beane Freeman L.E. Insecticide use and breast cancer risk among Farmers’ wives in the agricultural health study. Environmental Health Perspectives. 2017;125(9) doi: 10.1289/EHP1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission (2018). Pesticides database. Available <http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=homepage&language=EN>. (Accessed 26 June 2018).
- FAO (Food and Agriculture Organization of the United Nations) (2014). Total diet study as a tool to assess chemical contamination in foods. Application in Sub-Saharan Africa. Available: <http://www.fao.org/fileadmin/user_upload/agns/pdf/Highlights/SubAfricaHighlight-LR.pdf>. (Accessed 6 April 2018).
- FSANZ (2002). The 20th Australian total diet survey: a total diet survey of pesticide residues and contaminants. Canberra and Wellington: Food Standards Australia and New Zealand. Supplementary information. Part 3. Pesticides in food results. Available <http://www.foodstandards.gov.au/publications/Pages/20thaustraliantotaldietsurveyjanuary2003/20thaustraliantotaldietsurveyfullreport/Default.aspx> (Accessed 26 June 2018).
- Gautier L., Mallet J.F. Editions Herme; 2006. Le vrai goût du Mali. Collection Gastronomie. [Google Scholar]
- Gimou M.M., Charrondiere U.R., Leblanc J.C., Pouillot R. Dietary exposure to pesticide residues in Yaounde: The Cameroonian total diet study. Food Additives & Contaminants. 2008;25(4):458–471. doi: 10.1080/02652030701567475. [DOI] [PubMed] [Google Scholar]
- Huan Z., Xu Z., Jiang W., Chen Z., Luo J. Effect of Chinese traditional cooking on eight pesticides residue during cowpea processing. Food Chemistry. 2015;170:118–122. doi: 10.1016/j.foodchem.2014.08.052. [DOI] [PubMed] [Google Scholar]
- Ingenbleek L., Jazet E., Dzossa A.D., Adebayo S.B., Ogungbangbe J., Dansou S.…Coulibaly S. Methodology design of the regional Sub-Saharan Africa Total Diet Study in Benin, Cameroon, Mali and Nigeria. Food and Chemical Toxicology. 2017;109:155–169. doi: 10.1016/j.fct.2017.08.017. ISSN 0278-6915. [DOI] [PubMed] [Google Scholar]
- Ingenbleek L., Sulyok M., Adegboye A., Hossou S.E., Koné A.Z., Oyedele A.D.…Leblanc J.C. Regional Sub-Saharan Africa total diet study in Benin, Cameroon, Mali and Nigeria reveals the presence of 164 mycotoxins and other secondary metabolites in foods. Toxins (Basel). 2019;11(1):54. doi: 10.3390/toxins11010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingenbleek L., Veyrand B., Adegboye A., Hossou S.E., Koné A.Z., Oyedele A.D.…Leblanc J.C. Polycyclic aromatic hydrocarbons in foods from the first regional total diet study in Sub-Saharan Africa: Contamination profile and occurrence data. Food Control. 2019;103:133–144. ISSN 0956-7135. [Google Scholar]
- Lehmann E., Turrero N., Kolia M., Konaté Y., de Alencastro L.F. Dietary risk assessment of pesticides from vegetables and drinking water in gardening areas in Burkina Faso. Science of the Total Environment. 2017;601–602:1208–1216. doi: 10.1016/j.scitotenv.2017.05.285. [DOI] [PubMed] [Google Scholar]
- Madubike, F. (2013). All Nigerian recipes. Cookbook, Mass Market Paperback. ISBN-13: 978-8461617548.
- Mahugija J.A.M., Kayombo A., Peter R. Pesticide residues in raw and processed maize grains and flour from selected areas in Dar es Salaam and Ruvuma, Tanzania. Chemosphere. 2017;185:137–144. doi: 10.1016/j.chemosphere.2017.07.014. ISSN 0045-6535. [DOI] [PubMed] [Google Scholar]
- Merviel P., Cabry R., Chardon K., Haraux E., Scheffler F., Mansouri N.…Benkhalifa M. Impact of oocytes with CLCG on ICSI outcomes and their potential relation to pesticide exposure. Journal of Ovarian Research. 2017;10:42. doi: 10.1186/s13048-017-0335-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery M.P., Kame F., Saldana T.M., Alavanja M.C.R., Sandler D.P. Incident diabetes and pesticide exposure among licensed pesticide applicators: Agricultural health study 1993–2003. American Journal of Epidemiology. 2008;167(10):1235–1246. doi: 10.1093/aje/kwn028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Quezada M.T., Lucero B.A., Iglesias V.P., Muñoz M.P., Cornejo C.A., Achu E.…Villalobos M. Chronic exposure to organophosphate (OP) pesticides and neuropsychological functioning in farm workers: A review. International Journal of Occupational and Environmental Health. 2016;22(1):68–79. doi: 10.1080/10773525.2015.1123848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nougadère A., Sirot V., Kadar A., Fastier A., Truchot E., Vergnet C.…Leblanc J.C. Total diet study on pesticide residues in France: Levels in food as consumed and chronic dietary risk to consumers. Environment International. 2012;45:135–150. doi: 10.1016/j.envint.2012.02.001. ISSN 0160-4120. [DOI] [PubMed] [Google Scholar]
- Nya-Njike P. Editions l'Harmattan; 1998. L'Art Culinaire Camerounais. ISBN-13:978e2738457486. [Google Scholar]
- Pearson M., Metcalfe C., Jayamanne S., Gunnell D., Weerasinghe M., Pieris R.…Bandara P. Effectiveness of household lockable pesticide storage to reduce pesticide self-poisoning in rural Asia: A community-based, cluster-randomised controlled trial. Lancet (London, England) 2017;390(10105):1863–1872. doi: 10.1016/S0140-6736(17)31961-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouokam G.B., Lemnyuy-Album W., Ndikontar A.S., Sidatt M.E.H. A pilot study in Cameroon to understand safe uses of pesticides in agriculture, risk factors for Farmers’ exposure and management of accidental cases. Toxics. 2017;5(4):30. doi: 10.3390/toxics5040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford S.A., Panuwet P., Hunter R.E., Barr D.B., Ryan P.B. Degradation of organophosphorus and pyrethroid insecticides in beverages: Implications for risk assessment. Toxics. 2018;6(1):11. doi: 10.3390/toxics6010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANTE 11813 (2017). Analytical quality control and method validation procedures for pesticide residues analysis in food and feed.
- Schmidt R.J., Kogan V., Shelton J.F., Delwiche L., Hansen R.L., Ozonoff S.…Tancredi D.J. Combined prenatal pesticide exposure and folic acid intake in relation to autism spectrum disorder. Environmental Health Perspectives. 2017;125(9) doi: 10.1289/EHP604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton J.F., Geraghty E.M., Tancredi D.J., Delwiche L.D., Schmidt R.J., Ritz B.…Hertz-Picciotto I. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: The CHARGE study. Environmental Health Perspectives. 2014;122(10):1103–1109. doi: 10.1289/ehp.1307044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starling A.P., Umbach D.M., Kamel F., Long S., Sandler D.P., Hoppin J.A. Pesticide use and incident diabetes among wives of farmers in the Agricultural Health Study. Occupational and Environmental Medicine. 2014;71(9):629–635. doi: 10.1136/oemed-2013-101659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturza J., Silver M.K., Xu L., Li M., Mai X., Xia Y.…Meeker J. Prenatal exposure to multiple pesticides is associated with auditory brainstem response at 9 months in a cohort study of Chinese infants. Environment International. 2016;92–93:478–485. doi: 10.1016/j.envint.2016.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueker M.E., Silva V.M., Moi G.P., Pignati W.A., Mattos I.E., Silva A.M.C. Parenteral exposure to pesticides and occurence of congenital malformations: Hospital-based case–control study. BMC Pediatrics. 2016;16:125. doi: 10.1186/s12887-016-0667-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyemura S.A., Stopper H., Martin F.L., Kannen V. A perspective discussion on rising pesticide levels and colon cancer burden in Brazil. Frontiers in Public Health. 2017;5:273. doi: 10.3389/fpubh.2017.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinakpon-Gbaguidi V. Nouvelle Presse Publications; 2003. Saveurs du Benin et de la Sous-region. ISBN-13: 978-9991940908. [Google Scholar]
- Wagner-Schuman M., Richardson J.R., Auinger P., Braun J.M., Lanphear B.P., Epstein J.N.…Froehlich T.E. Association of pyrethroid pesticide exposure with attention-deficit/hyperactivity disorder in a nationally representative sample of U.S. children. Environmental Health. 2015;14:44. doi: 10.1186/s12940-015-0030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Wang Z., Zhang Y., Wang J., Guo R. Pesticide residues in market foods in Shaanxi Province of China in 2010. Food Chemistry. 2013;138(2–3):2016–2025. doi: 10.1016/j.foodchem.2012.11.116. ISSN 0308-8146. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) (2010). The WHO recommended classification of pesticides by hazard and guidelines to classification: 2009. Available <http://www.who.int/ipcs/publications/pesticides_hazard_2009.pdf?ua=1> (Accessed 20 June 2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.