Highlights
-
•
Formation of disinfection byproducts (DBPs) by NaOCl and PAA washing was compared.
-
•
Wash water and lettuce were analyzed for 45 conventional and emerging DBPs.
-
•
PAA formed much less chlorinated DBPs than NaOCl in wash water and lettuce.
-
•
PAA formed slightly less aldehyde DBPs than NaOCl in wash water and lettuce.
-
•
The DBPs formation patterns varied in wash water compared to washed lettuce.
Keywords: Free chlorine, Peracetic acid, Disinfection byproducts (DBPs), Food sanitization, Produce washing, DBP formation potential, Emerging DBPs
Abstract
Sodium hypochlorite (NaOCl) and peracetic acid (PAA) are being used for sanitization in food processing, but their chemical behaviors regarding disinfection byproducts (DBPs) formation during washing processes are still largely unknown. This study compared these two sanitizers in simulated washing processes for fresh-cut lettuce. Different doses of sanitizers were applied, and the wash water and washed lettuce were extracted and analyzed for 45 conventional and emerging DBPs of concern. Overall, washing by PAA generated much less DBPs than washing by NaOCl in both wash water and lettuce. Interestingly, the formation potentials of different groups of DBPs varied considerably in wash water versus in washed lettuce. This study is among the first to compare the two sanitizers for that many DBPs in both produce and wash water. The comprehensive data will facilitate the development of safer produce sanitization processes, and guide further research on DBPs in food.
1. Introduction
Chlorine-containing disinfectants, such as sodium hypochlorite (NaOCl), have long been widely used and considered inexpensive and effective for cleaning and sanitization in food processing environments including wash water and food contact surfaces (FAO/WHO, 2008). One of the main concerns with using chlorine-based disinfectants is the reactivity of chlorine with organic matter to generate disinfection by-products (DBPs) with potential health hazards (Hrudey, 2009). At the same time, the reactions that lead to DBP formation consume disinfectants and lower their efficacy in inactivating pathogens, thereby increasing the chances of microbial contamination in the food products (Cardador and Gallego, 2012, Fan and Sokorai, 2015, Gomez-Lopez et al., 2014, Gomez-Lopez et al., 2013, Lopez-Galvez et al., 2010, Shen et al., 2016).
Peracetic acid (PAA, CH3C( O)OOH) is the peroxide of acetic acid, and it is commercially available as a quaternary equilibrium mixture containing PAA, hydrogen peroxide (H2O2), acetic acid and water. PAA can be produced from the reaction of acetic acid or acetic anhydride with H2O2, using sulfuric acid as a catalyzing agent (Luukkonen & Pehkonen, 2017). PAA is considered a highly effective disinfectant and has been used in different disinfection applications including food and beverage processing, as well as cooling tower water, wastewater and storm water treatment (Block, 2001, Kitis, 2004, Luukkonen and Pehkonen, 2017). Studies have shown that PAA has significant biocidal effects on bacteria, followed by viruses, bacterial spores and then protozoan cysts (Baldry, 1983, Baldry et al., 1995, Briancesco et al., 2005, Freese et al., 2002, Liberti and Notarnicola, 1999, Rudd and Hopkinson, 1989). PAA is considered to be advantageous over chlorine disinfectants because it does not promote formation of chlorinated DBPs (Monarca et al., 2002).
To date, the limited data reporting DBPs in food is mostly about trihalomethanes (THMs) and haloacetic acids (HAAs); however, other types of DBPs, including nitrogenous DBPs (N-DBPs), nitrosamines (NISAMs), other carbonaceous DBPs (C-DBPs) and aldehydes, may also be generated. THMs (chloroform, bromodichloromethane, chlorodibromomethane and bromoform, total at 80 μg/L) and HAAs (chloro-, bromo-, dichloro-, dibromo- and trichloroacetic acids, total at 60 μg/L) are currently regulated by the USEPA for the maximum contaminant levels (MCL) allowed in the drinking water, due to their cancer risks (Villanueva et al., 2007). THMs and HAAs, however, cannot fully account for the magnitude of increased risk of developing human cancer observed in epidemiological studies. The other emerging DBPs including haloacetonitriles, halonitromethanes, haloacetamides, and NISAMs possess higher genotoxicity and cytotoxicity, and likely are responsible for some of the additional health risk (Bull et al., 2011, Hebert et al., 2010, Muellner et al., 2007, Plewa et al., 2008, Plewa and Wagner, 2009, Wagner et al., 2012). Once formed, the stability of DBPs and transfer of DBPs between the processing water and food may vary depending on the chemical structure of DBPs, food surfaces, water chemistry, washing conditions and contact time. Most of these processes remain poorly understood, and require more research to better understand the reactions involved and influencing factors.
Even though PAA is known to have a lower tendency to form chlorinated DBPs than chlorine, the formation potential of DBPs still exists with PAA. An earlier study by Booth and Lester (1995) reported transformation of phenol to mono-chlorinated or mono-brominated phenols when phenol was treated by PAA in the presence of excess chloride or bromide ions. The authors proposed that PAA could oxidize bromide to hypobromous acid (HOBr), which reacted with phenol to brominated phenols. In contrast, the authors argued that oxidation of chloride by PAA to hypochlorous acid (HOCl) is more unlikely to occur and, instead, generation of free chlorine radicals that led to formation of chlorinated phenols was more likely (Booth & Lester, 1995). Furthermore, since H2O2 is always present in PAA solutions, the recent study by Shah, Liu, Salhi, Hofer, and von Gunten (2015) found that different H2O2 concentrations relative to the PAA concentration in the PAA solutions could lead to quite different DBP formation patterns. When the system was with [H2O2] < [PAA] and a high bromide concentration, most brominated THMs and HAAs were formed. In contrast, when the system was with [H2O2] > [PAA], much less brominated THMs and HAAs were formed, which was due to the ability of H2O2 to reduce HOCl/HOBr to chloride or bromide. Other research demonstrated that when PAA reacted with amino acids, phenols, and other aromatic substances in treated wastewater, about 10–30 μg/L of aldehydes were formed (Crathorne et al., 1991). In general, research on the formation of DBPs by PAA treatment is significantly limited thus far, and this is particularly so for PAA application in the food washing processes.
Given that information regarding potential DBPs in produce after sanitizer washing is still quite limited, the objective of this study was to obtain a better understanding of the formation potential and distribution of DBPs in the wash water and fresh-cut lettuce after washing by PAA versus by free chlorine sanitizers. This study not only compared the two sanitizers, but also evaluated a wide range of DBPs including conventional and emerging DBPs of concern, with a total of 45 target DBPs as listed in Table 1. The target DBPs included 4 THMs, 9 HAAs, 11 C-DBPs, 8 N-DBPs, 8 NISAMs, and 5 aldehydes. Herein, the “C-DBPs” included carbonaceous DBPs other than THMs and HAAs, and the “N-DBPs” included nitrogenous DBPs excluding NISAMs. This study is among the first to compare the two sanitizers in formation potential for that many DBPs in both produce and wash water. Procedures to simulate fresh-cut lettuce washing under produce processing conditions were conducted in the lab using three different concentrations of PAA (45 mg/L, 85 mg/L, and 100 mg/L) and comparing with NaOCl (100 mg/L as free chlorine). The wash water and washed fresh-cut lettuce were then extracted and analyzed for the target DBPs. The new knowledge gained by this study is important to develop better sanitizer washing methods for produce and protect human health from the risks of DBPs.
Table 1.
The 45 target DBPs and their abbreviations in this study.
| Group | Target compound | Abbreviation | Group | Target compound | Abbreviation |
|---|---|---|---|---|---|
| THMs | Chloroform | CF | HAAs | Chloroacetic acid | MCAA |
| Bromoform | BF | Bromoacetic acid | MBAA | ||
| Bromodichloromethane | BDCM | Dichloroacetic acid | DCAA | ||
| Dibromochloromethane | DBCM | Trichloroacetic acid | TCAA | ||
| N-DBPsa | Bromonitromethane | BNM | Bromochloroacetic acid | BCAA | |
| Trichloronitromethane | TCNM | Dibromoacetic acid | DBAA | ||
| Dichloroacetonitrile | DCAN | Bromodichloroacetic acid | BDCAA | ||
| Trichloroacetonitrile | TCAN | Chlorodibromoacetic acid | CDBAA | ||
| Bromochloroacetonitrile | BCAN | Tribromoacetic acid | TBAA | ||
| Dibromoacetonitrile | DBAN | Aldehydes | Formaldehyde | FD | |
| 2,2-dichloroacetamide | DCAAm | Acetaldehyde | AD | ||
| Trichloroacetamide | TCAAm | Benzaldehyde | BD | ||
| C-DBPsb | Chloral hydrate | CH | Glyoxal | GX | |
| 1,1-dichloro-2-propanone | DCPN | Methyl glyoxal | MG | ||
| 1,1,1-trichloro-2-propanone | TCPN | NISAMs | N-nitrosodimethylamine | NDMA | |
| 1,1,3,3-tetrachloro-2-propanone | TetraCPN | N-nitrosomethylethylamine | NMEA | ||
| 1,1,1,3,3-pentachloro-2-propanone | PentaCPN | N-nitrosodiethylamine | NDEA | ||
| 1,2-dibromo-3-chloropropane | DBCPN | N-nitrosodi-n-propylamine | NDPA | ||
| Trichloroethylene | TriCE | N-nitrosomorpholine | NMOR | ||
| 1,1,1-trichloroethane | TCE | N-nitrosopyrollidine | NPYR | ||
| Tetrachloroethylene | TCEL | N-nitrosopiperidine | NPIP | ||
| Carbon tetrachloride | CTC | N-nitrosodi-n-butylamine | NDBA | ||
| 1,2-dibromoethane | DBE |
Nitrogenous DBPs excluding NISAMs.
Carbonaceous DBPs excluding THMs and HAAs.
2. Materials and methods
2.1. Standards and reagents
The sanitizers used in the study included a sodium hypochlorite solution containing 5% free chlorine (Acros Organics, Morris Plains, NJ, USA) and a peracetic acid mixed solution containing 15% PAA and 10% hydrogen peroxide (H2O2) (VigorOx® 15F & V, PeroxyChem, Philadelphia, PA, USA). Table 1 lists the full and abbreviated names of all the DBPs analyzed in this study. Sources of standards of DBPs and deuterated N-nitrosodimethylamine (NDMA-d6) were similar to those described in Lee, Huang, and Zhu (2018). Formaldehyde solution (37 wt% in H2O), acetaldehyde, glyoxal solution (40 wt% in H2O), methylglyoxal solution (40% in H2O), and benzaldehyde were obtained from Sigma-Aldrich. Other chemicals including o-(2,3,4,5,6-pentafluorobenzyl)hydroxylamine hydrochloride, decafluorobiphenyl, 2-bromobutanoic acid, 2′,4′,5′-trifluoroacetophenone, methyl tert-butyl ether (MTBE), methanol (MeOH), dichloromethane (DCM) and n-hexane (HEX) were obtained from Sigma-Aldrich (St. Louis, MO, USA). 4-Bromofluorobenzene, ammonium sulfate and anhydrate sodium sulfate were purchased from Fisher Scientific (Pittsburgh, PA, USA). The ENVIRO-CLEAN 521 cartridges were purchased from United Chemical Technologies (Levittown, PA, USA). The purity of all chemicals and standards was greater than 95%, and all solvents were of HPLC grade. High purity deionized (DI) water was generated by a Milli-Q water purification system (EMD Millipore, Burlington, MA, USA).
2.2. Simulated washing process
Iceberg lettuce was purchased from a local supermarket and transported back to the lab immediately. The lettuce was stored in a 5 °C refrigerator for up to three days before the experiments. The simulated washing processes were performed by using a salad spinner with occasional agitation. The first two layers of lettuce leaves were discarded, and the rest of the leaves were cut into about 2 cm × 2 cm pieces. Then, 200 g chopped lettuce were soaked in 2 L of tap water (∼20 °C) for 10 min as the pre-wash step, followed by collecting the after-pre-wash water and rinsed lettuce, respectively. In the next step, the rinsed lettuce was submerged in a 2-L, 5 °C and pH 6 (controlled by 1.0 M phosphate buffer) sanitizer solution (with either PAA or NaOCl) for 15 min. The sanitizer solutions were prepared by diluting the sanitizer stock solutions with DI water and added with phosphate buffer to set the desired pH. The sanitizer solutions contained 45, 85, and 100 mg/L of PAA or 100 mg/L of NaOCl. At the end of 15 min, the sanitizer solution was drained and collected. Finally, the lettuce was soaked in 2 L of tap water again for 5 min as the post-wash step, and then both the after-post-wash water and washed lettuce were collected by draining. All the collected wash water samples, including after pre-wash, after sanitizer wash, and after post-wash, were dosed with excess ammonium chloride to quench the residual chlorine. The quenched wash water samples were stored in a 5 °C refrigerator. The washed fresh-cut lettuce was collected in the Whirl-Pak™ sterile bags (Fisher Scientific, Pittsburgh, PA, USA) and stored in a −4 °C freezer to effectively retard any residual oxidant reactions. All the samples were extracted within three days for DBPs analysis.
2.3. Analytical methods
The initial and residual chlorine concentrations in the wash water were analyzed by the N,N-diethyl-p-phenylenediamine (DPD) colorimetric method (Method 4500-CI G) (APHA-AWWA-WEF, 1998), and the initial and residual PAA concentrations were analyzed by the PAA Vacu-vials® Kit with a SAM photometer (CHEMetrics). The pH of the water samples was measured using an Accumet® Research AR20 pH meter (Fisher Scientific).
2.3.1. Analysis of DBPs in wash water
The analysis of THMs, HAA, C-DBP, N-DBPs and NISAMs in lettuce wash water followed the methods reported by Lee et al. (2018). For THMs, C-DBPs and N-DBPs, the method utilized liquid–liquid extraction (LLE) followed by gas chromatography electron capture detection (GC/ECD). For HAAs, LLE and derivatization followed by GC/ECD were used. A HP 6890 GC/ECD was used to analyze THMs, C-DBPs, N-DBPs and HAAs using a DB-5MS column (30 m × 0.25 mm × 1 μm), with confirmatory analysis done by gas chromatography mass spectrometry (GC/MS) (Agilent 6890N GC/5975 MSD with NIST 2.0 MS database) equipped with the same type of DB-5MS column. For NISAMs, solid phase extraction (SPE) followed by large volume injection GC/MS were used. A HP 6890 GC/Agilent 5973 MSD equipped with a large volume injector and a Supelco Equity-1701 column (30 m × 0.25 mm × 0.25 μm) was used to analyze NISAMs. Details of instrumental conditions were reported in Lee et al. (2018). Overall, the methods yielded excellent linearity, with detection limits (MDLs) in the range of 0.11–0.21 μg/L for THMs, 0.14–0.31 μg/L for HAAs, 0.14–0.58 μg/L for C-DBPs, 0.07–0.20 μg/L for N-DBPs, and 10.4–19.4 ng/L for NISAMs in lettuce wash water. The average recoveries of these DBPs in lettuce wash water ranged from 42 to 147%.
The analysis of five aldehydes in lettuce wash water followed the EPA Method 556 (USEPA, 1998). Briefly, LLE was performed on a 20-mL volume of wash water, which was adjusted to pH 4 with potassium hydrogen phthalate (KHP) before adding the surrogate standard 2′,4′,5′-trifluoroacetophenone. To the sample, 1 mL of 15 mg/mL freshly prepared o-(2,3,4,5,6-pentafluorobenzyl)-hydroxylamine (PFBHA) was added, and then the sample was placed in a top-sealed heating block at 45 ± 2 °C for 1 h and 45 min to derivatize the aldehydes to oxime derivatives. Afterwards, to the derivatized sample, 50 μL of concentrated sulfuric acid was added, followed by extraction with 4 mL of hexane containing 400 μg/L internal standard (1,2-dibromopropane). After shaking for 3 min, the hexane layer (top layer) was transferred to a vial containing 3 mL 0.2 N sulfuric acid by using a Pasteur pipet. After shaking for another 30 s, the solvent layer was transferred to an autosampler vial for analysis.
The extracted aldehydes were analyzed by a HP 6890 GC/ECD with a DB-5MS column (30 m × 0.25 mm × 1 μm). The GC/ECD conditions were as follows: injection volume was 2 μL with the inlet temperature at 220 °C; the GC oven program was set at 50 °C for 1 min, then increased to 220 °C by 4 °C/min, and then increased to 250 °C by 20 °C/min and held for 10 min; and the ECD detector was set at 300 °C with the make-up gas (N2) at 30 mL/min. Overall, the analysis of aldehydes showed good linearity (R2 = 0.9982–0.9999, 1–40 μg/L), MDLs in the range of 0.13–1.88 μg/L, and average recoveries in the range of 152–241% in lettuce wash water (Table S1). The higher recoveries of aldehydes were expected because the presence of aldehydes in both air and water is common, and the reaction of natural organic matter with oxidative agents used in disinfection can lead to the formation of aldehydes (Ivancev-Tumbas and Dalmacija, 2001, Krasner et al., 1989). Furthermore, the amino acids in lettuce can be significant aldehydes precursors (Mitch & Schreiber, 2008), contributing to a higher recovery in extraction. Although the recoveries of all five aldehydes in lettuce wash water exceeded 100%, the overall relative standard deviation (RSD) was less than 7%, indicating that recovery was consistent and reliable (Table S1).
2.3.2. Analysis of DBPs in lettuce
To measure DBPs in lettuce, the analysis of THMs, HAA, C-DBP and N-DBPs followed the methods by Lee, Huang, and Zhu (submitted). The cut lettuce samples (frozen, 10 g) were homogenized using a food processor, mixed with 10 mL DI water and subjected to LLE with specific cleanup and derivatization (for HAAs) steps, followed by GC/ECD analysis. The DBPs were also analyzed and confirmed by GC/MS. The specific extraction and instrumental conditions are detailed in Lee et al. (submitted). The MDLs of these DBPs in lettuce were in the range of 0.67–3.81 ng/g for THMs, 0.81–9.48 ng/g for HAAs, 0.34–6.21 ng/g for C-DBPs, 0.28–8.62 ng/g for N-DBPs, with average recoveries of 40–124%.
To analyze NISAMs in lettuce, 20 g of cut lettuce (frozen) were homogenized and then mixed with 10 mL DI water. The resulted mixture was subjected to LLE by adding 5 mL DCM and 10 min intensive shaking. The DCM layer was collected after centrifugation, transferred to a glass tube, and further concentrated to 0.5 mL using an automated concentrator (CentriVap™ Benchtop Vacuum Concentrators, Labconco™). The final extract volume was adjusted to 1.0 mL by DCM with the internal standard (NDMA-d6) and analyzed by large volume injection GC/MS. The MDLs for individual NISAMs were estimated based on the signal-to-noise ratio (S/N) = 3/1 to be around 0.1 ng/g (for NDMA, NDEA, NDPA, NMOR and NPYR) and 0.25 ng/g (for NMEA). However, little or no signal (S/N < 2) of NPIP and NDBA could be found in the spike samples after LLE, suggesting strong adsorption or decomposition of these compounds in the lettuce matrix. Thus, the results of NPIP and NDBA in lettuce could not be obtained. The average recoveries were 26% for NDMA, 52% for NMEA, 81% for NDEA, 82% for NDPA, 52% for NMOR and 57% for NPYR. Even though the recovery spanned a broad range, the overall RSD was<8%, indicating that recovery was consistent and reliable (Table S2).
To analyze aldehydes in lettuce, 10 g of cut lettuce (frozen) were homogenized and then mixed with 10 mL of DI water. Then, LLE was applied to the homogenized lettuce mixture following the same procedures described above for lettuce wash water. After the derivatization step, extraction by hexane was applied by intensive shaking for 5 min and sonication for 5 min. Then, the hexane layer (top layer) was transferred and mixed with 3 mL 0.2 N sulfuric acid. The final solvent layer was then transferred to an autosampler vial for analysis. Overall, the MDLs of aldehydes in lettuce were in the range of 0.10–9.03 ng/g, with average recoveries of 52–249% (RSD% < 11%) (Table S1). Due to the background levels of aldehydes already present in the lettuce and matrix effects, the average recovery varied widely among the aldehydes. Even so, the recovery of each aldehyde was consistent based on the small RSD (<11%), confirming the reliability of the method.
It is noted that due to the matrix effects, some of the target compounds have higher or lower recoveries. In order to consider the matrix effects for the reported values, all the data including wash water and lettuce were corrected by the corresponding analytical recovery, which was obtained by always conducting spiking samples along with the experiments. The target DBPs were spiked into DI water versus lettuce wash water (without sanitizer), and into homogenized unwashed lettuce versus washed lettuce (without sanitizer) to assess matrix effect and obtain recovery.
3. Results and discussion
3.1. Background concentrations of DBPs in tap water
Because the pre-wash and post-wash were performed by tap water, it was important to determine the background levels of DBPs in the tap water. Fig. S1 in the Supplementary Information shows the total concentrations of different groups of DBPs detected in the tap water. The data represented two tap water samples collected from the same faucet in the lab on two different dates when the lettuce washing was conducted. The total THMs varied from 34 to 73 μg/L and more than 80% of the concentration was contributed by CF. The total HAAs was 34 ± 4.9 μg/L, followed by 15 ± 0.1 μg/L of total C-DBPs and 6.8 ± 0.6 μg/L of total N-DBPs. All five target aldehydes were found in the tap water at 18 μg/L in total concentration, with FD (7.9 μg/L) and AD (5.4 μg/L) at the highest concentrations. The total NISAMs was at 37 ng/L and the amount only contributed by NPIP (37 ng/L). NDBA and NDEA were detected but at concentrations too low to be quantified. After 10 min of pre-wash of lettuce, the concentrations of THMs and NISAMs increased only slightly, possibly due to release from lettuce, and/or increased organic load and their reactions with residual chlorine in the tap water to form DBPs. Overall, the concentrations of DBPs in the pre-wash tap water were strongly related to the background levels existing in the tap water.
3.2. Formation of DBPs in lettuce wash water by washing with PAA and NaOCl
After tap water pre-wash, the rinsed fresh-cut lettuce was washed by either PAA or NaOCl solutions (5 °C, pH 6) for 15 min. It was found that the lettuce exhibited a PAA demand of 33, 31 and 17 ± 3 mg/L when 100, 85 and 45 mg/L of PAA were used, respectively, and a chlorine demand of 45.7 ± 4.0 mg/L when 100 mg/L of NaOCl was used, in the experimental setting of 200 g of lettuce in 2 L of wash water with sanitizers. The results in Fig. 1 demonstrated that HAAs, N-DBPs and C-DBPs formed significantly using 100 mg/L NaOCl as the sanitizer, reaching concentrations of 222 ± 2.3, 145 ± 6.2 and 49 ± 2.1 μg/L, respectively. These concentrations were 10, 21, and 4 times, respectively, of those found in the tap water pre-wash. In contrast, the formation of HAAs (<2.2 μg/L), N-DBPs (<0.5 μg/L) and C-DBPs (<MDL) was quite low or below MDLs when washing with PAA at either 45, 85 or 100 mg/L doses. The concentrations of specific DBPs detected after tap water pre-wash and different sanitizer washings are detailed in Table S3. Considering the levels of these DBPs in the tap water and that the tap water pre-washed lettuce might possibly contain some of the DBPs residues from the tap water, the formation potential of HAAs, N-DBPs and C-DBPs by PAA could be considered negligible.
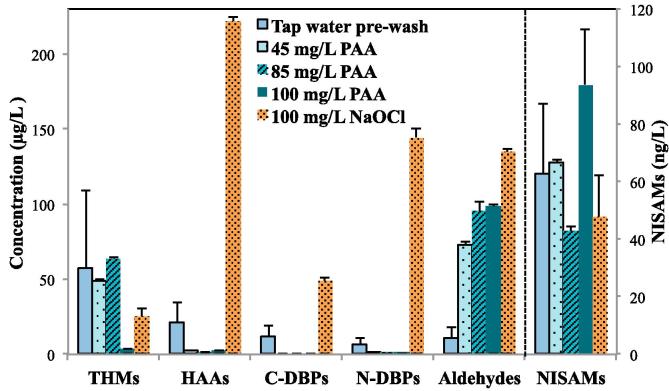
Fig. 1.
Concentrations of DBPs in the wash water from tap water pre-wash of fresh-cut lettuce (n = 4), and then washing by 45, 85, 100 mg/L PAA or 100 mg/L NaOCl sanitizer solution (n = 2 for each experiment). The tap water pre-wash was at 20 °C for 10 min and the sanitizer wash was at 5 °C for 15 min. The sanitizer solution was controlled at pH 6.0 using phosphate buffer.
Compared to the tap water pre-wash, washing by PAA or NaOCl both formed considerably more aldehydes, at 73–99 μg/L by PAA and 134 ± 2.6 μg/L by NaOCl for the total aldehyde concentrations (Fig. 1 and Table S3). The total aldehyde concentration increased generally when the PAA concentration was increased; these results agreed with the previous study which suggested that the formation of aldehydes was directly proportional to PAA dosage (Dell'Erba, Falsanisi, Liberti, Notarnicola, & Santoroa, 2007). Washing by 100 mg/L NaOCl generated about 27% more total aldehydes compared to washing by the same dose of PAA. By running Student’s t-test at 95% confidence interval (α = 0.050), the formation of aldehydes by washing with the same dose of NaOCl and PAA had statistically significant difference. Therefore, PAA appears to have lower potential than NaOCl in forming aldehyde DBPs.
The results in Fig. 1 and Table S3 also revealed that washing by PAA or NaOCl did not increase the levels (in ng/L) of NISAMs except when washing by 100 mg/L of PAA. The formation concentrations of total NISAMs were at 43–94 ng/L when washing by different doses of PAA, compared to 47.8 ± 14.7 ng/L when washing by 100 mg/L of NaOCl. The statistical analysis (Student’s t-test at 95% confidence interval) of comparing the differences among pre-wash (tap water) and wash by NaOCl (100 mg/L) and PAA (100, 85 and 45 mg/L) indicated that the washing by 100 mg/L NaOCl and 100 mg/L PAA had significant difference. These results suggested that the higher dosage of PAA has slightly higher potential than NaOCl to form NISAM DBPs.
Due to the high concentrations of THMs in the tap water pre-wash, no significant formation of THMs was found by washing with either PAA or NaOCl (Fig. 1 and Table S3). The lower level of THMs after 100 mg/L NaOCl wash than that of tap water pre-wash might be due to the lower pH (pH 6.0) of the sanitizer solution than the pH (pH 6.5–6.6) of tap water. Previous studies have indicated that less THMs were formed during chlorination when the water was at a lower pH condition (Ichikawa et al., 1985, Liang and Singer, 2003, Navalon et al., 2008, Rodrigues et al., 2007). Interestingly, while the levels of THMs after washing by 45 mg/L or 85 mg/L of PAA were similar to that by tap water pre-wash, the concentration of THMs (mainly CF) became much lower after washing by 100 mg/L of PAA (Fig. 1 and Table S3). These results implied that PAA, at a higher concentration, might promote decay of THMs; however, more research is needed to discern this phenomenon to obtain a better understanding.
3.3. Formation of DBPs in fresh-cut lettuce after sanitizer washing
The results shown in the previous section demonstrated that DBPs could be formed in the wash water when using either PAA or NaOCl as the sanitizer for fresh-cut lettuce. Thus, it was important to also evaluate the levels of DBPs formed in the washed lettuce. Fig. 2 and Table S4 show the concentrations of DBPs in the lettuce with or without the washing process by different sanitizers. The lettuce purchased from the local supermarket already contained some THMs (91.6 ng/g, 100% from CF), HAAs (27.6 ng/g, 79% from MCAA, followed by TCAA and DCAA), N-DBPs (53.7 ng/g, 100% from DCAAm), C-DBPs (only DBCPN was detectable but the concentration was too low to be quantified), aldehydes (155 ng/g, 61% form AD, followed by GX, FD, MG and BD), and NISAMs (2.5 ng/g, 93% from NMOR and 7% from NDMA). The sources of these DBPs in the lettuce were unknown and might be related to their previous washing processes.
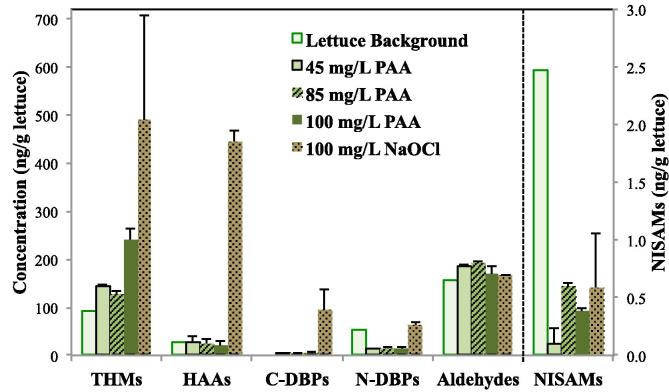
Fig. 2.
Concentrations of DBPs in fresh-cut lettuce with and without (lettuce background) washing (n = 2 for each experiment). The washing procedures included tap water pre-wash, then sanitizer (PAA or NaOCl) solution wash, and final tap water post-wash (detailed in Materials and Methods).
After the washing procedure in this study, the levels of HAAs, C-DBPs and N-DBPs in lettuce remained similar to or less than the background levels when washed by PAA, but increased significantly, especially for HAAs, when washed by NaOCl. These results were comparable to the findings in the wash water, which showed significant formation of HAAs, C-DBPs and N-DBPs in the wash water by NaOCl but not by PAA wash. These results also indicated that HAAs, C-DBPs and N-DBPs could be expected in both wash water and washed lettuce.
Compared to the background levels, washing by either PAA or NaOCl did not increase aldehydes in lettuce in the total or individual compound concentrations. For NISAMs, all of the sanitizer washings led to NISMAs at below 1 ng/g, lower than the background level. Combining with the findings in the wash waters, the results indicated that NISAMs could only be formed in the wash water by PAA or NaOCl, and had low tendency to form in or transfer from the wash water to fresh-cut lettuce.
For THMs, CF was the only trihalomethane detected in fresh-cut lettuce with or without sanitizer washing (Table S4); this finding was not surprising considering that CF was also at concentrations overwhelmingly higher than the other THMs in the wash waters (Table S3). The formation of CF in lettuce increased from 145 to 242 ng/g with increasing PAA dose (Fig. 2). By running the 95% confidence interval Student’s t-test, the results indicated that the CF increase at 45–85 mg/L dose of PAA was statistically insignificant and was statistically significant when PAA dose was increased to 100 mg/L. Washing by 100 mg/L NaOCl generated the highest level (490 ng/g) of CF in the lettuce, about twice as much as that formed by 100 mg/L of PAA. Previous research (Ichikawa et al., 1985) also demonstrated that washing fresh produce by NaOCl could form 770–4,500 ng/g of CF in different vegetables. Their higher CF concentrations than the results of this study were likely due to different reaction conditions including a higher chlorine dose (2,000 mg/L), higher pH (7), higher temperature (20 °C) and longer washing time (20 min) in that study.
After washing by sanitizers, the fresh-cut lettuce was subjected to post-wash by tap water. Table S5 shows the levels of DBPs detected in the post-wash water. The results in the post-wash water were compared with those from the pre-wash water because both utilized tap water (comparison shown in Fig. S2). The DBPs in the post-wash water could come from DBPs in the tap water, DBPs washed off from the lettuce, and DBPs formed from residual oxidant during post-wash period. A higher level of DBPs in the post-wash water implied that some DBPs were washed off from the lettuce by the post-wash step. For lettuce that was washed by PAA, the concentrations of most groups of DBPs in the post-wash were similar to those found in the pre-wash, with the exception of aldehydes. The level of aldehydes in the post-wash was 2–5 times higher than that in the pre-wash, suggesting that some aldehydes were washed off by the post-wash step and/or additional formation in the post-wash step. For lettuce that were washed by NaOCl, the concentrations of HAAs and N-DBPs were much higher in the post-wash than in the pre-wash, while the concentrations of THMs, C-DBPs, NISAMs and aldehydes were comparable or only slightly higher in the post-wash compared to the pre-wash. Considering the relatively high concentrations of HAAs, N-DBPs, THMs and C-DBPs in lettuce upon NaOCl wash, the post-wash results might suggest that HAAs and N-DBPs were more likely to be washed off from lettuce than THMs and C-DBPs.
3.4. Distribution of DBPs in wash water and lettuce after NaOCl and PAA washing
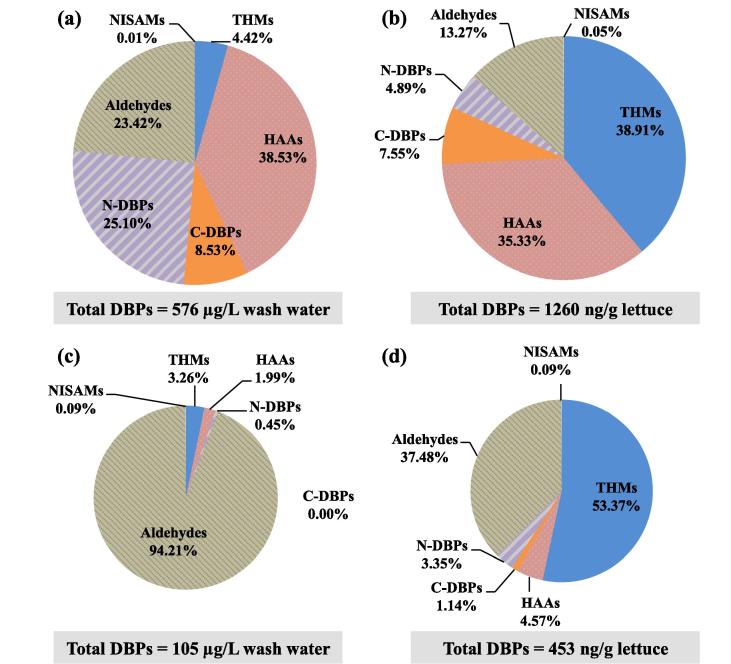
The previous sections discussed the concentrations of different groups of DBPs formed in the wash water and lettuce by PAA and NaOCl. In this section, the distribution of different groups of DBPs and individual DBP compounds relative to the total DBPs formed after different washings are discussed to further compare the significance of the various DBPs. As shown in Fig. 3a and b, washing by 100 mg/L of NaOCl generated total DBPs of 576 μg/L in the wash water and 1260 ng/g in the lettuce. In the wash water, the relative abundance (in %) of the different groups of DBPs was HAAs > N-DBPs > aldehydes > C-DBPs > THMs > NISAMs, with HAAs, N-DBPs and aldehydes accounting for greater than 87% of the total DBPs (Fig. 3a). The most dominant DBP compounds in each DBP group were DCAA for HAAs, DCAN for N-DBPs, AD for aldehydes, CH for C-DBPs, CF for THMs, and NPIP for NISAMs (Table S3). However, in the lettuce, the distribution was THMs > HAAs > aldehydes > C-DBPs > N-DBPs > NISAMs, with THMs, HAAs and aldehydes accounting for greater than 87% of the total DBPs (Fig. 3b). The most dominant DBP compounds in each DBP group in the lettuce were also not exactly the same as those in the wash water, in that DCPN was the most dominant for C-DBPs, DCAAm for N-DBPs, and NMOR for NISAMs (Table S3). In comparing wash water versus lettuce, the results indicated that THMs were more important in lettuce, N-DBPs were more important in wash water, while HAAs were important in both wash water and lettuce. These trends could be affected by different DBP formation potentials in water versus in lettuce, and by transfer of DBPs between the water and lettuce phases, which require further research to elucidate the processes.
Fig. 3.
Distribution of DBPs formed in wash water and lettuce by using different sanitizers. (a) wash water and (b) washed lettuce by 100 mg/L NaOCl; (c) wash water and (d) washed lettuce by 100 mg/L PAA.
Evidently, washing by 100 mg/L of PAA generated much less DBPs, at 105 μg/L total DBPs in the wash water and 453 ng/g total DBPs in the lettuce (Fig. 3c and d), about 5.5 times and 2.8 times lower than those by the same dose of NaOCl. Over 94% of the DBPs in the wash water were aldehydes (Fig. 3c), with FD as the most dominant compound (Table S4). THMs (53%) and aldehydes (37%) were two main groups of DBPs in the lettuce washed by 100 mg/L of PAA, with CF and AD as the most dominant compounds, respectively (Fig. 3d and Table S4).
4. Conclusions
The potential formation of DBPs in the wash water and washed lettuce by using PAA and NaOCl sanitizers was evaluated and compared in this study. Notably, this study evaluated a wide range of 45 DBPs belonging to various structural classes and thus provided a comprehensive data set concerning the issue of DBPs in produce. Many data in this study are among the first ever reported on the formation of emerging DBPs other than THMs and HAAs in washed produce and wash water.
Overall, the study results clearly demonstrated that washing by PAA generated much less DBPs than washing by NaOCl. Compared to NaOCl, PAA had negligible formation potential for HAAs, N-DBPs and C-DBPs, and slightly lower formation potential for aldehydes. For THMs, the higher background levels in tap water and in unwashed lettuce affected the experimental observations, but in general it could be concluded that PAA had a lower tendency to form THMs than NaOCl. Both PAA and NaOCl showed relatively low formation potential for NISAMs, with PAA slightly higher than NaOCl. The majority of DBPs formed by PAA were aldehydes in the wash water, and aldehydes and THMs in the washed lettuce. The majority of DBPs formed by NaOCl were HAAs, N-DBPs and aldehydes in the wash water, and THMs, HAAs and aldehydes in the lettuce. Interestingly, the distributions of DBP compounds were different in the wash water and in the lettuce. While HAAs were important in both wash water and lettuce, THMs were more likely to be present in the lettuce than in the wash water, and N-DBPs exhibited the opposite tendency.
The findings of this study significantly improved the current knowledge regarding potential formation of traditional and emerging DBPs in produce. These results will be useful in facilitating the development of safer produce washing processes and sanitizer applications, and guiding future research on DBPs in food.
Acknowledgements
This study was funded by a research grant from PeroxyChem, LLC, and was supported partially by resources available through the grant (11317808) from the U.S. Department of Agriculture National Institute of Food and Agriculture. The authors thank Dr. Guangxuan Zhu at Georgia Tech for laboratory assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2018.100003.
Contributor Information
Wan-Ning Lee, Email: wlee313@gatech.edu.
Ching-Hua Huang, Email: ching-hua.huang@ce.gatech.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- APHA-AWWA-WEF. (1998). Standard methods for the examination of water and wastewater (22th ed.). Washington, DC.
- Baldry M.G.C. The bactericidal, fungicidal and sporicidal properties of hydrogen peroxide and peracetic acid. Journal of Applied Bacteriology. 1983;54(3):417–423. doi: 10.1111/j.1365-2672.1983.tb02637.x. [DOI] [PubMed] [Google Scholar]
- Baldry M.G.C., Cavadore A., French M.S., Massa G., Rodrigues L.M., Schirch P.F.T., Threadgold T.L. Effluent disinfection in warm climates with peracetic acid. Water Science and Technology. 1995;31(5–6):161–164. [Google Scholar]
- Block S.S. 5th ed. Lippincott Williams & Wilkins; Philadelphia: 2001. Disinfection, sterilization, and preservation. [Google Scholar]
- Booth R.A., Lester J.N. The potential formation of halogenated by-products during peracetic acid treatment of final sewage effluent. Water Research. 1995;29(7):1793–1801. [Google Scholar]
- Briancesco R., Veschetti E., Ottaviani M., Bonadonna L. Peracetic acid and sodium hypochlorite effectiveness in reducing resistant stages of microorganisms. Central European Journal of Public Health. 2005;13(3):159–162. [PubMed] [Google Scholar]
- Bull R.J., Reckhow D.A., Li X.F., Humpage A.R., Joll C., Hrudey S.E. Potential carcinogenic hazards of non-regulated disinfection by-products: Haloquinones, halo-cyclopentene and cyclohexene derivatives, N-halamines, halonitriles, and heterocyclic amines. Toxicology. 2011;286(1–3):1–19. doi: 10.1016/j.tox.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Cardador M.J., Gallego M. Effect of the chlorinated washing of minimally processed vegetables on the generation of haloacetic acids. Journal of Agricultural and Food Chemistry. 2012;60(29):7326–7332. doi: 10.1021/jf302591u. [DOI] [PubMed] [Google Scholar]
- Crathorne, B., Fawell, J., Irving, T., Harris, N., Denny, S., Whitmore, T., Horth, H., James, H., Roddie, B., Smith, D. J., & Taylor, L. (1991). Sewage disinfection: By-Product formation, ecotoxicology and microbiological efficacy, Report NR 2727. United Kingdom: Water Research Centre: Medmenham.
- Dell'Erba A., Falsanisi D., Liberti L., Notarnicola M., Santoroa D. Disinfection by-products formation during wastewater disinfection with peracetic acid. Desalination. 2007;215(1–3):177–186. [Google Scholar]
- Fan X.T., Sokorai K.J. Formation of trichloromethane in chlorinated water and fresh-cut produce and as a result of reaction with citric acid. Postharvest Biology and Technology. 2015;109:65–72. [Google Scholar]
- FAO/WHO. (2008). Benefits and risks of the use of chlorine-containing disinfectants in food production and food processing. Report of a joint FAO/WHO Expert Meeting, Ann Arbor, MI, USA. Food and Agriculture Organization of the United Nations/World Health Organization.
- Freese, S. D., Nozaic, D. J., Bailey, I., & Trollip, D. (2002). Alternative disinfectants for wastewater effluents: Viable or prohibitively expensive? Water Sa Special Edition. In Wisa Biennial Conference 2002. (pp. 23–32).
- Gomez-Lopez V.M., Lannoo A.S., Gil M.I., Allende A. Minimum free chlorine residual level required for the inactivation of Escherichia coli 0157:H7 and trihalomethane generation during dynamic washing of fresh-cut spinach. Food Control. 2014;42:132–138. [Google Scholar]
- Gomez-Lopez V.M., Marin A., Medina-Martinez M.S., Gil M.I., Allende A. Generation of trihalomethanes with chlorine-based sanitizers and impact on microbial, nutritional and sensory quality of baby spinach. Postharvest Biology and Technology. 2013;85:210–217. [Google Scholar]
- Hebert A., Forestier D., Lenes D., Benanou D., Jacob S., Arfi C.…Levi Y. Innovative method for prioritizing emerging disinfection by-products (DBPs) in drinking water on the basis of their potential impact on public health. Water Research. 2010;44(10):3147–3165. doi: 10.1016/j.watres.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Hrudey S.E. Chlorination disinfection by-products, public health risk tradeoffs and me. Water Research. 2009;43(8):2057–2092. doi: 10.1016/j.watres.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Ichikawa T., Yamanaka Y., Fujii M. Formation of chloroform and carbon tetrachloride in foods treated with sodium hypochlorite. Chemosphere. 1985;14(9):1319–1326. [Google Scholar]
- Ivancev-Tumbas I., Dalmacija B. Effects of coagulation processes on aldehydes formation in groundwater treated with common oxidative agents. Water Research. 2001;35(16):3950–3958. doi: 10.1016/s0043-1354(01)00111-7. [DOI] [PubMed] [Google Scholar]
- Kitis M. Disinfection of wastewater with peracetic acid: A review. Environment International. 2004;30(1):47–55. doi: 10.1016/S0160-4120(03)00147-8. [DOI] [PubMed] [Google Scholar]
- Krasner S.W., Mcguire M.J., Jacangelo J.G., Patania N.L., Reagan K.M., Aieta E.M. The occurrence of disinfection by-products in United-States drinking water. Journal American Water Works Association. 1989;81(8):41–53. [Google Scholar]
- Lee W.N., Huang C.H., Zhu G. Analysis of 40 conventional and emerging disinfection by-products in fresh-cut produce wash water by modified EPA methods. Food Chemistry. 2018;256:319–326. doi: 10.1016/j.foodchem.2018.02.134. [DOI] [PubMed] [Google Scholar]
- Lee, W. N., Huang, C. H., & Zhu, G. (submitted). Analysis of a wide range of conventional and emerging disinfection by-products in fresh-cut produce. Food Chemistry, manuscript submitted for publication.
- Liang L., Singer P.C. Factors influencing the formation and relative distribution of haloacetic acids and trihalomethanes in drinking water. Environmental Science and Technology. 2003;37(13):2920–2928. doi: 10.1021/es026230q. [DOI] [PubMed] [Google Scholar]
- Liberti L., Notarnicola M. Advanced treatment and disinfection for municipal wastewater reuse in agriculture. Water Science and Technology. 1999;40(4–5):235–245. [Google Scholar]
- Lopez-Galvez F., Allende A., Truchado P., Martinez-Sanchez A., Tudela J.A., Selma M.V., Gil M.I. Suitability of aqueous chlorine dioxide versus sodium hypochlorite as an effective sanitizer for preserving quality of fresh-cut lettuce while avoiding by-product formation. Postharvest Biology and Technology. 2010;55(1):53–60. [Google Scholar]
- Luukkonen T., Pehkonen S.O. Peracids in water treatment: A critical review. Critical Reviews in Environmental Science and Technology. 2017;47(1):1–39. [Google Scholar]
- Mitch W.A., Schreiber I.M. Degradation of tertiary alkylamines during chlorination/chloramination: Implications for formation of aldehydes, nitriles, halonitroalkanes, and nitrosamines. Environmental Science and Technology. 2008;42(13):4811–4817. doi: 10.1021/es703017z. [DOI] [PubMed] [Google Scholar]
- Monarca S., Richardson S.D., Feretti D., Grottolo M., Thruston A.D., Zani C.…Alberti A. Mutagenicity and disinfection by-products in surface drinking water disinfected with peracetic acid. Environmental Toxicology and Chemistry. 2002;21(2):309–318. [PubMed] [Google Scholar]
- Muellner M.G., Wagner E.D., McCalla K., Richardson S.D., Woo Y.T., Plewa M.J. Haloacetonitriles vs. regulated haloacetic acids: Are nitrogen-containing DBPs more toxic? Environmental Science and Technology. 2007;41(2):645–651. doi: 10.1021/es0617441. [DOI] [PubMed] [Google Scholar]
- Navalon S., Alvaro M., Garcia H. Carbohydrates as trihalomethanes precursors. Influence of pH and the presence of Cl- and Br- on trihalomethane formation potential. Water Research. 2008;42(14):3990–4000. doi: 10.1016/j.watres.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Plewa M.J., Muellner M.G., Richardson S.D., Fasano F., Buettner K.M., Woo Y.T.…Wagner E.D. Occurrence, synthesis, and mammalian cell cytotoxicity and genotoxicity of haloacetamides: An emerging class of nitrogenous drinking water disinfection byproducts. Environmental Science and Technology. 2008;42(3):955–961. doi: 10.1021/es071754h. [DOI] [PubMed] [Google Scholar]
- Plewa M.J., Wagner E.D. Mammalian cell cytotoxicity and genotoxicity of disinfection by-products. Water Research Foundation report. 2009 [Google Scholar]
- Rodrigues P.M.S.M., Esteves da Silva J.C.G., Antunes M.C.G. Factorial analysis of the trihalomethanes formation in water disinfection using chlorine. Analytica Chimica Acta. 2007;595(1–2):266–274. doi: 10.1016/j.aca.2006.12.031. [DOI] [PubMed] [Google Scholar]
- Rudd T., Hopkinson L.M. Comparison of disinfection techniques for sewage and sewage effluents. Journal of the Institution of Water and Environmental Management. 1989;3(6):612–618. [Google Scholar]
- Shah A.D., Liu Z.Q., Salhi E., Hofer T., von Gunten U. Peracetic acid oxidation of saline waters in the absence and presence of H2O2: Secondary oxidant and disinfection byproduct formation. Environmental Science and Technology. 2015;49(3):1698–1705. doi: 10.1021/es503920n. [DOI] [PubMed] [Google Scholar]
- Shen C., Norris P., Williams O., Hagan S., Li K. Generation of chlorine by-products in simulated wash water. Food Chemistry. 2016;190:97–102. doi: 10.1016/j.foodchem.2015.04.146. [DOI] [PubMed] [Google Scholar]
- USEPA . US EPA; 1998. Method 556: Determination of carbonyl compounds in drinking water by pentafluorobenzylhydroxylamine derivatization and capillary gas chromatography with electron capture detection. [Google Scholar]
- Villanueva C.M., Cantor K.P., Grimalt J.O., Malats N., Silverman D., Tardon A.…Kogevinas M. Bladder cancer and exposure to water disinfection by-products through ingestion, bathing, showering, and swimming in pools. American Journal of Epidemiology. 2007;165(2):148–156. doi: 10.1093/aje/kwj364. [DOI] [PubMed] [Google Scholar]
- Wagner E.D., Hsu K.M., Lagunas A., Mitch W.A., Plewa M.J. Comparative genotoxicity of nitrosamine drinking water disinfection byproducts in Salmonella and mammalian cells. Mutation Research-Genetic Toxicology and Environmental Mutagenesis. 2012;741(1–2):109–115. doi: 10.1016/j.mrgentox.2011.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.