Highlights
-
•
Caffeine, theophylline, theobromine (methylxanthines) are common ingredients of many stimulating drinks.
-
•
The antioxidant ability of methylxanthines was studied in vitro and in vivo.
-
•
Under acute oxidative stress, methylxanthines could enhance the survival rate of Caenorhabditis elegans.
-
•
Lifespan extension was observed in Caenorhabditis elegans.
-
•
Lower concentration of methylxanthines has no toxicity.
Keywords: Caenorhabditis elegans, Pro-oxidant effect, Caffeine, Theophylline, Theobromine, DAF-16/FOXO, SKN-1
Abstract
Caffeine and related purine alkaloids are common ingredients of many stimulating drinks. Studies have shown that lower concentrations of caffeine have a protective role in aging-related disorders. However, the associated mode of action of caffeine and its related methylxanthines is still not clear. In this study, we demonstrated that caffeine and theophylline promote longevity in Caenorhabditis elegans. Lifespan studies with the wild type, DAF-16 and SKN-1 mutant strains indicated that the methylxanthines-mediated lifespan extension in C. elegans was independent of DAF-16/FOXO and SKN-1. All the tested methylxanthines could protect C. elegans against acute oxidative stress. At early stages of life, an increase of ROS (reactive oxygen species) induced the translocation of DAF-16 and SKN-1, resulting in upregulation of several antioxidant genes, for example, sod-3p::GFP, gst-4p::GFP, gcs-1p::GFP; and downregulation of hsp-16.2p::GFP. RT-PCR corroborates the upregulation of gst-4 and skn-1 genes. The expression of DAF-16 decreased although its nuclear translocation was induced.
1. Introduction
Caffeine, along with the purine-related alkaloids theobromine and theophylline, are present in many stimulating drinks, such as coffee, green tea, chocolate and some soft drinks. These methylxanthines are structurally similar. Theophylline and theobromine are isomers and their NH group is substituted in caffeine by a N-CH3 group (Xia, Ni, & Kokot, 2013), shown in Fig. 1.
Fig. 1.
Chemical structures of methylxanthines.
It has been reported that caffeine has a beneficial effect on a number of health disorders, including depression, liver disease, and type 2 diabetes mellitus (Hall et al., 2015, Higdon and Frei, 2006, Rivera-Oliver and Díaz-Ríos, 2014, Saab et al., 2014). Epidemiological studies suggest that coffee consumption has a neuroprotective role in Parkinson’s disease (PD) and Alzheimer’s disease (AD) (Eskelinen and Kivipelto, 2010, Liu et al., 2012). Furthermore, studies have proven that a moderate intake of caffeine may be beneficial in preventing the progression of non-alcoholic fatty liver disease (NAFLD). The reason may be that stimulation of lipid breakdown decreases the amount of stored fat in fatty liver (Sinha et al., 2014). Theophylline is a common drug mainly used to treat respiratory disorders, such as asthma (Barnes, 2013). Theobromine is not widely used.
Reactive oxygen species (ROS) are by-products of aerobic metabolism. ROS are a double-edged sword, as ROS are essential to the organism but can also exert detrimental effects on cell macromolecules (Heidler et al., 2010, Mattson, 2008). It is widely believed that low levels of ROS modulate the innate immune response and function as signaling molecules involved in a mitochondria-to-nucleus signaling pathway (Balaban et al., 2005, Wei and Kenyon, 2016). On the other hand, unbalanced levels of ROS can, for example, elicit the oxidation of the DNA base guanosine to 8-oxoguanosine, leading to point mutations. These mutations can impair important proteins in cells disrupting the normal cell function and even inducing cancer. An antioxidant defense system composed by enzymatic and nonenzymatic antioxidants is required to neutralize the overproduction of ROS.
The nematode Caenorhabditis elegans has been widely used as one of the most powerful model to tackle various biological questions (Yang & Hekimi, 2010). Specifically due to transparency throughout its complete life cycle, easy maintenance and easy manipulation of growth conditions, its short life cycle and lifespan (approximately 18 days at 20 °C), Caenorhabditis elegans has also been successfully used as a good choice in high-throughput drug screenings and in aging studies (O'Reilly, Luke, Perlmutter, Silverman, & Pak, 2014).
Aging is an inevitable process in all organisms. Insulin/insulin-like growth factor-1 signaling (IIS) was the first aging regulatory pathway described (Kenyon, 2011). The gene daf-2 codes for the homolog of the insulin/insulin-like growth factor-1 receptors in C. elegans. When the activity of DAF-2 is reduced or inhibited in nematodes, the transcription factor DAF-16 is released and translocated to the nucleus, promoting stress resistance and lifespan extension. In addition to daf-16, skn-1 encodes the C. elegans homolog of the mammalian nuclear respiration factor 2 (Nrf2). SKN-1 regulates more than 200 genes encodes cytoprotective phase II detoxification and antioxidant enzymes, including glutamate-cysteine ligase subunits and glutathione-S-transferase, which collectively synthesize glutathione (GSH) (Lu, 2009). The modulation of IIS and SKN-1/Nrf2 pathways by plant secondary metabolites have been shown to enhance the innate antioxidant defense system response, and to contribute to the reestablishment of the antioxidative-oxidative balance (Leonov et al., 2015).
As coffee and related stimulating drinks belong to the most widely consumed beverages, it is relevant to further understand the impact of caffeine, theophylline, and theobromine on stress resistance and aging. In this study, we aimed to evaluate whether and how caffeine, theophylline, and theobromine can promote longevity in C. elegans. Different C. elegans strains were used to detect the effect of these compounds on the expression of stress and lifespan extension related genes. Our results may provide useful information for understanding longevity and stress resistance induced by methylxanthines in stimulating drinks.
2. Methods
2.1. DPPH assay
The in vitro antioxidant ability of methylxanthines was measured by detecting the decrease in the absorption of the stable free radical DPPH (2,2-diphenyl-1,picrylhydrazyl) (Sigma-Aldrich GmbH, Steinheim, Germany), as described by Blois (1958) (Blois, 1958). Briefly, 100 μl of serially diluted methylxanthines were mixed with 100 μl of 200 μM DPPH (diluted in methanol). The reaction ran in the dark for 30 min. The absorption of the mixture was measured at 517 nm in a microplate reader (Tecan Group Ltd., Männedorf, Switzerland). The following equation was used to calculate the radical scavenging ability of methylxanthines:
Here A0 means the absorption of only DPPH, and A1 is the absorption in the presence of methylxanthines. The polyphenol from green tea, EGCG, was used as a positive control (Nikoo, Regenstein, & Ahmadi Gavlighi, 2018). All measurements were performed in triplicate.
2.2. C. elegans – strains and maintenance
C. elegans strains N2 (wild type), CF1553 [(pAD76) sod-3p::GFP], TJ356 [daf-16p::daf-16a/b::GFP + rol-6(su1006)], CF1038 [daf-16 (mu86)], LD1 [skn-1b/c::GFP + rol-6(su1006)], LD1171 [gcs-1p::GFP + rol-6(su1006)], CL2166 [(pAF15)gst-4p::GFP::NLS], TJ375 [hsp-16.2::GFP(gp1sI)], EU1 [skn-1(zu67) IV/nT1(IV;V)] were purchased from the Caenorhabditis Genetics Center (CGC, University of Minnesota, Minneapolis, MN) and cultured in NGM (nematode growth medium), which is seeded with Escherichia coli OP50 as a food source (CGC, University of Minnesota, Minneapolis, MN). The worms were incubated at 20 °C as described previously (Brenner, 1974). For assays that demand a liquid medium, S-medium was employed, which was complemented with E. coli OP50 (OD600 = 0.8–1.2). Caffeine, theophylline and theobromine were dissolved in S-medium prior to the assays.
2.3. Measurement of intracellular ROS
Synchronized L1 larval worms (N2 grown in S-medium) were treated with 1 mM caffeine, 5 mM caffeine, 1 mM theophylline, 5 mM theophylline, and 1 mM theobromine (due to lower solubility of theobromine, 5 mM theobromine could not be obtained) for 48 h, while a control group was left untreated. All groups were then treated with 50 μM CM-H2DCFDA (Sigma-Aldrich GmbH, Steinheim, Germany) and incubated in the dark for 1 h at 20 °C. Worms were washed once with M9 buffer to remove any excess dye and then mounted on a glass slide with a drop of 10 mM sodium azide (AppliChem GmbH, Darmstadt, Germany) for paralysis. CM-H2DCFDA is taken up by the cells and then oxidized by ROS into a fluorescent dye (DCF). Then the fluorescent intensity is related to the ROS level in C. elegans. Using a BIOREVO BZ-9000 fluorescence microscope (Keyence Deutschland GmbH, Neu-Isenburg, Germany) equipped with a mercury lamp, the slides were photographed (λex 480/20 nm, λem 510/38 nm, a 10× bjective lens, a constant exposure time) and analyzed. The relative fluorescence of the whole body was determined densitometrically using ImageJ (National Institutes of Health, Bethesda, MD). The results are presented as the percent mean fluorescent intensity compared to the untreated control. All the experiments were carried out at least three times and compared by one-way ANOVA followed by Dunnett’s method.
2.4. Survival assay under oxidative stress
Synchronized wild-type C. elegans (N2) at the L1 larval stage were treated as described above, while a control group was left untreated. After 48 h, all the groups (each contained 60 worms in total) were exposed to 80 µM of the pro-oxidant juglone (5-hydroxy-1,4-naphthoquinone) (Sigma-Aldrich GmbH, Steinheim, Germany) for 24 h. Dead and live worms were counted afterwards. The worms were considered dead when they did not respond to a gentle touch with a platinum wire. The survival rate is presented as the mean of three independent experiments and compared by one-way ANOVA followed by Dunnett’s multiple comparisons method, using Graphpad Prism Version 6.01 (GraphPad Software, La Jolla, California, USA).
2.5. Lifespan assay
For lifespan assays, caffeine, theophylline, and theobromine were added into NGM to have the fixed concentration of methylxanthines as described above. Then 100 µl of S-medium containing 0.8–1.2 E. coli and corresponding concentration of methyxanthines were added on the freshly prepared NGM plates. All the plates were left at room temperature until S-medium dried then they could be used for the lifespan assay. L1 worms (N2, CF1038, and EU1) were transferred to NGM plates prepared as described above. During the reproductive period, the worms were transferred daily to freshly prepared plates to separate the adults from their progeny; thereafter, the transfer was carried out every second day. Worms that no longer responded to a gentle touch with the platinum wire were scored dead and excluded from the plates. Worms with internally hatched progeny were censured. The results are presented as percentage of survival, mean and median lifespan using the Kaplan-Meier method, SPSS 22.0. Differences between groups were determined by the log-rank (Mantel-Cox) test followed by the Gehan-Breslow-Wilcoxon test using Graphpad Prism.
2.6. Intracellular localization of DAF-16::GFP and SKN-1::GFP
The strain TJ356 (with DAF-16::GFP) was used to detect the intracellular DAF-16 localization. Synchronized L1 stage TJ356 worms were grown in S-medium, divided into 6 groups and treated as described above for 24 h at 20 °C. The strain LD1 was used to monitor the intestinal nuclear localization of SKN-1::GFP. Synchronized L1 larval stage worms (LD1) were divided into 6 groups and treated as described above for 48 h at 20 °C. For each assay, nematodes were collected and mounted onto glass slides, anaesthetized with 10 mM sodium azide and capped with coverslips. The subcellular distributions of DAF-16::GFP and SKN-1::GFP were photographed and analyzed by fluorescence microscopy.
2.7. Expression of stress response genes
Synchronized L1 worms of the transgenic strains carrying a GFP reporter for sod-3 (CF1553), gcs-1 (LD1171), gst-4 (CL2166) were treated with caffeine, theophylline, and theobromine or left untreated as described above for 48 h. For hsp-16.2 (TJ375 strain), the worms were treated for 48 h, and then subjected to 20 μM juglone for a further 24 h. GFP fluorescence images of randomly selected animals were photographed and analyzed using ImageJ. The results are presented as percent mean fluorescent intensity compared to the untreated control. All the experiments were carried out at least three times and compared by one-way ANOVA followed by Dunnett’s method.
2.8. Quantitative real-time PCR
Synchronized L1 worms of the wild type (N2) were exposed to 5 mM caffeine, 5 mM theophylline, 1 mM theobromine or left untreated. The worms were then incubated in S-medium supplemented with E. coli OP50 for 48 h at 20 °C. Worms were washed with M9 buffer three times and total RNA was extracted using a Universal RNA Purification Kit (Roboklon, Berlin, Germany). The concentration of total RNA was measured spectrophotometrically, and purity was checked at 260 nm/280 nm and 260 nm/230 nm. For reverse transcription (RT), 500 ng of total RNA from control and each treatment group were used (QuantiTect Reverse Transcription Kit, Qiagen, Hilden, Germany) to produce cDNA. For the quantitative real-time PCR (qRT-PCR), the cDNA was diluted 100-fold in nuclease-free water. The quantitative real-time PCR was performed with a Roche LightCycler® 96. Data were analyzed by the LightCycler®96 System. cdc-42 was used as a housekeeping gene. The PCR primers are listed in Supplementary Table 2. These data were analyzed by a two-tailed Student’s test.
2.9. Reproduction assay and body length
Synchronized L1 stage N2 animals were individually transferred to NGM plates prepared as described in the lifespan assay. The NGM plates were freshly seeded with E.coli OP50. The adult animals were transferred to freshly prepared NGM plates until the reproductive period was over. The eggs or the hatched progeny were counted. The mean brood size was compared by one-way ANOVA followed by Dunnett (post-hoc). For the body length measurement, around 30 wild-type L4 larvae were randomly selected and transferred to the solid NGM plates containing the corresponding concentration of compounds. On the first day of adult life, images of individual nematodes were prepared and the body length was determined using the software BZ-II Analyzer (Keyence Corp.). The results were presented as body length in micrometers (mean ± SEM).
3. Results
3.1. Methylxanthines increased lifespan of wild type C. elegans
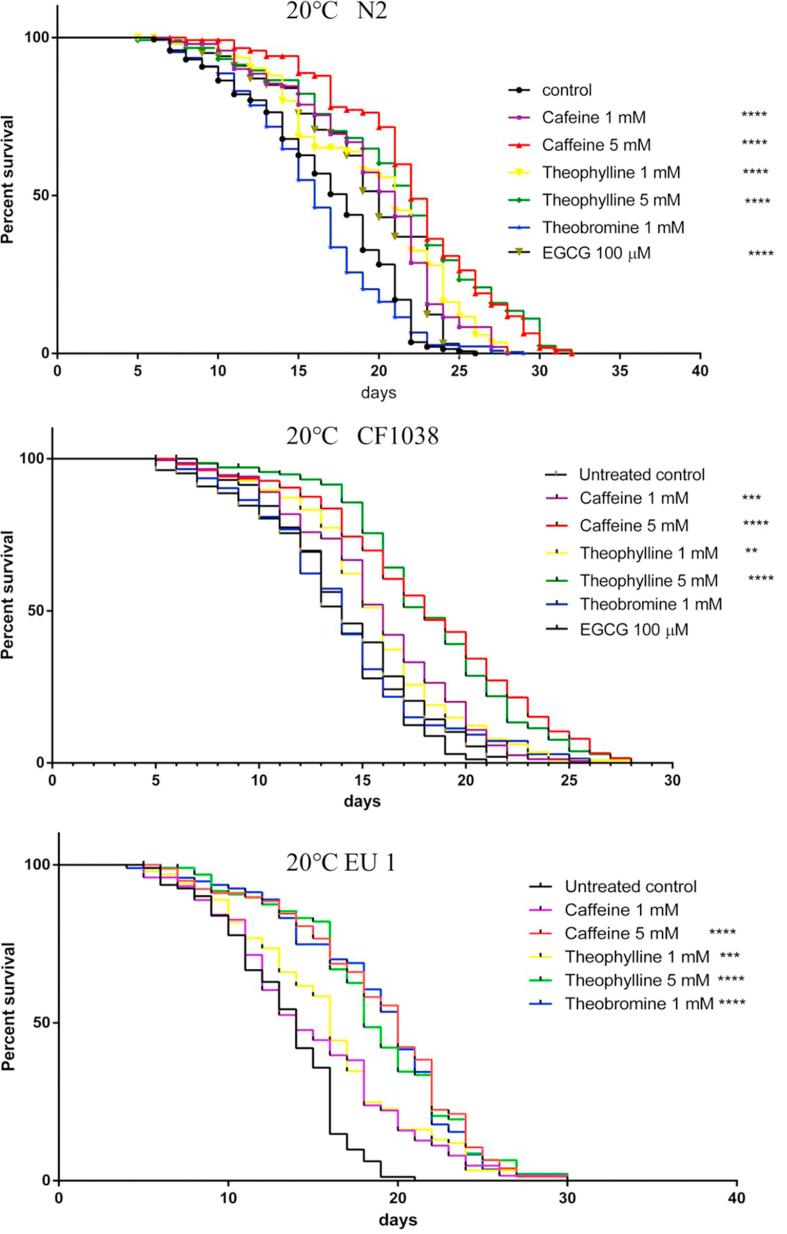
To determine whether caffeine, theophylline and theobromine affect the longevity of nematodes, worms were maintained throughout their whole lives on nematode growth medium (NGM) plates containing different concentrations of methylxanthines, i.e. 1 mM and 5 mM caffeine; 1 mM and 5 mM theophylline and 1 mM theobromine. Except for 1 mM theobromine, the other methylxanthines could prolong the lifespan (Fig. 2 and Supplementary Table 1), for example, 5 mM caffeine and theophylline could both increase the lifespan of N2 by 22.22%.
Fig. 2.
Effects of methyxanthines on the lifespan of wild-type (N2), daf-16 mutant (CF1038), and skn-1 mutant (EU1) worms. Lifespan experiments were performed at least three times. Additional information is shown in Supplementary Table 1.
In C. elegans, DAF-16 and SKN-1 play important roles in many regulatory pathways and are involved in lifespan-extending interventions (Blackwell, Steinbaugh, Hourihan, Ewald, & Isik, 2015a). In order to understand if the observed lifespan extension is dependent on DAF-16 or not, lifespan assays using a DAf-16 strain (CF1038) were carried out (Fig. 2). The results demonstrated that, compared to the untreated control, the lifespan of the DAF-16 mutant strain was extended when exposed to caffeine and theophylline, indicating that the action of caffeine and theophylline is not strictly dependent on the DAF-16 pathway. Furthermore, to investigate the involvement of SKN-1 in the lifespan extension, assays with a SKN-1 strain (EU1) were carried out (Fig. 2). Methylxanthines also prolonged the lifespan of SKN-1 mutant worms, indicating that SKN-1 was also not required for lifespan extension. Theobromine 1 mM had no effect on the lifespan of the wild type (N2) and daf-16 mutant (CF1038) but extended the lifespan of the skn-1 mutant by 42.86%.
3.2. Methylxanthines improve the stress resistance of C. elegans under oxidative stress
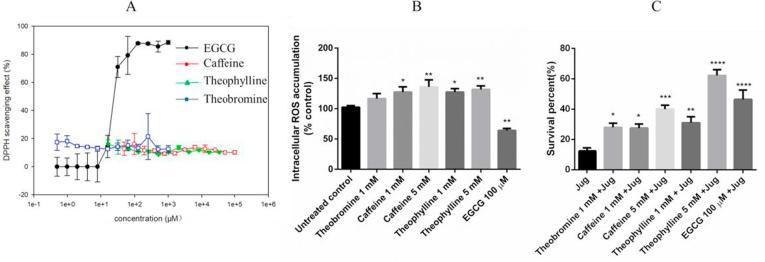
To explore how methylxanthines enhance the stress resistance of C. elegans under induced oxidative stress, the free radical-scavenging abilities of methylxanthines were evaluated. Methylxanthines do not exhibit an antioxidant activity in vitro (shown in Fig. 3A). In another set of experiments, we explored the effect of methylxanthines on intracellular ROS levels in wild type worms. 100 μM EGCG, which has the ability to scavenge the superoxide anion in vivo, was used as a positive control (Nikoo et al., 2018). The results showed that caffeine and theophylline increased the ROS levels in C. elegans. 5 mM caffeine increased the ROS levels by 35.9 ± 11.66%, and 5 mM theophylline by 31.8 ± 5.85%, while 100 μM EGCG decreased the ROS levels by 35.9 ± 2.41 (Fig. 3B). In basal stress conditions, the methylxanthines work as pro-oxidants.
Fig. 3.
Antioxidant effects of methylxanthines. They protected against oxidative stress in wild type nematodes. A, in vitro evaluation of antioxidant activity using DPPH assay. B, in vivo anti-oxidant activity was detected by the quantification of intracellular ROS levels in N2 worms. C, the effect of methylxanthines on stress resistance (% survival) under a lethal dose of juglone. The experiments were carried out at least three times, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and compared to the untreated control by one-way ANOVA following Dunnett.
A stress assay was carried out to detect the effects of methylxanthines on the survival rate of the worms under acute oxidative stress. Juglone, a free radical generator, converts oxygen to a superoxide anion and consequently increases intracellular oxidative stress (Przybysz, Choe, Roberts, & Strange, 2009). Worms were exposed to 80 µM juglone after 48 h of pretreatment with methylxanthines. The results showed that pretreatment with purine alkaloids improved the worm’s resistance to oxidative stress. Under oxidative stress, 5 mM of caffeine and theophylline showed strong protective effects. The survival rate of these worms was significantly higher (61.11 ± 22.75% and 67.78 ± 4.19%, respectively) than in the juglone group (12.42 ± 2.09%) (Fig. 3C).
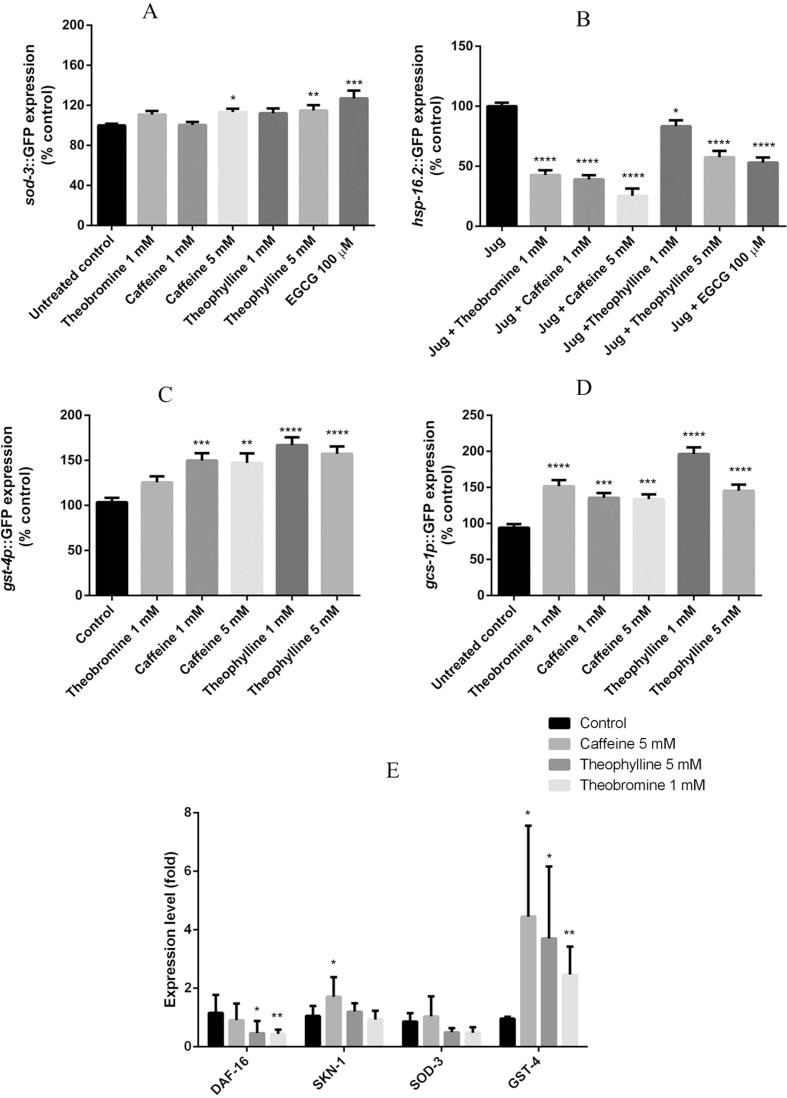
3.3. Methylxanthines induced DAF-16 and SKN-1 translocation
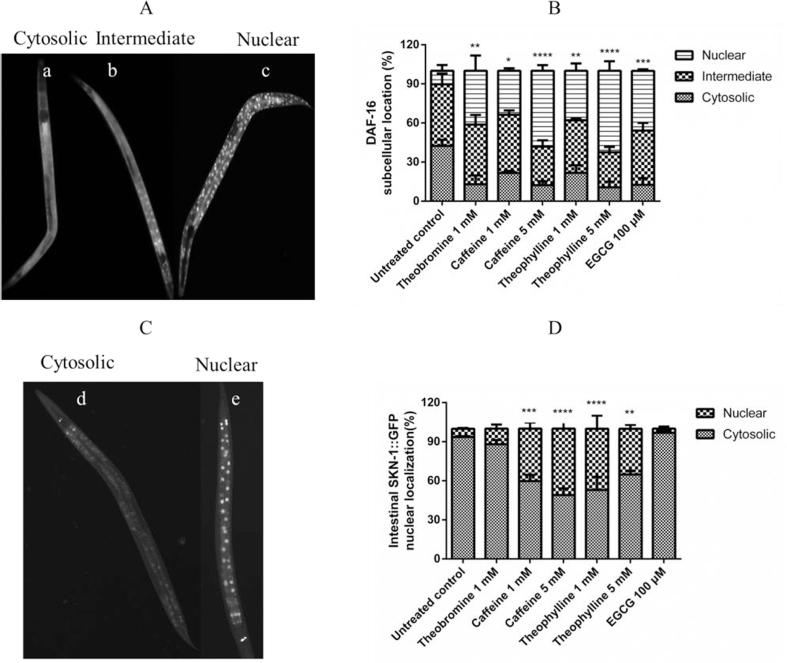
DAF-16/FOXO and SKN-1 are the main transcription factors involved in the regulation of stress-resistance and lifespan extension related genes (Park, Tedesco, & Johnson, 2009). Under basal stress conditions, DAF-16/FOXO remains inactive in the cytosol. Its translocation to the nucleus can be stimulated by different kinds of stress, for example, oxidative stress, resulting in influencing the expression of a variety of genes related to stress response (Mukhopadhyay, Oh, & Tissenbaum, 2006). To evaluate whether methylxanthines exert their stress-reducing effects via the DAF-16/FOXO pathway, the mutant strain TJ356 was treated with methylxanthines. The results showed that a higher percentage of methylxanthine-treated worms exhibited a nuclear location pattern of DAF-16::GFP than the negative control (shown in Fig. 4 B). The worms treated with 5 mM theophylline showed the highest significant fraction of 62.5 ± 4.24% DAF-16 nuclear localization and 5 mM caffeine showed 58.17 ± 2.53%, compared with 10.32 ± 2.55% observed among the negative control group. Treatments with 1 mM theobromine, 1 mM caffeine, and 1 mM theophylline also induced DAF-16::GFP translocation, but not to a remarkable extent. EGCG 100 μM was used as a positive control.
Fig. 4.
Methylxanthines induced DAF-16/FOXO and SKN-1 translocation to the nucleus and regulated target genes. A, three levels to determine the pattern of the DAF-16::GFP subcellular translocation. B, methylxanthines induced significant translocation of DAF-16::GFP. C. Two patterns of the SKN-1::GFP subcellular translocation. D, methylxanthine treatment induced a significant translocation of SKN-1::GFP in LD1. Data are presented as mean ± SEM, experiments were repeated at least three times, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and compared to the untreated control by one-way ANOVA following Dunnett.
The transgenic strain LD1 [skn-1b/c::GFP + rol-6(su1006)], which has a skn-1 promoter fused with a GFP reporter, was used to test whether methylxanthines could induce the translocation of SKN-1 (Dehghan et al., 2017). The results showed that caffeine and theophylline remarkably translocalized SKN-1 to the nuclei of the intestine (Fig. 4D). 1 mM caffeine induced 40.26 ± 2.75% SKN-1 translocation; and theophylline 1 mM induced 47.00 ± 5.81%. For 1 mM theobromine and 100 µM EGCG, no significant translocation was observed (shown in Fig. 4).
3.4. Regulation and expression of stress-resistant genes
SOD-3 (superoxide dismutase 3), which scavenges superoxide free radicals, is one of the DAF-16 target genes (Lee, Kennedy, Tolonen, & Ruvkun, 2003). CF1553, which has a sod-3 promoter fused with a GFP reporter, was used to further study the effects of methylxanthines on the expression of protective genes. Compared with the negative control group, an upregulation was detected among worms treated with 5 mM caffeine (13.3 ± 3.37%) and 5 mM theophylline (14.8 ± 5.36%) (Fig. 5A).
Fig. 5.
Effect of methyxanthines on stress resistant genes. A, higher concentrations of methylxanthines increased sod-3 expression in CF1553. B, hsp:GFP was suppressed by methylxanthines. C, methylxanthine treatment increased gst-4 expression in mutant CL2166. D, methylxanthine treatment increased gcs-1 expression in LD1171. Data are presented as mean ± SEM. Experiments were carried out at least three times, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and compared to the untreated control by one-way ANOVA following Dunnett. E, expression of candidate genes. *p < 0.05, **p < 0.01, and a two-tailed Student’s test were used to analyze the results. Data are presented as mean ± SD.
TJ375, which has a hsp-16.2 promoter fused with a GFP reporter, does not express detectable GFP under basal stress conditions (Peixoto et al., 2016). However, after induced oxidative stress or heat shock, the adult worms exhibit a detectable GFP expression in the pharynx. The expression of hsp-16.2::GFP induced by 20 μM juglone was decreased by 60.97 ± 3.47% (1 mM caffeine), 74.66 ± 6.05% (5 mM caffeine), 16.64 ± 4.92% (1 mM theophylline), 42.39 ± 5.24% (5 mM theophylline), and 57.28 ± 4.00% (1 mM theobromine) respectively, as compared with the negative control (Fig. 5B).
Activated SKN-1 is translocated into the nucleus, inducing the transcription of genes involved in oxidative stress response, including glutamate-cysteine ligase and glutathione S-transferases (Park et al., 2009). The mutant strains LD1171 (gcs-1 promoter fused with GFP reporter) and CL2166 (gst-4 promoter fused with GFP reporter) were used to investigate the expression of gcs-1 and gst-4. As shown in Fig. 5C, D, a higher expression level was detected among worms treated with methylxanthines as compared to control. The expression of gcs-1 was increased by 33.6 ± 6.78% (5 mM caffeine) and by 45.3 ± 8.66% (5 mM theophylline), respectively and the expression of gst-4 was increased by 47.1 ± 10.55% (5 mM caffeine) and by 57.3 ± 8.17% (5 mM theophylline), respectively.
Quantitative real-time PCR was performed to detect the expression levels of DAF-16, SKN-1, SOD-3, and GST-4 in C. elegans. As shown in Fig. 5, the expression of DAF-16 was downregulated 2.51-fold when the organisms were treated with 5 mM theophylline, and 2.64-fold when they were treated with 1 mM theobromine. SKN-1 was increased 1.63-fold in 5 mM caffeine treatment. SOD-3 expression was not affected. The expression of GST-4 in C. elegans treated with 1 mM theobromine was increased by 3.13-fold, with 5 mM caffeine 6.67-fold, and with 5 mM theophylline 5.24-fold.
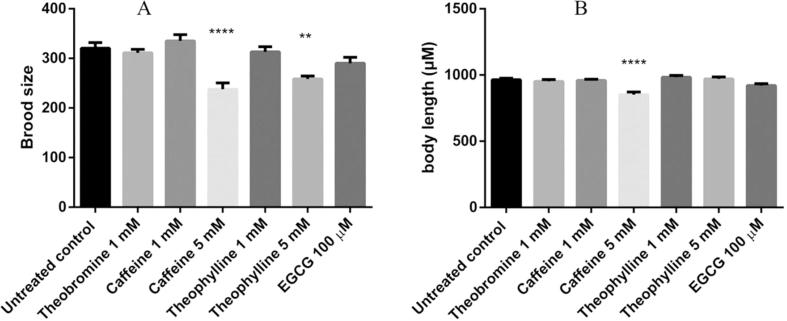
3.5. Higher concentrations of caffeine and theophylline reduced body length and brood size
Body length and brood size are toxicity markers in C. elegans (Bischof et al., 2006, Mohan et al., 2010). Our results showed that 5 mM caffeine and theophylline exposure reduced brood size (Fig. 6). For the body length, 5 mM caffeine exposure reduced the mean body length, while 5 mM theophylline had no effect on the body length of the adult worms. Lower concentrations of methylxanthines did not affect the body length and brood size, indicating that such concentrations had no toxicity.
Fig. 6.
Effect of methylxanthines on markers of toxicity in C. elegans. A, higher concentration of methylxanthines reduced the brood size. Data are presented as mean ± SEM. B, 5 mM caffeine treatment decreased the body length of worms. Data are presented as mean (μM) ± SEM. Experiments were carried out at least three times, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and compared to the untreated control by one-way ANOVA following Dunnett.
4. Discussion
Caffeine has been reported to exert lifespan extension of C. elegans in a dose-dependent manner (Bridi et al., 2015, Sutphin et al., 2012). A plant extract which mainly contained caffeine had also been demonstrated to play a positive role in lifespan extension in C. elegans (Peixoto et al., 2017). However, the effects of theophylline and theobromine have not been reported before.
In this study, we showed that caffeine and theophylline could extend the lifespan of the wild-type nematode C. elegans. For example, 5 mM caffeine and theophylline could increase the lifespan extension by 22.2%. However, in our study, DAF-16 and SKN-1 were apparently not essential for the induced lifespan extension. Here, we demonstrated that the lifespans of daf-16 mutant (CF1038) and skn-1 mutant (EU1) were both increased by caffeine and theophylline (Fig. 2). Our study also provides evidence that caffeine and theophylline exert similar effects on the lifespan of C. elegans, shown in Supplementary Table 1. While for theobromine, although it is the isomer of theophylline, no effect on the lifespan of wild type (N2) and daf-16 (CF1038) mutant was observed, it could only extend the lifespan of the skn-1 (LD1) mutant.
Bridi et al. (2015) observed a caffeine-induced lifespan extension at 5 mM and suggested that it was partly dependent on DAF-2, but not on DAF-16, as the daf-2 mutant exposed to caffeine showed a slight decrease in lifespan while the daf-16 mutant showed an increased life expectancy (Bridi et al., 2015). Furthermore, Bridi and coworkers suggested that adenosine signaling could also play an important role in the caffeine induced lifespan extension (Bridi et al., 2015), as caffeine is a known adenosine receptor antagonist and inhibitor of cAMP phosphodiesterase. In the studies of Sutphin et al. (2012) and Lublin et al. (2011), 5 mM caffeine failed to increase lifespan of a daf-16 mutant strain, thus indicating that it acts through the DAF-16 pathway to promote extended longevity (Lublin et al., 2011, Sutphin et al., 2012). The reason behind the non-sensical results might be related to the differences in the methodology applied. Bridi et al. (2015) proved that lifelong expose (from L1 stage) to 5 mM caffeine was needed to extend lifespan. While in Stuphin’s work, the worms were treated from the L4 stage. And in their work, 5 mM caffeine could not extend the lifespan of daf-16 mutants, however, 10 mM extended the lifespan of daf-16 (RNAi) mutants by 19.7%. The author suggested that a small mechanistic component of the life span extension in the presence of caffeine is independent from IIS. Another variable lifespan experiment was the usage of Amp/FUDR, which was not used in our and Bridi’s studies.
Methylxanthines had no antioxidant activity in vitro (Fig. 3A). The result of ROS accumulation in vivo demonstrated that the methylxanthines increased the ROS level to some degree (Fig. 3B). It was reported before that caffeine could trigger ROS generation in osteoblasts (Lu, Lai, & Chan, 2008). Although methylxanthines could not scavenge radicals in vitro and increased the ROS level in C. elegans, they could increase the survival rate under acute oxidative stress induced by juglone (Fig. 3C).
DAF-16 and SKN-1 regulate the expression of genes related to stress resistance (Blackwell et al., 2015, Hesp et al., 2015). In a short time-frame, caffeine and theophylline exposure significantly induced DAF-16 and SKN-1 translocation to the nucleus (Fig. 4A and B). SOD-3 is a mitochondrial enzyme, which is important for C. elegans to balance the ROS level. Our results indicated that higher concentrations of caffeine and theophylline could slightly increase the expression of SOD-3 (Fig. 5A). HSP-16.2, which is categorized as a small HSP, is induced when the organisms are under heat shock or oxidative stress (Strayer, Wu, Christen, Link, & Luo, 2003). Pretreatments with methylxanthines resulted in downregulation of HSP-16.2 expression, compared to the untreated control (Fig. 5B). The intestine not only mediates digestion, but also is a major detoxification organ, it is believed that many functions of SKN-1 have been linked to its intestinal expression (Blackwell et al., 2015a). In intestine cells, SKN-1 accumulates in nuclei and activates target genes in response to environmental stress. We showed here that Phase II detoxification genes gst-4 and gcs-1 were upregulated after exposure to methylxanthines (Fig. 5C and D). We hypothesized that the reported increase in the ROS levels might play a beneficial role in the regulation of anti-stress genes.
Quantitative PCR demonstrated that SKN-1 was highly expressed after caffeine treatment. And its target gene gst-4 was also highly expressed when treated with methylxanthines, which could explain the higher survival rate after oxidative stress (Fig. 3C). Significantly lower levels of DAF-16 were observed in theophylline and theobromine treatment, with a slight decrease in caffeine treatment. In our experiments worms were exposed to methylxanthines from the L1 larval stage to the adult stage (48 h after L1), the lower expression of DAF-16 could possibly explain the reduced brood size and shorter body length (Tissenbaum, 2018) (Fig. 6).
In C. elegans, brood size and body length reduction have been indicated as toxicity markers (Peixoto et al., 2016). In our study, higher concentrations of caffeine and theophylline interfered with the worm’s reproduction and body length (Fig. 6). The ability of toxic compounds to exert beneficial effects at low doses is called hormesis (Mattson, 2008). Compounds that act in a hormetic way typically increase mitochondrial activity and ROS formation, activating the innate stress response system (Ristow & Zarse, 2010). Since this sequence of phenomena has been shown to increase the lifespan of C. elegans, we hypothesize that caffeine and theophylline might act through hormesis to promote longevity in C. elegans. Indeed, a previous study demonstrated that lower concentrations of caffeine could be beneficial to C. elegans inducing lifespan extension, while higher concentrations of caffeine decreased lifespan (Sutphin et al., 2012).
Our study confirmed a longevity promoting effect and a modulation of oxidative stress response of caffeine and theophylline. Although our study suggests the role of hormesis in the observed activities, further studies are needed to fully explain the connection between the modulation of stress resistance related genes during the early life of C. elegans and the lifespan extension promoted by methylxanthines, which might include the inhibition of adenosine receptors and of cAMP phosphodiesterase, which are active in several pathways.
Acknowledgements
H.L. thanks the Chinese Scholarship Council (CSC) for a PhD fellowship. We also thank Douglas Fear for improving the English in the manuscript. We acknowledge financial support by Deutsche Forschungsgemeinschaft (Germany) within the funding programme Open Access Publishing, by the Baden-Württemberg Ministry of Science, Research and the Arts and by Ruprecht-Karls-Universität Heidelberg.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2019.100005.
Contributor Information
Mariana Roxo, Email: roxo@stud.uni-heidelberg.de.
Xinlai Cheng, Email: x.cheng@uni-heidelberg.de.
Michael Wink, Email: wink@uni-heidelberg.de.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Balaban R.S., Nemoto S., Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120(4):483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Barnes P.J. Theophylline. American Journal of Respiratory and Critical Care Medicine. 2013;188(8):901–906. doi: 10.1164/rccm.201302-0388PP. [DOI] [PubMed] [Google Scholar]
- Bischof L.J., Huffman D.L., Aroian R.V. Assays for toxicity studies in C. elegans with Bt crystal proteins. Springer; 2006. pp. 139–154. [DOI] [PubMed] [Google Scholar]
- Blackwell T.K., Steinbaugh M.J., Hourihan J.M., Ewald C.Y., Isik M. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radical Biology Medicine. 2015;88:290–301. doi: 10.1016/j.freeradbiomed.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1199. [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridi J.C., de Almeida Barros A.G., Sampaio L.R., Ferreira J.C.D., Soares F.A.A., Romano-Silva M.A. Lifespan extension induced by caffeine in Caenorhabditis elegans is partially dependent on adenosine signaling. Front Aging Neuroscience. 2015:7. doi: 10.3389/fnagi.2015.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghan E., Zhang Y., Saremi B., Yadavali S., Hakimi A., Dehghani M.…Lin R. Hydralazine induces stress resistance and extends C. elegans lifespan by activating the NRF2/SKN-1 signalling pathway. Nature Communications. 2017;8(1):2223. doi: 10.1038/s41467-017-02394-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskelinen M.H., Kivipelto M. Caffeine as a protective factor in dementia and Alzheimer’s disease. Journal of Alzheimer’s Disease. 2010;20(s1):S167–S174. doi: 10.3233/JAD-2010-1404. [DOI] [PubMed] [Google Scholar]
- Hall S., Desbrow B., Anoopkumar-Dukie S., Davey A.K., Arora D., McDermott C.…Grant G.D. A review of the bioactivity of coffee, caffeine and key coffee constituents on inflammatory responses linked to depression. Food Research International. 2015;76:626–636. doi: 10.1016/j.foodres.2015.07.027. [DOI] [PubMed] [Google Scholar]
- Heidler T., Hartwig K., Daniel H., Wenzel U. Caenorhabditis elegans lifespan extension caused by treatment with an orally active ROS-generator is dependent on DAF-16 and SIR-2.1. Biogerontology. 2010;11(2):183–195. doi: 10.1007/s10522-009-9239-x. [DOI] [PubMed] [Google Scholar]
- Hesp K., Smant G., Kammenga J.E. Caenorhabditis elegans DAF-16/FOXO transcription factor and its mammalian homologs associate with age-related disease. Experimental Gerontology. 2015;72:1–7. doi: 10.1016/j.exger.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Higdon J.V., Frei B. Coffee and health: A review of recent human research. Critical Reviews in Food Science and Nutrition. 2006;46(2):101–123. doi: 10.1080/10408390500400009. [DOI] [PubMed] [Google Scholar]
- Kenyon C. The first long-lived mutants: Discovery of the insulin/IGF-1 pathway for ageing. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2011;366(1561):9–16. doi: 10.1098/rstb.2010.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.S., Kennedy S., Tolonen A.C., Ruvkun G. DAF-16 target genes that control C. elegans life-span and metabolism. Science. 2003;300(5619):644–647. doi: 10.1126/science.1083614. [DOI] [PubMed] [Google Scholar]
- Leonov A., Arlia-Ciommo A., Piano A., Svistkova V., Lutchman V., Medkour Y., Titorenko V.I. Longevity extension by phytochemicals. Molecules. 2015;20(4):6544–6572. doi: 10.3390/molecules20046544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Guo X., Park Y., Huang X., Sinha R., Freedman N.D.…Chen H. Caffeine intake, smoking, and risk of Parkinson disease in men and women. American Journal of Epidemiology. 2012;175(11):1200–1207. doi: 10.1093/aje/kwr451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S.C. Regulation of glutathione synthesis. Molecular Aspects of Medicine. 2009;30(1):42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P.-Z., Lai C.-Y., Chan W.-H. Caffeine induces cell death via activation of apoptotic signal and inactivation of survival signal in human osteoblasts. International Journal of Molecular Sciences. 2008;9(5):698–718. doi: 10.3390/ijms9050698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lublin A., Isoda F., Patel H., Yen K., Nguyen L., Hajje D.…Mobbs C. FDA-approved drugs that protect mammalian neurons from glucose toxicity slow aging dependent on cbp and protect against proteotoxicity. PLoS One. 2011;6(11) doi: 10.1371/journal.pone.0027762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M.P. Hormesis defined. Ageing Research Reviews. 2008;7(1):1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan N., Chen C.-S., Hsieh H.-H., Wu Y.-C., Chang H.-C. In vivo imaging and toxicity assessments of fluorescent nanodiamonds in Caenorhabditis elegans. Nano Letters. 2010;10(9):3692–3699. doi: 10.1021/nl1021909. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay A., Oh S.W., Tissenbaum H.A. Worming pathways to and from DAF-16/FOXO. Experimental Gerontology. 2006;41(10):928–934. doi: 10.1016/j.exger.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Nikoo M., Regenstein J.M., Ahmadi Gavlighi H. Antioxidant and antimicrobial activities of (-)-epigallocatechin-3-gallate (EGCG) and its potential to preserve the quality and safety of foods. Comprehensive Reviews in Food Science and Food Safety. 2018 doi: 10.1111/1541-4337.12346. [DOI] [PubMed] [Google Scholar]
- O'Reilly L.P., Luke C.J., Perlmutter D.H., Silverman G.A., Pak S.C. C. elegans in high-throughput drug discovery. Advanced Drug Delivery Reviews. 2014;69:247–253. doi: 10.1016/j.addr.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.K., Tedesco P.M., Johnson T.E. Oxidative stress and longevity in Caenorhabditis elegans as mediated by SKN-1. Aging Cell. 2009;8(3):258–269. doi: 10.1111/j.1474-9726.2009.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto H., Roxo M., Krstin S., Röhrig T., Richling E., Wink M. An anthocyanin-rich extract of acai (Euterpe precatoria Mart.) increases stress resistance and retards aging-related markers in Caenorhabditis elegans. Journal of Agriculture and Food Chemistry. 2016;64(6):1283–1290. doi: 10.1021/acs.jafc.5b05812. [DOI] [PubMed] [Google Scholar]
- Peixoto H., Roxo M., Röhrig T., Richling E., Wang X., Wink M. Anti-aging and antioxidant potential of paullinia cupana var. sorbilis: Findings in Caenorhabditis elegans indicate a new utilization for roasted seeds of guarana. Medicines. 2017;4(3):61. doi: 10.3390/medicines4030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybysz A.J., Choe K.P., Roberts L.J., Strange K. Increased age reduces DAF-16 and SKN-1 signaling and the hormetic response of Caenorhabditis elegans to the xenobiotic juglone. Mechanisms of Ageing and Development. 2009;130(6):357–369. doi: 10.1016/j.mad.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow M., Zarse K. How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis) Experimental Gerontology. 2010;45(6):410–418. doi: 10.1016/j.exger.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Rivera-Oliver M., Díaz-Ríos M. Using caffeine and other adenosine receptor antagonists and agonists as therapeutic tools against neurodegenerative diseases: A review. Life Sciences. 2014;101(1):1–9. doi: 10.1016/j.lfs.2014.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab S., Mallam D., Cox G.A., 2nd, Tong M.J. Impact of coffee on liver diseases: A systematic review. Liver International. 2014;34(4):495–504. doi: 10.1111/liv.12304. [DOI] [PubMed] [Google Scholar]
- Sinha R.A., Farah B.L., Singh B.K., Siddique M.M., Li Y., Wu Y.…Zhou J. Caffeine stimulates hepatic lipid metabolism by the autophagy-lysosomal pathway in mice. Hepatology. 2014;59(4):1366–1380. doi: 10.1002/hep.26667. [DOI] [PubMed] [Google Scholar]
- Strayer A., Wu Z., Christen Y., Link C.D., Luo Y. Expression of the small heat-shock protein Hsp16-2 in Caenorhabditis elegans is suppressed by Ginkgo biloba extract EGb 761. FASEB Journal. 2003;17(15):2305–2307. doi: 10.1096/fj.03-0376fje. [DOI] [PubMed] [Google Scholar]
- Sutphin G.L., Bishop E., Yanos M.E., Moller R.M., Kaeberlein M. Caffeine extends life span, improves healthspan, and delays age-associated pathology in Caenorhabditis elegans. Longevity and Healthspan. 2012;1:9. doi: 10.1186/2046-2395-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissenbaum H.A. Current topics in developmental biology. Elsevier; 2018. DAF- 16: FOXO in the context of C. elegans; pp. 1–21. [DOI] [PubMed] [Google Scholar]
- Wei Y., Kenyon C. Roles for ROS and hydrogen sulfide in the longevity response to germline loss in Caenorhabditis elegans. Proceedings of the National Academy of Sciences United States of America. 2016;113(20):E2832–E2841. doi: 10.1073/pnas.1524727113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z., Ni Y., Kokot S. Simultaneous determination of caffeine, theophylline and theobromine in food samples by a kinetic spectrophotometric method. Food Chemistry. 2013;141(4):4087–4093. doi: 10.1016/j.foodchem.2013.06.121. [DOI] [PubMed] [Google Scholar]
- Yang W., Hekimi S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biology. 2010;8(12) doi: 10.1371/journal.pbio.1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.