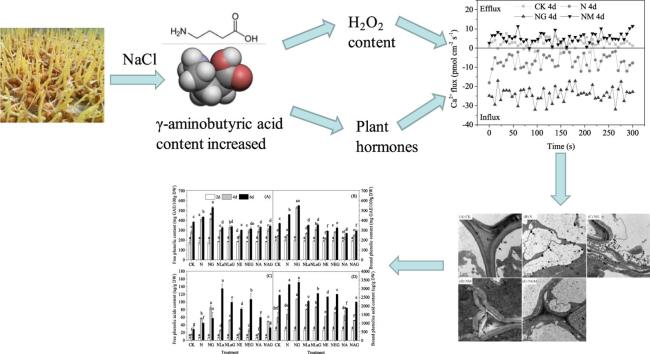
Graphical abstract
Keywords: GABA, Ca2+, Phenolics, NaCl stress, Germinated hulless barley
Highlights
-
•
GABA and Ca2+ were essential for phenolics accumulation.
-
•
GABA treatment induced an increase in calcium and calmodulin (CaM) content.
-
•
GABA treatment induced Ca2+ influx in barley root tips cells.
-
•
Ca2+ was involved in GABA signal transduction for phenolics accumulation.
Abstract
In this study, in order to investigate the role of Ca2+ in GABA signal transduction involved in phenolics accumulation in barley seedlings under NaCl stress, the seedlings were treated with exogenous GABA and its synthesis inhibitor, 3-mercaplopropionic acid (3-MP), as well as Ca2+ channel blockers La3+, Ca2+ chelator EGTA, and Ca2+ release channel inhibitor 2-aminoethoxydiphenyl borate (2-APB). The results showed that GABA significantly enhanced phenolics, calcium and calmodulin content. It also induced Ca2+ influx in barley root tips cells, and altered the distribution of Ca2+, making calcium precipitates more uniform and intensive. While, 3-MP treatment led to opposite changes, which suggested that GABA was essential for calcium content increase. In addition, accumulation of phenolics was inhibited by LaCl3, EGTA and 2-APB treatments, and this inhibition could be alleviated partly by exogenous GABA. Taken together, Ca2+ was involved in GABA signal transduction for phenolics accumulation in barley seedlings under NaCl stress.
1. Introduction
Calcium, as an essential mineral nutrient in plants, plays an important role in maintaining the stability of cell wall and cell membranes, and regulating the transport of inorganic ions (Ali, 2003). Moreover, Ca2+ serves as a ubiquitous and significant second messenger in the signal transduction networks of plants (Anil & Rao, 2001). Under stress, plants can initiate a series of physiological and biochemical processes by improving the concentration of free Ca2+ in the cytoplasm and combining Ca2+ with calmodulin (CaM), thus playing an important role in the transmission, response and adaptation of plants to stress (And & Wayne, 2003). Castañeda and Pérez (1996) demonstrated that foliar application of 1 μM CaCl2 increased phenylalanine ammonia-lyase (PAL) activity and caused subsequent accumulation of phenolics in Citrus limon. The calcium also proved to elevate the activity of PAL involved in phenolics metabolism and increase total soluble phenolic content in potato tubers (Ngadze, Coutinho, Icishahayo, & Waals, 2014). Exogenous Ca2+ improved phenolic compound contents and PAL activity of germinated wheat under high salinity (Yücel & Heybet, 2016). These results suggested that Ca2+ is closely related to phenolic compounds metabolism and the related enzyme activity.
γ-aminobutyric acid (GABA) is a non-protein amino acid and its physiological function is very extensive in higher plant. GABA is related to a series of important life activities in plants, such as pH regulation, carbon/nitrogen nutrient balance, growth and development, stress response and the like (Kinnersley & Turano, 2000). Besides, GABA involvement in signal transmission in different plants has been verified (Gilliham & Tyerman, 2016). GABA has been proven to be a signal molecule for pollen tube growth and guidance in Arabidopsis (Renault et al., 2011). Shi et al. (2010) also proposed that GABA as a signal molecule in regulating genes expression in plants under salt stress. Moreover, it was recently found that GABA is considered negatively regulated aluminum-activated malate transporter channel activity, which led to the changes in plant growth (Ramesh et al., 2015). Therefore, GABA was considered to play a dual role as both a metabolite and a legitimate plant-signaling molecule (Bouché & Fromm, 2004). Previously, we proposed a view that GABA acting as an important signal molecule in the metabolism of phenolics accumulation in germinated hulless barley under NaCl stress (Ma et al., 2019). However, signal transduction usually needs the involvement of downstream signaling molecules. Although GABA has been proven to be a signaling molecule for phenolic compound enrichment, the mechanism remains unclear.
As is well known, external stimulation induces increased intracellular Ca2+ concentration and activates the glutamate carboxylase (GAD), thereby inducing the increased GABA content to regulate physiological responses in plants (Yang, Yin, Guo, & Gu, 2013). In addition, the physiological role of Ca2+ in GABA has been demonstrated in many animals. GABA has been shown to regulate Ca2+ signaling in developing rat cerebellar Purkinje neurons (Eilers, Plant, Marandi, & Konnerth, 2010). GABA was also found to increase intracellular Ca2+ concentrations in mouse gonadotropin-releasing hormone neurons, through a calcium-induced calcium release mechanism involving internal calcium storage and inositol-1,4,5-trisphosphate receptors (Constantin, Jasoni, Wadas, & Herbison, 2010). Dave and Bordey (2010) reported that GABA depolarized granule cell precursors via GABAA receptors, which led to increased calcium levels in these cells. However, the GABA receptor has not been found in plants yet, and the relationship between the Ca2+ signaling pathway and GABA is still controversial. Recent studies have shown that GABA regulated Ca2+ signaling in several plants. GABA existed in the style of a concentration gradient, which activated the calcium permeable channels, resulting in oscillation of Ca2+ in tobacco pollen and affecting the level and distribution of Ca2+ in cells of pollen tube in tobacco (Yu, Liang, He, & Sun, 2006). Subsequent experiments confirmed that GABA might regulate Ca2+ channels and K+ channels via the activation of putative GABA-B type receptor on tobacco pollen tube cell membrane, and lead to Ca2+ influx and K+ outflux to modulate tobacco pollen tube polar growth and morphogenesis (Zhao, Zhao, Gong, Qin, & Yu, 2013). Nevertheless, relatively little is known about a causal relationship between Ca2+ signaling pathway and GABA-induced phenolic accumulation under NaCl stress. Whether Ca2+ is involved in GABA signaling for phenolics accumulation in germinated barley under NaCl stress has not been confirmed.
To address this issue, we have now investigated the plasmalemma Ca2+ channel blockers La3+, the Ca2+ chelator ethylene glycol tetraacetic acid (EGTA), and the Ca2+ release channel inhibitor 2-aminoethoxydiphenyl borate (2-APB) on phenolic components accumulation in germinated hulless barley under NaCl stress. The effect of GABA on endogenous calcium and CaM level, intracellular Ca2+ distribution, and the Ca2+ flux of barley root tip tissue were also investigated. The purpose of all these experiments was to explore supporting evidence for Ca2+ involved in GABA signal transduction for phenolics accumulation in germinated hulless barley under NaCl stress.
2. Materials and methods
2.1. Material and experimental design
Barley seeds were supplied by the Institute of Agricultural Sciences of the Yangtze River Bank (Jiangsu, China). They were sterilized by soaking in 0.5% NaClO for 10 min, washed and then steeped in distilled water at 25 °C for 6 h. Soaked seeds were then cultivated in a growth chamber with distilled water at 25 °C for 2 days, firstly. Then different treatments were performed from the 3rd day as follows: (1) CK: distilled water; (2) N: 60 mM NaCl; (3) NG: 60 mM NaCl + 0.5 mM GABA; (4) NLa: 60 mM NaCl + 5 mM LaCl3; (5) NLaG: 60 mM NaCl + 5 mM LaCl3 + 0.5 mM GABA; (6) NE: 60 mM NaCl + 0.4 mM EGTA; (7) NEG: 60 mM NaCl + 0.4 mM EGTA + 0.5 mM GABA; (8) NA: 60 mM NaCl + 0.1 mM 2-APB; (9) NAG: 60 mM NaCl + 0.1 mM 2-APB + 0.5 mM GABA; (10) NM: 60 mM NaCl + 0.1 mM 3-MP; (11) NGM: 60 mM NaCl + 0.5 mM GABA + 0.1 mM 3-MP. The barley seedlings under different treatments were collected at 2 d, 4 d and 6 d of germination for further analysis.
2.2. Extraction and determination of free and bound phenolics
The free and bound phenolics were extracted following the method of Ma et al. (2019). The total phenolic content was assayed according to the Folin-Ciocalteu colorimetric method (Ma et al., 2019).
The content of phenolic acids was measured as described by Ma et al. (2019) using high performance liquid chromatography (HPLC, Shimadzu LC-20A, Japan) fitted with a SPD-M20A diode array detector and an Gemini C18 110A column (4.6 mm × 150 mm, 5 µm; Phenomenex, Torrance, California, USA).
2.3. Determination of calcium and CaM content
The total calcium and water soluble calcium contents were measured by inductively coupled plasma optical emission spectroscopy (ICP-OES, Optima 2100 DV, PerkinElmer, USA) according to the method of Meyer and Popp (1997). The total calcium was extracted with nitric acid. Specifically, 5 ml of concentrated nitric acid was added to 0.2 g of dried samples for microwave digestion. After 1 h, the cooled liquid was transferred to a 50 ml volumetric bottle. And for water soluble calcium, 0.6 g dried samples were extracted with 50 ml of ultrapure water at 30 °C overnight. Results are presented as mg/g DW.
The CaM content was measured as Hu et al. (2007) described. The fresh barley seedlings were ground and homogenized in Tris-HCl buffer (pH 7.5, including 1 mM EGTA, 0.5 mM phenylmethylsulfonyl fluoride (PMSF), 20 mM NaHCO3 and 150 mM NaCl) at 1:8 (w/v). The determination of CaM content was performed by enzyme-linked immunosorbent assay (ELISA, catalog no. MBE21030, Nanjing Jiancheng Bioengineering Institute, Jiangsu, China). The result was presented as ng/g FW.
2.4. Determination of Ca2+-ATPase activity
The extraction of crude mitochondria was performed as Jin et al. (2013) described. Ten fresh barley seedlings were homogenized using Tris-HCl buffer (pH 7.5, including 0.25 M sucrose, 0.3 M mannite, 1 mM EDTA, 0.5 g/l polyvinyl pyrrolidone and 4 mM dithiothreitol) at 1:4 (w/v). After centrifugation (4000 × g, 15 min), the supernatant was centrifuged again (16,000 × g, 25 min). The sediment was washed using 10 mM of Tris-HCl buffer (pH 7.2, including 0.25 M sucrose, 0.3 M mannite, 1 mM EDTA and 1 mM dithiothreitol) and then centrifuged (4000 × g, 15 min). The supernatant was centrifuged again. The final sediment was dissolved in 1.5 ml washing buffer (Tris-HCl buffer, pH 7.2, including 0.25 M sucrose, 0.3 M mannite, 1 mM EDTA and 1 mM dithiothreitol) and used for the subsequent assay. The reaction mixture contained 0.75 ml Tris-HCl buffer (30 mM, pH 8.0, including 3 mM MgSO4, 0.2 mM Na3VO4, 50 mM NaNO3, 50 mM KCl, 4 mM Ca(NO3)2 and 0.1 mM ammonium molybdate), 0.1 ml of the supernatant, and 0.15 ml ATP-Tris (30 mM, pH 8.0). One Ca2+-ATPase activity unit (U) was defined as the release of 1 nmol of inorganic phosphorus per minute in absorbance at 660 nm.
2.5. Subcellular location of Ca2+
The subcellular location of Ca2+ in barley seedlings was assayed by the antimonite precipitation technique and transmission electron microscopy (TEM) as described by Zhou, Wang, Yang, Wang, and Gu (2017)). The leaf and root segments were immersed in a fixing solution for 6 h and were then rinsed with phosphate buffer (pH 7.6), then, fixed with 1% osmium tetroxide for 2 h, and rinsed with distilled water for 2 times. After dehydration in an acetone system (50% acetone for 10 min, 70% acetone for 10 min, 90% acetone for 10 min, 100% acetone for 10 min, 100% acetone for 10 min), part of the pure dehydrating agent was discarded and a small amount of Epon812 epoxy resin (02659-AB, Structure Probe, Inc.) was added to blend, and the materials were soaked at room temperature for 4 h. The infiltrated materials were cut into pieces of about 1 mm3 and placed in the embedded mold. After the embedding agent was added, the polymerization was carried out at 25 °C for 24 h, 45 °C for 24 h and 60 °C for 24 h.
2.6. Measurement of Ca2+ flux
The Ca2+ flux in the root tip cells of germinated barley was determined non-invasively with a scanning ion-selective electrode technique (SIET), as described previously (Tang et al., 2014). This test was carried out by NMT Physiolyzer® (Younger USA. LLC. Model: NMT100-YG) at the Institute of Soil Science, Chinese Academy of Sciences (Nanjing, China). The Ca2+-selective microelectrodes were prepared as described by Wang et al. (2010) The roots were carefully soaked in a testing liquid (pH 6.0, including 0.1 mM KCl, 0.1 mM CaCl2, 0.1 mM MgCl2, 0.5 mM NaCl, 0.3 mM MES, 0.2 mM Na2SO4) for 20 min before the test began to ensure the stability of ion fluxes. For measurement of Ca2+ flux in the root tip cells of barley seedlings under different treatments (CK, N, NG, NM), three seedlings at each treatment were analyzed, and at least three roots in each seedling were tested. All readings were 300 s in duration.
2.7. Western blot
The barley seedlings used for Western blot assays were sampled on day 6. The membranes were incubated with primary antibody (anti-CaMK, anti-CDPK and anti-CCaMK, own-made) overnight at 4 °C and then washed 4 times for 5 min each with TBST and were then incubated with secondary goat polyclonal antibody conjugated to horseradish peroxidase (goat anti-mouse IgG, 1:5000; goat anti-rabbit IgG, 1:5000, Thermo Pierce) for 1 h at 25 °C. The subsequent experiment was carried out according to the method of Jiao et al. (Jiao, Wang, Yang, Tian, & Gu, 2016).
2.8. Gene expression assay (quantitative real-time PCR, QRT-PCR)
The gene expression was performed as Ma et al. (2019) described. Total RNA was extracted using a Takara MiniBEST Plant RNA Extraction Kit. For first-strand cDNA synthesis, moderate amounts of total RNA were reverse transcribed, using the PrimeScriptH RT reagent Kit (Takara, DaLian, China; Code: DRR037A). Three PCRs per sample were performed for quantitative assays using the SYBR® Premix Ex TaqTM (TAKARA: RR420A) in the ABI 7500 sequence detection system (Applied Biosystems, Foster City, CA, USA). ΔΔCt was used as the calculation method. The 4 days-barley seedlings treated with distilled water was used as a comparison. The sequence-specific primers for QRT-PCR analysis were as follows; Ca2+-ATPase: 5ˊ-CCACCGTCATCTGCTCCGAC −3ˊ and 5ˊ-ATTGCACACAGCTGCGACCT-3ˊ, Actin: 5ˊ-TCGTGAGAAGATGACCCAGA-3ˊ and 5ˊ-CCGAGTCCAGCACAATACCT-3ˊ.
2.9. Statistical analysis
Significant differences were tested using SPSS 18.0 (SPSS Inc., Chicago, USA). The variables with three or more replications were compared through Duncan's multi-range test. Differences at p < 0.05 were considered as significant among treatments.
3. Results
3.1. Changes of total phenolic and phenolic acids content
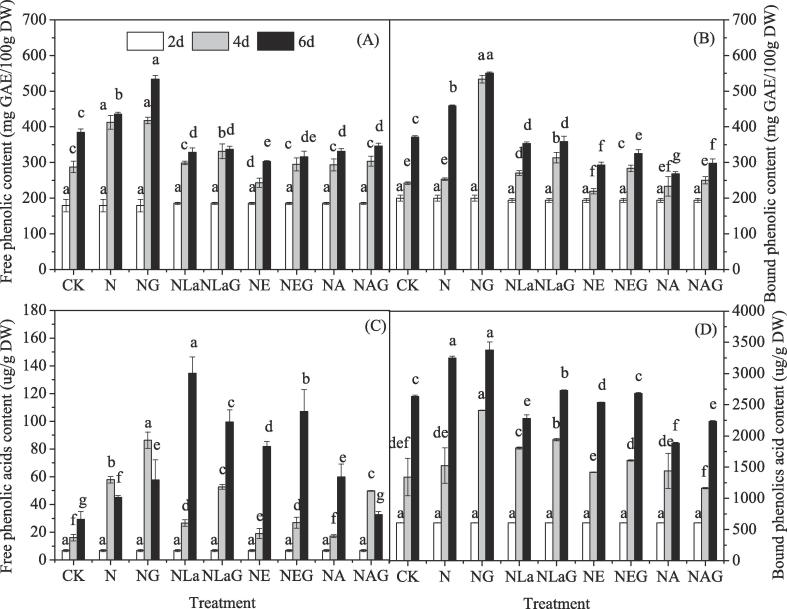
In this study, the contents of total phenolic (Fig. 1A, B) and phenolic acids (Fig. 1C, D) of barley seedlings were detected. The highest free and bound phenolic content was observed on day 4 after GABA plus NaCl treatment, which was 12% and 20% higher than that of NaCl-treated alone (Fig. 1). However, the plasmalemma Ca2+ channel blockers La3+, Ca2+ chelator EGTA and Ca2+ release channel inhibitor 2-APB treatments exhibited a negative effect on phenolic accumulation. While, the application of GABA could alleviate this inhibition largely. Compared with that of LaCl3, EGTA and 2-APB-treated alone, LaCl3 + GABA, EGTA + GABA and 2-APB + GABA treatments increased total phenolic content by 13%, 25% and 5%, respectively, at the 4th day of germination. Interestingly, there was no difference in total phenolic content among LaCl3, EGTA and 2-APB treatments with or without GABA treatment at the 6th day of germination.
Fig. 1.
Changes in total phenolic and phenolic acids content of germinated hulless barley under different treatments. Bars represent standard error of means (n ≥ 3) and means with different letters are significantly different among different treatments (p < 0.05). CK means the barley was sprayed with distilled water; N means 60 mM NaCl treatment; NG means 60 mM NaCl + 0.5 mM GABA treatment; NLa means 60 mM NaCl + 5 mM LaCl3 treatment; NLaG means 60 mM NaCl + 5 mM LaCl3 + 0.5 mM GABA treatment; NE means 60 mM NaCl + 0.4 mM EGTA treatment; NEG means 60 mM NaCl + 0.4 mM EGTA + 0.5 mM GABA treatment; NA means 60 mM NaCl + 0.1 mM 2-APB treatment; NAE means 60 mM NaCl + 0.1 mM 2-APB + 0.5 mM GABA treatment.
The similar result was observed in total phenolic acids content. CaCl2 treatment increased the total phenolic acids content by 77% and 12%, respectively, after germination for 4 and 6 days compared with that of NaCl treatment. As shown in Fig. 1, bound phenolic acids accounted for more than 94% of total phenolic acids content. LaCl3, EGTA, and 2-APB treatments reduced the total phenolic acids content by 27%, 21% and 41%, respectively, after germination for 6 days although the total free phenolic acids content increased when compared with that of NaCl used alone. Similarly, LaCl3, EGTA and 2-APB plus GABA treatments relieved the reduction of total phenolic acids content.
3.2. Changes of total calcium, water soluble calcium and CaM content
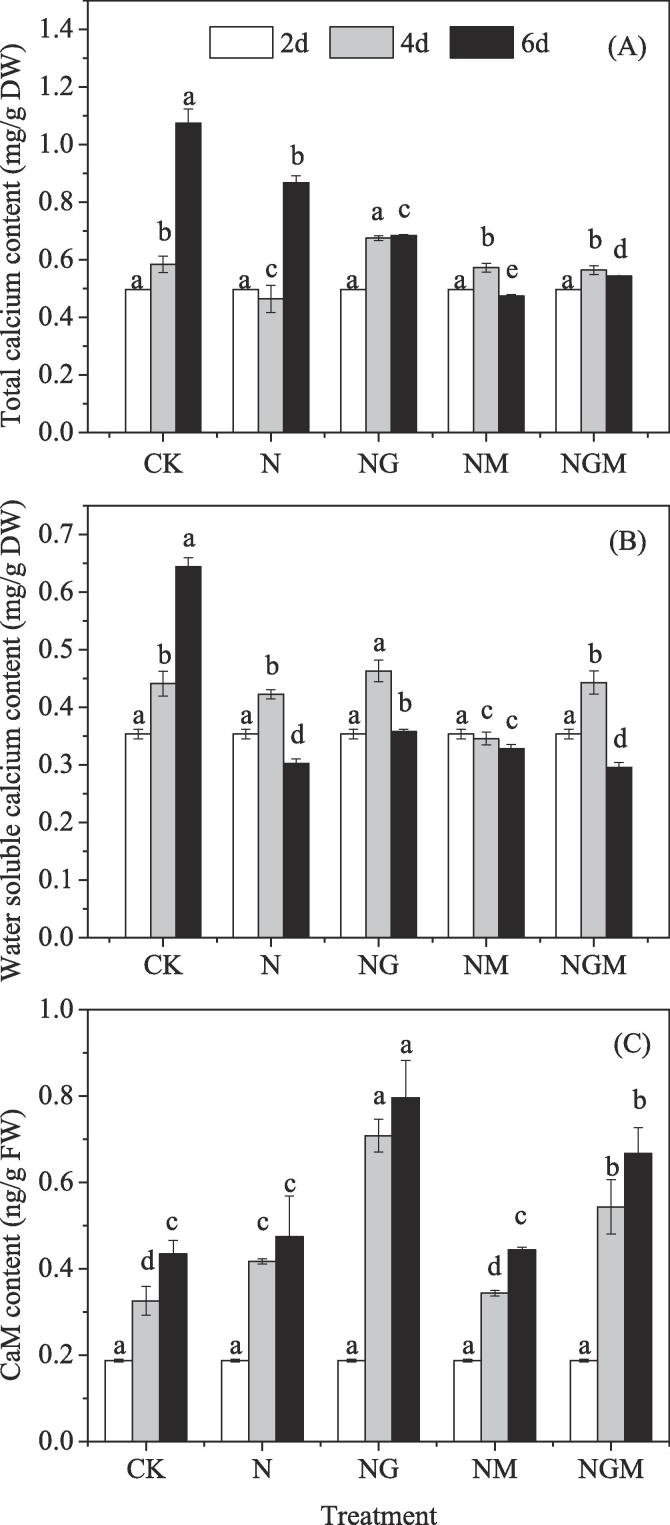
The influence of different treatments on the content of total calcium, water soluble calcium and CaM in barley seedlings is shown in Fig. 2. With increasing treatment time, NaCl stress significantly decreased the total calcium and water soluble calcium content compared with the control. However, the application of GABA plus NaCl significantly increased the total calcium content by 46% at the 4th day, but decreased the total calcium content at the 6th day of germination (Fig. 2A). And water soluble calcium content increased during the whole progress of germination compared with that of NaCl used alone (Fig. 2B). While, 3-MP treatment significantly down-regulated the total calcium and soluble calcium content compared with that of NaCl-treated seedlings. The addition of GABA alleviated this inhibition effectively.
Fig. 2.
Changes in total calcium content (A), water soluble calcium content (B) and CaM content (C) of germinated hulless barley under different treatments. Bars represent standard error of means (n ≥ 3) and means with different letters are significantly different among different treatments (p < 0.05). CK means the barley was sprayed with distilled water; N means 60 mM NaCl treatment; NG means 60 mM NaCl + 0.5 mM GABA treatment; NM means 60 mM NaCl + 0.1 mM 3-MP treatment; NGM means 60 mM NaCl + 0.5 mM GABA + 0.1 mM 3-MP treatment.
The CaM content increased continually during the germination (Fig. 2C), and it increased rapidly under GABA application, which was 70% and 68% higher than that of NaCl used alone at 4 and 6 days of germination. The 3-MP application significantly weakened the positive effects of NaCl stress on CaM content by 18% at the 4 days of germination compared with the control. The GABA treatment reversed the attenuation by 58% and 50% at 4 and 6 days of germination compared with that of 3-MP-treated seedlings.
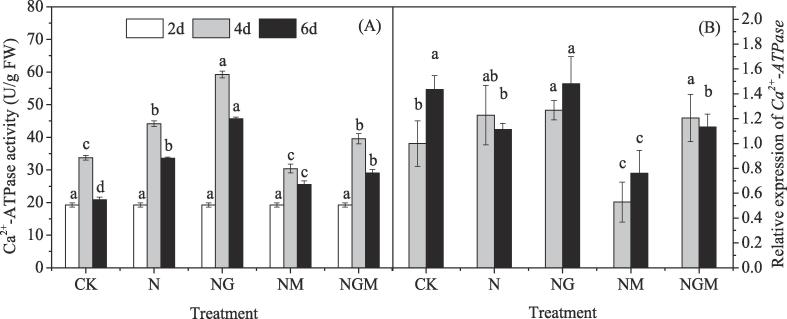
3.3. Changes of activity and gene expression of Ca2+-ATPase
The Ca2+-ATPase activity increased first and decreased afterward during germination (Fig. 3A). Compared with the control, Ca2+-ATPase activity was significantly improved by NaCl treatment. GABA application enhanced this increase, which was 35% and 34% higher than that of the NaCl-treated seedlings at 4 and 6 days of germination. In contrast, application of 3-MP alone significantly reduced Ca2+-ATPase activity during germination compared with the NaCl-treated seedlings, with a maximum reduction of 31% observed in Ca2+-ATPase activity at 4 days after germination. 3-MP plus GABA treatment reversed the inhibition effectively. Interestingly, the variation in gene expression (Fig. 3B) exhibited inconsistent trends with the activity of Ca2+-ATPase (Fig. 3A). NaCl stress had no significant effect on the expression of Ca2+-ATPase at 4 days but decreased it at 6 days of germination compared with the control. GABA treatment induced elevation of Ca2+-ATPase expression by 33% at the 6th day of germination, but showed no significant increase at the 4th day compared with NaCl-treated barley seedlings. Similarly, 3-MP treatment significantly down-regulated the expression of Ca2+-ATPase by 57% and 31% at 4 and 6 days of germination compared with that of NaCl alone. While, the addition of GABA alleviated this inhibition effectively.
Fig. 3.
Changes in activity and relative expression of Ca2+-ATPase of germinated hulless barley under different treatments. Bars represent standard error of means (n ≥ 3) and means with different letters are significantly different among different treatments (p < 0.05). CK means the barley was sprayed with distilled water; N means 60 mM NaCl treatment; NG means 60 mM NaCl + 0.5 mM GABA treatment; NM means 60 mM NaCl + 0.1 mM 3-MP treatment; NGM means 60 mM NaCl + 0.5 mM GABA + 0.1 mM 3-MP treatment.
3.4. Subcellular location of Ca2+ in leaf and root of germinated barley
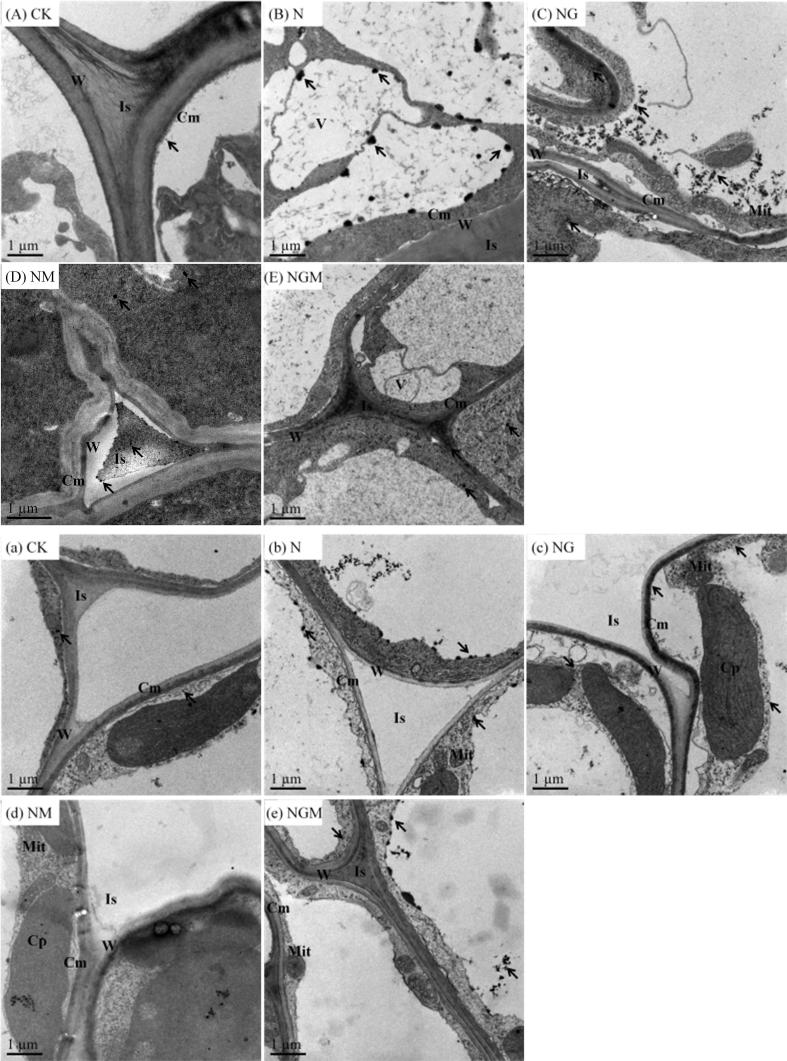
The distribution and accumulation of calcium precipitates in barley root cells underwent remarkable changes under different treatments (Fig. 4). The localization of calcium precipitates after NaCl treatment altered obviously. There were little calcium precipitates located in intercellular spaces, but a small number of precipitates were close to the cell wall in the control barley seedlings (Fig. 4A). NaCl stress significantly increased the number of precipitates in the cytosol and cell walls. In addition, the precipitates in the cytosol were large, sparse and appeared as globular-to-granular, and most of them were uniformly distributed in the cytoplasm other than close to the cell wall (Fig. 4B). After GABA treatment, the most significant change was the presence of calcium precipitates in intercellular spaces. Besides, the precipitates in the cytosol were smaller but intensive compared with the NaCl-treated seedlings, and most of them were close to the cell wall (Fig. 4C). Few precipitates were detected in the cytosol and the intercellular spaces under 3-MP treatment, and they were small, sparse and not close to the cell wall (Fig. 4D). While the number of precipitates increased slightly in the cytosol and intercellular spaces under 3-MP plus GABA treatment, and most precipitates were close to the cell wall (Fig. 4E).
Fig. 4.
Ca2+ location in barley root and leaf cells after germination for 6 days under different treatments. W: cell wall; Is: intercellular space; V: vacuole; Cm: cell membrane; Mit: mitochondria; Cp: chloroplast. Arrows represent the Ca. Capital letters (A-E) represent Ca2+ location in barley root cells, and lowercase letters (a-e) represent Ca2+ location in barley leaf cells. CK means the germinated barley was sprayed with distilled water; N means 60 mM NaCl treatment; NG means 60 mM NaCl + 0.5 mM GABA treatment; NM means 60 mM NaCl + 0.1 mM 3-MP treatment; NGM means 60 mM NaCl + 0.5 mM GABA + 0.1 mM 3-MP treatment.
The distribution and accumulation of calcium precipitates in barley leaf cells showed different changes under all the treatments (Fig. 4). NaCl stress induced many small calcium precipitates accumulating in the cell walls and cell membranes during germination (Fig. 4b). GABA application made the precipitates in the cytoplasm smaller and more dispersed (Fig. 4c). After treatment with 3-MP, precipitates in leaf cells were virtually undetected (Fig. 4d). However, 3-MP plus GABA treatment significantly increased the number of precipitates in the cytosol (Fig. 4e).
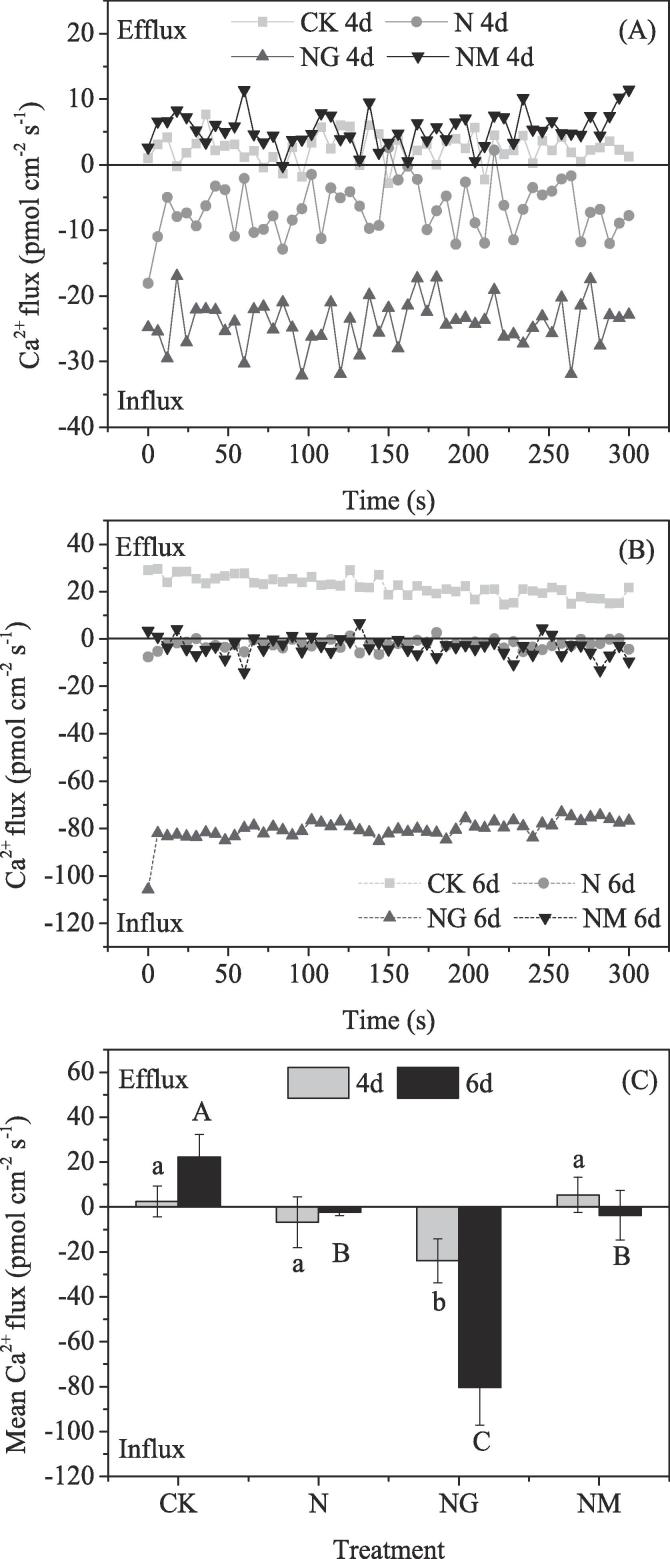
3.5. Changes of Ca2+ flux at the barley tip
The results indicated that Ca2+ efflux of control barley seedlings increased gradually with prolonged germination time (Fig. 5). Compared with the control, NaCl treatment caused a shift of Ca2+ efflux towards an influx in tip root cells of barley seedlings. GABA application induced a strong and steady net Ca2+ influx, which reached 24 pmol cm−2 s−1 after 4 d and 80 pmol cm−2 s−1 after 6 d of germination (Fig. 5C). It was noticeable that, GAD inhibitor (3-MP) used in our experiment resulted in different trends during germination time, which markedly enhanced the efflux rate of Ca2+ at the 4th day compared with NaCl alone, but led to a weak Ca2+ influx at the 6th day of germination.
Fig. 5.
Changes in net Ca2+ flux between 0 and 300 s in measuring solutions after different treatment. (A) Time course of net Ca2+ flux of barley tip cells measured using a Ca2+-selective microelectrodes. The barley was treated with different treatments for four days. (B) Time course of net Ca2+ flux of barley tip cells measured using a Ca2+-selective microelectrodes. The barley was treated with different treatments for six days. (C) Effect of different treatments on mean value (n ≥ 6) of Ca2+ flux between 0 and 300 s at the barley tip cells. Upward deflection corresponds to an increase in net Ca2+ efflux. Barley roots were incubated in the measuring solution to equilibrate for 20 min ahead. Steady-state ion fluxes were then recorded until the values variation amplitude is relatively stable. Bars represent standard error of means (n = 6) and means with different letters are significantly different among different treatments (p < 0.05). CK means the barley was sprayed with distilled water; N means 60 mM NaCl treatment; NG means 60 mM NaCl + 0.5 mM GABA treatment; NM means 60 mM NaCl + 0.1 mM 3-MP treatment.
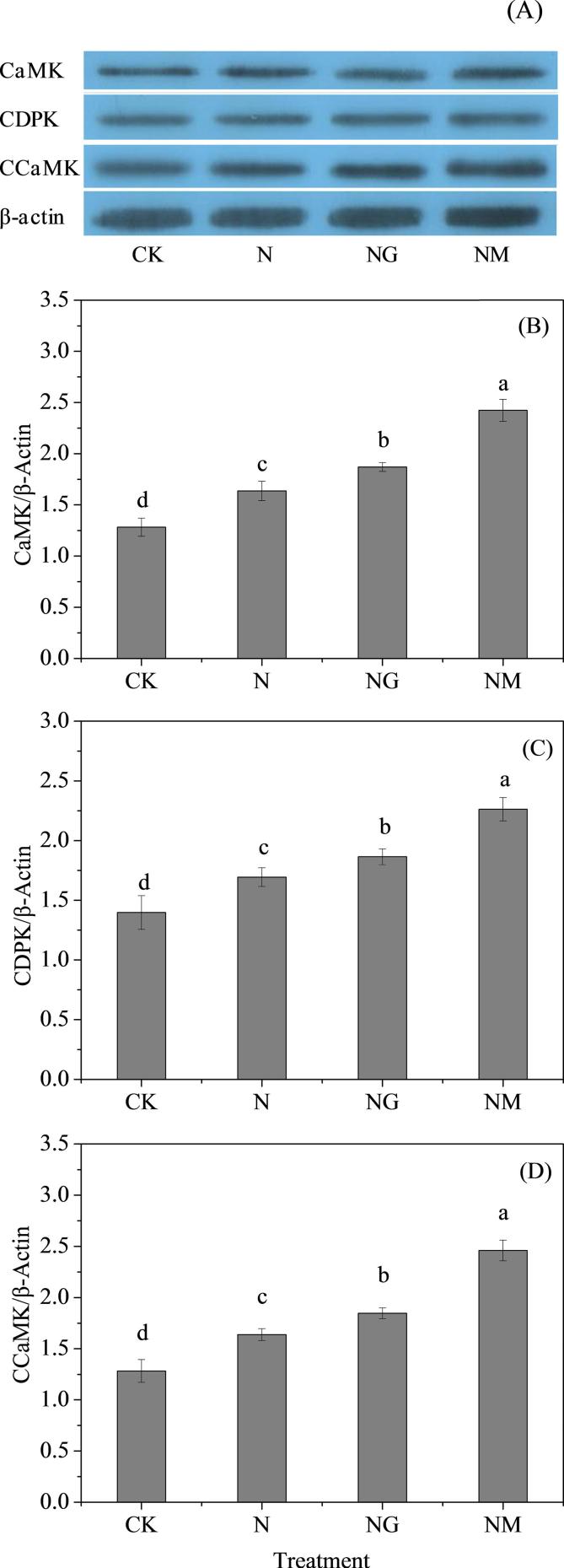
3.6. Changes of protein expression of CaMK, CDPK and CCaMK
The protein expression of calcium-binding proteins including calmodulin dependent protein kinase (CaMK), Ca2+ dependent protein kinase (CDPK) and Ca2+ and calmodulin dependent protein kinase (CCaMK) were detected at the 6th day of germination. Compared with the control, NaCl treatment significantly induced elevation of protein expression of CaMK, CDPK and CCaMK (Fig. 6). Exogenous GABA plus NaCl treatment could significantly up-regulated the protein expression of calcium-binding proteins compared with that of NaCl-treated seedlings. The protein expression of CaMK, CDPK and CCaMK in seedlings treated with 3-MP plus NaCl were both significantly improved compared with that of NaCl alone (Fig. 6).
Fig. 6.
Changes in relative expression of CaMK (A), CDPK (B) and CCaMK (C) of germinated hulless barley under different treatments. Bars represent standard error of means (n ≥ 3) and means with different letters are significantly different among different treatments (p < 0.05). CK means the barley was sprayed with distilled water; N means 60 mM NaCl treatment; NG means 60 mM NaCl + 0.5 mM GABA treatment; NM means 60 mM NaCl + 0.1 mM 3-MP treatment.
4. Discussion
In the present study, our results indicated that GABA plus NaCl treatment significantly increased the contents of total phenolic (Fig. 1A, B) and phenolic acids (Fig. 1C, D) of barley seedlings. However, the LaCl3, EGTA and 2-APB treatment exhibited a negative effect on phenolic accumulation. While, this inhibition could be alleviated largely by GABA application. The results indicated that Ca2+ was involved in GABA-promoted phenolics biosynthesis. Previous studies have confirmed that Ca2+ improved phenolic compound content and phenylalanine ammonia-lyase activity in germinated wheat (Yücel & Heybet, 2016), and GABA increased phenolic compound contents and up-regulated the activity of related enzymes in germinated barley (Ma et al., 2019). However, whether GABA can regulate Ca2+ signaling in germinated barley remains unclear. To address this issue, we investigated the effect of GABA on endogenous calcium content and intracellular Ca2+ distribution in barley seedlings.
NaCl stress significantly increased the number of calcium precipitates in cytosol and cell walls and induced an obvious net Ca2+ influx in barley root cells compared with the control seedlings. The results were consistent with previous studies, which reported that high salt triggered an increase in [Ca2+]cyt in Arabidopsis (Tracy, Gilliham, Dodd, Webb, & Tester, 2008) and Populus euphratica (Xuan et al., 2015). GABA application significantly enhanced the contents of total calcium and water soluble calcium in barley seedlings during the whole process of germination compared with that of NaCl used alone (Fig. 2). While, GABA synthesis inhibitor, 3-MP treatment significantly down-regulated their contents. The changes were consistent with that of the distribution and accumulation of calcium precipitates in barley root cells (Fig. 4). GABA treatment induced elevation of the number of precipitates in intercellular spaces, cytosol and cell walls, but few precipitates were detected in the cytosol and the intercellular spaces under 3-MP treatment (Fig. 4). The above results confirmed that GABA was essential for calcium content increase in barley seedlings under NaCl stress. The reason might be that GABA treatment induced a strong and steady net Ca2+ influx in tip root cells of barley seedlings (Fig. 5). Similar results were found in previous research, which indicated that GABA could specially facilitate Ca2+ influx across the membrane on the tip of tobacco pollen tubes (Zhao et al., 2013) and integrate multiple signal pathways to modulate tobacco pollen tube growth (Yu et al., 2014). However, there is still controversy in understanding the roles of chemical signals in NaCl stress, particularly in changes of GABA induced Ca2+ flux and their possible regulation of NaCl-induced phenolics accumulation of germinated hulless barley seedlings. It was reported that H2O2 activated the plasma membrane Ca2+ channels (Jian et al., 2010), and the experiment that exogenous H2O2 application to Arabidopsis thaliana root epidermis resulting in dose-dependent transient increases in net Ca2+ influx confirmed this view (Demidchik, Shabala, & Davies, 2007), which suggested that H2O2 functions as an upstream component in the [Ca2+]cyt signaling network (Demidchik et al., 2007). Our study observed that GABA or NaCl treatment induced elevation of H2O2 content compared with the control during the whole process of germination (Fig. S1), which might be one of the reasons for GABA induced Ca2+ influx. In addition, GABA plus NaCl treatment was also found to increase ABA content in barley seedlings (Fig. S2A). As is well known, Ca2+ was a key second messenger in ABA signaling. Previous work showed that Ca2+ and reactive oxygen species (ROS) were required for ABA-induced antioxidant defense in maize plants, and that Ca2+-CaM played an important role in ABA signaling (Shucheng, 2010). Therefore, ABA and H2O2 involved in GABA-induced cytosolic Ca2+ ([Ca2+]cyt) increased in barley seedlings. In addition, other plant hormones-IAA and GA3 was also detected in barley seedlings under different treatments (Fig. S2B and C). Auxin reportedly induced oscillations in [Ca2+]cyt with a period of 20–30 min superimposed on an increase in Zea mays coleoptiles (Felle, 1988) and steady-state changes in root hairs of Sinapis alba (Tretyn, Wagner, & Felle, 1991). Compared with the control, the levels of GA3 increased under GABA with or without NaCl treatment might be closely related to the increases in [Ca2+]cyt (Bush, 1995). Taken together, GABA may regulate Ca2+ flux by activating related plant hormones, and H2O2 is involved in this process.
The activity of Ca2+-ATPase from barley seedlings was markedly affected by NaCl plus GABA treatment. When the seedlings were grown under deficiency of GABA, the activity of Ca2+-ATPase was significantly decreased. While the inhibition was reversed effectively by the 3-MP plus GABA treatment (Fig. 3A). Similar change was found in the expression of Ca2+-ATPase under different treatments (Fig. 3B). As reported, Ca2+-ATPase, which has a low capacity for Ca2+ transport, is responsible for maintaining [Ca2+]cyt homeostasis in the resting cell (Kendal, 2001). NaCl treatment, as abiotic stress, stimulated the increase of [Ca2+]cyt level. Excessive [Ca2+]cyt is cytotoxic, therefore, higher Ca2+-ATPase is required to maintain a submicromolar [Ca2+]cyt level by removing Ca2+ to either the apoplast or the lumen of intracellular organelles to improve plant stress resistance and maintain plant normal growth and development (White & Broadley, 2003). Treatment with 3-MP prevented the influx of extracellular Ca2+ (Fig. 5), resulting in a low [Ca2+]cyt concentration, and thus requiring only low enzyme activity.
The addition of GABA also triggered an obvious increase of CaM content and the 3-MP treatment significantly decreased the CaM content (Fig. 2C). The reduction of CaM content by 3-MP treatment could be partially alleviated by application of GABA. The results suggested that this re-triggered CaM formation might be due to the function of GABA in inducing Ca2+ increase at the early stage of germination after GABA application. In addition, CaM reportedly modulated ROS production and acted as a molecular link between Ca2+ and ROS signal pathways in plant growth and development (Tang et al., 2014). Thus, the increased Ca2+ or CaM contents triggered H2O2 production, which might inversely affect CaM content (Hu et al., 2008). In plants, Ca2+ can combine with CaM to form Ca2+-CaM complex. CaM is a highly conserved regulatory Ca2+ sensor, which is widely found in various eukaryotic cells (Snedden & Fromm, 2010). Although CaM has no catalytic activity of its own, the Ca2+-CaM complex interacts with transcription factors through binding and regulation of the activity of many target proteins or indirectly triggering cellular responses by regulating the expression of genes encoding downstream effectors (Kim, Chung, & Yun, 2009). Our study indicated that the expression of calcium and/or Ca2+-binding proteins (CaMK, CDPK and CCaMK) was significantly up-regulated under GABA with or without NaCl treatment compared with the control throughout the germination period (Fig. 6). Exogenous GABA markedly increased the gene expression CDPK in Nicotiana tabacum, which demonstrated that CDPK was responding to GABA as a common signaling pathway (Yu et al., 2014).
5. Conclusions
This study confirmed that GABA significantly enhanced the calcium and CaM content, altered the distribution of Ca2+, and induced Ca2+ influx in barley root tips cells under NaCl stress. In addition, Ca2+ was verified to be essential for phenolic components enrichment under GABA plus NaCl treatment. Taken together, Ca2+ was involved in GABA signal transduction for phenolic accumulation in germinated hulless barley under NaCl stress.
Acknowledgments
Acknowledgements
Financial support was provided by the National Natural Science Foundation of China (Grant No. 31871725) and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Notes
The authors declare no competing financial interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2019.100023.
Contributor Information
Yan Ma, Email: 2015208008@njau.edu.cn.
Pei Wang, Email: wangpei@njau.edu.cn.
Zhenxin Gu, Email: guzx@njau.edu.cn.
Yang Tao, Email: yang.tao@njau.edu.cn.
Chang Shen, Email: shenchang@njau.edu.cn.
Yulin Zhou, Email: yljoe@njau.edu.cn.
Yongbin Han, Email: hanyongbin@njau.edu.cn.
Runqiang Yang, Email: yangrq@njau.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ali R.M. Response of salt stressed barley seedlings to phenylurea. Plant Soil & Environment. 2003;49(4):158–162. [Google Scholar]
- And P.K.H., Wayne R.O. Calcium and plant development. Annual Review of Plant Biology. 2003;36(36):397–439. [Google Scholar]
- Anil V.S., Rao K.S. Calcium-mediated signal transduction in plants: A perspective on the role of Ca2+ and CDPKs during early plant development [Review] Journal of Plant Physiology. 2001;158(10):1237–1256. [Google Scholar]
- Bouché N., Fromm H. GABA in plants: Just a metabolite? Trends in Plant Science. 2004;9(3):110–115. doi: 10.1016/j.tplants.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Bush D.S. Calcium Regulation in plant cells and its role in signaling [Review] Annual Review of Plant Physiology & Plant Molecular Biology. 1995;46(1):95–122. [Google Scholar]
- Castañeda P., Pérez L.M. Calcium ions promote the response of Citrus limon against fungal elicitors or wounding. Phytochemistry. 1996;42(3):595–598. [Google Scholar]
- Constantin S., Jasoni C.L., Wadas B., Herbison A.E. Gamma-aminobutyric acid and glutamate differentially regulate intracellular calcium concentrations in mouse gonadotropin-releasing hormone neurons. Endocrinology. 2010;151(1):262–270. doi: 10.1210/en.2009-0817. [DOI] [PubMed] [Google Scholar]
- Dave K.A., Bordey A. GABA increases Ca2+ in cerebellar granule cell precursors via depolarization: Implications for proliferation. Iubmb Life. 2010;61(5):496–503. doi: 10.1002/iub.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V., Shabala S., Davies J. Spatial variation in H2O2 response of Arabidopsis thaliana root epidermal Ca2+ flux and plasma membrane Ca2+ channels. Plant Journal. 2007;49(3):377–386. doi: 10.1111/j.1365-313X.2006.02971.x. [DOI] [PubMed] [Google Scholar]
- Eilers J., Plant T.D., Marandi N., Konnerth A. GABA-mediated Ca2+ signalling in developing rat cerebellar Purkinje neurones. Journal of Physiology. 2010;536(2):429–437. doi: 10.1111/j.1469-7793.2001.0429c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle H. Auxin causes oscillations of cytosolic free calcium and pH in Zea mays coleoptiles. Planta. 1988;174(4):495–499. doi: 10.1007/BF00634478. [DOI] [PubMed] [Google Scholar]
- Gilliham M., Tyerman S.D. Linking metabolism to membrane signaling: The GABA-malate connection. Trends in Plant Science. 2016;21(4):295–301. doi: 10.1016/j.tplants.2015.11.011. [DOI] [PubMed] [Google Scholar]
- Hu X., Jiang M., Zhang J., Zhang A., Lin F., Tan M. Calcium-calmodulin is required for abscisic acid-induced antioxidant defense and functions both upstream and downstream of H2O2 production in leaves of maize (Zea mays) plants. New Phytologist. 2007;173(1):27–38. doi: 10.1111/j.1469-8137.2006.01888.x. [DOI] [PubMed] [Google Scholar]
- Hu X., Wang W., Li C., Zhang J., Lin F., Zhang A. Cross-talks between Ca2+/CaM and H2O2 in abscisic acid-induced antioxidant defense in leaves of maize plants exposed to water stress. Plant Growth Regulation. 2008;55(3):183–198. [Google Scholar]
- Jian S., Wang M.J., Ding M.Q., Deng S.R., Liu M.Q., Lu C.F. H2O2 and cytosolic Ca2+ signals triggered by the PM H+-coupled transport system mediate K+/Na+ homeostasis in NaCl-stressed Populus euphratica cells. Plant Cell & Environment. 2010;33(6):943–958. doi: 10.1111/j.1365-3040.2010.02118.x. [DOI] [PubMed] [Google Scholar]
- Jiao C., Wang P., Yang R., Tian L., Gu Z. IP3 mediates NO-cGMP-induced isoflavone accumulation in soybean sprouts under UV-B radiation. Journal of Agricultural & Food Chemistry. 2016;64(44) doi: 10.1021/acs.jafc.6b02633. [DOI] [PubMed] [Google Scholar]
- Jin P., Zhu H., Wang J., Chen J., Wang X., Zheng Y. Effect of methyl jasmonate on energy metabolism in peach fruit during chilling stress. Journal of the Science of Food & Agriculture. 2013;93(8):1827–1832. doi: 10.1002/jsfa.5973. [DOI] [PubMed] [Google Scholar]
- Kendal Vacuolar H+/Ca2+ transport: Who's directing the traffic? Trends in Plant Science. 2001;6(3):100–104. doi: 10.1016/s1360-1385(00)01863-x. [DOI] [PubMed] [Google Scholar]
- Kim Min C., Chung Sik W., Yun Dae Jin. Calcium and calmodulin-mediated regulation of gene expression in plants. Molecular Plant. 2009;2(1):13–21. doi: 10.1093/mp/ssn091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnersley A.M., Turano F.J. Gamma aminobutyric acid (GABA) and plant responses to stress. Critical Reviews in Plant Sciences. 2000;19(6):479–509. [Google Scholar]
- Ma Y., Wang P., Wang M., Sun M., Gu Z., Yang R. GABA mediates phenolic compounds accumulation and the antioxidant system enhancement in germinated hulless barley under NaCl stress. Food Chemistry. 2019;270:593–601. doi: 10.1016/j.foodchem.2018.07.092. [DOI] [PubMed] [Google Scholar]
- Meyer A., Popp M. Free Ca2+ in tissue saps of calciotrophic CAM plants as determined with Ca2+-selective electrodes. Journal of Experimental Botany. 1997;48(307):337–344. [Google Scholar]
- Ngadze E., Coutinho T.A., Icishahayo D., Waals J.E.V.D. Effect of calcium soil amendments on phenolic compounds and soft rot resistance in potato tubers. Crop Protection. 2014;62(4):40–45. [Google Scholar]
- Ramesh S.A., Tyerman S.D., Xu B., Bose J., Kaur S., Conn V. GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nature Communications. 2015;6(7879):1–9. doi: 10.1038/ncomms8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault H., Amrani A.E., Palanivelu R., Updegraff E.P., Yu A., Renou J.P. GABA accumulation causes cell elongation defects and a decrease in expression of genes encoding secreted and cell wall-related proteins in Arabidopsis thaliana. Plant & Cell Physiology. 2011;52(5):894–908. doi: 10.1093/pcp/pcr041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S.Q., Zheng S., Jiang Z.P., Qi L.W., Sun X.M., Li C.X. Effects of exogenous GABA on gene expression of Caragana intermedia roots under NaCl stress: Regulatory roles for H2O2 and ethylene production. Plant Cell & Environment. 2010;33(2):149–162. doi: 10.1111/j.1365-3040.2009.02065.x. [DOI] [PubMed] [Google Scholar]
- Shucheng Abscisic acid activates a Ca2+-calmodulin-stimulated protein kinase involved in antioxidant defense in maize leaves. Acta Biochimica et Biophysica Sinica. 2010;42(9):646–655. doi: 10.1093/abbs/gmq064. [DOI] [PubMed] [Google Scholar]
- Snedden W.A., Fromm H. Calmodulin as a versatile calcium signal transducer in plants [Review] New Phytologist. 2010;151(1):35–66. doi: 10.1046/j.1469-8137.2001.00154.x. [DOI] [PubMed] [Google Scholar]
- Tang W., Tu L., Yang X., Tan J., Deng F., Hao J. The calcium sensor GhCaM7 promotes cotton fiber elongation by modulating reactive oxygen species (ROS) production. New Phytologist. 2014;202(2):509–520. doi: 10.1111/nph.12676. [DOI] [PubMed] [Google Scholar]
- Tracy F.E., Gilliham M., Dodd A.N., Webb A.A., Tester M. NaCl-induced changes in cytosolic free Ca2+ in Arabidopsis thaliana are heterogeneous and modified by external ionic composition. Plant Cell & Environment. 2008;31(8):1063–1073. doi: 10.1111/j.1365-3040.2008.01817.x. [DOI] [PubMed] [Google Scholar]
- Tretyn A., Wagner G., Felle H.H. Signal transduction in Sinapis alba root hairs-auxins as external messengers. Journal of Plant Physiology. 1991;139(139):187–193. [Google Scholar]
- Wang Y., Chen T., Zhang C., Hao H., Liu P., Zheng M. Nitric oxide modulates the influx of extracellular Ca2+ and actin filament organization during cell wall construction in Pinus bungeana pollen tubes. New Phytologist. 2010;182(4):851–862. doi: 10.1111/j.1469-8137.2009.02820.x. [DOI] [PubMed] [Google Scholar]
- White P.J., Broadley M.R. Calcium in Plants. Annals of Botany. 2003;92(4):487–511. doi: 10.1093/aob/mcg164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan Z., Shen Z., Jian S., Yu Y., Deng S., Li Z. NaCl-elicited, vacuolar Ca2+ release facilitates prolonged cytosolic Ca2+ signaling in the salt response of Populus euphratica cells. Cell Calcium. 2015;57(5–6):348–365. doi: 10.1016/j.ceca.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Yang R., Yin Y., Guo Q., Gu Z. Purification, properties and cDNA cloning of glutamate decarboxylase in germinated faba bean (Vicia faba L.) Food Chemistry. 2013;138(2–3):1945–1951. doi: 10.1016/j.foodchem.2012.11.050. [DOI] [PubMed] [Google Scholar]
- Yu G., Liang J., He Z., Sun M. Quantum dot-mediated detection of gamma-aminobutyric acid binding sites on the surface of living pollen protoplasts in tobacco. Chemistry & Biology. 2006;13(7):723–731. doi: 10.1016/j.chembiol.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Yu G.H., Zou J., Feng J., Peng X.B., Wu J.Y., Wu Y.L. Exogenous γ-aminobutyric acid (GABA) affects pollen tube growth via modulating putative Ca2+-permeable membrane channels and is coupled to negative regulation on glutamate decarboxylase. Journal of Experimental Botany. 2014;65(12):3235–3248. doi: 10.1093/jxb/eru171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yücel C.N., Heybet E.H. Salicylic acid and calcium treatments improves wheat vigor, lipids and phenolics under high salinity. Acta Chimica Slovenica. 2016;63(4):738–746. doi: 10.17344/acsi.2016.2449. [DOI] [PubMed] [Google Scholar]
- Zhao Li, Zhao Zhi, Gong Han, Qin Yong, Yu Guang. In vivo dynamic Ca2+ and K+ oscillation responding to GABA reveals ion channels participating in tobacco pollen tube growth regulation. Chinese Journal of Cell Biology. 2013;35(5):668–675. [Google Scholar]
- Zhou T., Wang P., Yang R., Wang X., Gu Z. Ca2+ influxes and transmembrane transport are essential for phytic acid degradation in mung bean sprouts. Journal of the Science of Food and Agriculture. 2017;98:68–76. doi: 10.1002/jsfa.8680. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.