Highlights
-
•
Significant differences in characterization of starches were observed among them.
-
•
Imilla negra starch exhibited the highest apparent amylose and phosphorous content.
-
•
Imilla negra starch presented higher resistant starch content in cooked starch.
-
•
Loćka starch had the lowest apparent amylose content and higher crystallinity.
Keywords: Andean native potato, Starch, FTIR, Gelatinization, Crystallinity
Abstract
Three varieties of native potato (Imilla blanca, Imilla negra and Loc’ka) that grow in the Andean region at more than 3800 m.a.s.l. were selected fot the extraction and characterization or their starch. Instrumental techniques such as scanning electron microsocopic (SEM), differential scanning calorimetry (DSC), Fourier transformed infrarred spectroscopy (FTIR), X-ray diffraction, colorimetry and polarized light microscopy were used. The results showed that only Loc’kás starch had a unimodal granule size distribution, whereas Imilla negra and Imilla blanca starches showed two and three granule size populations, respectively. The starch from Imilla negra showed higher apparent amylose content, peak viscosity, phosphorous content and paste clarity. The starch from Imilla blanca showed high relative crystallinity, while Imilla blanca and Imilla negra had higher intensity ratios than that from Loc’ka, suggesting high molecular order. Cooked starch from Imilla negra showed higher resistant starch (RS) fraction than the other starches studied.
1. Introduction
The potato plant and its edible tuber were first domesticated in the Andean highlands of Peru. Along with other Andean crops, potatoes were used by pre-columbian and the Incas people; and have since spread globally and became a staple crop in many countries. In the Andean highlands of Peru there are more than 3500 varieties of native potato that are well-adapted to harsh environmental conditions (over 3800 m.a.s.l.). These potatoes have been selected over the centuries with respect to their textures, shapes and colours, and they are produced with minimal or zero agrochemical inputs. Such potatoes are of a heritage of great value and only a few reach the markets. Producers of organic foods are commonly seek organic ingredients; therefore, native starches with functional properties relevant to such applications should be identified.
Starch is widely used in the food industry as an ingredient in processed products (e.g. sauces, soups, snacks, bread) with functions such as gelling, stabilising, thickening, binding, texture modification and as a bulking agent. Starches singificantly vary in physicochemical, functional and structural properties between and within botanical species, and even when the same plant cultivar is grown under different environmental conditions (Yusuph et al., 2003, Šimková et al., 2013). Among starches, potato starch is preferred because its paste is characterized by high clarity and neutral taste (Alvani, Qi, Tester, & Snape, 2011). This clarity is attributed to the high content of phosphate esters on the amylopectin chain (Šimková et al., 2013). In addition, because of its granule size, purity, amylose and amylopectin chain lengths, the ability to exchange certain cations with corresponding effects on rheological behaviour, and, to form thick viscoelastic gels upon heating and subsequent cooling, the potato starch is considered important and unique (Vasanthan, Bergthaller, Driedger, Yeung, & Sporns, 1999).
The starches isolated from various potato cultivars grown in different parts of the world have been previously characterized with respect to their physicochemical properties, pasting properties during cooking in a stress rheometer, thermal properties (assessed by differential scanning calorimetry, DSC), morphology (evaluated by environmental scanning electron microscopy, ESEM), size distribution (assessed by laser diffraction analysis), X-ray diffraction pattern and percentage of crystallinity of granule starch, organization of the external region of the granules starch (assessed by FTIR-ATR spectroscopy). The functionality and potential digestibility (native and processed) of potato starches are not affected by cultivars (Alvani et al., 2011). Previous studies (Šimková et al., 2013) have shown that amylose and phosphorous contents affect starch digestibility and influence the different functional properties of potato starches. A study ont the physicochemical properties of dry matter and isolated starch suggested that different growing conditions may influence crystallinity and molecular structures; such information is useful with respect to the potential uses of the starch (Chung et al., 2014).
Some potential applications of native and modified starches isolated from different sources are indicated as follows: modified maize starches used in dispersions with calcium caseinate and in the casein matrix presented the best results to be used as fat replacers in reduced-fat cheese (Diamantino et al., 2018); soluble enzyme-resistant dextrins, produced by microwave heating of potato starch acidified with small amounts of hydrochloric and citric acids, could be used in the soft drink industry because of their highly-soluble and low-viscous (Jochym & Nebesny, 2017).
Furthermore, there are several studies regarding the potential applications of starch based composites: starch nanoparticles were obtained using normal corn starch via simple dispersion in water and dry heating treatment without physical fragmentation (Miskeen, Park, & Kim, 2018), starch nanoparticles were extracted from purified waxy and non-waxy barley granules by applying acidic hydrolysis and proved that waxy starch characterized by granules of smaller dimensions produced nanoparticles (Del Buono et al., 2019).
To the best of our knowledge, there are no reports regarding the presence of starch in potato cultivars growing in high altitudes. This study aims to physically and functionally characterize the starches extracted from three varieties of potatoes grown in the Andean region at 3862 m.a.s.l.
2. Material and methods
2.1. Materials
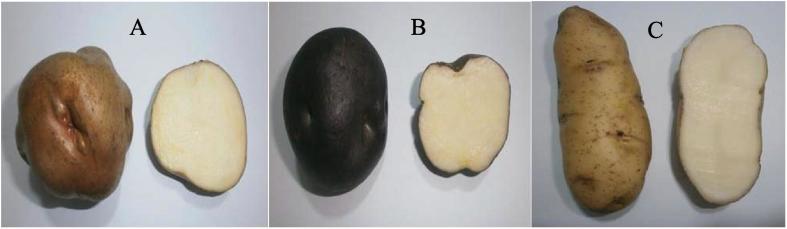
The starches used in this study were isolated from native potato’s varieties grown in Ilave (Puno, Peru) at 3862 m.a.s.l., and harvested from the fields of a local farmer, Constantino Flores. The varieties included Imilla blanca (Solanum tuberosum subsp. andigena), Imilla negra (Solanum tuberosum subsp. andigena) and Loc’ka (Solanum × juzepczukii) (Fig. 1) in June 2015. The fresh potatoes were transported to the laboratory in Lima, Peru, where starch isolation was performed inmediately. All chemical reagents were of analytical grade, and used without further purification.
Fig. 1.
Andean native potatoes from Ilave (Puno): A) Imilla blanca, B) Imilla negra and C) Loc’ka.
2.2. Starch isolation
Starch was isolated from each potato variety by using a slight modification of Singh, McCarthy, Singh, and Moughan (2008) method. Potatoes were washed, scrubbed in water and peeled manually, with eyes and bruises pitted out. Immediately, after peeling, the tubers were manually cut into small cubes (approximately 4 cm3) and soaked in a 0.075% (w/v) aqueous solution of sodium metabisulphite for 30 min, pieces with dark spots were discarded. A representative sample of 3 kg was ground (macerated) in a blender with 3 L of distilled water. The juice was filtered through a muslin cloth and the residue remaining on the cloth was washed with distilled water until only a small amount of starch passed through the muslin cloth. The filtrate was collected in a vessel and left undisturbed for four hours and the residue on the muslin cloth was discarded. A solid layer of starch settled in the vessel, after which the supernatant was decanted. The starch layer was again suspended with two volumes of distilled water (for washing) and then left at rest to allow its sedimentation. This procedure was repeated four or five times, until the supernatant became transparent. The starch cake was collected and dried at 40 °C to about 10% moisture content using an air oven (Venticell 111, MMM-Group, Germany). Dried starches were ground gently in a lab grinder and packed in double Ziploc plastic bags until further analysis.
2.3. Chemical composition
AOAC methods were used to examine starch: moisture content (950.46), total protein (984.13), fat (203.05), ash (942.05), crude fibre (962.09), phosphorous content (965.17) and carbohydrates content, which is determined by subtracting the total percentage of other components from 100 (AOAC, 2005). The apparent amylose content was measured according to the method of McGrance, Cornell, and Rix (1998). Starch purity was measured as the total starch percentage by enzymatic hydrolysis.
2.4. Colour parameters
The colour of each starch was determined using a calibrated spectrocolorimeter (CM-5, Konica Minolta, Japan). The information given by the parameters L* (lightness), a* (redness) and b* (yellowness) is generally used to express the total colour of the powder samples.
2.5. Light and polarized light microscopy
The samples were placed on a microscope slide, mixed with a drop of distilled water and fixed to slide with a coverslip. The starch granules were observed by light and polarized light microscopy (Eclipse 80i, Nikon Corp., Tokyo, Japan) with an objective lens of 20×.
2.6. Morphology and size granules
The morphology of the starch granules was assessed using (E-SEM, EVO LS10, Carl Zeiss Microscopy GmbH, Jena, Germany) with a resolution of 5 nm in high vacuum mode. The samples were fixed to a conductive carbon tape of double glue and placed in the microscope cells. The analysis was performed at 20 kV electron acceleration voltage to obtain images with a backscattering electron signal at 1000× magnification. The images obtained were processed using Carl Zeiss Microscopy software. The particle size distribution was determined using a laser diffraction analyzer with the Hydro EV accessory (Mastersizer 3000, Malvern Instruments Ltd., Worcestershire, UK). Starch powders were dispersed in deionized water at 3000 rpm for 2 min. In this study, the refractive index used for water and starch was 1.36. Particle size was expressed as the median diameter of the granules, which is defined as the diameter for which 50% of the particles (by volume) are larger.
2.7. X-ray diffraction
The starches were conditioned in a desiccator over saturated potassium sulfate solution for 5 days. The X-ray diffraction patterns of the samples were measured using a diffractometer (Ultima IV, Rigaku Corporation, USA) with an ultra D/tex detector operating at 35 kV and 15 mA and equipped with a CuKα radiation source at a wavelength of 0.154186 nm. The intensities were measured in the 3.0–37.0° range on a 2θ scale with a step size of 0.02°.
2.8. FTIR spectroscopy
Prior to FTIR analysis, the starches were conditioned at 23 ± 2 °C and 0% relative humidity for 10 days in a desiccator. FTIR analysis was carried out according to the method described by Hoyos-Leyva et al. (2017) with slight modificiations. We used a Vertex 70 FT-IR (Bruker Optik GmbH., Ettlingen, Germany) spectrometer, which was equipped with a cadmium mercury telluride detector and KBr beam splitter using an ATR accessory with a diamond crystal at an angle of incidence of 45°. The spectra were collected from 256 scans at a resolution of 4 cm−1 over the 4000–400 cm−1 wavenumber region. For each sample, three spectra were taken and averaged, and finally, processing was performed using the OPUS software (version 7.0).
2.9. Thermal properties
The thermal properties of the potato starches were measured using a DSC (Q20, TA Instrument, New Castle, NJ, USA), which had previously been calibrated with indium. The samples (2.2 mg db) were weighed directly into aluminium DSC pans, to which 7 mL of deionized water was added. After sealing, the pans were left to equilibrate for 1 h at room temperature before the analysis was performed. A sample was subjected to a heating ramp of 30–120 °C and at a heating rate of 10 °C/min. An empty aluminium pan was used as a reference. The onset (To), peak (Tp), conclusion (Tc) temperatures, and gelatinization enthalpy (ΔH) were estimated directly by the instrument software.
2.10. Pasting profile
The pasting profiles of the starch suspensions were obtained with a stress rheometer (Ar-1500ex, TA Instruments, Dallas, TX, USA) using the starch pasting cell at a concentration of 8% (w/w, dry basis). The suspension was stirred with a vaned rotor at 500 s−1 during the test. First, the suspension was held at 50 °C for 1 min, then heated to 95 °C at 5 °C/min, held at 95 °C for 10 min, cooled to 60 °C at 5 °C/min, and finally held at 60 °C for 10 min. The pasting temperature (PT), peak viscosity (PV), minimum viscosity (MV), final viscosity (FV), breakdown viscosity (BDV), and setback viscosity (SBV) of the starch pastes were recorded.
2.11. Paste clarity
Following a slight modification of the method described by Bello-Pérez, Contreras-Ramos, Romero-Manilla, Solorza-Feria, and Jiménez-Aparicio (2002), the paste clarity of the starches was determined by measuring their light transmittance (%). An aqueous dispersion of potato starch sample 1% (w/v) in a screw-cap tubes were heated in a water bath (95 °C) for 30 min with continuous mixing at 5 min intervals using a vortex mixer (75 rpm). The cooked paste was then cooled to room temperature and its transmittance was measured at 650 nm using a UV–visible spectrophotometer (Spectronic® 20 Genesys™, Spectronics Instruments, USA). The transmittances of the pastes stored at 4 °C was also determined daily for four consecutive days.
2.12. Digestibility on native and gelatinized starches
The digestibility of samples was measured according to Englyst, Kingman, and Cummings (1992) procedure, with some modifications. Briefly, 200 mg (dry basis) of starch samples was placed into a glass tube and dispersed in 1 mL sodium acetate buffer (0.5 mol/L, pH 5.2). After the suspension was equilibrated at 37 °C for 5 min, six glass balls (5 mm diameter) and 5 mL of enzyme solution (α-amylase and amyloglucosidase mixed in a proportion of 120 U/80 U/mL) were added in the flask. Then, the samples were immersed in a shaking–water bath for hydrolysis at 37 °C and 160 rpm. Aliquots of 200 mL hydrolyzed solution were taken out at 20 min and 120 min intervals and placed into a plastic tube that had been treated in a boiling water bath to deactivate the enzyme. Subsequently, the solution was centrifuged at 10000×g for 5 min, and the glucose content in the supernatant was determined by the glucose oxidase method using a d-glucose assay kit (Megazyme, K-GLUC).
The proportions of different starch fractions, i.e. rapidly digestible starch (RDS), slowly digestible starch (SDS) and resistant starch (RS) were calculated according to the timeline of digestion using the following formulas:
where G20 and G120 represent the content of glucose released after 20 and 120 min, respectively. FG is the free glucose content of RS which is extracted with distilled water and measured in the supernatant and TG is the total glucose measured after the starch is thoroughly hydrolyzed to glucose. The determination of FG and TG was conducted using the glucose oxidase method as mentioned above. A glucose-to-starch conversion factor of 0.9 was used.
2.13. Statistical analysis
Data were analyzed using one-way ANOVA, followed by an LSD test, to enable post–hoc comparisons of the means and simple correlations (p < 0.05). The ANOVA and LSD tests were performed using Statgraphics software (Centurion XVI, Statgraphics Technologies, USA).
3. Results and discussion
3.1. Chemical characteristics
The moisture, protein, fat, ash, purity, amylose and phosphorous contents of the starches are shown in Table 1. The starches had moisture contents of approximately 10%, which is acceptable, given that up to 20% of moisture content is allowed in starches used commercially as raw materials. The protein content of the studied starches was ranged from 0.58% to 0.74%. These values were higher than those reported for different potato starches from UK (Alvani et al., 2011), two potato starches from the USA (Xu, Grizzard, Sismour, Bhardwaj, & Li, 2013). No starches contained fibre (data not shown) and only Loc’ka starch contained fat (0.07%). This fat content is smaller than that reported for Mexican potato starch (Jiménez-Hernández, Salazar-Montoya, & Ramos-Ramírez, 2007) and potato starch from Venezuela (Lovera, Pérez, & Laurentin, 2017). The ash content ranged from 0.22% to 0.29%, which is similar to the values reported for Indian potato starch and potato starch from Venezuela (Lovera et al., 2017). Note that the chemical composition of these starches is dependent on the potato variety and on the process of starch isolation. Starch purities were 91.7%, 92.2% and 89.7% for Imilla blanca, Imilla negra and Loc’ka starches, respectively; these values were higher than those reported for potato starch from Venezuela (Lovera et al., 2017), but similar to those reported for Marantha starch (Hoyos-Leyva et al., 2017).
Table 1.
Chemical characteristics, colour properties, mean diameter, short-range molecular order and crystallinity of Andean native potato starches.
| Starch | Imilla blanca | Imilla negra | Loc’ka |
|---|---|---|---|
| Moisture (%) | 9.2 ± 0.06a | 9.3 ± 0.13a | 10.2 ± 0.03b |
| Total protein (%, db) | 0.58 ± 0.01a | 0.58 ± 0.11a | 0.74 ± 0.05b |
| Fat (%, db) | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.07 ± 0.02b |
| Ash (%, db) | 0.26 ± 0.01ab | 0.29 ± 0.05b | 0.22 ± 0.01a |
| Phosphorous (mg/100 g) | 55.1 ± 0.04ª | 82.7 ± 5.62b | 55.7 ± 0.02ª |
| Starch purity (%, db) | 91.7 ± 2.5b | 92.2 ± 1.7b | 89.7 ± 0.6a |
| Apparent amylose (%, db) | 28.2 ± 0.88b | 30.0 ± 0.69c | 24.9 ± 0.19a |
| L* (lightness) | 96.82 ± 0.08b | 96.84 ± 0.14b | 96.59 ± 0.08a |
| a* (redness) | −0.27 ± 0.04a | −0.22 ± 0.01b | −0.18 ± 0.01c |
| b* (yellowness) | 1.88 ± 0.09b | 1.79 ± 0.11b | 1.66 ± 0.04ª |
| Mean diameter (µm) | 31.6 ± 0.1c | 13.6 ± 0.0a | 27.2 ± 0.6b |
| R (1047/1022 cm−1) 1,2 | 0.729 ± 0.000b | 0.726 ± 0.001ab | 0.725 ± 0.004a |
| R (995/1022 cm−1) 1,3 | 1.201 ± 0.003a | 1.207 ± 0.001a | 1.200 ± 0.005a |
| Relative crystallinity (%) | 36.3 | 34.4 | 35.3 |
db = dry basis.
Intensity ratio Values are means of three measurements ± SE. Means in rows not sharing the same letter are significantly different (p < 0.05).
Ratio of ordered crystalline domain to amorphous domain in starch.
Reflects helical organization within the crystalline lamella.
The amylose associated with the large branches of amylopectin chains comprise an amorphous region of granules, whereas amylopectin chains with short branches comprises a crystalline region. A higher proportion of amylose in the starch granules results in lower crystallinity (Cheetham & Tao, 1998). The apparent amylose contents of Imilla blanca, Imilla negra and Loc’ka starches were 28.4%, 30.0% and 24.5%, respectively; these are equivalent to total amylose content owing of the absence of lipids in these starches (Table 1). The amylose content range (24.5% to 30%) was higher than those reported for potato starches from India (17.2% to 23.7%) (Peshin, 2001) and for six potato starches from Japan (18.7% to 23.9%) (Karim et al., 2007), but similar to the range (23.4% to 31%) reported for potato starches from the USA (Wiesenborn, Orr, Casper, & Tacke, 1994).
The presence of phosphorous is an important influence on the clarity of potato starch pastes. This mineral is mainly present as phosphate monoesters, which are coupled to the amylopectin fraction via covalent bonds (Karim et al., 2007). The phosphorous content in potato starch is relatively high compared to the starches isolated from other botanical sources; in this study, the phosphorous contents of the starches were 55.1, 82.7 and 55.7 mg/100 g starch for Imilla blanca, Imilla negra and Loc’ka, respectively (Table 1). Imilla negra starch had the highest phosphorous content. The values for Imilla blanca and Loc’ka starches were comparable to the average phosphorous content (0.54 µg/mg) reported for 12 potato starches from the UK (Yusuph et al., 2003) and lower than the range (90–100 mg/100 g) reported for five potato starches from India (Peshin, 2001). Starch with high phosphate content, as Imilla negra starch, is favoured in some industrial applications, such as, paper making, flocculation effects in mining, and water treatment (Xu, Huang, Visser, & Trindade, 2017).
3.2. Colour properties
The colour parameters of the potato starches are shown in Table 1. The starches showed higher lightness (L*) than commercial potato starch (L* = 95.4), as well as low redness (a*) and yellowness (b*), because the isolated starches had an acceptable purity. The L* value indicates the level of lightness of the starch, with a value close to 100 indicating a strong white colour. Loc’ka starch had a lower lightness value than the others in this study, which can be attributed to its high protein content and low purity (Kim, Woo, & Chung, 2018). The a* values were close to zero, indicating that the starches showed a neutral colour. Imilla blanca and Imilla negra starches showed higher b* values (1.88 and 1.79, respectively) than Loc’ka starch (1.66), although these values are smaller than those reported for cowpea and mugbean starches from India (Kim et al., 2018).
3.3. Morphology and particle size distribution
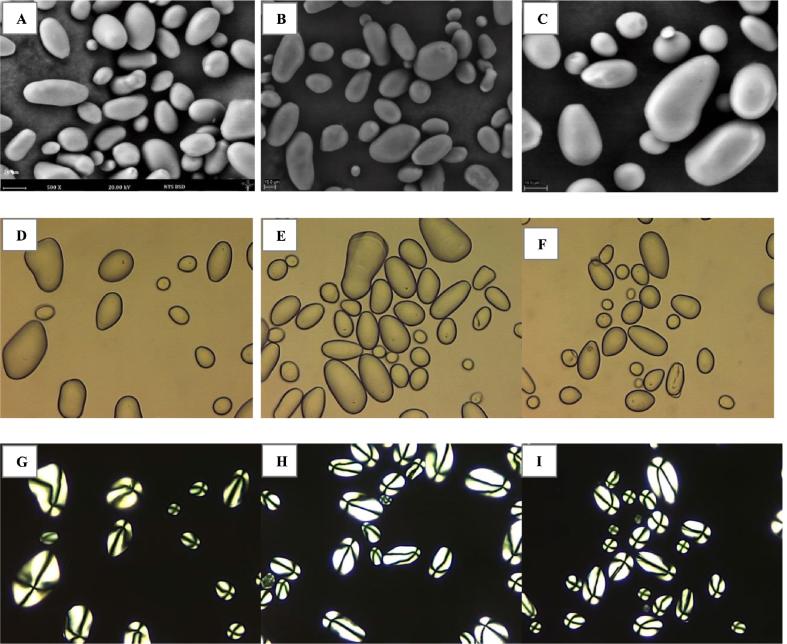
The morphologies of the potato starches, as evaluated by ESEM and polarized light and light microscopy, are shown in Fig. 2. All starches showed oval and ellipsoidal shapes (Hoover and Ratnayake, 2001, Kaur et al., 2002), smooth surfaces, absence pores, and birefringent granules (Fig. 2G, H, and I). Measurement of starch granule size distribution using laser diffraction is more accurate than that given by ESEM analysis as the laser diffraction analysis is performed using a large population of starch granules in water, whereas ESEM analysis uses only a small population of granules. The mean diameters of the starches are shown in Table 1 and the particle size distribution is shown in Fig. 1-Supplementary data. The particle size distribution of Loc’ka starch showed a unimodal distribution with a range of starch granules diameters of 11.2–51.8 µm, whereas the Imilla negra starch showed two granule size populations: a small population of 0.6–7 µm, and a main population of 12.7–58.9 µm. On the other hand, Imilla blanca starch showed three granule size population: two small populations of 0.013–0.36 µm, and 0.77–8.69 µm, and a main population of 12.7–272 µm. Some reports have shown that potato starch has a relatively broad granule size distribution. For example, granule size distributions of 5–100 µm (mean diameter of 20.6–30.9 µm) for 10 starches isolated from potato cultivars of UK (Alvani et al., 2011) and 17.99–23.06 µm for 12 starches isolated from potato cultivars of Scotland (Yusuph et al., 2003) have been reported. More typically, a range of 20–40 µm is found (Peshin, 2001). The big-granule starch, such as potato, could be used in the production of moulded objects up to 50% (w/w) of the plastic (Ellis et al., 1998).
Fig. 2.
Morphologies of Imilla blanca (A, D, G), Imilla negra (B, E, H) and Loc’ka starches (C, F, I), as observed by E-SEM (A, B, C), light microscopy (C, D, F) and polarized light microscopy (G, H, I).
3.4. Paste clarity
Paste clarity is known to be one of the most important functional properties of starch in certain food products. The results for paste clarity (measured as light transmittance at 650 nm) of potato starch gels at 4 °C gradually decreased during storage (Table 1-Supplementary data). The transmittance of Imilla negra starch gel decreased from 78.6 (0 h) to 16.6% (72 h), while those of Imilla blanca and Loc’ka starch gels showed initial transmittance values (0 h) of 50.2% and 59.3%, respectively, decreasing to 9.8% and 6% at 72 h. Cold storage induces retrogradation of amylose and debranched amylopectin in the starch gels, so the formed crystals decreased the clarity of the gels (Bello-Pérez et al., 2002). These values are similar to the transmittance value ranges reported for potato starches from India by Singh, Kaur, and Singh (2004a) (70.2%−9.2%) and those by Kaur et al. (2002) (65.6%−20.4%); however, they are greater than the range (57.4%−1.7%) reported for starch isolated from the commercial variety Única (Vargas, Martinez, & Velezmoro, 2016). Differences in transmittance values between starches can be due to several factors such as amylose content, lipid and protein contents, particle size of the granules, lengths of the amylose and amylopectin chains (Kaur et al., 2002) and content of phosphate monoesters (Karim et al., 2007, Ambigaipalan et al., 2011). In this study, Imilla negra starch showed the highest phosphorous content and the highest clarity; this may have been due to repulsion between the adjacent starch molecules caused by the negatively charged phosphate groups increasing the levels of the hydrated molecules, which promote high transparency (Paredes-López, Bello-Pérez, & López, 1994). Unlike the gels obtained from the starches isolated from other botanical sources, potato starches generally produces gels that are characterized by high transmittance; this is attributed to the high content of phosphate monoesters content of potato starch (Šimková et al., 2013).
3.5. X-ray diffraction and relataive crystallinity
X-ray diffraction was used to study the crystalline structure of the starch granules, which form a semi-crystalline system; consisting of crystalline and amorphous regions (Xu et al., 2013). The X-ray diffraction patterns showed the crystalline components of the starches as sharp peaks, whereas the amorphous components were presented as dispersive peaks (Zhou, Liu, Zhang, Chen, & Kong, 2016). Generally, differences in relative crystallinity between starches can be attributed to differences in crystal size, number of crystallite regions (influenced by amylopectin content and amylopectin chain length), orientation of the double helices within the crystalline domains and the extent of interaction between double helices (Ambigaipalan et al., 2011). Literature values for starch crystallinity range from 15% to 45% depending on not only the source and the moisture content of starch but also the technique used to determine this parameter (Alvani et al., 2011). The percentages of crystallinity in the potato starches were 36.3%, 34.4% and 35.3% for Imilla blanca, Imilla negra and Loc’ka, respectively (Table 1). These values were higher than those reported by Alvani et al. (2011) for ten starches isolated from potato cultivars of the UK, those reported by Vasanthan et al. (1999) for six starches isolated from potato varieties of Canada, and those reported by Yusuph et al. (2003) for 12 starches isolated from potato cultivars of Scotland. Differences in crystallinity level can be related to the amylose content in these starches, with previous reports suggesting that the crystallinity percentage is low in starches with high amylose content (Cheetham & Tao, 1998). Indeed, in the present study Imilla negra starch showed the lowest crystallinity and the highest amylose content. All starches exhibited B-type crystallinity, typical of tuber starches with extended amylopectin chains; such as, they could be less susceptible to digestion by enzymatic hydrolysis (Englyst et al., 1992, Jane et al., 1997). It was observed that starches showed a strong reflection peak of 17° at 2θ and several medium peaks about 15°, 20°, 22° and 24° at 2θ; these results agree with those of previous reports regarding the crystallinite structure of potato starch (Vasanthan et al., 1999, Alvani et al., 2011, Xu et al., 2013, Zhou et al., 2016). A unique peak of about 5° at 2θ, which is typically found in the B-type crystallinity pattern, was very weak; a similar result was reported by Xu et al. (2013) (Fig. 2-Supplementary data).
3.6. FTIR-ATR spectroscopy
The FTIR-ATR spectrum is sensitive to short-range order, defined as the double helical order, as opposed to a long-range order related to the packing of double helices detected by X-ray diffraction (Ambigaipalan et al., 2011). Since an IR beam penetrates about 2 µm into a starch granule (Ambigaipalan et al., 2011, Hoyos-Leyva et al., 2017, Sevenou et al., 2002), FTIR spectra mainly reflect a short-range order near the granule surface. Pronounced peaks of absorbance in the wavelengths between 1800 and 900 cm−1 were observed, representing the characteristics of the native starches. The peaks observed above 1800 cm−1 were in bands from 3000 to 2800 cm−1 and from 3500 to 3000 cm−1, which are characteristic of the stretching of C–H and O–H bonds, respectively. The FTIR-ATR data of the potato starches used in this are shown in Table 1. The bands in the region 1100–900 cm−1 are shown to be sensitive to changes in starch structure, particularly, the bands at 995, 1022 and 1047 cm−1 (Warren et al., 2016, Hoyos-Leyva et al., 2017). The absorbance band at 995 cm−1 is related to the water–starch interaction, and its absorption intensity is produced by C–O–H bending vibrations; in contrast, the absorbance bands at 1022 and 1047 cm−1 are characteristic of amorphous and crystalline structures in starch, respectively (van Soest et al., 1995, Ambigaipalan et al., 2011, Warren et al., 2016). Indeed, the ratio of 1047/1022 cm−1 ratio is used to express the ratio of ordered crystalline domains to amorphous domains in starches (van Soest et al., 1995), and the 995/1022 cm−1 ratio is shown to reflect a helical organization (alignment of helices at short-range order) within the crystalline lamella (Ambigaipalan et al., 2011). In this study, Loc’ka starch showed a lower intensity ratio of 1047/1022 cm−1 than the Imilla blanca and Imilla negra starches, suggesting that although the amount of ordered crystalline domains is higher (higher intensity of the 1047 cm−1 peak detected), the crystallites of Loc’ka starch were probably smaller and/or those of the double helices forming the crystallites were weakly associated within the crystalline lamella. This is reasonable, since the peak attributed to the amorphous domains (1022 cm−1) presented a higher intensity in Loc’ka starch; similar results were reported by Ambigaipalan et al. (2011) for faba bean starches. On the other hand, Imilla negra starch showed a higher intensity ratio 995/1022 cm−1 than Imilla blanca and Loćka starches, suggesting a more ordered helical organization inside the crystalline lamellae (Ambigaipalan et al., 2011, Warren et al., 2016).
3.7. Thermal properties
The thermal gelatinization properties, as assessed by DSC are shown in Table 2. Imilla blanca starch showed higher gelatinization temperatures than Imilla negra and Loc’ka starches, although the gelatinization enthalpy was similar. Increased gelatinization temperatures can be induced by high double-helical arrangement between amylose and amylopectin chains, as well as by strong interactions between amylose–amylose and amylopectin–amylopectin chains. The slow leaching of amylose chains during swelling or the formation of insoluble remnants during gelatinization (Hoover & Ratnayake, 2001), could result in a high gelatinization range (GR). The average transition temperatures To, Tp and Tc were 58.1, 61.9 and 68.3 °C, respectively; similar values were reported for different potato starches from India (Singh et al., 2004a, Singh et al., 2004b). The differences between the starches in terms of transition temperatures and gelatinization enthalphy by DSC, may be attributed to differences in their degree of crystallinity (Singh et al., 2004a). Potato starches with small particle sizes showed higher ΔH values and viceversa (Waterschoot, Gomand, & Delcour, 2016); as such, Imilla blanca and Imilla negra starches showed the smallest granule sizes and the highest ΔH values.
Table 2.
Gelatinization properties of native potato starches determined by differential scanning calorimetry.
| Starch | To (°C) | Tp (°C) | Tc (°C) | ΔH (J/g)* | GR |
|---|---|---|---|---|---|
| Imilla blanca | 59.5 ± 0.0c | 63.1 ± 0.1c | 69.2 ± 0.0b | 15.8 ± 0.1a | 9.7 ± 0.1a |
| Imilla negra | 58.1 ± 0.1b | 62.4 ± 0.1b | 69.5 ± 0.1b | 15.8 ± 0.1ª | 11.4 ± 0.1b |
| Loćka | 56.8 ± 0.1a | 60.2 ± 0.1a | 66.3 ± 0.2a | 15.6 ± 0.3ª | 9.5 ± 0.1a |
To = onset temperature; Tp = peak temperature; Tc = end temperature; ΔH = gelatinization enthalpy; GR = gelatinization range (Tc − To). Values represent the mean of three determinations ± SE. Means in columns not sharing the same letter are significantly different (p < 0.05).
3.8. Pasting properties
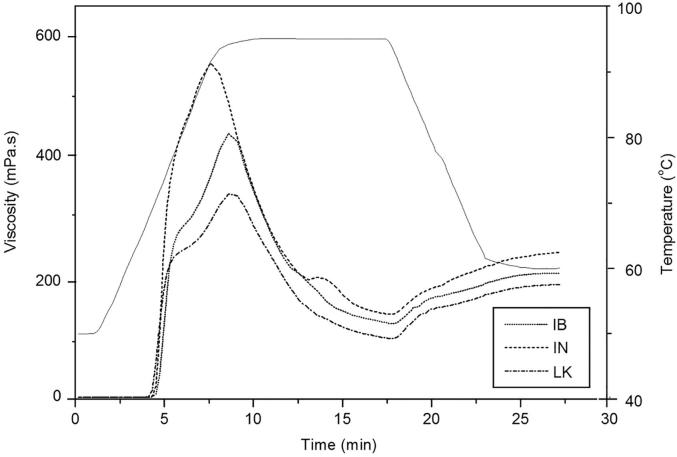
Fig. 3 shows the pasting profile for each potato starch studied. The PT of the starches was higher than the gelatinization onset temperature (To) measured by DSC. The PT of Imilla blanca starch was higher than those of Imilla negra and Loc’ka starches, which can be related to amylose content (Singh et al., 2008, Chung et al., 2014), as this retards starch swelling, thereby increasing the PT. PV and shear stability have been shown to be influenced by amylose content, proportion of amylopectin chain lengths and phosphorous content (Karim et al., 2007, Ambigaipalan et al., 2011). Higher PV values (6964–8197 mPa·s) have been reported for potato starches with different granule sizes (Waterschoot et al., 2016) and a potato starch from China (10235 mPa·s) (Zhou et al., 2016); therefore, the higher PV of Imilla negra (556.5 mPa·s) starch can be attributed to its high phosphorous content (Chung et al., 2014). Moreover, amylose is leached out of starch granules during the heating of dispersions as more water is transported into the granule, thus producing greater swelling; consequently, a higher PV. Breakdown viscosity is produced by disintegration of the starch granular structure via shear forces; higher values indicates that the starch has a lower tendency to resist shear force during heating (Zhou et al., 2016); in this study, the starches with higher amylose content showed higher breakdown viscosities. Imilla negra starch showed a higher breakdown viscosity (416.5 mPa·s) than other starches, indicating the susceptibility of these starch granules to the combined effects of temperature, excess water and shear rate. The setback and final viscosities were higher for Imilla negra starch than they were for Imilla blanca and Loc’ka starches. During the cooling of starch paste (decreasing temperature), leached amylose molecules rapidly aggregate and the formation of amylose junction zones is responsible for a setback and FV (Bello-Pérez, Sánchez-Rivera, Núñez-Santiago, Rodríguez-Ambriz, & Román-Gutierrez, 2010). This is consistent with the present results because the apparent amylose content of Imilla negra starch was greater (Table 1). Notably, for Imilla negra and Loc’ka starches, a two-stage viscosity development was observed; first, there was a rapid increase in viscosity and then, at a certain point, the slope of the curve changed, and viscosity development slowed down. This could be due to the transition of the system from diluted to concentrated regime. This similar behaviour for native potato starches was reported by Gomand et al., 2010, Waterschoot et al., 2014, Waterschoot et al., 2016.
Fig. 3.
Pasting profiles of Imilla blanca (IB), Imilla negra (IN), and Loc’ka (LK) starches.
3.9. In vitro digestibility
Data for the three digestion fractions defined by Englyst et al. (1992), i.e. RDS, SDS and RS, are shown in Table 3. The raw potato starches showed high RS fractions, which are typical of tuber starches. This resistance to enzymatic digestion is associated with starches whose granular crystalline organization corresponds to the B-type X-ray diffraction pattern (Englyst et al., 1992, Zhang et al., 2006). The heating starches with high water contents induces the disorganization of their components; in consequence, this increases their enzymatic susceptibility and hydrolysis rate (Zhang et al., 2006). The RS contents for raw starches ranged between 78.9% and 92.9%; and were similar to those reported for a commercial potato starch and for a commercial waxy potato starch (Lee, Kim, Choi, & Moon, 2012). The RS contents for cooked starches was ranged between 1.7% and 8.0%, which is similar to the amount was reported for a native potato starch from China (Zhou et al., 2016). The RSD contents were significantly different across the raw starches used in the present study, a finding that is similar to those reported by Lee et al. (2012). The RDS contents of cooked starches increased with a concomitant decrease in RS content. Nevertheless, Imilla negra cooked starch yielded the highest RS content (8.0%) and the lowest RDS content (77.3%); this pattern has been previously reported for potato starch (Zhang et al., 2006).
Table 3.
Rapidly and slowly digested starch (g·100 g−1) in raw and cooked starches isolated from native potato starches.
| Starch | Raw |
Cooked |
||||
|---|---|---|---|---|---|---|
| RDS | SDS | RS | RDS | SDS | RS | |
| Imilla blanca | 3.6 ± 0.1a | 3.5 ± 0.8a | 92.9 ± 0.7c | 82.5 ± 2.1ab | 14.8 ± 1.3a | 2.7 ± 0.8b |
| Imilla negra | 4.4 ± 0.1b | 4.7 ± 0.4a | 91.0 ± 0.5b | 77.3 ± 2.6 a | 14.6 ± 1.2a | 8.0 ± 1.4c |
| Loc’ka | 7.2 ± 0.1c | 13.9 ± 0.4b | 78.9 ± 0.5a | 84.6 ± 1.2b | 13.7 ± 1.3a | 1.7 ± 0.1a |
RDS = rapidly digested starch; SDS = slowly digested starch; RS = resistant starch. Values are means of four replicates ± SE. Dry matter basis. Means in column not sharing the same letter are significantly different (p < 0.05).
The SDS content of Loc’ka raw potato starch was significantly higher than that of Imilla negra and Imilla blanca starches. After gelatinization, these three starches yielded similar SDS contents. In raw starches, the increasing amylose content results in more resistance to enzyme digestion by α-amylase and amyloglucosidase; hence, the RS content increased whereas the SDS content decreased (Hong, Zeng, Buckow, & Han, 2018). It was reported that potato starches with high phosphorous content showed high RS content; nevertheless, the amylose content does not affect considerably digestibility of cooked starches (Absar et al., 2009, Lu et al., 2012). The interest in RS is related to its effects in the gastrointestinal tract, which in many ways are similar to these of dietary fibre (Fuentes-Zaragoza, Riquelme-Navarrete, Sánchez-Zapata, & Pérez-Álvarez, 2010). This is important from a nutritional perspective because as a food ingredient, potato starch is less susceptible to digestion by enzymatic hydrolysis (Jane et al., 1997). Thus, Imilla negra starch could be used in food industry applications such an additive with potential prebiotic effect.
4. Conclusions
The physicochemical, functional and morphological properties of Imilla blanca, Imilla negra and Loc’ka starches isolated from Andean native potatoes from Peru were assessed, and significant differences between these starches were found. Imilla negra starch exhibited higher apparent amylose content, phosphorous content, peak viscosity, clarity, gelatinization temperature, retrogradation and RS content in cooked starch. Furthermore, X-ray diffraction and FTIR-ATR analyses showed that all starches had high molecular order, suggesting high energy requirements for gelatinization. Therefore, owing to its high RS fraction, which is important for functional diets, Imilla negra starch shows a potential for application in the food industry as a functional ingredient or in the production of moulded objects. Finally, these results provide useful information about Andean native potatoes as the sources of starch for food industries, which possibly expand markets and promote the sustainable use of the Peruvian biodiversity.
Declaration of Competing Interest
The authors have declared no conflict of interest.
Acknowledgments
Acknowledgements
The authors are grateful to Programa Nacional de Innovación para la Competitividad y Productividad (Innovate Peru) for funding the projects N° 121-ELC-2014 and N° 139-PNICP-PIAP-2015.
This study is novel since it is the first to report starch properties of native potatoes from the Andean region of Peru.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2019.100030.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Absar N., Zaidul I.S.M., Takigawa S., Hashimoto N., Matsuura-Endo C., Yamauchi H., Noda T. Enzymatic hydrolysis of potato starches containing different amounts of phosphorus. Food Chemistry. 2009;112:57–62. [Google Scholar]
- Alvani K., Qi X., Tester R.F., Snape C.E. Physico-chemical properties of potato starches. Food Chemistry. 2011;125:958–965. [Google Scholar]
- Ambigaipalan P., Hoover R., Donner E., Liu Q., Jaiswal S., Chibbar R.…Seetharaman K. Structure of faba bean, black bean and pinto bean starches at different levels of granule organization and their physicochemical properties. Food Research International. 2011;44:2962–2974. [Google Scholar]
- AOAC (2005). Official Methods of Analysis of the AOAC (18th ed.) Maryland, USA, Association of Official Analytical Chemists.
- Bello-Pérez L., Contreras-Ramos S., Romero-Manilla R., Solorza-Feria J., Jiménez-Aparicio A. Propiedades químicas y funcionales del almidón modificado de plátano Musa paradisiaca L. (Var. Macho) Agrociencia. 2002;36:169–180. [Google Scholar]
- Bello-Pérez L.A., Sánchez-Rivera M.M., Núñez-Santiago C., Rodríguez-Ambriz S.L., Román-Gutierrez A.D. Effect of the pearled in the isolation and the morphological, physicochemical and rheological characteristics of barley starch. Carbohydrate Polymers. 2010;81:63–69. [Google Scholar]
- Cheetham N.W., Tao L. Variation in crystalline type with amylose content in maize starch granules: An X-ray powder diffraction study. Carbohydrate Polymers. 1998;36:277–284. [Google Scholar]
- Chung H.-J., Li X.-Q., Kalinga D., Lim S.-T., Yada R., Liu Q. Physicochemical properties of dry matter and isolated starch from potatoes grown in different locations in Canada. Food Research International. 2014;57:89–94. [Google Scholar]
- Del Buono D., Luzi F., Benincasa P., Kenny J.M., Torre L., Puglia D. Extraction of nanostructured starch from purified granules of waxy and non-waxy barley cultivars. Industrial Crops and Products. 2019;130:520–527. [Google Scholar]
- Diamantino V.R., Costa M.S., Taboga S.R., Vilamaior P.S.L., Franco C.M.L., Penna A.L.B. Starch as a potential fat replacer for application in cheese: Behaviour of different starches in casein/starch mixtures and in the casein matrix. International Dairy Journal. 2018;89:129–138. [Google Scholar]
- Ellis R.P., Cochrane M.P., Dale M.F.B., Duffus C.M., Lynn A., Morrison I.M.…Tiller S.A. Starch production and industrial use. Journal of the Science of Food and Agriculture. 1998;77:289–311. [Google Scholar]
- Englyst H.N., Kingman S.M., Cummings J. Classification and measurement of nutritionally important starch fractions. European Journal of Clinical Nutrition. 1992;46:S33–S50. [PubMed] [Google Scholar]
- Fuentes-Zaragoza E., Riquelme-Navarrete M.J., Sánchez-Zapata E., Pérez-Álvarez J.A. Resistant starch as functional ingredient: A review. Food Research International. 2010;43:931–942. [Google Scholar]
- Gomand S.V., Lamberts L., Visser R.G.F., Delcour J.A. Physicochemical properties of potato and cassava starches and their mutants in relation to their structural properties. Food Hydrocolloids. 2010;24:424–433. [Google Scholar]
- Hong J., Zeng X.-A., Buckow R., Han Z. Structural, thermodynamic and digestible properties of maize starches esterified by conventional and dual methods: Differentiation of amylose contents. Food Hydrocolloids. 2018;83:419–429. [Google Scholar]
- Hoover R., Ratnayake W. Current protocols in food analytical chemistry. Editorial John Wiley & Sons; Chicheste, UK: 2001. Determination of total amylose content of starch; pp. E2.3.1–E2.3.5. [Google Scholar]
- Hoyos-Leyva J.D., Alonso-Gomez L., Rueda-Enciso J., Yee-Madeira H., Bello-Perez L.A., Alvarez-Ramirez J. Morphological, physicochemical and functional characteristics of starch from Marantha ruiziana Koern. LWT-Food Science and Technology. 2017;83:150–156. [Google Scholar]
- Jane J., Wong K., McPherson A.E. Branch-structure difference in starches of A- and B-type X-ray patterns revealed by their Naegeli dextrins. Carbohydrate Research. 1997;300:219–227. [Google Scholar]
- Jiménez-Hernández J., Salazar-Montoya J.A., Ramos-Ramírez E.G. Physical, chemical and microscopic characterization of a new starch from chayote (Sechium edule) tuber and its comparison with potato and maize starches. Carbohydrate Polymers. 2007;68:679–686. [Google Scholar]
- Jochym K.K., Nebesny E. Enzyme-resistant dextrins from potato starch for potential application in the beverage industry. Carbohydrate Polymers. 2017;172:152–158. doi: 10.1016/j.carbpol.2017.05.041. [DOI] [PubMed] [Google Scholar]
- Karim A.A., Toon L.C., Lee V.P.L., Ong W.Y., Fazilah A., Noda T. Effects of phosphorous contents on the gelatinization and retrogradation of potato starch. Journal of Food Science. 2007;72:C132–C138. doi: 10.1111/j.1750-3841.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- Kaur L., Singh N., Sodhi N.S. Some properties of potatoes and their starches II. Morphological, thermal and rheological properties of starches. Food Chemistry. 2002;79:183–192. [Google Scholar]
- Kim Y., Woo K.S., Chung H.-J. Starch characteristics of cowpea and mungbean cultivars grown in Korea. Food Chemistry. 2018;263:104–111. doi: 10.1016/j.foodchem.2018.04.114. [DOI] [PubMed] [Google Scholar]
- Lee C.J., Kim Y., Choi S.J., Moon T.W. Slowly digestible starch from heat-moisture treated waxy potato starch: Preparation, structural characteristics, and glucose response in mice. Food Chemistry. 2012;133:1222–1229. [Google Scholar]
- Lovera M., Pérez E., Laurentin A. Digestibility of starches isolated from stem and root tubers of arracacha, cassava, cush–cush yam, potato and taro. Carbohydrate Polymers. 2017;176:50–55. doi: 10.1016/j.carbpol.2017.08.049. [DOI] [PubMed] [Google Scholar]
- Lu Z.-H., Donner E., Yada R.Y., Liu Q. The synergistic effects of amylose and phosphorus on rheological, thermal and nutritional properties of potato starch and gel. Food Chemistry. 2012;133:1214–1221. [Google Scholar]
- McGrance S.J., Cornell H.J., Rix C.J. A simple and rapid colorimetric method for the determination of amylose in starch products. Starch/Stärke. 1998;50:158–163. [Google Scholar]
- Miskeen S., Park E.Y., Kim J.-Y. Controlled fragmentation of starch into nanoparticles using a dry heating treatment under mildly acidic conditions. International Journal of Biological Macromolecules. 2018;123:810–816. doi: 10.1016/j.ijbiomac.2018.11.072. [DOI] [PubMed] [Google Scholar]
- Paredes-López O., Bello-Pérez L.A., López M.G. Amylopectin: Structural, gelatinization and retrogradation studies. Food Chemistry. 1994;50:411–418. [Google Scholar]
- Peshin A. Characterization of starch isolated from potato tubers (Solanum tuberosum L.) Journal of Food Science and Technology. 2001;38:447–449. [Google Scholar]
- Sevenou O., Hill S.E., Farhat I.A., Mitchell J.R. Organisation of the external region of the starch granule as determined by infrared spectroscopy. International Journal of Biological Macromolecules. 2002;31:79–85. doi: 10.1016/s0141-8130(02)00067-3. [DOI] [PubMed] [Google Scholar]
- Šimková D., Lachman J., Hamouz K., Vokál B. Effect of cultivar, location and year on total starch, amylose, phosphorous content and starch grain size of high starch potato cultivars for food and industrial processing. Food Chemistry. 2013;141:3872–3880. doi: 10.1016/j.foodchem.2013.06.080. [DOI] [PubMed] [Google Scholar]
- Singh J., Kaur L., Singh N. Effect of acetylation on some properties of corn and potato starches. Starch/Stärke. 2004;56:586–601. [Google Scholar]
- Singh N., Kaur L., Singh J. Relationships between various physicochemical, thermal and rheological properties of starches separated from different potato cultivars. Journal of the Science of Food and Agriculture. 2004;84:714–720. [Google Scholar]
- Singh J., McCarthy O.J., Singh H., Moughan P.J. Low temperature post-harvest storage of New Zealand Taewa (Maori potato): Effects on starch physico-chemical and functional characteristics. Food Chemistry. 2008;106:583–596. [Google Scholar]
- van Soest J.J.G., Tournois H., de Wit D., Vliegenthart J.F.G. Short-range structure in (partially) crystalline potato starch determined with attenuated total reflectance Fourier-transform IR spectroscopy. Carbohydrate Research. 1995;279:201–214. [Google Scholar]
- Vargas G., Martinez P., Velezmoro C. Propiedades funcionales de almidón de papa (Solanum tuberosum) y su modificación química por acetilación. Scientia Agropecuria. 2016;79:183–192. [Google Scholar]
- Vasanthan T., Bergthaller W., Driedger D., Yeung J., Sporns P. Starch from Alberta potatoes: wet-isolation and some physicochemical properties. Food Research International. 1999;32:355–365. [Google Scholar]
- Warren F.J., Gidley M.J., Flanagan B.M. Infrared spectroscopy as a tool to characterise starch ordered structure-a joint FTIR–ATR, NMR, XRD and DSC study. Carbohydrate Polymers. 2016;139:35–42. doi: 10.1016/j.carbpol.2015.11.066. [DOI] [PubMed] [Google Scholar]
- Waterschoot J., Gomand S.V., Delcour J.A. Impact of swelling power and granule size on pasting of blends of potato, waxy rice and maize starches. Food Hydrocolloids. 2016;52:69–77. [Google Scholar]
- Waterschoot J., Gomand S.V., Willebrords J.K., Fierens E., Delcour J.A. Pasting properties of blends of potato, rice and maize starches. Food Hydrocolloids. 2014;41:298–308. [Google Scholar]
- Wiesenborn D.P., Orr P.H., Casper H.H., Tacke B.K. Potato starch paste behavior as related to some physical/chemical properties. Journal of Food Science. 1994;59:644–648. [Google Scholar]
- Xu Y., Grizzard C., Sismour E., Bhardwaj H., Li Z. Resistant starch content, molecular structure and physicochemical properties of starches in Virginia-grown corn, potato and mungbean. Journal of Cereals and Oilseeds. 2013;4:10–18. [Google Scholar]
- Xu X., Huang X.-F., Visser R.G.F., Trindade L.M. Engineering potato starch with a higher phosphate content. PLoS One. 2017;12 doi: 10.1371/journal.pone.0169610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuph M., Tester R.F., Ansell R., Snape C.E. Composition and properties of starches extracted from tubers of different potato varieties grown under the same environmental conditions. Food Chemistry. 2003;82:283–289. [Google Scholar]
- Zhang G., Ao Z., Hamaker B.R. Slow digestion property of native cereal starches. Biomacromolecules. 2006;7:3252–3258. doi: 10.1021/bm060342i. [DOI] [PubMed] [Google Scholar]
- Zhou F., Liu Q., Zhang H., Chen Q., Kong B. Potato starch oxidation induced by sodium hypochlorite and its effect on functional properties and digestibility. International Journal of Biological Macromolecules. 2016;84:410–417. doi: 10.1016/j.ijbiomac.2015.12.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.