Highlights
-
•
Commercial oat products contained ten phenolic acids and three avenanthramides.
-
•
Commercial oats provide 15.79–25.05 mg total phenolic acids in a 40 g serving of oats.
-
•
The concentrations and compositions in the products were broadly similar.
-
•
Major component was ferulic acid (58–78.1%) in all products.
-
•
Oatbran concentrate has the highest levels of phenolic acids and avenanthramides.
Keywords: Oats, Avenanthramides, Phenolic acids, Ferulic acid, Oat products, Oat cakes, Oat bran
Abstract
Oats contain a range of phenolic acids and avenanthramides which may have health benefits. Analysis of 22 commercial oat products (oat bran concentrate, oat bran, flaked oats, rolled oats and oatcakes) using HPLC-DAD detected eleven bound and thirteen free + conjugated phenolic acids and avenanthramides. The oat products (excluding concentrate) provided between 15.79 and 25.05 mg total phenolic acids (9.9–19.33 mg bound, 4.96–5.72 mg free + conjugated) and between 1.1 and 2 mg of avenanthramides in a 40 g portion while an 11 g portion of oat concentrate provided 16.7 mg of total phenolic acids (15.17 mg bound, 1.53 mg free + conjugated) and 1.2 mg of avenanthramides. The compositions and concentrations of the components in the different products were broadly similar, with the major component being ferulic acid (58–78.1%). The results show that commercial oat products are a source of phenolic acids and avenanthramides for consumers.
1. Introduction
Epidemiological evidence suggests that regular consumption of whole grains is associated with a lower risk of chronic diseases, such as cardiovascular disease (CVD), type 2 diabetes and certain cancers (Reynolds et al., 2019). Meta-analyses of multiple human intervention studies provide strong evidence that regular oat intake lowers blood cholesterol, improves insulin sensitivity and post-prandial glycaemic control (Bao et al., 2014, Ho et al., 2016). Oats (Avena sativa) are wholegrain cereals which provide proteins, unsaturated fatty acids, vitamins, minerals (Singh, De, & Belkheir, 2013), phenolics (including avenanthramides that are unique to oats), and significant quantities of dietary fibre, including cellulose, arabinoxylan and β-glucan. (Menon et al., 2016). There is a solid body of evidence and a health claim by EFSA approving the cholesterol lowering properties of oat β-glucans. (Efsa Panel on Dietetic Products & Allergies, 2011, Ho et al., 2016). A diet rich in oats can provide phenolic acids and avenanthramides that may contribute to the beneficial health effects. Although there are mixed findings from different studies testing different foods, well designed randomized controlled trials investigating a variety of foods such as coffee and blueberries showed protective effects of circulating phenolics against CVD (Mills et al., 2017, Rodriguez-Mateos et al., 2016, Rodriguez-Mateos et al., 2013, Sawicki et al., 2016, Schar et al., 2015). Avenanthramides have also been shown to be bioavailable in humans and may exert anti-inflammatory, anti-proliferative and vasodilatory effects to protect against CVD and colon cancer (Bryngelsson et al., 2002, Chen et al., 2007, Liu et al., 2004, Nie et al., 2006, Xie et al., 2017).
Phenolic acids comprise one aromatic ring bearing an acid group and one or more hydroxyl groups (Li, Shewry, & Ward, 2008) and are present in the oat grain in three forms: as soluble free acids, as soluble conjugates esterified to low molecular weight components such as sugars, and as insoluble bound acids esterified to high molecular weight components, notably cell wall polysaccharides (dietary fibre and lignin) (Collins, 1986). Cereal phenolic acids consist of two major types: based on either hydroxycinnamic acid or hydroxybenzoic acid. Hydroxybenzoic acids in oats include protocatechuic, syringic, vanillic, p-hydroxybenzoic and gallic acids, while hydroxycinnamic acids in oats are ferulic, p-coumaric, o-coumaric, caffeic and sinapic acids (Supplementary Fig. 1) (Shewry et al., 2008). Avenanthramides consist of an amide conjugate of anthralinic acid and hydroxycinnamic acids. The 3 major types are esters of 5-hydroxyanthranilic acid with p-coumaric (2p aka A), and ferulic (2f aka B), and caffeic (2c aka C) acids which occur predominantly in the bran layer of the oat grain (Supplementary Fig. 1) (Bratt et al., 2003, Collins, 1989).
Several previous studies have reported the presence and amounts of phenolic acids and avenanthramides in oats (Dimberg et al., 2005, Mattila et al., 2005, Shewry et al., 2008). However, some of these studies analysed experimental material (including hulled grains) which would not be available to consumers while others analysed either avenanthramides or phenolic acids but not the two groups together. Therefore, the aim of this study was to determine the composition and amounts of phenolic acids and avenanthramides in a number of commercial oat samples, determining differences between products (oat bran, oat bran concentrate, rolled, flaked oats and oat cakes) and estimating the amounts consumed in typical portions of oat-based products in order to understand their contribution to dietary intakes of phenolics and to inform further clinical trials aiming to test the bioactivity of these compounds at normal intake levels.
2. Materials & methods
2.1. Chemicals and reagents
All individual phenolic acid and avenanthramide standards were obtained from Sigma-Aldrich (St. Louis, MO, USA). HPLC column and guard cartridges were obtained from Phenomenex (Torrance, CA, USA). Unless otherwise stated, all chemicals and reagents were obtained from Sigma-Aldrich or Fisher Scientific.
2.2. Oat samples
22 commercial oat products were purchased in local shops or online and comprised 5 oat brans, 5 oat flakes, 6 rolled oats, 5 oatcakes and one oat concentrate (Table 1). The samples were milled using a coffee grinder and kept at −20 °C until analysis to protect bioactive ingredients from degradation.
Table 1.
Brands, names, types and numbers of commercial oat products.
| Brand | Type | Number |
|---|---|---|
| Oatwell | Bran concentrate | 1 |
| White’s (Fine cut) | Bran | 2 |
| Mornflake | Bran | 3 |
| Sante (Otreby Owsiane) | Bran | 4 |
| Greenlands (organic) | Bran | 5 |
| M&S (Traditional British porridge oats) | Flaked | 6 |
| Sainsbury’s (SO organic Scottish oats) | Flaked | 7 |
| Sainsbury’s (Scottish porridge oats) | Flaked | 8 |
| Flahavan’s (Irish organic jumbo oats) | Flaked | 9 |
| Kupiec (plain) | Flaked | 10 |
| Quaker (Oats so simple original-sachets) | Rolled | 11 |
| Sainsbury’s (Taste the difference Scottish) | Rolled | 12 |
| Asda (Original porridge) | Rolled | 13 |
| Quaker (Jumbo oats) | Rolled | 14 |
| Quaker | Rolled | 15 |
| Scott’s (Porridge oats) | Rolled | 16 |
| Nairns (Fine milled, 85% oats) | Oatcake | 17 |
| Nairns (Organic, enriched with oatbran, 85% oats) | Oatcake | 18 |
| Savour Bakes (Scottish rough, 89% oats) | Oatcake | 19 |
| Clearspring (Organic, 83% oats) | Oatcake | 20 |
| Paterson’s (Rough, % *ng) | Oatcake | 21 |
| Stockan’s (Orkney, thin, 76% oats) | Oatcake | 22 |
ng (not given); proportion of oat (%) is not provided on the package of the product.
2.3. Extraction of free, conjugated and bound phenolic acids
Phenolic acids and avenanthramides were extracted in two separate fractions (i.e. free + conjugated and bound) (Supplementary Fig. 2), in three replicates, using the method of Schär et al. (2018). 2 mL of hexane were added to 20.0 ± 0.1 mg of milled oat samples for defatting. Solutions were vortexed for 30 s, sonicated for 10 min, vortexed and kept on a shaker for 50 min. Following centrifugation for 15 min at 5000 rcf, the supernatant was discarded and the wet samples were dried with a Savant™ SPD131DDA SpeedVac™ Concentrator (ThermoFisher Scientific, Waltham, MA, USA) for 10 min. The first extraction step was carried out by adding 80:20 ethanol/water (1 mL) and samples were vortexed until all of the flour was suspended. 0.375µg of internal standard (3-5,dichloro-4-hydroxybenzoic acid) was added and the samples were extracted for 10 min by sonication in a sonic bath. Samples were then heated to 80 °C for 15 min and centrifuged for 15 min at 5000g. The supernatant was removed into a test tube and evaporated with a SpeedVac. This extraction was repeated twice without the heating and the addition of internal standard. The combined supernatants were evaporated to dryness. For the extraction of free + conjugated fractions, the dried supernatant was hydrolysed with 2 M NaOH (400 μl) with 10 μM EDTA and 1% (w/v) ascorbic acid for 4 h and then acidified with 12 M HCl (125 μl) to pH 2 and kept at −20 °C overnight. For the extraction of the bound fraction, the pellet was dried in a Speedvac for 5 min, 30 μg internal standard added and hydrolysis was carried out with 2 M NaOH solution (800 μl), 10 μM EDTA and 1% (w/v) ascorbic acid for 16–18 h with gentle agitation. Following hydrolysis, samples were centrifuged for 15 min at 16,100g, supernatants were transferred to clean Eppendorf tubes and acidified with 12 M HCl (220 μl). Bound and free + conjugated fractions were extracted with ethyl acetate (3 × 800 μl), evaporated to dryness and re-dissolved in 2% (v/v) aqueous acetic acid (100 μl) using vortexing for 30 s, sonication for 1 min and shaking for an hour. Final solutions were centrifuged for 5 min at 16,100g and transferred to vials containing a low volume insert for HPLC analysis.
2.4. HPLC analysis
Phenolic acids and avenanthramides were identified and quantified using an Agilent 1100 series HPLC (Agilent Technologies Ltd, Santa Clara, CA, USA) equipped with a quaternary pump, autosampler, column thermostat, sample thermostat and photodiode array detector. Phenolic acids were separated on a Kinetex biphenyl column (100A 250 × 4.6 mm length, 5 μM particle size; Phenomenex) using a gradient elution programme developed in house for the separation of 18 different phenolic acids and 3 avenanthramides. Mobile phase A consisted of 0.1% (v:v) formic acid in HPLC grade water (A), while mobile phase B was 0.1% (v:v) formic acid in methanol. The following optimised gradient protocol was run: 0 min, 95% A; 20 min, 75% A; 25 min, 74% A; 30 min, 65% A; 40 min, 64% A; 53 min, 30% A; 56 min, 5% A; 61 min, 5% A; 62 min, 95% A; 65 min, 95% A. The flow rate of the mobile phase was 1.0 mL/min, the column temperature was 30 °C and the sample injection volume was 20 µl. Quantification of phenolic acids and avenanthramides were made at 280 nm. The retention time and absorbance of each compound was established by running standards (Supplementary Table 1). All standards were prepared as stock solutions at 1 mg/mL in methanol and stored at −80 °C. Phenolic acids and avenanthramides were quantified by calculating the ratio of the area of the analyte to the area of the internal standard (i.e. 3-5,dichloro-4-hydroxybenzoic acid). Quantification was based on 10 point linear calibration curves of phenolic acid and avenanthramide standards which covered the range present in the oat samples and had R2 values above 0.9 (Supplementary Table 1). The concentrations of individual components were stated as μg/g. Good reproducibility was observed between replicate analyses with an average of 9% for the coefficient of variation for all samples. All data were analysed using Agilent Chem Station software.
2.5. Statistical analysis
Means and standard errors of 3 replicates were calculated for each sample. Data were quantified and expressed as μg/g fresh weight. Calibration curves of phenolic acid and avenanthramide standards were created by MS Excel 2010.
3. Results and discussion
3.1. Identification of phenolic acids and avenanthramides in commercial oat products
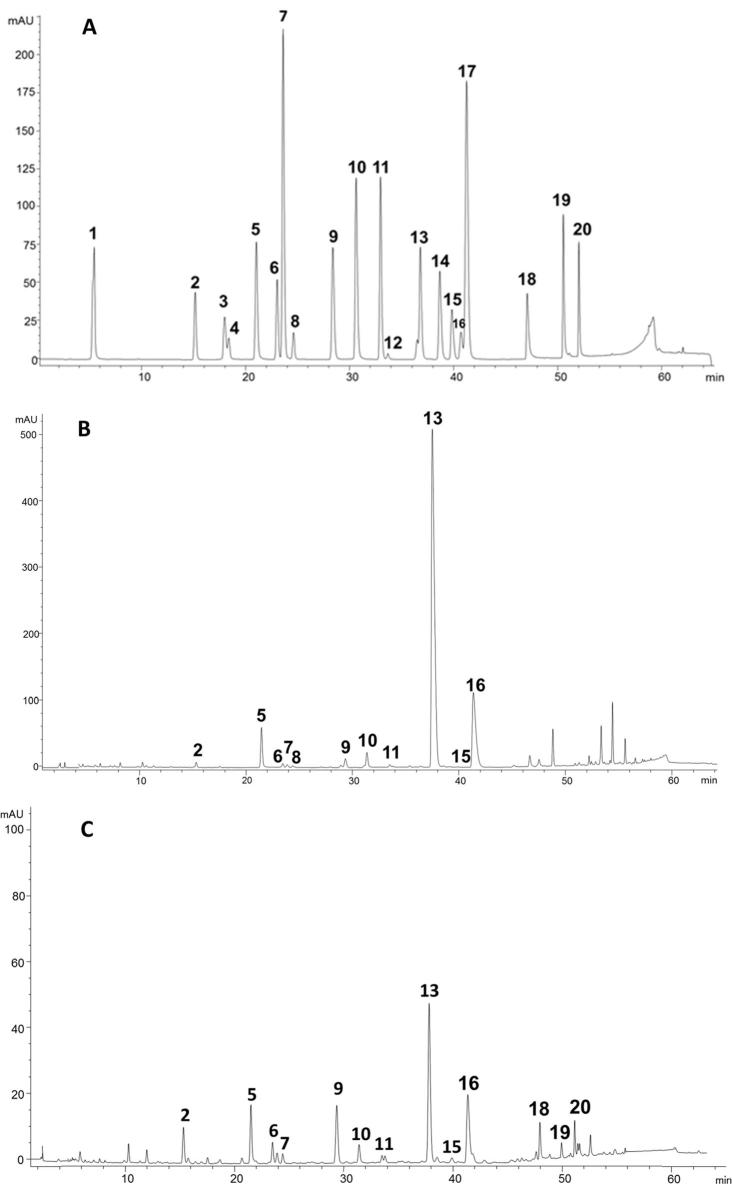
Standards of twenty different phenolic compounds (including phenolic acids and avenanthramides) which could be present in oat products were analysed by HPLC-DAD (Fig. 1A), measuring their absorbance maxima and retention times (Supplementary Table 1). Comparisons with these standards identified eleven components in the bound fractions and thirteen in the free + conjugated fractions extracted from the products. This is illustrated by the typical chromatograms for oat bran (product 3) in Fig. 1B and C. While both the bound and free + conjugated fractions contained 4-hydroxybenzoic acid, vanillic acid, caffeic acid, 4-hydroxybenzaldehyde, syringic acid, p-coumaric acid vanillin, ferulic acid and sinapic acid, the three avenanthramides (avenanthramide A, avenanthramide B and avenanthramide C) were only present in the free + conjugated fractions.
Fig. 1.
Chromatogram of phenolic acid and avenanthramide standards at 280 nm (A) and representative chromatograms of bound phenolic acids (B) and free + conjugated phenolic acids oat from sample 3 (Table 1) for at 280 nm (C). Compounds were identified based on retention time and absorbance spectra (Supplementary Table 1): 1, gallic acid; 2, 4-hydroxybenzoic acid; 3, 2,4-dihydroxybenzoic acid; 4, 4-hydroxyphenyl acetic acid; 5, caffeic acid; 6, vanillic acid; 7; 4-hydroxybenzaldehyde; 8, homovanillic acid; 9, syringic acid; 10, p-coumaric acid; 11, vanillin; 12, salicylic acid; 13, ferulic acid; 14, syringaldehyde; 15, sinapic acid; 16, 3-5, dichloro-4-hydroxybenzoic acid; 17, o-coumaric acid; 18, avenanthramide C; 19, avenanthramide A; 20, avenanthramide B.
3.2. Total contents of phenolic acids and avenanthramides
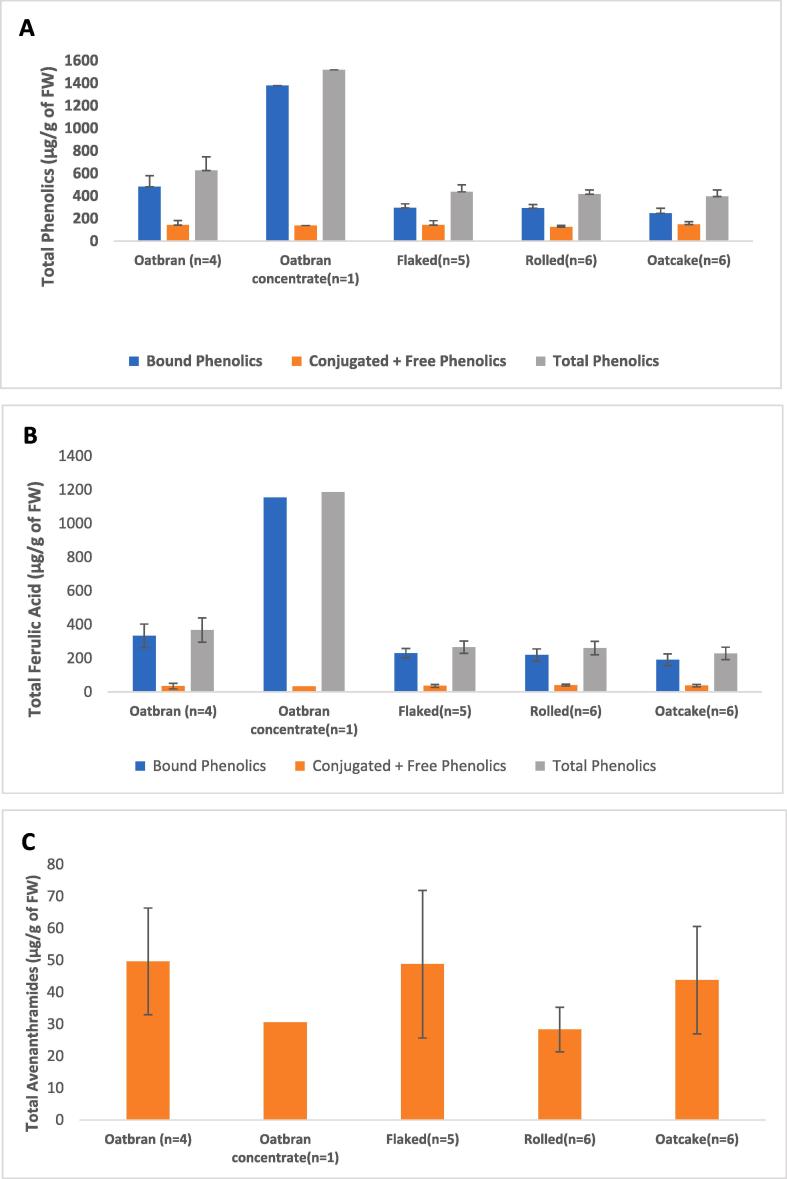
The mean total contents of phenolic acids and avenanthramides in the groups of oat products was highest for the oat bran concentrate (1518.6 μg/g) followed by oat bran (626.3 ± 60.1 μg/g), flaked oats (438.1 ± 27.0 μg/g), rolled oats (415.8 ± 14.9 μg/g) and oatcakes (394.8 ± 24.2 μg/g) (Fig. 2A). All the results are reported on a fresh weight (FW) basis. The bound phenolic acid fraction made the greatest contribution to the total phenolic contents of all products (62.7–90.8%) while the amounts of the free + conjugated fractions were lower in all products (9.2–37.2%). This is in agreement with other studies (Multari et al., 2018, Zeng et al., 2016). When the contents and compositions of phenolic acids and avenanthramides were compared within each group of oat products, the bran products showed the greatest variation in both free + conjugated (ranging from 100.9 to 194.9 μg/g) and bound (ranging from 359.5 to 592.4 μg/g) fractions, the free + conjugated fraction also varied between the samples of oat flakes (115.7–210 μg/g). Oat bran concentrate comprises mainly fibre and, in agreement with the literature, our analysis showed that this fraction was rich in bound phenolic acids (Peterson, 2001). The total contents of phenolic acids in the oat bran products were only slightly higher than those in the flaked oats, rolled oats and oatcake products. Similar results for different oat products were reported by Mattila et al. (2005) who suggested that the similar phenolic content of oat bran and other oat products might be due to the processing method which results in the bran containing some aleurone and starchy endosperm as well as outer layers of the grain (pericarp and testa) (Chen et al., 2018, Mattila et al., 2005).
Fig. 2.
The amounts of total, conjugated + free and bound phenolic acids (A), ferulic acid (B) and total avenanthramides (C) in oat products including oat bran, oat bran concentrate, flaked oats, rolled oats and oatcake. Data are expressed as the mean ± SEM. n indicates the number of each product analysed. FW, fresh weight.
Avenanthramides were only present as soluble forms with the mean total concentrations being highest in oat bran (49.6 ± 8.3 μg/g) followed by flaked oats (48.8 ± 10.3 μg/g), oatcakes (43.8 ± 6.9 μg/g), oat bran concentrate (30.6 μg/g) and rolled oats (28.3 ± 2.9 μg/g) (Fig. 2C). Avenanthramides have been reported to be heat stable under conditions of commercial processing and the levels of these compounds in oatcakes, which are mostly processed products in our sample range, were higher than in the oat bran concentrate which contained highest total levels of phenolic acids (Dinberg, Sunnerheim, Sundberg, & Walsh, 2001). The concentrations determined in this study were broadly consistent with those reported previously (Chen et al., 2018, Mattila et al., 2005, Pridal et al., 2018, Shewry et al., 2008). However, whereas Chen et al., 2018, Pridal et al., 2018 reported higher concentrations of avenanthramides in oat bran samples in comparison to whole oats, including rolled oats, Mattila et al. (2005) reported that commercial oat bran products produced by conventional milling techniques could not be discriminated from oat flakes based on their avenanthramide content, which is in agreement with our results. It should also be noted that the contents of phenolic acids and avenanthramides in oat grain will vary with the crop genotype and the growth conditions (including location and environment) (Li et al., 2017, Pridal et al., 2018).
3.3. Compositions and individual contents of phenolic acids and avenanthramides
The most abundant bound phenolic acids in all oat products analysed were ferulic acid, caffeic acid and sinapic acid (Table 2). The oat bran concentrate had the highest concentrations of these compounds in the bound fraction (1153.6 μg/g, 72.2 μg/g and 80.3 μg/g, respectively) whereas oatcakes had the lowest (191.1 ± 14.2 μg/g, 11.9 ± 0.9 μg/g and 21.7 ± 1.1 μg/g, respectively). Ferulic acid, sinapic acid and avenanthramide B were the major components in the free + conjugated fractions of all products, with rolled oats having the highest concentration of ferulic acid (40.8 ± 2.2 μg/g), oat bran concentrate the highest concentration of sinapic acid (36.2 μg/g) and oat bran the highest concentration of avenanthramide B (27.6 ± 4.6 μg/g).
Table 2.
Composition and contents of phenolic acids and avenanthramides in individual oat products.
| Concentration (μg/g of FW)a | Bran Conc. (1) | Bran (2) | Bran (3) | Bran (4) | Bran (5) | Flaked (6) | Flaked (7) | Flaked (8) | Flaked (9) | Flaked (10) | Rolled (11) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4-hydroxybenzoic acid | |||||||||||
| Free &Conjugated | 7.1 ± 0.1 | 4.1 ± 0.2 | 9 ± 0.1 | 3.67 ± 0.2 | 3.3 ± 0 | 4.6 ± 0.2 | 3.6 ± 0.1 | 5.1 ± 0.1 | 4.3 ± 0.1 | 4 ± 0 | 6.3 ± 0.3 |
| Bound | 15.5 ± 0.1 | 4.5 ± 0.2 | 8.5 ± 0.4 | 12.3 ± 0.7 | 10.7 ± 0.8 | 3.9 ± 0.4 | 3.2 ± 0 | 1.9 ± 0.2 | 3.5 ± 0.3 | 5.6 ± 0.2 | 3.9 ± 0.1 |
| Vanillic acid | |||||||||||
| Free& Conjugated | 5.5 ± 0 | 6.8 ± 0.2 | 10.7 ± 0.2 | 4 ± 0.6 | 4.5 ± 0.2 | 7.1 ± 0.4 | 9.5 ± 0.4 | 8.1 ± 0.2 | 6.3 ± 0.4 | 3.5 ± 0.1 | 9.6 ± 0.6 |
| Bound | 12.9 ± 0.3 | 6.9 ± 0.1 | 12.2 ± 0.3 | 16.9 ± 1.2 | 15.3 ± 1.1 | 5.6 ± 0.4 | 6.5 ± 1.4 | 9.2 ± 1 | 6.5 ± 0.5 | 3.4 ± 0.2 | 5.8 ± 4.2 |
| Caffeic acid | |||||||||||
| Free& Conjugated | 8.8 ± 0.1 | 1.1 ± 0.2 | 1.9 ± 0.1 | 19.3 ± 0.9 | 19.4 ± 0.4 | 1.4 ± 0 | 1.5 ± 0 | 1.9 ± 0 | 27.1 ± 0.3 | 11.8 ± 0.3 | 1.8 ± 0.1 |
| Bound | 72.7 ± 0.7 | 21.1 ± 0.9 | 33.2 ± 0.3 | 39 ± 3.8 | 32.5 ± 2.6 | 15.4 ± 0.4 | 17.9 ± 0.6 | 13.1 ± 0.8 | 15 ± 0.7 | 17 ± 0.1 | 17.4 ± 1.2 |
| 4-Hydorxybenzaldeyhde | |||||||||||
| Free& Conjugated | 2.6 ± 0 | 2.8 ± 0.1 | 3.8 ± 0 | 2.3 ± 0.1 | 2.5 ± 0 | 2.7 ± 0 | 3.5 ± 0.1 | 3.1 ± 0 | 3 ± 0.2 | 2.3 ± 0.1 | 3.6 ± 0.1 |
| Bound | 4.1 ± 0.1 | 2.2 ± 0.1 | 3.6 ± 0.2 | 4.5 ± 0.2 | 4.1 ± 0.2 | 1.6 ± 0 | 1.6 ± 0.1 | 1.4 ± 0 | 2.3 ± 0.1 | 1.6 ± 0 | 2.9 ± 0.1 |
| Syringic acid | |||||||||||
| Free& Conjugated | 10.7 ± 0.1 | 6.8 ± 0.9 | 13.1 ± 0.2 | 6.8 ± 0.3 | 8.6 ± 0.1 | 7.5 ± 0.5 | 8.5 ± 0.4 | 11.9 ± 0.1 | 10.7 ± 0.1 | 8.2 ± 0.3 | 10.2 ± 0.7 |
| Bound | 18.2 ± 0.4 | 5.1 ± 0.7 | 15.2 ± 0.7 | 20.8 ± 2 | 21.1 ± 1.8 | 5 ± 0.6 | 6.4 ± 0.1 | 5.2 ± 0.1 | 7.2 ± 0.4 | 6.8 ± 0.2 | 6.7 ± 1 |
| P-coumaric acid | |||||||||||
| Free& Conjugated | 2.3 ± 0 | 6.3 ± 0.5 | 6.8 ± 0.5 | 2.1 ± 0.1 | 2.1 ± 0.1 | 5.5 ± 1.4 | 3.1 ± 0.2 | 4.3 ± 0.1 | 3.3 ± 0.1 | 2.3 ± 0.1 | 4.1 ± 0.3 |
| Bound | 17.5 ± 1.3 | 49.4 ± 3.9 | 36.6 ± 32.7 | 5.7 ± 0.7 | 5.8 ± 0.9 | 6.8 ± 1.5 | 13.7 ± 5.2 | 3.5 ± 0.5 | 5.3 ± 0.3 | 2.7 ± 0.2 | 5.4 ± 0 |
| Vanillin | |||||||||||
| Free& Conjugated | 2.8 ± 0 | nq | 2 ± 0 | 2.7 ± 0 | 2.7 ± 0 | nq | nq | nq | 3.5 ± 0.2 | 2.7 ± 0 | nq |
| Bound | 4.5 ± 0.4 | 3.3 ± 0.1 | 3.9 ± 1 | 3 ± 0.2 | 2.4 ± 0.1 | 2.3 ± 0.1 | 2.2 ± 0 | 2.3 ± 0.4 | 2.5 ± 0.1 | 2 ± 0.2 | 2.1 ± 0.1 |
| Ferulic acid | |||||||||||
| Free& Conjugated | 32.8 ± 0.1 | 32.8 ± 2.8 | 58.6 ± 0.1 | 24 ± 1.9 | 23.1 ± 0.9 | 34.1 ± 0.8 | 40.8 ± 2.6 | 38.1 ± 0.7 | 44.2 ± 1.2 | 20.2 ± 0.8 | 50.6 ± 3.5 |
| Bound | 1153.6 ± 5.8 | 247.2 ± 8.2 | 348.7 ± 3.3 | 416.9 ± 29.7 | 317.1 ± 10.2 | 230.1 ± 10.3 | 255.7 ± 3.6 | 222.7 ± 7.9 | 251.8 ± 3.1 | 187.2 ± 6.2 | 262.7 ± 4.7 |
| Sinapic acid | |||||||||||
| Free& Conjugated | 36.2 ± 0.1 | 14.2 ± 0.8 | 32 ± 0.2 | 15.4 ± 1.1 | 17.1 ± 0.5 | 19.9 ± 0.4 | 21.7 ± 1.4 | 26.3 ± 0.4 | 25.9 ± 0.5 | 15.4 ± 0.1 | 8.7 ± 0.6 |
| Bound | 80.3 ± 1.3 | 20.6 ± 0.6 | 50.2 ± 0.4 | 74.1 ± 4.9 | 60 ± 3.3 | 23.3 ± 1.9 | 23.1 ± 0.7 | 19.5 ± 3.5 | 32.8 ± 1.6 | 20.5 ± 1.2 | 25.7 ± 2.1 |
| Avenanthramide-C | |||||||||||
| Free& Conjugated | 11.5 ± 0.2 | 2.2 ± 0.2 | 1.6 ± 0.2 | 11.6 ± 3.2 | 12.4 ± 1.8 | 1 ± 0.1 | 1 ± 0.1 | 1.4 ± 0.1 | 18.1 ± 0.4 | 18.5 ± 8.5 | 8.3 ± 0.4 |
| Avenanthramide-A | |||||||||||
| Free& Conjugated | 6.8 ± 0.2 | 8.5 ± 0.9 | 23.8 ± 4.7 | 12.9 ± 3.4 | 15.4 ± 2.4 | 14.9 ± 0.8 | 9.2 ± 1.3 | 15 ± 0.7 | 25.4 ± 0.8 | 18.7 ± 4.9 | 6.7 ± 0.3 |
| Avenanthramide-B | |||||||||||
| Free& Conjugated | 12.3 ± 0.3 | 15.3 ± 1.5 | 31.5 ± 1.5 | 26.6 ± 4.7 | 36.8 ± 6.9 | 25.7 ± 1.9 | 13.3 ± 2.5 | 18.7 ± 0.8 | 38.3 ± 1.5 | 24.5 ± 3.7 | 7.3 ± 1.2 |
| Total Free & Conjugated | 139.3 ± 0.5 | 100.9 ± 8.2 | 194.9 ± 2.6 | 129.5 ± 14.1 | 146.8 ± 9.4 | 124.4 ± 5.2 | 115.7 ± 8.3 | 134 ± 2.8 | 210 ± 1.9 | 130.3 ± 15.8 | 117.2 ± 7.1 |
| Total Bound | 1379.3 ± 6.9 | 359.5 ± 14.8 | 512.2 ± 36.9 | 592.4 ± 43 | 469 ± 19.1 | 294 ± 13.7 | 330.2 ± 2.8 | 278.8 ± 13.6 | 326.9 ± 3.7 | 246.7 ± 8.1 | 332.5 ± 13.1 |
| Total Phenolics | 1518.6 ± 277.3 | 460.5 ± 58.3 | 707 ± 80.3 | 721.9 ± 105 | 615.7 ± 72.2 | 418.2 ± 38.5 | 445.9 ± 48.1 | 412.8 ± 35.8 | 536.9 ± 26.2 | 377 ± 27.2 | 449.7 ± 48.6 |
| Concentration (μg/g of FW)a | Rolled (12) | Rolled (13) | Rolled (14) | Rolled (15) | Rolled (16) | Oatcake (17) | Oatcake (18) | Oatcake (19) | Oatcake (20) | Oatcake (21) | Oatcake (22) |
| 4-hydroxybenzoic acid | |||||||||||
| Free &Conjugated | 3.1 ± 0.2 | 5 ± 0.1 | 5.4 ± 0.3 | 6 ± 0.3 | 5.2 ± 0.7 | 4.7 ± 0.1 | 4.5 ± 0.2 | 4.1 ± 0.1 | 3.9 ± 0.2 | 4.9 ± 0.5 | 3.5 ± 0 |
| Bound | 2 ± 0.2 | 2.7 ± 0.5 | 3.5 ± 0.5 | 3.7 ± 0.1 | 3.9 ± 0.2 | 3 ± 0.1 | 3.7 ± 0.3 | 2.5 ± 0.2 | 2.8 ± 0.2 | 3.6 ± 0.3 | 2.6 ± 0.3 |
| Vanillic acid | |||||||||||
| Free& Conjugated | 8.6 ± 0.6 | 7.3 ± 0.2 | 7.2 ± 0.3 | 8.6 ± 0.3 | 7.8 ± 0.5 | 6.9 ± 0.1 | 8.3 ± 0.3 | 7.4 ± 0.2 | 7.5 ± 0.3 | 8.5 ± 0.6 | 5.6 ± 0 |
| Bound | 8.9 ± 1 | 5.8 ± 0.8 | 8.1 ± 1.2 | 6.4 ± 0.5 | 6.7 ± 0.4 | 6.3 ± ± 0.1 | 5.6 ± 0.1 | 5.8 ± 0.4 | 4.9 ± 0.4 | 7.6 ± 0.7 | 5.1 ± 0.4 |
| Caffeic acid | |||||||||||
| Free& Conjugated | 1.4 ± 0 | 19.4 ± 0.6 | 1.5 ± 0 | 1.7 ± 0 | 1.8 ± 0.1 | 8.5 ± 0.3 | 11.5 ± 0.5 | 10 ± 0.3 | 12.8 ± 0.5 | 16 ± 1.2 | 12.8 ± 0.2 |
| Bound | 14.7 ± 1 | 12.1 ± 2.4 | 15.1 ± 0.6 | 18.4 ± 0.4 | 16.4 ± 0.3 | 11 ± 0.4 | 11 ± 0.7 | 10.4 ± 0.6 | 11.2 ± 1.2 | 16.4 ± 1.3 | 11.2 ± 1.2 |
| 4-Hydroxybenzaldeyhde | |||||||||||
| Free& Conjugated | 3.3 ± 0.1 | 2.9 ± 0 | 3 ± 0.1 | 3.4 ± 0.1 | 3.2 ± 0.1 | 2.9 ± 0 | 3.5 ± 0.1 | 3.3 ± 0 | 3.3 ± 0.1 | 3.5 ± 0.1 | 2.6 ± 0 |
| Bound | 1.5 ± 0.1 | 2.2 ± 0.2 | 2.3 ± 0.1 | 2.4 ± 0.1 | 2.4 ± 0.1 | 2.1 ± 0 | 2.1 ± 0.1 | 2.2 ± 0.1 | 1.9 ± 0.1 | 2.6 ± 0.1 | 1.8 ± 0.1 |
| Syringic acid | |||||||||||
| Free& Conjugated | 7.7 ± 0.5 | 10.1 ± 0.4 | 8 ± 0.5 | 11 ± 0.7 | 9 ± 0.8 | 8.8 ± 0.2 | 11.8 ± 0.6 | 9.8 ± 0.2 | 12.2 ± 0.5 | 10.5 ± 0.8 | 7.3 ± 0.1 |
| Bound | 5.3 ± 0.7 | 4.7 ± 0.7 | 6.2 ± 0.7 | 7.8 ± 0.3 | 6.5 ± 0.5 | 4.5 ± 0.2 | 7.2 ± 0.7 | 4.9 ± 0.1 | 4.8 ± 0.4 | 5.9 ± 0.7 | 4.5 ± 0.5 |
| P-coumaric acid | |||||||||||
| Free& Conjugated | 3.1 ± 0.3 | 3.5 ± 0.1 | 3.8 ± 0.1 | 4.4 ± 0.1 | 4.6 ± 0.2 | 2.9 ± 0.1 | 4.2 ± 0.2 | 2.9 ± 0.1 | 8.2 ± 0.3 | 4 ± 0.2 | 3.2 ± 0 |
| Bound | 3.5 ± 0.7 | 2.6 ± 1.7 | 3.9 ± 0.4 | 5.5 ± 0.6 | 5.7 ± 0.7 | 4.1 ± 0.2 | 8.5 ± 0.3 | 3.3 ± 0.8 | 5.6 ± 1.1 | 7.4 ± 1.6 | 1.9 ± 0.4 |
| Vanillin | |||||||||||
| Free& Conjugated | 3.1 ± 0 | 4.6 ± 0.1 | 3.2 ± 0.1 | 3.5 ± 0.3 | 2.8 ± 0.4 | 4.3 ± 0.1 | 3.5 ± 0 | 3.2 ± 0.1 | 5.4 ± 0.1 | 3.6 ± 0.1 | 3.6 ± 0.1 |
| Bound | 2 ± 0.1 | 1.4 ± 0.7 | 3.5 ± 0.9 | 2.2 ± 0.1 | 2.1 ± 0.1 | 1.7 ± 0 | 1.8 ± 0.1 | 1.6 ± 0.1 | 1.4 ± 0.1 | 2 ± 0.1 | 1.4 ± 0.1 |
| Ferulic acid | |||||||||||
| Free& Conjugated | 38 ± 1.9 | 37.3 ± 0.7 | 35.5 ± 2.6 | 41.1 ± 1.5 | 42.1 ± 3.3 | 32.4 ± 0.5 | 42.5 ± 1.9 | 34.8 ± 0.6 | 48.3 ± 1.5 | 40.1 ± 2.8 | 27.2 ± 0.5 |
| Bound | 238.5 ± 22.3 | 175.7 ± 33.3 | 177.3 ± 21.1 | 243 ± 3.3 | 218.2 ± 8.3 | 181.7 ± 3.5 | 214.3 ± 10.4 | 198.9 ± 25.6 | 152.5 ± 17.9 | 242.7 ± 8.5 | 156.9 ± ± 16.1 |
| Sinapic acid | |||||||||||
| Free& Conjugated | 17.4 ± 0.8 | 22.7 ± 0.5 | 17.9 ± 1.1 | 25.9 ± 1 | 15.2 ± 6.7 | 17.3 ± 0.4 | 22.9 ± 1 | 25.4 ± 1.3 | 21.9 ± 0.6 | 26.1 ± 2 | 16.1 ± 0 |
| Bound | 24.2 ± 2.4 | 23 ± 3.5 | 22.8 ± 2.7 | 28.6 ± 1.1 | 25.1 ± 1.4 | 19.9 ± 0.5 | 23.3 ± 1.7 | 22.8 ± 1 | 18.9±2.2 | 25.9 ± 2.6 | 19.6 ± 1.1 |
| Avenanthramide-C | |||||||||||
| Free& Conjugated | 0.9 ± 0.1 | 9.4 ± 1.1 | 1.2 ± 0.1 | 1.5 ± 0.2 | 1.3 ± 0.3 | 5.8 ± 0.3 | 6.1 ± 0.9 | 10.7 ± 4.5 | 6.6 ± 0.4 | 23.3 ± 2.6 | 16.3 ± 4.2 |
| Avenanthramide-A | |||||||||||
| Free& Conjugated | 7.8 ± 0.5 | 8 ± 0.2 | 11.4 ± 0.4 | 12.6 ± 0.6 | 16.8 ± 1.7 | 5.3 ± 0.1 | 6.5 ± 0.6 | 10.7 ± 2.5 | 13.8 ± 1.4 | 18.9 ± 1.2 | 14.6 ± 4.4 |
| Avenanthramide-B | |||||||||||
| Free& Conjugated | 10.9 ± 0.6 | 12.3 ± 0.6 | 14 ± 0.9 | 19.1 ± 0.8 | 20.5 ± 3.1 | 11.5 ± 0.3 | 17.6 ± 1.8 | 23.7 ± 8.5 | 19.7 ± 2.2 | 26.5 ± 1.8 | 25 ± 10.4 |
| Total Free & Conjugated | 103.3 ± 3.8 | 142.4 ± 0.8 | 112.2 ± 5.7 | 138.8 ± 4.1 | 130.2 ± 15.9 | 111.2 ± 1.9 | 142.9 ± 7.8 | 140.4 ± 7.7 | 163.5 ± 6.7 | 185.8 ± 13 | 137.7 ± 18.4 |
| Total Bound | 300.8 ± 28 | 270 ± 43.6 | 242.6 ± 24.2 | 318 ± 5.8 | 287 ± 7.4 | 234.2 ± 4.5 | 277.5 ± 14.1 | 252.3 ± 28.5 | 203.9 ± 23.5 | 314.1 ± 14 | 205.1 ± ± 20.2 |
| Total Phenolics | 404.1 ± 45.9 | 412.4 ± 27.7 | 354.8 ± 31.2 | 456.8 ± 40.2 | 417.2 ± 35.9 | 345.4 ± 27.6 | 420.4 ± 30.9 | 392.7 ± 28.3 | 367.4 ± 14.2 | 499.9 ± 29.9 | 342.8 ± 19.4 |
aValues are expressed as means ± SEMs (n = 3). nq = not quantified.
The compositions of oat phenolics reported in other studies are largely in line with our findings, but there are few inconsistencies. Ferulic acid was reported to be the major component in both fractions by other workers (Mattila et al., 2005, Multari et al., 2018, Shewry et al., 2008). However, p-coumaric acid has also been reported to be highly abundant in other studies whereas the concentrations in our study were low (Bryngelsson et al., 2002, Kováčová and Malinová, 2007, Shewry et al., 2008). In agreement with Chen et al. (2018), avenanthramide-B was the predominant avenanthramide. However, the concentrations of avenanthramide-C reported here are lower than in other studies (Multari et al., 2018, Shewry et al., 2008). These inconsistencies may be due to differences in samples used (e.g. the samples analysed by Shewry et al. (2008) contained the hulls which are rich in p-coumaric acid), the methods used for extraction and analysis, and genetic and environmental factors.
3.4. Multivariate analysis of phenolic acid and avenanthramide composition
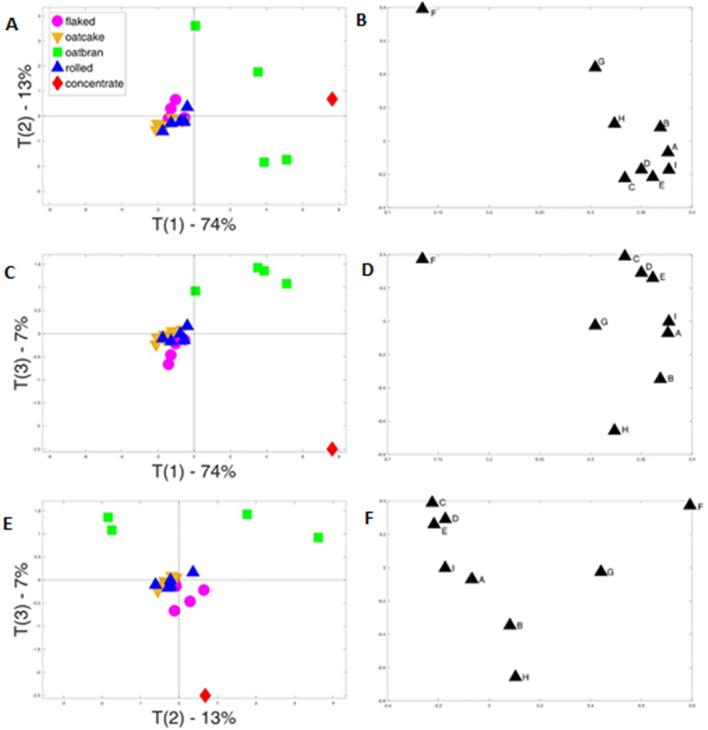
Principal component analyses (PCA) of the phenolics present in the bound and free + conjugated fractions are shown in Fig. 3, Fig. 4, respectively. Panels A, C and E are PCA score plots which show the grouping of oat products based on their overall phenolic acid compositions while panels B, D and F are the corresponding loading plots which show the individual phenolic acids responsible for the separation. For the bound fraction, all three score plots showed clear clustering of the rolled oats, oatcake and flaked oats in the centre of the plot, and separation of the single oat concentrate. Comparison of the score and loadings plots indicates that the latter separation mainly results from the very high content of ferulic acid (see also Table 2). The four bran samples do not cluster, but pairs of samples group together in all three plots and these pairs can be distinguished by their high and low contents of p-coumaric acid.
Fig. 3.
Principal component analysis of the contents of bound phenolic acids in different oat products. A, C and E are score plots and B, D and F the corresponding loading plots. Letters on loading plots represent phenolic acids: A, 4-hydroxybenzoic acid; B, caffeic acid; C, vanillic acid; D, 4-hydroxybenzaldehyde; E, syringic acid; F, p-coumaric acid; g, vanillin; h, ferulic acid; I, sinapic acid.
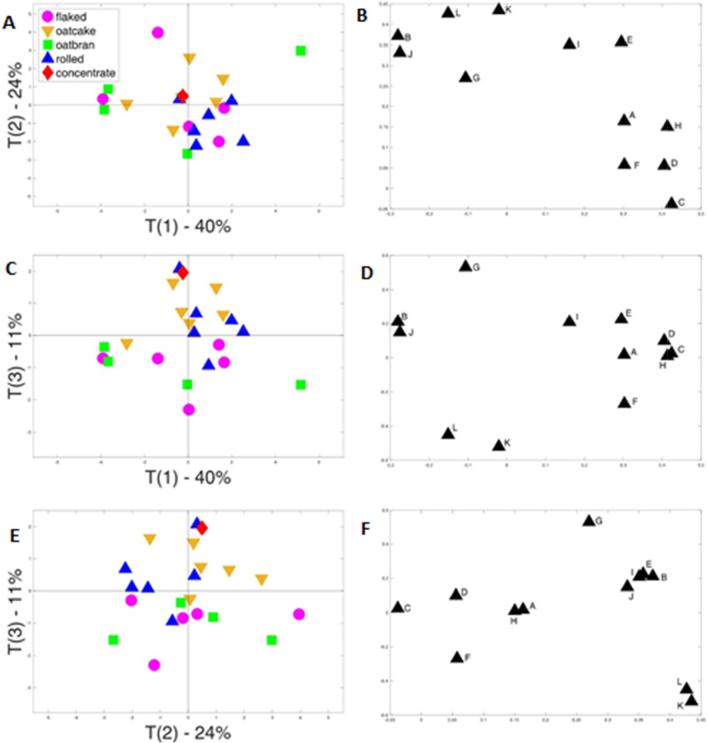
Fig. 4.
Principal component analysis of the contents of free + conjugated phenolic acids and avenanthramides in different oat products. A, C and E are score plots and B, D and F the corresponding loading plots. Letters on loading plots represent phenolic acids: A, 4-hydroxybenzoic acid; B, caffeic acid; C, vanillic acid; D, 4-hydroxybenzaldehyde; E, syringic acid; F, p-coumaric acid; g, Vanillin; h, Ferulic acid; I, sinapic acid; J, avenanthramide C; K, avenanthramide A; L, avenanthramide B.
By contrast, the free + conjugated fraction does not show any clear clustering (Fig. 4). Because bound phenolic acids represent about 80% of the total, PCA analysis of the total fraction was essentially similar to that of the bound fraction (not shown).
The greater variation in the contents of free + conjugated components compared to bound, both within and between the types of sample, may reflect wider variation in the composition of the raw material. For example, studies of wheat have shown that the growing conditions have a much greater impact on the content and composition of free and conjugated phenolic acids than on bound phenolic acids, with heritabilities calculated as 6.4%, 9.8% and 28.5%, respectively (Shewry et al., 2010). The solubility of these components could also lead to greater losses during processing.
3.5. Potential health benefits of phenolic acids and ferulic acid and intake from commercial oat products
It has been suggested that the consumption of dietary phenolics may reduce the risk of chronic diseases such as CVD, diabetes, cancer and neurodegenerative diseases (Shahidi & Yeo, 2018). The average daily intake of phenolic acids in Europe is 605 mg/day, with wholegrains contributing about 5.5% of the total, while the ferulic acid intake is 38 mg/day (Zamora-Ros et al., 2016). Previous studies carried out in humans reported significant improvements in vascular function following a single dose of phenolics in 375 mL of champagne (61.63 mg/l) and chlorogenic acids (89 and 310 mg/g) in 3.6 g of coffee (Mills et al., 2017, Vazour et al., 2010). Our study showed that the consumption of a 40 g portion of commercial oat products provides from 15.8 to 25.1 mg total phenolics (from 9.9 to 19.3 mg bound and from 4.9 to 5.7 mg free + conjugated phenolics) including 1.1–2 mg avenanthramides and 16.7 mg of total phenolics (15.2 mg of bound and 1.5 mg of free + conjugated phenolics) including 1.2 mg of avenanthramides in a 11 g portion of oat bran concentrate.
Ferulic acid is the major component present in all commercial oat products and our analyses show that between 9.1 and 14.7 mg of total ferulic acid (from 7.6 to 13.3 mg bound and from 1.4 and 1.6 mg free + conjugated fraction) can be provided in a 40 g portion of commercial oat products and 13.1 mg of total ferulic acid (12.7 mg of bound and 1.3 mg free + conjugated fraction) can be provided in a 11 g portion of oat bran concentrate. A number of studies have reported therapeutic effects of ferulic acid against diseases related to oxidative stress, with an increasing body of evidence for a role in the prevention or management of chronic disease (Kumar & Pruthi, 2014). For example, randomized controlled trials to determine the effect of coffee and cranberries on vascular health showed improvements on outcomes such as flow mediated dilatation (FMD) and these findings were correlated with the presence of ferulic acid metabolites (Mills et al., 2017, Rodriguez-Mateos et al., 2016). We would therefore predict that the consumption of oat phenolics, and ferulic acid in particular, may have similar health benefits.
In conclusion, this study showed that wholegrain oat products can provide a source of phenolic compounds. The total concentrations of phenolic acids and avenanthramides ranged from 394.8 ± 24.2 to 1518.6 μg/g, with oat bran concentrate having the highest content and the other wholegrain products and oat bran containing lower but similar amounts. However, the total contents of free + conjugated phenolic acids and avenanthramides were similar in all products. This is important as this fraction should be more bioaccessible and absorbed in the small intestine, whereas the bound fraction will only be released, at least partially, during fermentation in the colon. Consequently, similar potential health benefits could be delivered by all products analysed.
These data could be useful to inform studies of human dietary intake and interventions with oat products to better understand the health benefits of oats.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Acknowledgements
The authors acknowledge support from the Biotechnology and Biological Sciences Research Council of the UK (Grant BB/M002802/1 Development and application of phenolic-rich oats for the maintenance of cardiovascular health). Rothamsted Research receives grant-aided support from the Biotechnology and Biological Sciences Research Council (BBSRC) of the UK and the work reported here forms part of the Designing Future Wheat Institute Strategic Programme [BB/P016855/1].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2019.100047.
Contributor Information
Gulten Soycan, Email: gulten.soycan@pgr.reading.ac.uk.
Joanna Boberska, Email: joanna.boberska@pgr.reading.ac.uk.
Giulia Corona, Email: giulia.corona@roehampton.ac.uk.
Peter R. Shewry, Email: peter.shewry@rothamsted.ac.uk.
Jeremy P.E. Spencer, Email: j.p.e.spencer@reading.ac.uk.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Bao L., Cai X., Xu M., Li Y. Effect of oat intake on glycaemic control and insulin sensitivity: A meta-analysis of randomised controlled trials. British Journal of Nutrition. 2014;112:457–466. doi: 10.1017/S0007114514000889. [DOI] [PubMed] [Google Scholar]
- Bratt K., Sunnerheim K., Bryngelsson S., Fagerlund A., Engman L., Andersson R.E. Avenanthramides in oats (Avena sativa L.) and structure–antioxidant activity relationships. Journal of Agricultural and Food Chemistry. 2003;51:594–600. doi: 10.1021/jf020544f. [DOI] [PubMed] [Google Scholar]
- Bryngelsson S., Dinberg L.H., Kamal-Eldin A. Effects of commercial processing on levels of antioxidants in oats (Avena sativa L.) Journal of Agricultural and Food Chemistry. 2002;50:1890–1896. doi: 10.1021/jf011222z. [DOI] [PubMed] [Google Scholar]
- Chen C., Wang L., Wang R., Luo X., Li Y., Li J. Phenolic contents, cellular antioxidant activity and antiproliferative capacity of different varieties of oats. Food Chemistry. 2018;239:260–267. doi: 10.1016/j.foodchem.2017.06.104. [DOI] [PubMed] [Google Scholar]
- Chen C.Y., Milbury P.E., Collins F.W., Blumberg J.B. Avenanthramides are bioavailable and have antioxidant activity in humans after acute consumption of an enriched mixture from oats. Journal of Nutrition. 2007;137:1375–1382. doi: 10.1093/jn/137.6.1375. [DOI] [PubMed] [Google Scholar]
- Collins F.W. Oat phenolics: Structure, occurrence and function. In: Webster F.H., editor. Oats: Chemistry and technology. American Association of Cereal Chemists; St. Paul, MN: 1986. pp. 227–295. [Google Scholar]
- Collins F.W. Oat phenolics: Avenanthramides, novel substituted N-cinnamoylanthranilate alkaloids from oat groats and hulls. Journal of Agricultural and Food Chemistry. 1989;37:60–66. [Google Scholar]
- Dimberg L.H., Gissen C., Nilsson J. Phenolic compounds in oat grains (Avena sativa L.) grown in conventional and organic systems. Ambio. 2005;34:331–337. doi: 10.1639/0044-7447(2005)034[0331:pcioga]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Dinberg L., Sunnerheim K., Sundberg B., Walsh K. Stability of oat avenanthramides. Cereal Chemistry. 2001;78:278–281. [Google Scholar]
- Efsa Panel on Dietetic Products & Allergies Scientific Opinion on the substantiation of health claims related to beta-glucans from oats and barley and maintenance of normal blood LDL-cholesterol concentrations (ID 1236, 1299), increase in satiety leading to a reduction in energy intake (ID 851, 852), reduction of post-prandial glycaemic responses (ID 821, 824), and “digestive function” (ID 850) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal. 2011;9:2207. [Google Scholar]
- Ho H.V., Sievenpiper J.L., Zurbau A., Blanco Mejia S., Jovanovski E., Au-Yeung F. The effect of oat beta-glucan on LDL-cholesterol, non-HDL-cholesterol and apoB for CVD risk reduction: A systematic review and meta-analysis of randomised-controlled trials. British Journal of Nutrition. 2016;116:1369–1382. doi: 10.1017/S000711451600341X. [DOI] [PubMed] [Google Scholar]
- Kováčová M., Malinová E. Ferulic and coumaric acids, total phenolic compounds and their correlation in selected oat genotypes Czech. Journal of Food Science. 2007;25:325–332. [Google Scholar]
- Kumar N., Pruthi V. Potential applications of ferulic acid from natural sources. Biotechnology Reports. 2014;4:86–93. doi: 10.1016/j.btre.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.-P., Li M.-Y., Ling A.J., Hu X.-Z., Ma Z., Liu L. Effects of genotype and environment on avenanthramides and antioxidant activity of oats grown in northwestern China. Journal of Cereal Science. 2017;73:130–137. [Google Scholar]
- Li L., Shewry P.R., Ward J.L. Phenolic acids in wheat varieties in the HEALTHGRAIN diversity screen. Journal of Agricultural and Food Chemistry. 2008;56:9777–9784. doi: 10.1021/jf801069s. [DOI] [PubMed] [Google Scholar]
- Liu L., Zubik L., Collins F.W., Marko M., Meydani M. The antiatherogenic potential of oat phenolic compounds. Atherosclerosis. 2004;175:39–49. doi: 10.1016/j.atherosclerosis.2004.01.044. [DOI] [PubMed] [Google Scholar]
- Mattila P., Pihlava J.M., Hellstrom J. Contents of phenolic acids, alkyl- and alkenylresorcinols, and avenanthramides in commercial grain products. Journal of Agricultural and Food Chemistry. 2005;53:8290–8295. doi: 10.1021/jf051437z. [DOI] [PubMed] [Google Scholar]
- Menon R., Gonzalez T., Ferruzzi M., Jackson E., Winderl D., Watson J. Oats-from farm to fork. Advances in Food and Nutrition Research. 2016;77:1–55. doi: 10.1016/bs.afnr.2015.12.001. [DOI] [PubMed] [Google Scholar]
- Mills C.E., Flury A., Marmet C., Poquet L., Rimoldi S.F., Sartori C. Mediation of coffee-induced improvements in human vascular function by chlorogenic acids and its metabolites: Two randomized, controlled, crossover intervention trials. Clinical Nutrition. 2017;36:1520–1529. doi: 10.1016/j.clnu.2016.11.013. [DOI] [PubMed] [Google Scholar]
- Multari S., Pihlava J.M., Ollennu-Chuasam P., Hietaniemi V., Yang B., Suomela J.P. Identification and quantification of avenanthramides and free and bound phenolic acids in eight cultivars of husked oat (Avena sativa L) from Finland. Journal of Agricultural and Food Chemistry. 2018;66:2900–2908. doi: 10.1021/acs.jafc.7b05726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie L., Wise M.L., Peterson D.M., Meydani M. Avenanthramide, a polyphenol from oats, inhibits vascular smooth muscle cell proliferation and enhances nitric oxide production. Atherosclerosis. 2006;186:260–266. doi: 10.1016/j.atherosclerosis.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Peterson D.M. Oat antioxidants. Journal of Cereal Science. 2001;33:115–129. [Google Scholar]
- Pridal A.A., Bottger W., Ross A.B. Analysis of avenanthramides in oat products and estimation of avenanthramide intake in humans. Food Chemistry. 2018;253:93–100. doi: 10.1016/j.foodchem.2018.01.138. [DOI] [PubMed] [Google Scholar]
- Reynolds A., Mann J., Cummings J., Winter N., Mete E., Te Morenga L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. The Lancet. 2019;393:434–445. doi: 10.1016/S0140-6736(18)31809-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Mateos A., Feliciano R.P., Boeres A., Weber T., Dos Santos C.N., Ventura M.R. Cranberry (poly)phenol metabolites correlate with improvements in vascular function: A double-blind, randomized, controlled, dose-response, crossover study. Molecular Nutrition and Food Research. 2016;60:2130–2140. doi: 10.1002/mnfr.201600250. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Mateos A., Rendeiro C., Bergillos-Meca T., Tabatabaee S., George T.W., Heiss C. Intake and time dependence of blueberry flavonoid–induced improvements in vascular function: A randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. The American Journal of Clinical Nutrition. 2013;98:1179–1191. doi: 10.3945/ajcn.113.066639. [DOI] [PubMed] [Google Scholar]
- Sawicki C.M., McKay D.L., McKeown N.M., Dallal G., Chen C., Blumberg J.B. Phytochemical pharmacokinetics and bioactivity of oat and barley flour: a randomized crossover trial. Nutrients. 2016;8(12) doi: 10.3390/nu8120813. pii: E813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schär M.Y., Corona G., Soycan G., Dine C., Kristek A., Alsharif S.N.S. Excretion of avenanthramides, phenolic acids and their major metabolites following intake of oat bran. Molecular Nutrition and Food Research. 2018;62:1700499. doi: 10.1002/mnfr.201700499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schar M.Y., Curtis P.J., Hazim S., Ostertag L.M., Kay C.D., Potter J.F. Orange juice-derived flavanone and phenolic metabolites do not acutely affect cardiovascular risk biomarkers: A randomized, placebo-controlled, crossover trial in men at moderate risk of cardiovascular disease. American Journal of Clinical Nutrition. 2015;101:931–938. doi: 10.3945/ajcn.114.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi F., Yeo J. Bioactivities of phenolics by focusing on suppression of chronic diseases: A review. International Journal of Molecular Science. 2018;19:1573. doi: 10.3390/ijms19061573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewry P.R., Piironen V., Lampi A.-M., Edelmann M., Kariluoto S., Nurmi T. The HEALTHGRAIN wheat diversity screen: Effects of genotype and environment on phytochemicals and dietary fiber components. Journal of Agricultural and Food Chemistry. 2010;58:9291–9298. doi: 10.1021/jf100039b. [DOI] [PubMed] [Google Scholar]
- Shewry P.R., Piironen V., Lampi A.-M., Nyström L., Li L., Rakszegi M. Phytochemical and fiber components in oat varieties in the HEALTHGRAIN diversity screen. Journal of Agricultural and Food Chemistry. 2008;56:9777–9784. doi: 10.1021/jf801880d. [DOI] [PubMed] [Google Scholar]
- Singh R., De S., Belkheir A. Avena sativa (Oat), a potential neutraceutical and therapeutic agent: An overview. Critical Reviews in Food Science and Nutrition. 2013;53:126–144. doi: 10.1080/10408398.2010.526725. [DOI] [PubMed] [Google Scholar]
- Vazour D., Houseman E.J., George T., Corona G., Garnotel R., Jackson K.G. Moderate champagne consumption promotes an acute improvement in acute endothelial vascular function in healthy human volunteers. British Journal of Nutrition. 2010;103:1168–1178. doi: 10.1017/S0007114509992959. [DOI] [PubMed] [Google Scholar]
- Xie Z., Mui T., Sintara M., Ou B., Johnson J., Chu Y. Rapid quantitation of avenanthramides in oat-containing products by high-performance liquid chromatography coupled with triple quadrupole mass spectrometry (HPLC-TQMS) Food Chemistry. 2017;224:280–288. doi: 10.1016/j.foodchem.2016.12.079. [DOI] [PubMed] [Google Scholar]
- Zamora-Ros R., Knaze V., Rothwell J.A., Hémon B., Moskal A., Overvad K. Dietary polyphenol intake in Europe: The European Prospective Investigation into Cancer and Nutrition (EPIC) study. European Journal of Nutrition. 2016;55(4):1359–1375. doi: 10.1007/s00394-015-0950-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z., Liu C., Luo S., Chen J., Gong E. The profile and bioaccessibility of phenolic compounds in cereals influenced by improved extrusion cooking treatment. PLoS One. 2016;11 doi: 10.1371/journal.pone.0161086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.