Highlights
-
•
Suitable production of biofunctional ingredients from soy protein.
-
•
Bioactivities of hydrolysates improved significantly after in vitro digestion.
-
•
Bioactivities were stable despite a partial degradation of the bioactive peptides.
-
•
Corolase PP hydrolysis increased the soy protein isolate solubility.
Keywords: Soy protein, Bioactive peptides, ACE inhibitor, Antioxidant activity, Enzymatic hydrolysis, Simulated gastrointestinal digestion
Abstract
This work aimed to evaluate the digestive stability of the peptides previously identified from a Corolase PP soy protein hydrolysate (SPH) and to respond to the uncertainty about the merit of controlled hydrolysis. For this purpose, we applied an empirical and theoretical analysis, determining peptide sequences, oxygen radical scavenging (ORAC) and ACE inhibitory (iACE) activities, and the effect of hydrolysis on solubility. Results showed that during digestion most of SPH peptides were degraded as smaller ones. However, both SPH bioactivities improved significantly after digestion (3.9 ± 0.1 μmol TE/mg protein for ORAC and IC50 = 52 ± 4μg protein/mL for iACE) with similar values for soy protein isolate (SPI). With respect to solubility, the controlled hydrolysis considerably increased this functional property. In conclusion, the results indicated that controlled enzymatic hydrolysis of SPI with Corolase PP produced an ingredient more apt to be incorporated in certain nutritional or nutraceutical formulations.
1. Introduction
Many of the physiological and functional properties of proteins are attributed to biologically active peptides normally encoded in the parental protein sequence (Rizzello et al., 2016). Recent research has shown that peptides from different sources, such as dairy products, plants, animals, and seafood have a wide range of bioactivities. >3200 different bioactive peptides have been reported in the database BIOPEP (Minkiewicz, Dziuba, Iwaniak, Dziuba, & Darewicz, 2008), based on either in vitro or in vivo studies. Most of the bioactive peptides exhibit specific bioactivity, but some peptides have been found to exhibit multifunctional properties. The largest number of bioactive peptides isolated to date comes from milk proteins. However, soy is an important alternative source of these bioactive compounds (Hartmann and Meisel, 2007, Wang and Gonzalez De Mejia, 2005). In this regard, different studies have shown that the consumption of soy protein has several positive effects on health, including antioxidant and antihypertensive activities, cholesterol and body fat reduction, prevention of osteoporosis, and reduction of the incidence of stomach, colorectal and breast cancer (Singh, Yadav, & Vij, 2017).
Among the mentioned bioactivities, antioxidant capacity is considered one of the most important (Coscueta et al., 2016, Rizzello et al., 2016). Oxidative processes have detrimental effects on human health and on the quality of food. Due to the potential risks presented by synthetic antioxidants for health (e.g., butylated hydroxyanisole -BHA, butylated hydroxytoluene -BHT, propyl gallate -PG and tertiary butylhydroquinone -TBHQ), the use of natural antioxidants has gained increasing interest (Shahidi & Zhong, 2015). It has been reported that peptides exhibit antioxidant activity due to their properties to eliminate free radicals, donate electrons and/or chelate metals (Shahidi et al., 2015). On the other hand, antihypertensive capacity is a bioactivity that has been valorised with a special interest in the compounds present in food. Hypertension has established itself as a major chronic health problem in epidemic proportions. In mammals, one of the most important instruments to maintain the homeostasis of blood pressure and water and salt balance is the renin-angiotensin system. Angiotensin-I converting enzyme (ACE) is a key factor in the mentioned system to regulate blood pressure since its inhibition allows controlling hypertension. Synthetic inhibitors, such as captopril, enalapril, alacepril, and lisinopril, are commercially available, but their use is restricted due to possible adverse effects (García-Mora et al., 2017, Lee et al., 2010). Therefore, special attention has been paid to the inhibitory effects of some biological nutraceuticals, such as bioactive peptides, on ACE (Capriotti et al., 2015, Coscueta et al., 2016, Esteve et al., 2015). These peptides are very valuable because they are easily absorbed in the body, thus representing a great alternative to synthetic drugs (Lee et al., 2010). In the last years, the antioxidant and antihypertensive potential of soy protein fractions have been reported, together with the isolation and structural characterization of the most active peptides (Capriotti et al., 2015, González-Montoya et al., 2016).
Previously, we identified peptides derived from soy protein with potential antioxidant and antihypertensive activities (Coscueta et al., 2016). Such peptides were obtained from the enzymatic hydrolysis of soy protein isolate (SPI), using for the first time the commercial enzyme Corolase PP. The bioactive capacities of the hydrolysates obtained presented values comparable or superior to those reported in other works with different enzymes and substrates (Coscueta et al., 2016). However, bioactive peptides can undergo physiological transformations during the passage through the gastrointestinal tract that determines their bioavailability and activity in the organism (Escudero, Mora, & Toldrá, 2014). Therefore, the effective inclusion of bioactive peptides into the diet requires that their active sequences resist the gastrointestinal digestion, in order to preserve their structure and reach the target sites in the intestine where they exert their physiological effects. At present, information related to stability of bioactive peptides after gastrointestinal digestion is lacking (Wang, Yadav, Smart, Tajiri, & Basit, 2015), so we considered to obtain this relevant information for peptides obtained with Corolase PP.
In this context, the purpose of this work is to analyse and characterize the peptides generated during the in vitro simulated gastrointestinal digestion of soy protein hydrolysate (SPH) from Corolase PP hydrolysis. Thus, the digestive stability of the peptides previously identified as well as the identification of other potentially bioactive sequences released or encrypted in the resulting peptides must be explored. For this purpose, the bioactive motif identification tools from the BIOPEP server will be applied for an empirical and theoretical analysis. In addition, considering that in vitro simulated gastrointestinal digestion of intact soy protein has been demonstrated to significantly increase the antioxidant and ACE inhibition activities, the hypothesis about the utility of the previous soy protein isolate hydrolysis is formulated.
2. Materials and methods
2.1. Materials
Defatted white soybean flour was obtained from Molinos Río de la Plata (San Lorenzo, Argentina). Pepsin (800–1000 U/mg protein), pancreatin (4 × USP), angiotensin-I converting enzyme (peptidyl-dipeptidase A, EC 3.4.15.1, 5.1 U/mg), bile salts and trolox (6- hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid) were obtained from Sigma-Aldrich (St. Louis, MO, USA) and used without further purification. The commercial enzymatic complex Corolase PP was purchased from AB Enzymes GmbH (Darmstadt, Germany). Fluorescein [3′, 6′-dihydroxyspiro (isobenzofuran-1 [3H], 9′ [9H] -xanten)-3-one] was purchased from Fisher Scientific (Hanover Park, IL). AAPH [2,2′-azobis (2-amidi-nopropane) dihydrochloride] was purchased from Aldrich (Milwaukee, WI). The tripeptide Abz-Gly-Phe(NO2)-Pro was obtained from Bachem Feinchemikalien (Bubendorf, Switzerland). Tris [tris (hydroxymethyl) aminomethane] was obtained from Fluka (Gmbh, Germany).
2.2. Preparation of soy protein isolate
Soy protein isolate (SPI) was prepared from defatted soybean flour by aqueous extraction as explained previously (Coscueta et al., 2016), under controlled conditions of time and temperature: 70 °C for 1 h. Then the extract was clarified by centrifugation at 2370×g for 45 min. The supernatant was precipitated by acidification to pH 4.5 with 2 M HCl (Merck, Damdstadt, Germany) and the insoluble fraction was collected by centrifugation at 2370×g for 30 min. The precipitate was freeze dried.
2.3. Enzymatic hydrolysis
Following the methodology described in our previous work (Coscueta et al., 2016), the SPI was hydrolysed with Corolase PP at a ratio of enzyme: substrate (10 mg enzyme/g SPI- 1% m/m). The enzymatic hydrolysis was conducted at optimal conditions previously established, i.e. 50°C and pH 8.0 for 4 h (Coscueta et al., 2016). The enzyme was inactivated by heat treatment at 80 °C for 20min, and the supernatant was separated from precipitate by centrifugation at 2370×g for 45 min. Finally, the SPI hydrolysate (SPH) was freeze dried.
2.4. In vitro simulated gastrointestinal digestion
In vitro simulated gastrointestinal digestion was performed for SPH and SPI. Since the focus was on perceiving protein digestion, the simulation of the digestive passage that takes place in the mouth and esophagus, where carbohydrates are mainly affected, was not performed. Three independent in vitro digestion experiments were performed for each sample, taking 280 mg (SPH, SPI) for each experience. 6 mL of acidified water (pH 2.0) was added in each case, the pH being adjusted to 2.0 with 1 N HCl. Then the system volume was completed up to 7 mL with the same acidified water, brought to 37 °C and stirred at 130 rpm for 20 min to temper the digestive process beforehand. Before starting the in vitro digestion (time 0), 1 mL of supernatant was removed from each experiment. The initial stage of the in vitro simulated gastrointestinal digestion began at the digestion step in the stomach, the gastric juice being simulated with pepsin at 25 mg/mL, prepared in 0.1 N HCl (Aura, 2005). To each experiment, 0.3 mL of this “gastric juice” was added and left at 37 °C shaking at 130 rpm. After 60 min, the simulating gastric passage was terminated by increasing the pH to 6.5 with 100 mM NaHCO3 solution. An aliquot of 1 mL of each experiment was then withdrawn, representing the end sample of such stage. For the intestinal stage, pancreatic juices were simulated with a 2 mg/mL of pancreatin solution and 12 mg/mL bile salts diluted in 100 mM NaHCO3 solution (Laurent, Besançon, & Caporiccio, 2007). An aliquot of 1.5 mL of this pancreatic solution was added to each experiment, the temperature was brought back to 37 °C and the stirring was lowered to 45 rpm. This stage was extended for 90 min and was finalized by freezing at −30 °C. The final digests were called DSPI for SPI and DSPH for SPH. Finally, initial SPI and SPH solutions as well DSPI and DSPH were nanofiltered with membranes of 3000 Da pore (Amicon® Ultra-4, Millipore), for the subsequent permeate analysis. The protein content of the permeates was estimated by Pierce BCA Protein Assay kit following the manufacturer’s instructions. The albumin was used as standard and the determination of peptide concentration was based on their actual dry weight.
2.5. Analysis of total antioxidant activity
Oxygen radical absorbance capacity (ORAC) assay was based on that proposed by Contreras et al. (2011). Briefly, the reaction was carried out at 40 °C in 75 mM phosphate buffer (pH 7.4) and the final assay mixture (200 μL) contained fluorescein (70 nM), AAPH (14 mM), and either antioxidant (Trolox (9.98 × 10−4–7.99 × 10−3 μmol/mL) or sample (SPI, SPH and their digests at different concentrations)). The fluorescence was recorded during 137 min (104 cycles). A FLUOstar OPTIMA plate reader (BMG Labtech, Offenburg, Germany) with 485 nm excitation and 520 nm emission filters was used. The equipment was controlled by the FLUOstar Control software version (1.32 R2) for fluorescence measurement. Black polystyrene 96-well microplates (Nunc, Denmark) were used. AAPH and Trolox solutions were prepared daily and fluorescein was diluted from a stock solution (1.17 mM) in 75 mM phosphate buffer (pH 7.4). All reaction mixtures were prepared in triplicate and at least three independent runs were performed for each sample. Final ORAC values were expressed as μmol TE (Trolox equivalent)/mg protein.
2.6. Measurement of ACE-inhibitory activity
The ACE-inhibitory effect was measured using the fluorimetric assay of Sentandreu and Toldrá (2006), as modified by Quirós, del Contreras, Ramos, Amigo, and Recio (2009). The method is based on the ability of ACE to hydrolyse a specific substrate (o-aminobenzoylglycyl-p-nitro-l-phenylalanyl-l-proline or ABz-Gly-Phe(NO2)-Pro) generating fluorescent o-aminobenzoyl-glycine. A commercial angiotensin-I converting enzyme (EC 3.4.15.1, 5.1 U/mg) was diluted in 5 mL of a glycerol solution in 50% ultra-pure water. This solution was maintained at −20 °C until use. The stock solution was then diluted 1:24 with a 150 mM Tris buffer solution, pH 8.3, containing 1 μM of ZnCl2, reaching a concentration of 42 mU/mL in the final reaction solution. 40 μL of the working solution of ACE were added to each well of the reaction microplate, and then adjusted to 80 μL by adding both ultra-pure water for the control and the sample to be analysed. The enzymatic reaction was initiated by adding 160 μL of a 0.45 mM solution of ABz-Gly-Phe(NO2)-Pro, dissolved in 150 mM Tris buffer (pH 8.3), containing 1.125 M NaCl, mixing immediately and incubating at 37 °C. The microplate was automatically shaken before the first reading, and the fluorescence generated was measured for 30 min. The inhibitory activity on ACE (iACE) was expressed as the concentration capable of inhibiting the 50% of the enzymatic activity (IC50). A non-linear modelling of the obtained data was used to calculate the IC50 values, which were expressed as mean ± SD. The measurements were made with the FLUOstar OPTIMA plate reader with excitation filters at 350 nm and emission at 420 nm. Black 96-well polystyrene microplates (Nunc, Denmark) were used for the assay.
2.7. Mass spectrometry analysis
Peptides from SPH, DSPI and DSPH were detected and acquired by a MALDI-TOF/TOF mass spectrometer (4800 Plus MALDI TOF/TOF Analyzer, SCIEX, Framingham, MA). Each spectrum was the accumulated sum of at least 2000 laser shots within the ion range at m/z 700–5000. The samples were co-crystallized with HCCA matrix (4-hydroxycinnamic-α-cyano acid), in a stainless steel MALDI sample plate (Opti-TOF 384- Well insert, AB SCIEX, Framingham, MA) at room temperature. MS data was acquired in reflector positive mode. Some of the ion peptide peaks were further selected for MS/MS sequencing analysis. Combined Peptide Mass Fingerprint (PMF) + MS/MS analysis was performed with the Mascot software (Matrix Science, UK) using the UniProt protein sequencing database for Glycine max taxonomic selection. Methionine oxidation was selected as a variable modification.
To determine the bioactivity, all the identified peptides were searched against a database available online, BIOPEP (Minkiewicz et al., 2008). For SPH peptides which coincided with those reported in our previous study (Coscueta et al., 2016), the frequency of bioactive fragments occurrence in peptide sequence (A) was analysed:
where a is the number of fragments with a given activity in a peptide sequence, and N is the number of amino acid residues of the peptide (Dziuba, Iwaniak, & Minkiewicz, 2003).
2.8. Solubility
The protein solubility of SPI and SPH in water was determined over a pH range of 3.0–7.0, following the methodology of Lamsal, Jung, and Johnson (2007). SPI and SPH were suspended in deionized water at a ratio protein/water of 1 g/100 g. The pH of the dispersion was adjusted with either 2 M NaOH or HCl, as appropriate. The dispersions were then stirred at room temperature for 1 h and finally they were centrifuged at 20 °C and 5000×g for 30 min. The protein content in the supernatant was measured by the Pierce BCA Protein Assay kit, using bovine serum albumin as standard. Solubility was expressed as a percentage of the original protein present in the supernatant. The experiments were done in triplicate.
2.9. Statistical analysis
Statistical analysis was carried out with the aid of RStudio V 1.0.143. For significant main effects, means were considered to be different at a significance level of 0.05. Data was expressed as means of three replicates.
3. Results and discussion
3.1. Bioactivity analysis during in vitro simulated gastrointestinal digestion
During the digestive process, the gastrointestinal proteolytic enzymes may release bioactive peptides that are encoded inactive in the native structure of the parent protein (Capriotti et al., 2015, Escudero et al., 2014, Hartmann and Meisel, 2007). Likewise, both these new bioactive peptides and those present in the dietary matrix before their ingestion can be degraded by these same enzymes and intestinal microbial peptidases, increasing or reducing their biological activities. In order to exert their effect in vivo, it is important that the bioactive peptides resist this degradation or improve their capacities in the process, so as to reach the bloodstream in an active form (Escudero et al., 2014, Wang et al., 2015). To analyse empirically the effects of in vitro simulated gastrointestinal digestion on SPI and SPH, antioxidant (ORAC) and ACE inhibitory (iACE) activities were analysed. Since in the data obtained the response variables are recorded more than once on the same experimental unit, the analysis is considered as “repeated measures”, applying then the procedure of Generalized Linear Models, GLM (Vittinghoff, Glidden, Shiboski, & McCulloch, 2011). It is important to note that the analyses were performed on the nanofiltration permeate (3000 Da cut-off) of the SPH as well on the digests, enriching in bioactive peptides (Coscueta et al., 2016) while, in the case of digests, the digestive enzymes were subtracted.
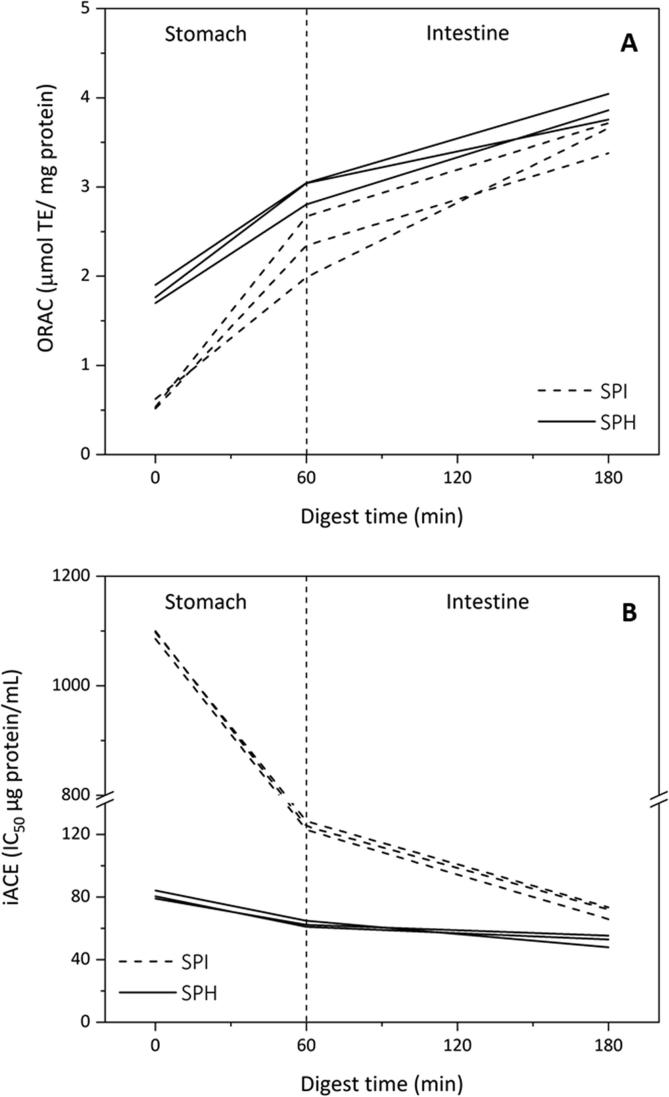
Adjusted GLMs were related to ORAC and iACE with three categorical predictive factors: experimental units, substrate type (SPI or SPH) and digestion stage. Since the P-value for each model was <0.05, there were statistically significant relationships between the observed responses and the predictor variables. The coefficients of determination (R2) indicated that the models, thus adjusted, explained 98.97% (ORAC) and 99.99% (iACE) of the variability. When considering the general digestion process, irrespective of substrate, we found significant differences between the different stages (beginning of the digestion at oral phase, end of the gastric phase, and final of the digestion at intestinal phase) for both responses (ORAC, iACE). As seen in Fig. 1A, the scavenging capacity increased significantly during the digestion of both substrates (SPI and SPH). In relation to iACE, Fig. 1B shows how the IC50 of the digests decreased, which indicates an increase in their ACE inhibitory capacity, i.e. potential antihypertensive activity. Although there was a change in both bioactivities throughout the digestion, the change was more marked during the first 60 min (gastric phase).
Fig. 1.
Variation of bioactivity during the time of in vitro simulated gastrointestinal digestion. The lines display the observations of ORAC (A) and iACE (B) for each experimental unit.
When considering the substrates separately, significant differences between processes for SPI and SPH were observed for both analysed responses. As Fig. 1A shows, the change in the ORAC values was significantly greater for the SPI, which was evident by the higher slope of gastric stage plot. Likewise, at the end of the in vitro digestion, there were no significant differences (P > 0.080) between the ORAC values shown by DSPI (3.6 ± 0.2 μmol TE/mg protein) and DSPH (3.9 ± 0.1 μmol TE/mg protein). For iACE, despite the significance of the potential increase of said bioactivity the IC50 of SPH only showed a slight variation during digestion, decreasing from 81 ± 3 μg protein/mL to 52 ± 4 μg protein/mL. In contrast, for SPI, iACE evidenced a radical change (Fig. 1B), decreasing its IC50 from 1095 ± 8 μg protein/mL to 70 ± 4 μg protein/mL. Although both can be considered highly inhibitory, at the end, DSPH had a significant higher iACE (P < 0.005) than DSPI.
When compared with similar studies reported in bibliography, the ORAC values presented in this study are positioned between the most relevant values. For example, the ORAC values at the end of the digestion were lower (3.9 ± 0.1 μmol TE/mg protein) than those reported by Ranamukhaarachchi, Meissner, and Moresoli (2013) under similar conditions (5.2 μmol TE/mg protein). However, the difference between these results may be due to the different origin and quality of the starting substrate. In our case, the SPI was produced in the laboratory from soybean flour of industrial quality, while a commercial standardized high-quality preparation of SPI was used in the cited work, and therefore different purity grades were studied. On the other hand, the digests produced in our study showed a greater antioxidant capacity compared to the digests of intact amaranth protein isolate (Ipepa) and hydrolysed with Alcalase (Hpepa), produced by Orsini Delgado, Tironi, and Añón (2011). The authors reported ORAC values of 1.23 ± 0.03 μmol TE/mg protein (Ipepa) and 1.20 ± 0.04 μmol TE/mg protein (Hpepa), which are not different (P > 0.05) as in our case. Also, DSPI and DSPH presented scavenging values higher than those corresponding to other naturally occurring antioxidant food components and ingredients, such as crude bran (3.1 μmol TE/mg), ground clove (0.9 μmol TE/mg), dry oregano (1.8 μmol TE/mg) and ground cinnamon (1.3 μmol TE/mg) (Ranamukhaarachchi et al., 2013). Moreover, antioxidant activities (ORAC) of pure peptides derived from the in vitro digestion of amaranth protein from 0.1 to 4.9 μmol TE/mg peptide have been recently reported (Orsini Delgado et al., 2016), these values being within the same order or lower than those obtained in our work.
In the case of iACE, when contrasting with other similar studies, we also consider that our results are promising. In 2009 (Quirós et al., 2009), peptides obtained from 3000 Da-permeate-water soluble extract of milks fermented with Enterococcus faecalis were subjected to in vitro simulated gastrointestinal digestion. During this process, some of the peptides increased their ACE inhibitory capacity while others decreased it. Those that improved their bioactivity were partially hydrolysed by digestive enzymes, while those that decreased their activity were hydrolysed into several much smaller peptides. The highest activities were between 14 and 428 μg peptide/mL a range that includes the results of the present work. Recently, García-Mora et al. (2017) identified peptides derived from lentil hydrolysates with Savinase and synthesized those with the highest ACE inhibitory potential. These pure peptides were subjected to in vitro simulated gastrointestinal digestion to analyse their stability. iACE was observed to increase with in vitro digestion, such as our study, with reported values between 19 and 149 μg peptide/mL, comparable with those of DSPI and DSPH. Similarly, Escudero et al. (2014) analysed peptides synthesized from structures identified after the in vitro digestion of ham, thus finding the best values of iACE between 7 and 166 μg peptide/mL. By considering that the bioactive capacities of pure peptides are always markedly higher than that of original total hydrolysates, these results reinforce the relevant bioactivity in our digests.
According to previous studies, the antioxidant and ACE inhibitory activities can be maintained after digestion, especially if the peptides are small (Escudero et al., 2014, Orsini Delgado et al., 2011, Quirós et al., 2009). Both, the oxygen radical scavenging and the ACE inhibitory activities of SPH improved significantly after in vitro digestion. This suggests that peptides present in SPH are resistant to digestion in the gastrointestinal tract or are partially degraded in smaller peptides, thus maintaining its biological activity. To address this issue more deeply, a structural analysis of the peptides produced during digestion was carried out.
3.2. Overview of the identified peptides
Peptides arising from both SPH and the in vitro simulated gastrointestinal digestion were sequenced by MS/MS to identify their nature and then analysed to find the presence of potentially bioactive sequences in their structures. Composition of the peptides identified, ranged from 8 to 25 amino acid residues (i.e. between 1054 Da and 2998 Da) for SPH and 6–23 amino acid residues (i.e. between 699 Da and 2562 Da) for the digests. These values are similar to those reported in other studies after a similar digestion of soy seed, soy milk protein (Capriotti et al., 2015), and different animal proteins (Bauchart et al., 2006, Escudero et al., 2014). Roberts, Burney, Black, and Zaloga (1999) established that adult animal gastrointestinal tracts can absorb physiologically significant amounts of polypeptides with chain length from 3 to 51 amino acids. A highly studied soybean peptide, lunasin (5000 Da), has been reported in human plasma after ingestion of soy products (Dia, Torres, de Lumen, Erdman, & Gonzalez de Mejia, 2009). Thus, the size of peptides identified in the present work is still compatible with absorption by the intestinal lumen, thus allowing the peptides to exert a nutritional or bioactive function.
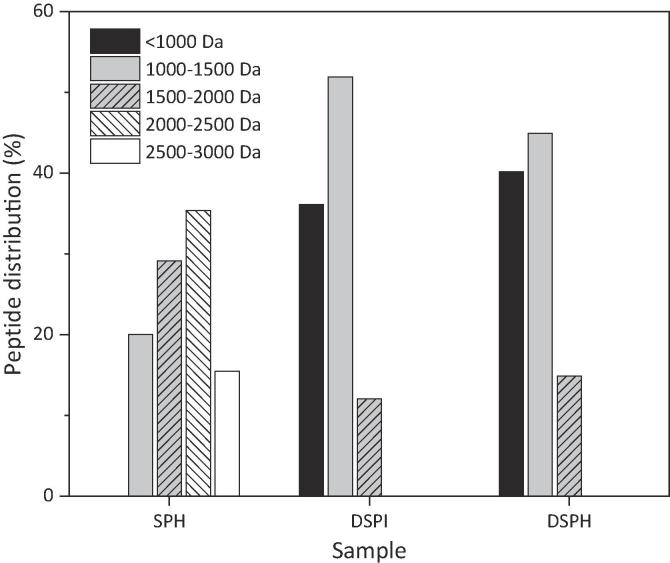
The analysis of the three systems under investigation (SPH, DSPI, and DSPH) led to the identification of extensive number of 1410 non-redundant peptides, distributed as follow: 549 peptides in SPH, 398 in DSPI and 463 in DSPH. As seen in Fig. 2, peptides released after in vitro simulated gastrointestinal digestion were clustered based on their molecular mass (Mm, Da). They showed similar profiles for the two systems. Most of peptides (>80%) present in DSPI and DSPH exhibited Mm below 1500 Da, although the 1000–1500 Da group was predominant. In the case of the SPH, the profile was different with a larger proportion of peptides above 1500 Da (Fig. 2). Also, intact SPI after being subjected to the in vitro digestion (DSPI) presented a peptide profile like DSPH in terms of percentage, but with an increase in the peptide fraction below 1000 Da for this last one (Fig. 2). Further comparison between the three investigated systems was performed considering the overlap between the identified peptides. The analysis of peptide sequence profiles showed the presence of 81 peptides common to both DSPI and DSPH, while the SPH did not present any sequence shared with the digests.
Fig. 2.
Percentage of peptides present in each sample (SPH, DSPI and DSPH), clustered according to molecular mass.
These results indicated that through the digestive process most of the peptides present in the SPH were degraded to smaller peptides, with a distribution pattern similar to that of the SPI digest. Besides, the differences observed in peptide sequences cannot be attributed to different parent protein sequences since they are identical. However, as described previously (Capriotti et al., 2015), the release of unique peptides from the digested samples, could be attributed to the nature of the peptidases used in the gastrointestinal digestion. The lack of specificity in protein hydrolysis is due to the random hydrolysis of pepsin and pancreatin that produce relatively large peptides for the first enzyme and peptides that differ in amino acid sequence for the second one.
3.3. Potential bioactivity of the identified peptides
In our previous study (Coscueta et al., 2016), we identified five oligopeptides with potential bioactive capacities: IRHFNEGDVLVIPPGVPY, IRHFNEGDVLVIPPGVPYW, IYNFREGDLIAVPTG, VSIIDTNSLENQLDQMPRR, and YRAELSEQDIFVIPAG. In the present study, these peptides with marked hydrophobic character were again obtained by hydrolysis of soy protein isolate with Corolase PP. The analysis of their sequences with the BIOPEP database (Table 1) showed that all of them exhibited some potential bioactivities such as the antioxidant and inhibitory capacities of ACE and dipeptidyl peptidase IV. Taking into consideration that this study focuses on the antioxidant and ACE inhibitory activities, Table 1 shows that the later stands out for its frequency of occurrence of bioactive fragments in the peptide sequence (A). This increases the probability of obtaining mainly ACE inhibitor peptides from the precursors.
Table 1.
Oligopeptides previously identified in the SPH with their potential bioactivities, and the corresponding frequencies of occurrence of bioactive fragments in the peptide sequence (A).
| Peptide | Activity | A |
|---|---|---|
| IRHFNEGDVLVIPPGVPY | ACE inhibitor | 0.5556 |
| Antiamnestic | 0.0556 | |
| Antioxidative | 0.1111 | |
| Antithrombotic | 0.0556 | |
| Dipeptidyl peptidase IV inhibitor | 0.8889 | |
| Hypotensive | 0.0556 | |
| Inhibitor | 0.0556 | |
| Regulating | 0.0556 | |
| Stimulating | 0.1111 | |
| IRHFNEGDVLVIPPGVPYW | ACE inhibitor | 0.5789 |
| Antiamnestic | 0.0526 | |
| Antioxidative | 0.1053 | |
| Antithrombotic | 0.0526 | |
| Dipeptidyl peptidase IV inhibitor | 0.8947 | |
| Hypotensive | 0.0526 | |
| Inhibitor | 0.0526 | |
| Regulating | 0.0526 | |
| Stimulating | 0.1053 | |
| IYNFREGDLIAVPTG | ACE inhibitor | 0.8 |
| Antioxidative | 0.0667 | |
| Dipeptidyl peptidase IV inhibitor | 0.6667 | |
| Stimulating | 0.0667 | |
| VSIIDTNSLENQLDQMPRR | ACE inhibitor | 0.1053 |
| Dipeptidyl peptidase IV inhibitor | 0.5263 | |
| Stimulating | 0.0526 | |
| YRAELSEQDIFVIPAG | ACE inhibitor | 0.375 |
| Activating ubiquitin-mediated proteolysis | 0.0625 | |
| Antioxidative | 0.0625 | |
| Dipeptidyl peptidase IV inhibitor | 0.5625 | |
| Stimulating | 0.0625 |
The sequences of all the peptides identified for each sample were analysed with the bioactive motif identification tools of the BIOPEP database, considering sequences with more than two amino acids. The main potential bioactivities identified correspond to antioxidant and ACE inhibitory capacities. Table S1 (Supplementary Material) shows all the identified peptides that possess either antioxidant or ACE inhibitory motifs. Although the five oligopeptides mentioned above were partially degraded during in vitro digestion, their major bioactive fragments were retained in the resulting structures. This indicates that, despite the structures were not very stable under the conditions of the digestive system, probably their bioactivities are maintained and can even improve.
Furthermore, we identified a peptide present in both DSPI and DSPH that was previously reported in other works (Capriotti et al., 2015, Chen et al., 1995). This peptide whose sequence is LLPHHADADY, comes from the alpha subunit of beta-conglycinin (position 200–209 in the complete sequence), and has a significant antioxidant activity. Chen et al. (1995) attributed this property to the presence of histidine residues in the amino acid sequence, mainly the LLPHH fragment. In the case of SPH, we observed that such peptide was encrypted in other oligopeptides and released during in vitro simulated gastrointestinal digestion.
Many other recognized bioactive motifs were still encrypted in oligopeptides obtained by in vitro digestion for both DSPI and DSPH. However, considering that other exopeptidases and endopeptidases also act at in vivo gastrointestinal digestion, as well as those excreted by the microbial flora present in the intestinal lumen, it is likely that these oligopeptides were degraded, releasing many of these encrypted bioactive sequences (Capriotti et al., 2015).
3.4. Hydrolyse or not hydrolyse?
When analysed the antioxidant and ACE inhibitory properties generated by in vitro simulated gastrointestinal digestion, both substrates (SPI and SPH) were observed to present interesting characteristics in addition to the widely known nutritional ones. This allows us to affirm that the pre-hydrolysis with Corolase PP does not clearly improve the analysed bioactivities of the intact soy protein isolate. Therefore, these bioactive properties should not be considered a categorical parameter to contrast and decide the application of either SPI or Corolase PP SPH in nutritional or nutraceutical formulations. When an ingredient is incorporated into a product, other technologically important parameters, such as the functional properties, should be considered. These functional properties are: solubility, water retention capacity, foaming, gelling, emulsifying and fat binding properties (Zayas, 1997). These properties are attributed to both intrinsic factors (molecular structure and composition) and extrinsic factors (temperature, pH and presence of chemical compounds). When these factors are modified the functional properties of the proteins also change. Particularly, in our study we analysed the solubility.
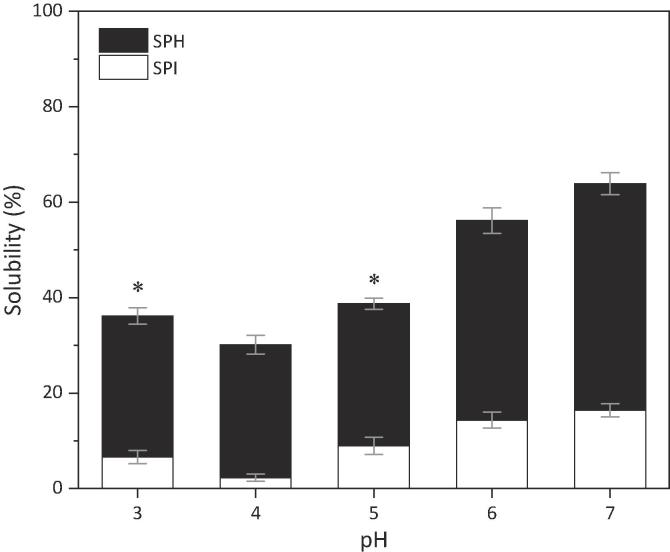
Fig. 3 shows the water solubility profile of SPH and its corresponding control (SPI). As expected, the solubility of both SPI and SPH was greater on both sides of the isoelectric point (pH 4.5). The hydrolysis with Corolase PP increased the SPI solubility, the SPH solubility at pH 7 being approximately 4-fold higher than that of SPI. It is known that partial denaturation of the final product occurs in the isolation process of the soy protein (Lamsal et al., 2007), due to the aggressive conditions of acid precipitation during the separation of globulins from the whey compounds. However, our results showed that this can be reversed by enzymatic hydrolysis with Corolase PP, which agrees with other studies (Lamsal et al., 2007, Panyam and Kilara, 1996, Tsumura et al., 2005). The increase in solubility with hydrolysis can be attributed to the increase in the number of ionizable amino and carboxyl groups, exposed after the peptide bond cleavage (Lamsal et al., 2007, Panyam and Kilara, 1996), that leads, among other things, to the production of soluble peptides (Lamsal et al., 2007, Tsumura et al., 2005). Lamsal et al. (2007) found that the hydrolysis of SPI with bromelain not only increased the protein solubility, but also modified its rheological properties, thus producing ingredients suitable for applications in baby foods, yogurts and soups. In another study, SPI was hydrolysed with different commercial enzymes. It was found an increase in protein solubility at pH 7.0 and 4.5, as well as an increase in the emulsifying capacity and the ability to undergo thermal aggregation (Kim, Park, & Rhee, 1990).
Fig. 3.
Water solubility profiles of SPI and SPH at different pHs. Bars represent means of three independent experiments. Error bars represent sample standard deviation (SD). SPI and SPH present significant differences between their solubility means (P < 0.05) for each pH value. The pH levels that share the symbol (⁎) do not present significant difference between their solubility means (P > 0.05) within each system (SPI and SPH). Statistical analysis of the variance (Two-Way ANOVA) was performed, with Tukey's post-hoc test.
Based on our results, we consider that the controlled enzymatic hydrolysis of SPI with Corolase PP is a very useful tool for generating ingredients with improved functional properties, thus conserving nutritional and bioactive qualities of the starting material. On the other hand, for industries that require ingredients with more concentrated or refined bioactive peptides, the enzymatic hydrolysis followed by adequate downstream operations is an effective and sustainable way to obtain them. Therefore, “hydrolyse or not hydrolyse” depends on the purpose but we may assure that several advantages are introduced via hydrolysis if food applications are sought.
4. Conclusions
Our in vitro results allow us to think of an in vivo scenario in which the peptides present in the studied hydrolysate, administered orally, could maintain their sequence integrity in the gastrointestinal tract or break up into new smaller bioactive peptides able to reach the bloodstream. At the end of the in vitro simulated gastrointestinal digestion, DSPI and DSPH exhibit similar antioxidant capacity and different ACE inhibitory activity each other. DSPI presents ORAC values of 3.6 ± 0.2 μmol TE/mg protein and iACE (IC50) of 70 ± 4 μg protein/mL. DSPH presents ORAC values of 3.9 ± 0.1 μmol TE/mg protein and iACE (IC50) of 52 ± 4 μg protein/mL.
From a technological point of view, the hydrolysis of soy protein with Corolase PP increases the solubility of the intact SPI between pH 3–7. For example, at pH 7 the solubility of SPH was approximately 4-fold higher than that of SPI. Finally, it can be highlighted that the enzymatic hydrolysis of the SPI with Corolase PP is a suitable tool to produce ingredients with a better solubility than the intact SPI, maintaining the nutritional and bioactive properties. However, other functional properties should be further studied as well as to validate through in vivo studies the bioactive capacities established.
Acknowledgments
Author E. R. Coscueta acknowledges BiValBi – Biotechnologies to Valorise the regional Biodiversity in Latin America (Refª PIRSES-GA-2013-611493 BI_1) – for the research fellowship administered for funding this work, as well as CONICET for PhD fellowship. We would also like to thank the scientific collaboration of CBQF under the FCT project UID/Multi/50016/2013.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2019.100006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Aura, A. M. (2005). In vitro digestion models for dietary phenolic compounds. VTT Publications. VTT Technical Research Centre of Finland.
- Bauchart C., Rémond D., Chambon C., Patureau Mirand P., Savary-Auzeloux I., Reynès C., Morzel M. Small peptides (<5 kDa) found in ready-to-eat beef meat. Meat Science. 2006;74(4):658–666. doi: 10.1016/j.meatsci.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Capriotti A.A.L., Caruso G., Cavaliere C., Samperi R., Group P., Ventura S.…Laganà A. Identification of potential bioactive generated by simulated gastrointestinal digestion of soybean seeds and soy milk proteins. Journal of Food Composition and Analysis. 2015;44:205–213. [Google Scholar]
- Chen H.-M., Muramoto K., Yamauchi F. Structural analysis of antioxidative peptides from soybean β-conglycinin. Journal of Agricultural and Food Chemistry. 1995;43(3):574–578. [Google Scholar]
- Contreras M. del M., Hernández-Ledesma B., Amigo L., Martín-Álvarez P.J., Recio I. Production of antioxidant hydrolyzates from a whey protein concentrate with thermolysin: optimization by response surface methodology. LWT – Food Science and Technology. 2011;44(1):9–15. [Google Scholar]
- Coscueta E.R., Amorim M.M., Voss G.B., Nerli B.B., Picó G.A., Pintado M.E. Bioactive properties of peptides obtained from Argentinian defatted soy flour protein by Corolase PP hydrolysis. Food Chemistry. 2016;198:36–44. doi: 10.1016/j.foodchem.2015.11.068. [DOI] [PubMed] [Google Scholar]
- Dia V.P., Torres S., de Lumen B.O., Erdman J.W., Gonzalez de Mejia E. Presence of lunasin in plasma of men after soy protein consumption. Journal of Agricultural and Food Chemistry. 2009;57(4):1260–1266. doi: 10.1021/jf803303k. [DOI] [PubMed] [Google Scholar]
- Dziuba J., Iwaniak A., Minkiewicz P. Computer-aided characteristics of proteins as potential precursors of bioactive peptides SO POLIMERY. Polimery. 2003;48(1):50–53. [Google Scholar]
- Escudero E., Mora L., Toldrá F. Stability of ACE inhibitory ham peptides against heat treatment and in vitro digestion. Food Chemistry. 2014;161:305–311. doi: 10.1016/j.foodchem.2014.03.117. [DOI] [PubMed] [Google Scholar]
- Esteve C., Marina M.L., García M.C. Novel strategy for the revalorization of olive (Olea europaea) residues based on the extraction of bioactive peptides. Food Chemistry. 2015;167:272–280. doi: 10.1016/j.foodchem.2014.06.090. [DOI] [PubMed] [Google Scholar]
- García-Mora P., Martín-Martínez M., Angeles Bonache M., González-Múniz R., Peñas E., Frias J., Martinez-Villaluenga C. Identification, functional gastrointestinal stability and molecular docking studies of lentil peptides with dual antioxidant and angiotensin I converting enzyme inhibitory activities. Food Chemistry. 2017;221:464–472. doi: 10.1016/j.foodchem.2016.10.087. [DOI] [PubMed] [Google Scholar]
- González-Montoya M., Ramón-Gallegos E., Robles-Ramírez M. del C., Mora-Escobedo R. Evaluation of the antioxidant and antiproliferative effects of three peptide fractions of germinated soybeans on breast and cervical cancer cell lines. Plant Foods for Human Nutrition. 2016;71(4):368–374. doi: 10.1007/s11130-016-0568-z. [DOI] [PubMed] [Google Scholar]
- Hartmann R., Meisel H. Food-derived peptides with biological activity: From research to food applications. Current Opinion in Biotechnology. 2007;18(2):163–169. doi: 10.1016/j.copbio.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Kim S.Y., Park S.-W.P., Rhee K.C. Functional properties of proteolytic enzyme modified soy protein isolate. Journal of Agricultural and Food Chemistry. 1990;38(1970):651–656. [Google Scholar]
- Lamsal B.P., Jung S., Johnson L.A. Rheological properties of soy protein hydrolysates obtained from limited enzymatic hydrolysis. LWT – Food Science and Technology. 2007;40(7):1215–1223. [Google Scholar]
- Laurent C., Besançon P., Caporiccio B. Flavonoids from a grape seed extract interact with digestive secretions and intestinal cells as assessed in an in vitro digestion/Caco-2 cell culture model. Food Chemistry. 2007;100(4):1704–1712. [Google Scholar]
- Lee S.H., Qian Z.J., Kim S.K. A novel angiotensin I converting enzyme inhibitory peptide from tuna frame protein hydrolysate and its antihypertensive effect in spontaneously hypertensive rats. Food Chemistry. 2010;118(1):96–102. [Google Scholar]
- Minkiewicz P., Dziuba J., Iwaniak A., Dziuba M., Darewicz M. BIOPEP database and other programs for processing bioactive peptide sequences. Journal of AOAC International. 2008;91(4):965–980. http://www.ncbi.nlm.nih.gov/pubmed/18727559 Retrieved from. [PubMed] [Google Scholar]
- Orsini Delgado M.C., Nardo A., Pavlovic M., Rogniaux H., Añón M.C., Tironi V.A. Identification and characterization of antioxidant peptides obtained by gastrointestinal digestion of amaranth proteins. Food Chemistry. 2016;197:1160–1167. doi: 10.1016/j.foodchem.2015.11.092. [DOI] [PubMed] [Google Scholar]
- Orsini Delgado M.C., Tironi V.A., Añón M.C. Antioxidant activity of amaranth protein or their hydrolysates under simulated gastrointestinal digestion. LWT – Food Science and Technology. 2011;44(8):1752–1760. [Google Scholar]
- Panyam D., Kilara A. Enhancing the functionality of food proteins by enzymatic modification. Trends in Food Science & Technology. 1996;7(4):120–125. [Google Scholar]
- Quirós A., Contreras M. del M., Ramos M., Amigo L., Recio I. Stability to gastrointestinal enzymes and structure-activity relationship of β-casein-peptides with antihypertensive properties. Peptides. 2009;30(10):1848–1853. doi: 10.1016/j.peptides.2009.06.031. [DOI] [PubMed] [Google Scholar]
- Ranamukhaarachchi S., Meissner L., Moresoli C. Production of antioxidant soy protein hydrolysates by sequential ultrafiltration and nanofiltration. Journal of Membrane Science. 2013;429:81–87. [Google Scholar]
- Rizzello Carlo G., Tagliazucchi Davide, Babini Elena, Sefora Rutella Giuseppina, Taneyo Saa Danielle L., Gianotti Andrea. Bioactive peptides from vegetable food matrices: Research trends and novel biotechnologies for synthesis and recovery. Journal of Functional Foods. 2016;27:549–569. [Google Scholar]
- Roberts P.R., Burney J.D., Black K.W., Zaloga G.P. Effect of chain length on absorption of biologically active peptides from the gastrointestinal tract. Digestion. 1999;60(4):332–337. doi: 10.1159/000007679. [DOI] [PubMed] [Google Scholar]
- Sentandreu M.Á., Toldrá F. A rapid, simple and sensitive fluorescence method for the assay of angiotensin-I converting enzyme. Food Chemistry. 2006;97(3):546–554. [Google Scholar]
- Shahidi F., Rodriguez-Amaya D.B., Makahleh A., Saad B., Bari M.F., Allen K.E.…Embuscado M.E. In: Handbook of Antioxidants for Food Preservation. Shahidi F., editor. Elsevier; 2015. doi: 10.1016/C2013-0-16454-9. [Google Scholar]
- Shahidi F., Zhong Y. Measurement of antioxidant activity. Journal of Functional Foods. 2015;18:757–781. [Google Scholar]
- Singh B.P., Yadav D., Vij S. Soybean bioactive molecules: current trend and future prospective. In: Mérillon J.-M., Ramawat K.G., editors. Bioactive Molecules in Food. Springer International Publishing; 2017. pp. 1–29. https://doi.org/10.1007/978-3-319-54528-8_4-1. [Google Scholar]
- Tsumura K., Saito T., Tsuge K., Ashida H., Kugimiya W., Inouye K. Functional properties of soy protein hydrolysates obtained by selective proteolysis. LWT – Food Science and Technology. 2005;38(3):255–261. [Google Scholar]
- Vittinghoff E., Glidden D.V., Shiboski S.C., McCulloch C.E. Second Edn. Springer Science & Business Media; 2011. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. [Google Scholar]
- Wang J., Yadav V., Smart A.L., Tajiri S., Basit A.W. Toward oral delivery of biopharmaceuticals: an assessment of the gastrointestinal stability of 17 peptide drugs. Molecular Pharmaceutics. 2015;12(3):966–973. doi: 10.1021/mp500809f. [DOI] [PubMed] [Google Scholar]
- Wang W., Gonzalez De Mejia E. A new frontier in soy bioactive peptides that may prevent age-related chronic diseases. Comprehensive Reviews in Food Science and Food Safety. 2005;4(4):63–78. doi: 10.1111/j.1541-4337.2005.tb00075.x. [DOI] [PubMed] [Google Scholar]
- Zayas J.F. Springer Berlin Heidelberg; Berlin, Heidelberg: 1997. Functionality of Proteins in Food. https://doi.org/10.1007/978-3-642-59116-7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.