Abstract
Objective
Retinopathy of prematurity (ROP) is a vasoproliferative disorder that is one of the main preventable causes of blindness among preterm neonates. This study aimed to determine the incidence of ROP and investigate the relationship between perinatal risk factors and ROP development.
Methods
This retrospective, non-interventional, non-comparative, hospital-based study was conducted at a tertiary-level neonatal intensive care unit. A total of 163 consecutive patients who met the inclusion criteria were recruited in this study.
Results
ROP prevalence was 0.01. During the study period, 44 patients developed ROP (27%), and 119 (73%) did not. Stage I ROP was detected in 8 patients (4.9%); stage II ROP without plus-disease in 26 patients (16%); stage II disease with comorbidities in 1 patient (0.6%); and stage III disease in 9 patients (5.5%). None of the patients showed stage IV and V disease. The mean gestational age was 27.7 ± 2.08 weeks in babies who had ROP and 29.59 ± 1.80 weeks in the other group. Neonates with ROP required more frequent blood transfusion (average, 4.89 ± 3.164 transfusions) compared to their counterparts who received an average of 1.19 ± 1.733 transfusions. Intracranial haemorrhage was identified in 55 (33.7%) patients, of whom 14.1% had ROP. Moreover, neonatal seizures occurred in 23 (14.11%) babies and were more common among babies who had ROP (n = 14).
Conclusion
This study identified key factors associated with ROP, such as intracranial haemorrhage with or without neonatal seizures and a high frequency of blood transfusions.
Keywords: Incidence of ROP, Neonates, Prematurity, Retinopathy of prematurity, Risk factors of ROP
الملخص
أهداف البحث
اعتلال الشبكية الخداجي هو اضطراب تكاثر الأوعية الدموية عند الخدج وهو أحد الأسباب الرئيسة للعمى التي يمكن الوقاية منها بين حديثي الولادة. تهدف هذه الدراسة إلى تحديد معدل الإصابة ودراسة العلاقة بين عوامل الخطر المحيطة بالولادة ونشوء اعتلال الشبكية الخداجي.
طرق البحث
أجريت هذه الدراسة الاستعادية، وهي غير تداخلية، وغير مقارِنة، في وحدة الرعاية المركزة لحديثي الولادة في مستشفى من المستوى الثالث. أدرج ١٦٣ مريضا ممن تنطبق عليهم المعايير المحددة في الدراسة على التوالي.
النتائج
كان معدل انتشار اعتلال الشبكية الخداجي ٠.٠١ خلال فترة الدراسة، حيث تم اكشاف اعتلال الشبكية الخداجي عند ٤٤ مريضا (٢٧ ٪) بينما لم تظهر أي أعراض للمرض عند ١١٩ (٧٣ ٪). تم الكشف عن المرحلة الأولى لاعتلال الشبكية الخداجي عند ٨ مرضى (٤.٩٪)، والمرحلة الثانية بدون مرض زائد عند ٢٦ (١٦ ٪)، والمرحلة الثانية مع أمراض مصاحبة عند مريض واحد (٠.٦٪)، والمرحلة الثالثة عند ٩ أطفال (٥.٥ ٪). ولم يكن هناك مرضى تم الإبلاغ عنهم في المرحلتين الرابعة والخامسة. كما كان متوسط عمر الحمل هو ٢٧.٧ أسبوعا (±٢.٠٨) عند الرضع الذين أصيبوا باعتلال الشبكية الخداجي، بينما كان متوسط عمر الحمل ٢٩.٥٩ (± ١.٨٠) في المجموعة الأخرى. احتاج حديثو الولادة المصابون باعتلال الشبكية الخداجي إلى نقل الدم بشكل متكرر؛ بمعدل ٤.٨٩ (±٣.١٦) مرة، مقارنةً بنظائرهم الذين تلقوا في المتوسط ١.١٩(± ١.٧٣) مرة. كما تم تشخيص نزف داخل الجمجمة عند ٥٥ مريضا (٣٣.٧ ٪)، منهم ١٤.١٪ لديهم اعتلال الشبكية الخداجي. علاوة على ذلك، حدثت نوبات تشنجات لحديثي الولادة عند ٢٣ (١٤.١٪) من الأطفال، وكان أكثر شيوعا بين الأطفال الذين لديهم اعتلال الشبكية الخداجي (العدد = ١٤).
الاستنتاجات
حددت هذه الدراسة العوامل الرئيسة المرتبطة باعتلال الشبكية الخداجي من مثل حدوث نزف داخل الجمجمة مع أو بدون نوبات تشنجات لحديثي الولادة، بالإضافة إلى تواتر عمليات نقل الدم.
الكلمات المفتاحية: حديثي الولادة, معدل الإصابة باعتلال الشبكية الخداجي, الخدج, اعتلال الشبكية الخداجي, عوامل خطر الإصابة باعتلال الشبكية الخداجي.
Introduction
Retinopathy of prematurity (ROP), previously known as retro-lental fibroplasia of the newborn owing to its end-stage appearance, was first reported by Terry et al., in 1942. It was initially presumed that the development of ROP is linked to the elaborate and unmonitored use of oxygen supplementation in the treatment of respiratory distress in preterm babies, who are kept in closed incubators; this association was described by Patz at al., in 1952.1 Currently, ROP is defined as a vasoproliferative disorder that occurs in preterm babies due to the immaturity of their retinas, which are susceptible to various insults.2 It is considered to be one of the leading causes of potentially preventable childhood blindness worldwide and continues to be a challenge in neonatology.2, 3
ROP stage I is characterized by a thin line demarcating the vascularized and non-vascularized segments of the retina. This line further progresses to a recognizable ridge in stage II. Stage III disease is diagnosed when areas of extraretinal vascularization are identified. The more advanced clinically severe stages III and IV are defined by partial retinal detachment and complete retinal detachment, respectively. Plus-disease represents dilatation and tortuosity of the retinal vessels and is generally considered a sinister sign.4 In clinical practice, eye examination findings are documented separately based on the recommendations of the International Classification of Retinopathy of Prematurity (ICROP) revisited, which was published in 2005.4 These recommendations were revised and updated from the initial ICROP guidelines published in 1984 and 1987.5, 6
The incidence of ROP varies between developed and developing countries. This difference is mainly attributable to differences in the resources and facilities directed toward care of preterm babies. With the recent advances in neonatal care, the survival of premature infants has markedly improved, leading to an increase in the number of neonates at risk of developing ROP. Furthermore, despite judicious control of oxygen therapy and advances in oxygen monitoring devices, the incidence of ROP is on the rise again, suggesting that the disease has a more complex aetiology than what was originally supposed.7
Hussain et al., in 1999, reported that the overall incidence of all stages of ROP was around 21.3%.8 More recently, Hwang et al., in 2015, reported the incidence of ROP to be around 34.1%.9 However, there is limited data on the incidence of ROP in Africa and the sub-Saharan regions as the disease is essentially only recognized in urban areas with neonatal intensive care unit (NICU) facilities capable of caring for premature babies.
The incidence of ROP in Oman and the KSA ranged from 19% to 39%, and the link between low birth weight and gestational age and the probability of ROP development has been established. As a result, many screening programs to detect and track such patients were established or are being developed to decrease the incidence of ROP and limit the disease progression10,11. This study aims to investigate the prevalence and incidence of ROP in a tertiary NICU centre and examine the factors influencing ROP development. In addition, this study attempts to detect the relationship between ROP and perinatal risk factors. To our knowledge, no reports addressing these topics in the United Arab Emirates have been published recently. Therefore, this study also aimed to compare the prevalence of ROP in the United Arab Emirates with those in neighbouring countries.
Materials and Methods
A retrospective cohort study was conducted in the Dubai Hospital NICU between August 2012 and December 2014. Over the study period, 4657 babies were born in Dubai Hospital whereas 611 neonates were admitted to the NICU, including babies who were born in Dubai Hospital or in other hospitals and subsequently transferred to the NICU at Dubai Hospital. A total of 163 neonates met the inclusion criteria for birth weight and gestational age. We included preterm neonates with gestational age at birth of no more than 32+0 weeks completed that were born in Dubai Hospital or outside Dubai Hospital but transferred to its NICU before completing 24 h of age during the study period. Patients transferred after completing 24 h were excluded due to the considerable lack of consistently informative referral letters from other regional hospitals. Other exclusion criteria were death before the first scheduled ROP screening.
Upon fulfilment of the inclusion criteria, patient data were extracted from both hardcopy and electronic medical records. Hardcopy and electronic medical records of the mothers were also retrieved. Patient information collected included data regarding gestational age, gender, multiple gestation, mode of delivery, birth weight, respiratory support (duration of mechanical ventilation and oxygen administration as well as the need for surfactant therapy), presence of congenital heart disease or intracranial haemorrhage, neonatal seizures, culture-proven sepsis, number of packed red blood cell (PRBC) transfusions received, total duration of stay in the NICU, the highest ROP stage recorded for either eye, and whether any treatment was required. Maternal data for prenatal complications included information regarding the presence of pre-gestational or gestational diabetes mellitus (DM), pregnancy-induced hypertension (PIH) with or without preeclampsia, and premature rupture of the membranes (PROM). To ensure confidentiality of the collected information, data were stored in a password-protected database that was only accessible to the primary investigator.
Our local ROP screening protocol specifies a schedule for the first fundal examination based on the postmenstrual age for all neonates admitted to the NICU, which is identified as the calculated gestational age at birth in weeks + days using the best obstetrical estimate, based on the study by Palmar et al., in 1991.9 Patients were examined regularly at 1–2-week intervals by an experienced senior specialist ophthalmologist, depending on the departmental rota, for detection of ROP. Retinal examinations were performed using an indirect binocular ophthalmoscope. The eyes were dilated with a combination of cyclopentolate 0.1% and phenylephrine 0.1% eye drops applied 1 h before the examination. Efforts were made to minimize the discomfort and systemic effects of the examination by using topical anaesthetic agents such as tetracin, pacifiers, and oral sucrose according to the NICU neonatal pain assessment protocol used locally. After the ophthalmological examination was completed, findings were documented for each eye separately following the recommendations of ICROP revisited in 2005.4 On the basis of the ICROP guidelines, retinopathy findings were described in terms of (1) the location of retinal involvement by zone; (2) the extent of retinal involvement by clock hour; (3) the stage or severity of retinopathy at the junction of the vascularized and avascular retina (stage I–V); and (4) the presence or absence of dilated and tortuous posterior pole vessels (plus-disease).4
Statistical analysis
Statistical analysis was performed using the IBM Statistical Package for the Social Sciences software (SPSS) v.21. Results were presented in terms of confidence intervals at 95%, and a P value of 0.05 was used as the threshold for statistical significance. Independent-sample t-test was used for continuous variables and the chi-squared test was used for categorical variables. The maximum severity of ROP in either eye of the infant was considered as the stage of ROP.
Results
Out of 163 patients enrolled in the study, 44 developed ROP (27%) and 119 (73%) did not develop ROP in any of the serial ophthalmological screening examinations. In the entire study population, 81 patients (50%) were males and 82 (50%) were females. Of the patients who developed ROP, 18 (40.9%) were males and 26 (59.1%) were females, and among those who did not develop ROP, 63 (52.9%) were males and 56 (47.1%) were females.
Stage I disease was detected in 8 patients (4.9%); stage II without plus-disease in 26 patients (16%); stage II with plus-disease in 1 patient (0.6%); and stage III with plus-disease in 9 patients (5.5%). None of the patients showed stage IV and V disease throughout the study period. Treatment was required for 9 patients, of which 1 had ROP stage II with plus-disease, while the remaining 8 had stage III ROP with plus-disease. Intravitreal Avastin® (bevacizumab) was the mode of treatment in 6 babies, while the other patients received peripheral retinal laser ablation.
Only 2 (1%) of the study subjects were born at or before 24+0 weeks, while 44 patients (27%) were born at a gestational age of 24+1 to 27+6 weeks, and the majority of the patients (117; 72%) were born at more than 28+0 weeks of gestation. In the ROP group, the mean gestational age was 27.7 ± 2.08 weeks while the mean gestational age in the no-ROP group was 29.59 ± 1.80 weeks (P < 0.0001). Patients with ROP had a mean birth weight of 851.20 ± 262.61 g while those who did not develop ROP were heavier at birth, with a mean birth weight of 1261.16 ± 276.75 g (P < 0.0001) (Table 1).
Table 1.
Relationship and associations between ROP (any stage) and risk factors (ROP, n = 44; no ROP, n = 119).
| No ROP mean (±SD) | ROP mean (±SD) | P value | |
|---|---|---|---|
| Birth weight (grams) | 1261.16 (±276.75) | 851.20 (±262.61) | <0.0001 |
| Gestational age (weeks) | 29.59 (±1.80) | 27.7 (±2.08) | <0.0001 |
| Surfactant therapy | 45 (37.8%) | 22 (50%) | 0.209 |
| Mechanical ventilation duration (days) | 4.38 (±12.33) | 27.64 (±27.65) | <0.0001 |
| Oxygen therapy duration (days) | 13.36 (±13.79) | 27.34 (±16.43) | <0.0001 |
| Number of PRBC transfusions | 1.19 (±1.733) | 4.89 (±3.164) | <0.0001 |
| Neonatal Seizures | 9 (7.56%) | 14 (31.8%) | 0.0002 |
| Length of hospitalization (days) | 49.55 (±25.64) | 106.75 (±102.56) | <0.0001 |
ROP, retinopathy of prematurity; PRBC, packed red blood cell.
Patients who developed ROP were mechanically ventilated for longer time periods (27.64 ± 27.65 days) and required subsequent oxygen therapy for longer periods as well (27.34 days ± 16.43) in comparison to patients who had no ROP (P < 0.0001). Overall, 41% (n = 67) of the patients required surfactant administration for management of respiratory distress syndrome, of which 22 patients developed ROP (of any stage), while 45 did not develop ROP (Table 1).
A significant link was found between the need for PRBC transfusion and the cumulative amount of blood received, when the 2 groups were compared, with 41 (93%) infants who developed ROP requiring PRBC transfusion and 65 (54.6%) infants belonging to the no-ROP population requiring PRBC transfusion (P < 0.0001). On average, babies in the ROP group received 4.89 ± 3.164 transfusions, while their counterparts received 1.19 ± 1.733 transfusions (Table 1).
The relationship between the presence of a congenital heart pathology and the development of ROP was found to be statistically significant (P < 0.0001). This finding was particularly highlighted by the association of patent ductus arteriosus (PDA) and any stage of ROP, in which 26 patients with ROP developed PDA (59.1%) while only 27 patients without ROP (22.7%) showed PDA (P = 0.004).
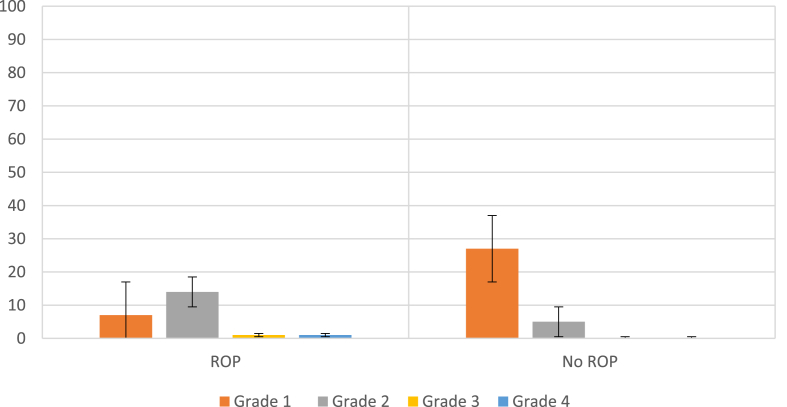
Out of the 163 patients studied, 55 developed intracranial bleeding, with grade I bleeding detected in 34 (61.8%) patients; grade II bleeding, in 19 (34.6%) patients; and grade III and grade IV bleeding, in one patient each (1.8% per group). Patients who did not develop ROP had either grade I (27 infants; 22.68%) or grade II (5 infants; 4.2%) intracranial bleeding, with the majority of the patients in this group (87 patients; 73.1%) showing normal brain US findings. In contrast, the ROP group contained 7 patients (15.9%) with grade I intracranial bleeding, 14 patients (31.81%) with grade II bleeding, and one patient each with grade III and IV bleeding (2.27% per group). A significant link was noted between intracranial bleeding and ROP development (P = 0.0030) (Figure 1). Seizures were documented in 23 patients (14.11%) across the 2 groups, of which 14 showed ROP and 9 showed no ROP (P = 0.0002) (Table 1).
Figure 1.
Grade of intracranial bleeding in relation to the presence of retinopathy of prematurity (ROP, n = 44; no ROP, n = 119).
Patients who developed ROP had a significantly longer hospital stay than those who did not develop ROP (106.75 ± 102.56 vs. 49.55 ± 25.64 days, respectively; P < 0.0001). Longer periods of hospitalization led to complications such as hospital-acquired infections. In the ROP group, 23 patients (52.3%) had culture-proven sepsis, while the non-ROP group contained only 18 (15.1%) patients who showed culture-proven sepsis (P = 0.0001).
Overall, throughout the study population, the majority of mothers (121; 74.2%) received antenatal care referred to in the current study as “Booked” to deliver in Dubai Hospital. For these mothers, initial obstetric evaluations were carried out in Dubai Health Authority primary health clinics, and at about 20 weeks of gestation, the mothers were transferred to the care of the obstetricians in Dubai Hospital. Being born to a booked mother did not show a statistically significant association across the 2 study arms.
Caesarean section (C-section) was the commonest mode of delivery (107 births; 65.6%), while vaginal delivery accounted for 56 births (34.4%). Indications for C-section included both maternal causes (preeclampsia, previous C-section) and foetal causes (breech presentation, foetal distress, multiple births). In the no-ROP group, 38 patients (31.9%) were born via normal vaginal delivery (NVD), while 18 (40.9%) patients who had ROP of any stage were born via the same mode (Table 2).
Table 2.
List of reported maternal complications during pregnancy (ROP, n = 44; no ROP, n = 119).
| Group |
Total | |||
|---|---|---|---|---|
| ROP any stage | No ROP | |||
| Maternal Complications | None | 13 | 38 | 51 (31%) |
| DM | 14 | 34 | 48 (29%) | |
| PIH | 3 | 18 | 21 (13%) | |
| PROM | 2 | 12 | 14 (9%) | |
| Others | 12 | 17 | 29 (18%) | |
| Total | 44 | 119 | 163 | |
ROP, retinopathy of prematurity; DM, diabetes mellitus; PIH, pregnancy-induced hypertension; PROM, premature rupture of the membranes. “Others” includes hypothyroidism, oligohydramnios, and antepartum haemorrhage.
On the basis of the number of foetuses conceived, the 2 groups of patients (patients with and without ROP) were further subdivided and grouped into singleton and multiple births, including twins, triplets, and quadruplets. Of the patients who had ROP, 23 (52.3%) were born of singleton pregnancies and 21 (47.3%) were products of multiple gestation, and among those who did not have ROP, 69 (57.9%) were born of singleton pregnancies, whereas 50 (42.1%) were products of multiple gestation. There was no statistically significant relationship between the development of ROP and the number of foetuses (P = 0.51) (Table 2).
Discussion
Our results showed that prematurity and low birth weight were by far the most important risk factors for ROP development, as they reflect the immaturity of the retina. However, improvement in prenatal care could reduce the incidence of ROP,12 with emerging evidence suggesting that some physiologic events that result in ROP start before delivery2, 13, 14.
Blumenfeld et al., in 1998, reported that multiple gestations were an independent risk factor for the development of ROP, with no relation to the severity.15 However, Motta et al., in 2011, identified multiple gestation as a predictor both for the development and the severity of ROP.16 This relationship could be attributed to the fact that newborns born as products of multiple gestation are more likely to be more premature compared to those who are products of singleton pregnancies. In contrast, Riazi–Esfahani et al., in 2008, showed no significant difference in the rate or severity of ROP between neonates from multiple gestation and singleton pregnancies, which is consistent with our findings.17
Manzonui et al., studied the potential link between the mode of delivery and the development of threshold ROP in a prospective cohort study of 174 extremely low birth weight (ELBW) neonates and concluded that vaginal delivery was a statistically significant and independent predictor for the development of ROP. The cause underlying this association, however, remains undetermined and is a possible subject for future studies.18 However, data from our centre showed no significant correlation between mode of delivery and ROP development.
Blood transfusion has been linked to ROP since it has been assumed that the lower affinity of adult haemoglobin in transfused blood is responsible for greater oxygen delivery to the retina.19 In addition, packed cell transfusions contain 1 mg iron/ml, and a few transfusions can double the body iron content in the first week alone. Preterm babies lack optimal protection against free iron due to their low levels of transferrin, which results in generation of free radicals and subsequent damage to the retinas.20 This could explain the highly significant association between PRBCs and the development of ROP in our population. Furthermore, the cumulative amount of blood received was also significantly linked to the development of ROP.21
The presence of an association between gender and the development of ROP remains a matter of debate. In a population-based cohort study conducted in Australia and New Zealand as a part of the neonatal network audit for high-risk infants born either with VLBW or at gestational age less than 32 weeks, there was a statistically significant association between male gender and the development of clinically severe ROP (defined as stage III or IV disease).22 On the other hand, Higgins et al., in 1991, conducted an animal-based experimental study in which gender did not predict the development of ROP resulting from hyperoxaemia. Similar findings were also demonstrated by Saeidi et al., in 2009, who also reported a lack of gender predilection, which is consistent with our results as aforementioned23,24.
Our results showed that sepsis increases the risk of ROP development; this might be partly due to systemic inflammation. Manzoni et al., in 2007, found a statistically significant and independent association between fungal septicaemia and development of ROP only in VLBW patients and the lack of a similar association in patients with higher birth weight.19
In a nationwide study conducted in Sweden that included infants born after less than 27 weeks of gestation in the period from 2004 through 2007, 34.8% of the study population developed severe ROP, with the study results suggesting that sepsis might be a moderate risk factor for ROP development (odds ratio = 1.4; confidence interval, 0.9–2.2).25 On the other hand, a study by Kavurt et al., in 2014, demonstrated a statistically significant association between ROP and sepsis in babies born before 32 weeks of gestation.26 In this study, blood cultures positive for bacteria and fungi showed statistical significance in comparisons between patients with ROP and those who did not develop ROP.
In our practice, all patients diagnosed with stage III ROP or plus-disease were treated either with intravitreal Avastin® (bevacizumab) injections or laser photocoagulation therapy. At the time of discharge from the NICU, all premature babies were given follow-up appointments to the ophthalmology clinic, and they underwent frequent follow-up examinations until full vascularization of the retinas was achieved. In the event of further progression of the disease, the patients were promptly treated.
Recommendations
In anaemic patients, the authors recommend other lines of management such as administration of erythropoietin to be considered before PRBC administration. Additionally, the introduction of an individualized screening program as well as proper training of healthcare professionals is of utmost importance, since this will lead to early management of ROP and possible better outcomes.
Limitations of the study
One of the main limitations of this study was the fact that it was performed retrospectively. Additionally, an observational bias may have occurred due to interobserver differences since ROP screening was done by different ophthalmologists. Furthermore, the current study was conducted at one centre, and a multicentre collaboration may have improved our understanding of the disease pathophysiology.
Conclusion
Our study shows, that in addition to the degree of prematurity (reflected by the gestational age) and birth weight, other variables showing a significant association with ROP that could predict the development of ROP were (1) the development of intracranial haemorrhage or neonatal seizures, (2) need for PRBC transfusions, (3) longer duration of mechanical ventilation, and (4) subsequent oxygen therapy requirement prior to weaning to room air. In addition, the length of stay was observed to be significantly longer in patients who developed ROP.
Source of funding
The authors have no source of funding to declare in relation to this study.
Conflict of interest
The authors have no conflict of interest to declare
Ethical approval
Ethical approval was obtained from the IRB before study commencement.
Authors' contributions
AAN1 and AA conceived the idea and worked on data collection. AAN2, and SAN performed the data entry and wrote the paper. RAK worked on the data analysis. MAJ reviewed and edited the final draft. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Patz A., Hoeck L., De La Cruz E. Studies on the effect of high oxygen administration in retrolental fibroplasia*. Am J Ophthalmol. 1952;35(9):1248–1253. doi: 10.1016/0002-9394(52)91140-9. [DOI] [PubMed] [Google Scholar]
- 2.Hellström A., Smith L., Dammann O. Retinopathy of prematurity. Lancet. 2013;382(9902):1445–1457. doi: 10.1016/S0140-6736(13)60178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blencowe H., Lawn J., Vazquez T., Fielder A., Gilbert C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res. 2013;74(S1):35–49. doi: 10.1038/pr.2013.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123(7):991. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 5.An international classification of retinopathy of prematurity. Arch Ophthalmol. 1984;102(8):1130–1134. doi: 10.1001/archopht.1984.01040030908011. [DOI] [PubMed] [Google Scholar]
- 6.Aaberg T. An international classification of retinopathy of prematurity. Arch Ophthalmol. 1987;105(7):906. doi: 10.1001/archopht.1987.01060070041024. [DOI] [PubMed] [Google Scholar]
- 7.Wong H., Santhakumaran S., Statnikov Y., Gray D., Watkinson M., Modi N. Retinopathy of prematurity in English neonatal units: a national population-based analysis using NHS operational data. Arch Dis Child Fetal Neonatal Ed. 2013;99(3):F196–F202. doi: 10.1136/archdischild-2013-304508. [DOI] [PubMed] [Google Scholar]
- 8.Hussain N., Clive J., Bhandari V. Current incidence of retinopathy of prematurity, 1989–1997. Pediatrics. 1999;104(3) doi: 10.1542/peds.104.3.e26. e26-e26. [DOI] [PubMed] [Google Scholar]
- 9.Hwang J., Lee E., Kim E. Retinopathy of prematurity among very-low-birth-weight infants in Korea: incidence, treatment, and risk factors. J Korean Med Sci. 2015;30(Suppl 1):S88. doi: 10.3346/jkms.2015.30.S1.S88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binkhathlan A., Almahmoud L., Saleh M., Srungeri S. Retinopathy of prematurity in Saudi Arabia: incidence, risk factors, and the applicability of current screening criteria. Br J Ophthalmol. 2008;92(2):167–169. doi: 10.1136/bjo.2007.126508. [DOI] [PubMed] [Google Scholar]
- 11.Jacob M., Sawardekar K., Ayoub H., Busaidi I. Validation of the existing modified screening criteria for detection of all cases of Retinopathy of Prematurity in preterm babies – 11 year study from a governorate referral hospital in Oman. Saudi Journal of Ophthalmology. 2016;30(1):3–8. doi: 10.1016/j.sjopt.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu T., Zhang L., Tong Y., Qu Y., Xia B., Mu D. Retinopathy of prematurity among very low-birth-weight infants in China: incidence and perinatal risk factors. Investigative Opthalmology & Visual Science. 2018;59(2):757. doi: 10.1167/iovs.17-23158. [DOI] [PubMed] [Google Scholar]
- 13.Chow P., Yip W., Ho M., Lok J., Lau H., Young A. Trends in the incidence of retinopathy of prematurity over a 10-year period. Int Ophthalmol. 2018;39(4):903–909. doi: 10.1007/s10792-018-0896-0. [DOI] [PubMed] [Google Scholar]
- 14.Lynch A., Wagner B., Hodges J., Thevarajah T., McCourt E., Cerda A. The relationship of the subtypes of preterm birth with retinopathy of prematurity. Am J Obstet Gynecol. 2017;217(3):354.e1–354.e8. doi: 10.1016/j.ajog.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 15.Blumenfeld L., Siatkowski R., Johnson R., Feuer W., Flynn J. Retinopathy of prematurity in multiple-gestation pregnancies. Am J Ophthalmol. 1998;125(2):197–203. doi: 10.1016/s0002-9394(99)80092-0. [DOI] [PubMed] [Google Scholar]
- 16.Fortes Filho J., Motta Coblentz. Multiple pregnancies and its relationship with the development of retinopathy of prematurity (ROP) Clin Ophthalmol. 2011:1783. doi: 10.2147/OPTH.S25431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riazi-Esfahani M., Alizadeh Y., Karkhaneh R., Mansouri M., Kadivar M., Nili Ahmadabadi M. Retinopathy of prematurity: single versus multiple-birth pregnancies. J Ophthalmic Vis Res. 2008;3(1):47–51. [PMC free article] [PubMed] [Google Scholar]
- 18.Manzoni P., Farina D., Maestri A., Giovannozzi C., Leonessa M., Arisio R. Mode of delivery and threshold retinopathy of prematurity in pre-term ELBW neonates. Acta Paediatr. 2007;96(2):221–226. doi: 10.1111/j.1651-2227.2007.00091.x. [DOI] [PubMed] [Google Scholar]
- 19.Cooke R., Clark D., Hickey-Dwyer M., Weindling A. The apparent role of blood transfusions in the development of retinopathy of prematurity. Eur J Pediatr. 1993;152(10):833–836. doi: 10.1007/BF02073381. [DOI] [PubMed] [Google Scholar]
- 20.Stutchfield C., Jain A., Odd D., Williams C., Markham R. Foetal haemoglobin, blood transfusion, and retinopathy of prematurity in very preterm infants: a pilot prospective cohort study. Eye. 2017;31(10):1451–1455. doi: 10.1038/eye.2017.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dani C., Reali M., Bertini G., Martelli E., Pezzati M., Rubaltelli F. The role of blood transfusions and iron intake on retinopathy of prematurity. Early Hum Dev. 2001;62(1):57–63. doi: 10.1016/s0378-3782(01)00115-3. [DOI] [PubMed] [Google Scholar]
- 22.Darlow B., Cust A., Donoghue D. Improved outcomes for very-low-birthweight infants: evidence from a New Zealand national population-based data. Obstet Gynecol Surv. 2003;58(8):520–522. doi: 10.1136/fn.88.1.F23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins R., Yu K., Sanders R., Nandgaonkar B., Rotschild T., Rifkin D. Diltiazem reduces retinal neovascularization in a mouse model of oxygen induced retinopathy. Curr Eye Res. 1999;18(1):20–27. doi: 10.1076/ceyr.18.1.20.5390. [DOI] [PubMed] [Google Scholar]
- 24.Saeidi R., Hashemzadeh A., Ahmad S., Rahmani S. Prevalence and predisposing factors of retinopathy of prematurity in very Low¬birth¬weight infants discharged from NICU. Iran J Pediatr. 2009;19(1):59–63. [Google Scholar]
- 25.Ohlin A., Björkman L., Serenius F., Schollin J., Källén K. Sepsis as a risk factor for neonatal morbidity in extremely preterm infants. Acta Paediatr. 2015;104(11):1070–1076. doi: 10.1111/apa.13104. [DOI] [PubMed] [Google Scholar]
- 26.Kavurt S., Özcan B., Aydemir O., Bas A., Demirel N. Risk of retinopathy of prematurity in small for gestational age premature infants. Indian Pediatr. 2014;51(10):804–806. doi: 10.1007/s13312-014-0506-9. [DOI] [PubMed] [Google Scholar]