Abstract
Objective
Invasive microorganisms and free radicals are responsible for the delayed healing of various infections. It is necessary to discovery of novel molecules that are effective against invasive microorganisms and inhibit free radicals. Therefore, a series of metal complexes of 2-amino-4-substituted phenylthiazole Schiff bases were synthesized.
Methods
Structural characterization of the synthesized molecules was performed by elemental analysis, FT/IR, 1H NMR, UV–Vis spectrophotometry, LC-MS, XRD, and SEM. The antimicrobial activities of all the synthesized molecules were investigated by an agar well diffusion method. An acute oral toxicity study of the synthesized ligands and their metal complexes was conducted according to OECD guidelines. The DPPH assay was used to evaluate the radical-scavenging activities of the compounds.
Results
Results of the oral acute toxicity study revealed that the synthesized analogues are safe up to a dose of 2000 mg/kg body weight. The complexes bis[{4-((4-bromo-3-methylphenyl)diazenyl)-2-((4-phenylthiazol-2-ylimino)methyl)phenoxy}]cobalt (6a) and bis[4-{(4-bromo-3-methylphenyl)diazenyl}-2-{(4-(4-chlorophenyl)thiazol-2-ylimino)methyl}phenoxy]cobalt (6d) exhibited significant antibacterial activities against drug-resistant bacterial strains as well as potent radical-scavenging properties.
Conclusion
The results justify that the chelation of metals with Schiff base ligands enhances their biological activities against drug-resistant microbial strains.
Keywords: Antimicrobial, Antioxidant, Magnetic susceptibility, Metal complexes, Schiff base
الملخص
أهداف البحث
المسئول عن تأخر الشفاء من الأمراض المعدية المختلفة هو الكائنات الحية الدقيقة الغازية والشوارد الحرة. هناك حاجة لجزيئات مهجنة جديدة تبقى فاعلة ضد الكائنات الحية الدقيقة الغازية ويمكن أن تمنع الشوارد الحرة. تم توليف سلسلة من ٢-أمينو-٤-فينيل ثيازول المستبدل والمعتمد على قاعدة ”شيف“ لتحمل المركبات المعدنية.
طرق البحث
تم تأكيد البيئة الهيكلية للجزيئات المركبة بواسطة تحليل العناصر. كما تم التحقيق من النشاط المضاد للميكروبات لجميع الجزيئات المركبة بواسطة طريقة أجار للانتشار الجيد. وكذلك تم تحديد دراسة السمية الحادة للتوليفات والمركبات المجمعة بواسطة خط التوجيه. وتم التحقق من نشاط الكسح الجذري.
النتائج
وفقآ لنتائج دراسة السمية الحادة عن طريق الفم، فإن النظائر المؤلفة هي آمنة إلى جرعة تصل ٢٠٠٠ مجم⁄كجم من وزن الجسم. أظهرت المجمعات "٦أ" و "٦د" نشاطا مضادا للجراثيم كبيرا ضد سلالات من البكتيريا مقاومة للدواء فضلا عن خاصية الكسح الجذري المحتملة.
الاستنتاجات
تثبت هذه الدراسة أن خالبات المعادن مع روابط قاعدة "شيف هي المسئولة عن تعزيز النشاط البيولوجي ضد مقاومة السلالات الميكروبية.
الكلمات المفتاحية: مضادات الميكروبات, مضادات الأكسدة, القابلية المغناطيسية, قاعدة شيف؛ طيفي
Introduction
Azo-linked Schiff bases exhibit diverse biological properties.1 Compounds having an azomethine (—CH N—) functional group are known as Schiff bases; they function as organic synthons for the synthesis of new molecules. They exhibit anticancer,2 anti-tubercular,3 antiviral,4 anticonvulsant,5 and antimicrobial activities.6 They are flexible and structurally similar to natural biological substances due to the presence of an imine (—N CH—) group.7
The azomethine group is a good electron donor, and forms stable complexes with transition metal ions.8 Both azo and azomethine groups in azo-linked Schiff bases are capable of forming coordination bonds with metal ions; however, metal ions interact preferentially with the azomethine group, leaving the azo group free.8 Coordination of an organic compound with a metal drastically changes its biological properties.9 Metal complexes of Schiff bases exhibit anticancer,10 antimicrobial,9, 10, 1 and antioxidant activities11 as well as decreased cytotoxicities compared to individual metal ions and Schiff bases.11 The treatment of infections is challenged by multidrug resistance in pathogenic organisms and oxidative stress. Hence, the development of newer molecules for the management of infections and oxidative stress is warranted.12, 13 Metal complexation alters the therapeutic efficiency of azo-linked ligands.14 Azo-linked Schiff bases contain both azomethine and azo groups. Coordination of metals to such groups is responsible for their versatile biological properties.8 Schiff bases derived from salicylaldehydes are known polydentate ligands which can coordinate to metals in both their deprotonated and neutral forms.15 Salicylaldehyde-bearing molecules exhibit, antimicrobial,15 antiviral,16 cytotoxic,17 DNA-binding,18 and antioxidant19 activities.
The objective of the study was to synthesize a series of metal complexes retaining both azo and azomethine groups and identify molecules that are effective against drug-resistant microbial strains and possess significant antioxidant properties, thereby acting as efficient anti-infective agents. Antimicrobial activities were evaluated using drug-resistant bacterial strains. In this study, different transitional metals were conjugated with Schiff base intermediates of 5-[(4-bromo-3-methylphenyl)diazenyl]-2-hydroxybenzaldehyde, (4e),6 and their biological properties were evaluated.
Materials and Methods
All the chemicals (Merck specialties Ltd., Mumbai, India) used were of synthesis grade. Melting points were determined by the open capillary method (Elico), and were uncorrected. An FT/IR spectrophotometer was used to record the IR spectra of the synthesized molecules (JASCO FT/IR 4100, JASCO, JAPAN). A mass spectrophotometer with a C6 column (150 mm × 4.6 mm, 5 μm) was used to determine the molecular mass (Shimadzu). 1H NMR spectra were recorded using tetramethylsilane as an internal standard (Bruker 1H NMR, 400 MHz), and chemical shifts (δ) were reported in ppm. UV and elemental analyses were performed using a JASCO V-630 spectrophotometer and a Perkin Elmer 2400 CHNS/O analyser, respectively. XRD analysis was performed using a Cu Kα X-ray source; step = 0.02(2θ), run 2θ = 2–80°, scanning speed = 2°/min (Shimadzu XRD 7000). Structure elucidation was performed using the Origin data analysis software. A scanning electron microscope (EGOMA 15 ZEISS) was used to study the structural environment. Magnetic susceptibility was analysed by a Faraday balance.
The synthesis of 5-[(4-bromo-3-methylphenyl)diazenyl]-2-hydroxybenzaldehyde (4e) was carried out as previously reported.6
Synthesis of Schiff base ligands5(a,b)
An ethanolic solution (50 mL) of 2-amino-4-substituted phenylthiazole 4 (a,b) was mixed with an aqueous solution (100 mL) of 4e in equimolar concentrations, and the mixture was refluxed in the presence of glacial acetic acid for 2 h at 70 °C to obtain Schiff base ligands 5 (a,b).20 The precipitates obtained were repeatedly washed with a mixture of ethanol and diethyl ether.
Synthesis of complexes6(a–f)
Each Schiff base ligand (2 mmol) was mixed with an equal proportion of respective metal chlorides (Co2+, Cu2+, and Ni2+; 1 mmol) and refluxed with ethanol for 2 h at 70 °C, followed by recrystallization in ethanol. The progression of the reaction was monitored by TLC with a solvent system containing ethyl acetate and cyclohexane (1:3).6
Antimicrobial activity
Antimicrobial activities of the synthesized molecules were studied by an agar well diffusion method using nutrient agar and Sabouraud dextrose agar media for bacterial and fungal pathogens, respectively.21 Freshly subcultured microbial strains of Klebsiella pneumoniae (MTCC 109) and Candida albicans (MTCC 3017) were procured from the Institute of Microbial Technology and Gene bank (IMTECH), Chandigarh, India. Escherichia coli (res) (resistant to norfloxacin, ofloxacin, ampicillin, cefixime, and nitrofurantoin) and Staphylococcus aureus (res) (resistant to norfloxacin, ofloxacin, ampicillin, and cefotaxime) were isolated by the Pharmaceutical Biotechnology Division of the University Department of Pharmaceutical Sciences, Utkal University, Bhubaneswar, Odisha, India. The faecal collected from Institute of Medical Sciences & SUM Hospital, Bhubaneswar, Odisha. Cryptococcus neoformans was obtained from the University Department of Pharmaceutical Sciences, Utkal University.22 Amoxicillin (Reference Antibiotic: RA) and ampicillin (RA1) were used as reference antibacterial antibiotics, and fluconazole (RA) was used as a reference antifungal antibiotic. Antimicrobial diffusion assays were performed using cell suspensions containing 1.5 × 106 CFU/mL employing the McFarland turbidity standard No. 0.5.23
Minimum inhibitory concentration (MIC)
Stock solutions of test and reference compounds were prepared in DMF at a concentration of 1 mg/mL. Further, five different concentrations (31.25–500 µg/mL) were prepared by two-fold dilution method and loaded into agar wells. Before incorporating the stock solution of test and reference compounds in to the agar wells, the agar plates were seeded with respective bacterial and fungal strains in a suitable concentration. The loaded petri plates were incubated at 37 °C for 18–24 h. After incubation, the MIC values were determined.21
Acute toxicity study
A fixed dose acute oral toxicity study was conducted according to OECD guideline no. 420 (2000) using female Wistar rats for a period of 14 days. Test compounds at doses of 5, 50, 300, and 2000 mg/kg body weight were administered to the rats with an interval of 24 h between each dose. The study was carried out in adherence to CPCSEA and IAEC guidelines (registration number 1171/C/08/CPCSEA and Ref. No. 60/SPS/IAEC/SOAU).21
Antioxidant activity
The free radical scavenging activities of the synthesized Schiff base analogues of 2-amino-4-substituted phenylthiazole were measured by the DPPH assay with some modifications.21 The absorbance of test compounds and standard butylated hydroxy toluene (BHT) at different concentrations was measured at 517 nm, and free radical inhibitory activities were calculated. A mixture of DPPH and methanol was considered as control.
| Inhibition (%) = [(Acont − Atest)/Atest] × 100 |
Acont = Absorbance of control
Atest = Absorbance of the test and standard samples
The IC50 value was graphically determined.
Statistical analysis
One-way analysis of variance using Dunnett's post hoc test was used for analysis of the data.21
Results
Schiff base ligands, 4-[(4-bromo-3-methylphenyl)diazenyl)-2-[(4-substituted phenylthiazol-2-ylimino)methyl]phenol, 5 (a–b), along with their metal complexes 6 (a–f) were synthesized as stated in Scheme I.
Scheme I.
Synthesis of metal complexes of Schiff bases derived from 2-amino-4-substituted phenylthiazole. i. NaNO2/HCl, 0–5 °C, diazotization; ii. 10% NaOH, coupling reaction; iii. Ethanol reflux, 2 h, 70 °C, Schiff base; iv. MCl2/x H2O, ethanol (M = Co2+, Cu2+, or Ni2+).
The physical properties of the synthesized analogues are reported in Table 1.
Table 1.
Physical characteristics of novel metal complexes of 2-amino-4-substituted phenylthiazole Schiff bases.
| Compounds | Chemical name | M. formula | LC-MS (RT, % area) | m/z | Rf | M. P. (°C) | Colour | Yield (%) |
|---|---|---|---|---|---|---|---|---|
| 4e | 5- [(4-Bromo-3-methylphenyl)diazenyl]-2-hydroxybenzaldehyde | C14H11BrN2O2 | 3.053, 95.57 | 319.01 (M + 1) | 0.8 | 180–90 | Dark red (powder) | 85 |
| 5a(Lig) | 4-[(4-bromo-3-methylphenyl)diazenyl)-2-[(4-phenylthiazol-2-ylimino)methyl]phenol | C23H17BrN4OS | 1.581, 99.93 | 477.3 (M−1) | 0.6 | 235–37 | Brown (crystal) | 74 |
| 5b(Lig) | 4-[(4-bromo-3-methylphenyl)diazenyl]-2-[(4-(4-chlorophenyl)thiazol-2- ylimino)methyl]phenol | C23H16BrClN4OS | 1.861, 89.73 | 512.3 (M + 1) | 0.5 | 242–45 | Brown (crystal) | 78 |
| 6a | Bis[{4-((4-bromo-3-methylphenyl)diazenyl)-2-((4-phenylthiazol-2-limino)methyl)phenoxy}] cobalt | C46H32Br2CoN8O2S2 | 1.761, 91.73 | 1009.3 (M + 1) | 0.6 | 258–60 | Brown (powder) | 43 |
| 6b | Bis[{4-((4-bromo-3-methylphenyl)diazenyl)-2-((4-phenylthiazol-2-ylimino)methyl)phenoxy}] copper, | C46H32Br2CuN8O2S2 | 1.351, 97.43 | 1013 (M + 1) | 0.7 | 262–65 | Brown (powder) | 49 |
| 6c | Bis[{4-((4-bromo-3-methylphenyl)diazenyl)-2-((4-phenylthiazol-2-ylimino)methyl)phenoxy}] nickel | C46H32Br2N8NiO2S2 | 1.661, 87.73 | 1008.79 (M−1) | 0.8 | 253–58 | Brown (powder) | 51 |
| 6d | Bis[4-{(4-bromo-3-methylphenyl)diazenyl}-2-{(4-(4-chlorophenyl)thiazol-2-ylimino)methyl}phenoxy]cobalt | C46H30Br2Cl2CoN8O2S2 | 1.561, 88.73 | 1079.1 (M + 1) | 0.7 | 238–40 | Brown (powder) | 53 |
| 6e | Bis[4-{(4-bromo-3-methylphenyl)diazenyl}-2-{(4-(4-chlorophenyl)thiazol-2-ylimino)methyl}phenoxy] copper, | C46H30Br2Cl2CuN8O2S2 | 1.356, 81.73 | 1085.31 (M + 1) | 0.8 | 246–50 | Brown (powder) | 73 |
| 6f | bis[4-{(4-bromo-3-methylphenyl)diazenyl}-2-{(4-(4-chlorophenyl)thiazol-2-ylimino)methyl}phenoxy] nickel | C46H30Br2Cl2NiN8O2S2 | 1.531, 79.73 | 1081.10 (M + 2) | 0.6 | 253–55 | Brown (powder) | 67 |
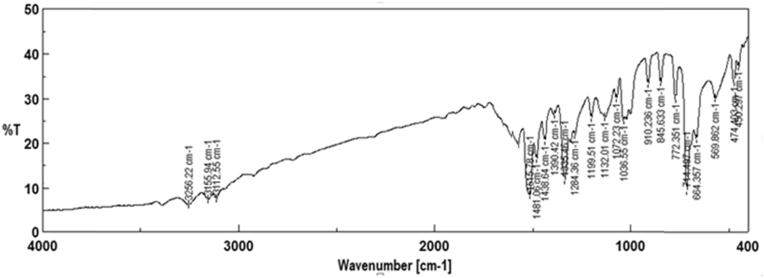
The far and near IR spectra of the newly synthesized compounds were studied in the frequency (ν) range of 4000–400 cm−1. A band at ν 1660 cm−1 indicated the presence of the carbonyl stretching of salicylaldehyde in 4e. A weak absorption band appeared at ν 1481–1477 cm−1 in the spectra of all the compounds due to the presence of an (—N N—) group. Broad, medium-intensity bands at ν 3155 and 3159 cm−1 were observed in the FT/IR spectra of Schiff bases 5 (a–b), indicating intra-molecular hydrogen bonding between hydroxyl groups and the nitrogen of azomethine. The FT/IR spectra of Schiff base ligands 5a(Lig) are reported in Figure 1. The spectra of the intermediate Schiff bases, 5a(Lig) and 5b(Lig), showed peaks indicating the azomethine group at ν 1615 and 1610 cm−1, respectively, whereas the spectra of the metal complexes 6 (a–f), derived from 5a(Lig) and 5b(Lig), showed moderate changes in the azomethine frequency. The phenolic (C—O) stretching vibrations of Schiff base ligands at ν 1132 and 1142 cm−1 were shifted in all the complexes towards the lower frequencies ν 1104 and 1115 cm−1.6 The IR spectra of the metal complexes showed bands at ν 493–455 and 597–535 cm−1 assigned to (M−N) and (M−O), respectively (Table 2).
Figure 1.
FT/IR spectra of 4-[(4-bromo-3-methylphenyl)diazenyl]-2-[(4-phenylthiazol-2-ylimino)methyl]phenol: 5a(Lig).
Table 2.
Spectral characterization of novel metal complexes of 2-amino-4-substituted phenylthiazole Schiff bases.
| Compound | UV–Vis λ max (nm) (Ethanol) |
Spectral Characterization |
Elemental Analysis Calculated (Found) % |
||||
|---|---|---|---|---|---|---|---|
| IR (cm−1) | 1H NMR (δ, ppm) | ||||||
| C | H | N | S | ||||
| 4e | 346 | 3444 (O—H str.), 2857, 2743 (CH str. of aldehyde), 1660 (C O str.), 1615 (C C str.), 1478 (—N N—), 1305, 1175 (SO2str.SO3H), 1137 (C—O str.); | (CDCl3, 400 MHz): 2.50 (s, 3H, CH3), 7.26 (d, 1H, salicylaldehyde, H-5), 7.69–7.78 (d, 2H, Ar H), 7.78 (s, 1H, Ar H), 8.20 (s, 1H, salicylaldehyde H-2), 8.17 (d, 1H, salicylaldehyde H-6), 10.38 (s, 1H, CHO), 11.34 (s, 1H, OH); |
52.69 (52.71) | 3.47 (3.48) | 8.78 (8.77) | – |
| 5a(Lig) | 403 | 3155 (O—H str. intra mol. H.), 1615 (C N str.), 1481 (—N N—), 1284 (OH bend), 1132 (C—O str.), 845 (C—Br str.); | (CDCl3, 400 MHz): 2.49 (s, 3H, CH3), 7.25 (d, 1H, salicylaldehyde H-6), 7.43–7.59 (m, 5H, ArH), 7.67 (d, 1H, diazenylphenyl H-5), 7.76 (s, 1H, diazenylphenyl H-2), 8.17 (s, 1H, —CH N), 8.18 (s, 1H, Thiazolyl H-5), 8.72 (d, 1H, salicylaldehyde H-5), 9.42 (s, 1H, salicylaldehyde H-3), 11.33 (s, 1H, OH); |
57.87 (57.83) | 3.59 (3.60) | 11.74 (11.71) | 6.72 (6.74) |
| 5b(Lig) | 411 | 3159 (O—H str. intra mol. H.), 1610 (C N str.), 1477 (—N N—), 1265 (OH bend.), 1142 (C—O str.), 841 (C—Br str.), 835 (1,4 disubst.); | (CDCl3, 400 MHz): 2.39 (s, 3H, CH3), 7.24 (d, 1H, salicylaldehyde H-6), 7.53–7.98 (d, 4H, ArH), 7.69 (d, 1H, diazenylphenyl H-5),7.71 (d, 1H, diazenylphenyl H-6), 7.76 (s, 1H, diazenylphenyl H-2), 8.13 (s, 1H, —CH N), 8.16 (s, 1H, Thiazolyl H-5), 8.73 (d, 1H, salicylaldehyde H-5), 9.13 (s, 1H, salicylaldehyde H-3), 11.29 (s, 1H, OH); | 53.97 (53.93) | 3.15 (3.21) | 10.95 (10.91) | 6.26 (6.28) |
| 6a | 375 | 1614 (C N str.), 1470 (—N N—), 1104 (C—O str.), 815 (C—Br str.), 457 (Co—N), 593(Co—O); | (DMSO-d6, 400 MHz): 2.50 (s, 3H, CH3), 7.25 (d, 1H, salicylaldehyde H-6), 7.44–7.60 (m, 10H, ArH), 7.66 (d, 1H, diazenylphenyl H-5), 7.77 (s, 1H, diazenylphenyl H-2), 8.17 (s, 1H, —CH N), 8.19 (s, 1H, Thiazolyl H-5), 8.72 (d, 1H, salicylaldehyde H-5), 9.45 (s, 1H, salicylaldehyde H-3); |
54.61 (54.33) | 3.19 (3.25) | 11.08 (11.19) | 6.34 (6. 31) |
| 6b | 392 | 1612 (C N str.), 1465 (—N N—), 1115 (C—O str.), 814 (C—Br str.), 455 (Cu—N), 535 (Cu—O) cm−1; | (DMSO-d6, 400 MHz): 2.50 (s, 3H, CH3), 7.26 (d, 1H, salicylaldehyde H-6), 7.39–7.66 (m, 10H, ArH), 7.67 (d, 1H, diazenylphenyl H-5), 7.77 (s, 1H, diazenylphenyl H-2), 8.17 (s, 1H, —CH N), 8.20 (s, 1H, Thiazolyl H-5), 8.79 (d, 1H, salicylaldehyde H-5), 9.63 (s, 1H, salicylaldehyde H-3); |
54.36 (54.39) | 3.17 (3.17) | 11.03 (11.11) | 6.31 (6.33) |
| 6c | 350 | 1611 (C N str.), 1482 (—N N—), 1105 (C—O str.), 816 (C—Br str.), 456 (Ni—N), 593 (Ni—O) cm−1; | (DMSO-d6, 400 MHz): 2.50 (s, 3H, CH3), 7.26 (d, 1H, salicylaldehyde H-6), 7.38–7.67 (m, 10H, ArH), 7.69 (d, 1H, diazenylphenyl H-5), 7.77 (s, 1H, diazenylphenyl H-2), 8.17 (s, 1H, —CH N), 8.19 (s, 1H, Thiazolyl H-5), 8.79 (d, 1H, salicylaldehyde H-5), 9.46 (s, 1H, salicylaldehyde H-3); |
54.62 (54.79) | 3.19 (3.37) | 11.08 (11.14) | 6.34 (6.45) |
| 6d | 345 | 1613 (C N str.), 1472 (—N N—), 1109 (C—O str.), 837 (C—Br str.), 463 (Co—N), 597 (Co—O); | (DMSO-d6, 400 MHz): 2.45 (s, 3H, CH3), 7.47 (d, 1H, salicylaldehyde H-6), 7.51–7.71 (m, 10H, ArH), 7.73 (d, 1H, diazenylphenyl H-5), 7.79 (s, 1H, diazenylphenyl H-2), 8.21 (s, 1H, —CH N), 8.28 (s, 1H, Thiazolyl H-5), 8.89 (d, 1H, salicylaldehyde H-5), 9.51 (s, 1H, salicylaldehyde H-3); |
51.13 (51.18) | 2.80 (2.79) | 10.37 (10.49) | 5.93 (5.97) |
| 6e | 375 | 1609 (C N str.), 1465 (—N N—), 1113 (C—O str.), 849 (C—Br str.), 493 (Cu—N), 583 (Cu—O); | (DMSO-d6, 400 MHz): 2.39 (s, 3H, CH3), 7.51 (d, 1H, salicylaldehyde H-6), 7.32–7.69 (m, 10H, ArH), 7.71 (d, 1H, diazenylphenyl H-5), 7.78 (s, 1H, diazenylphenyl H-2), 8.23 (s, 1H, —CH N), 8.29 (s, 1H, Thiazolyl H-5), 8.65 (d, 1H, salicylaldehyde H-5), 9.43 (s, 1H, salicylaldehyde H-3); |
50.91 (50.79) | 2.79 (2.89) | 10.33 (10.49) | 5.91 (5.93) |
| 6f | 377 | 1611 (C N str.), 1463 (—N N—), 1109 (C—O str.), 837 (C—Br str.), 487 (Ni—N), 588 (Ni—O). | (DMSO-d6, 400 MHz): 2.37 (s, 3H, CH3), 7.57 (d, 1H, salicylaldehyde H-6), 7.32–7.68 (m, 10H, ArH), 7.71 (d, 1H, diazenylphenyl H-5), 7.77 (s, 1H, diazenylphenyl H-2), 8.22 (s, 1H, —CH N), 8.26 (s, 1H, Thiazolyl H-5), 8.63 (d, 1H, salicylaldehyde H-5), 9.63 (s, 1H, salicylaldehyde H-3). |
51.14 (51.29) | 2.80 (2.83) | 10.37 (10.41) | 5.94 (5.97) |
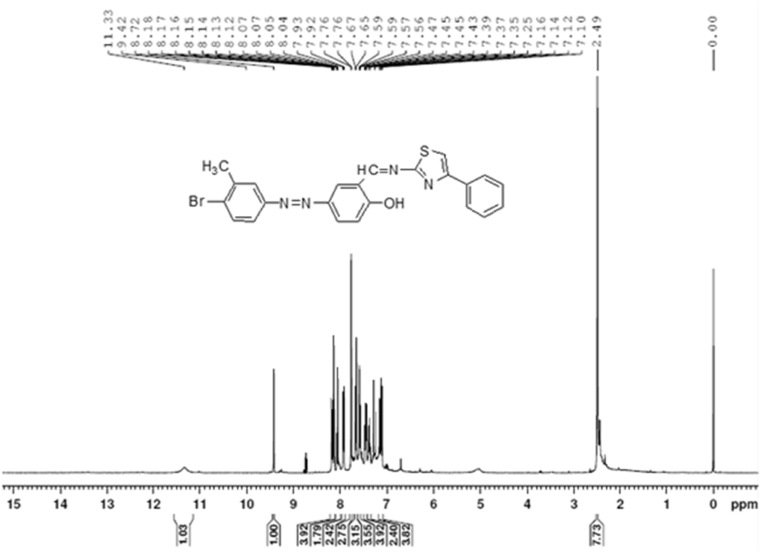
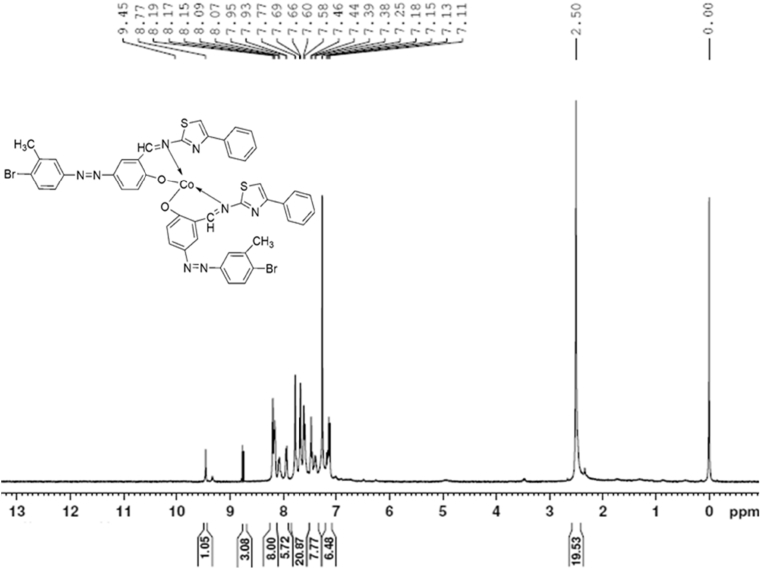
The 1H NMR spectra of 5a(Lig) and 6a are reported in Figure 2 and Figure 3, respectively. Singlet signals appeared at δ 11.34, 11.29, and 11.33 ppm in the spectra of 4e, 5a(Lig), and 5b(Lig) due to the presence of the phenolic —OH group of salicylaldehyde. None of the metal complexes showed signals corresponding to this phenolic —OH group, confirming the formation of metal complexes. Schiff bases 5a(Lig) and 5b(Lig) showed sharp singlet signals at δ 8.17 and 8.13 ppm, respectively, due to the azomethine group. The interaction of the nitrogen atom of the (—CH N–) group with the corresponding metal chlorides in all the complexes 6 (a–f) was responsible for the sharp singlet signals in the range of δ 8.17–8.23 ppm. The sharp singlet signals observed in the spectra of all the compounds, 4e, 5 (a–b), and 6(a–f), in the range of δ 2.39–2.45 ppm corresponded to methyl protons (Table 2).
Figure 2.
1H NMR spectra of 4-[(4-bromo-3-methylphenyl)diazenyl]-2-[(4-phenylthiazol-2-ylimino) methyl]phenol: 5a(Lig).
Figure 3.
1H NMR spectra of bis[{4-((4-bromo-3-methylphenyl)diazenyl)-2-((4-phenylthiazol-2-ylimino)methyl)phenoxy}]cobalt (6a).
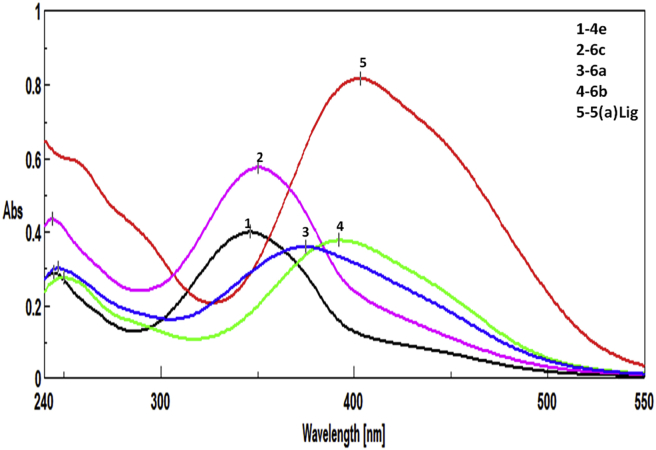
The λmax of all the synthesized molecules was studied on a UV–Vis spectrophotometer at concentrations of 10−5–10−6 M in ethanol. The Schiff base ligands and their metal complexes showed absorption bands in the range of 345–411 nm due to n−π* transitions indicating the presence of —N N— in all the molecules (Table 2). The solvatochromic effects of compounds 4e and 5a(Lig), and metal complexes 6(a–c) are illustrated in Figure 4. The predicted molecular weights of the synthesized compounds were confirmed by LC-MS (Table 1). Compound 5a(Lig) was predicted to have the molecular formula C23H17BrN4OS due to the molecular ion peak at 477.3 m/z (Figure 5). Elemental analysis of the synthesized molecules verified their molecular formulas.
Figure 4.
Solvatochromic effect of 4e, and 5a(Lig) and its metal complexes 6 (a–c) using ethanol.
Figure 5.
LCMS of 4-[(4-bromo-3-methylphenyl)diazenyl]-2-[(4-phenylthiazol-2-ylimino)methyl]phenol: 5a(Lig).
Structures of the Schiff base 5b(Lig) and its cobalt complex (6d) based on XRD analysis are shown in Figure 6, which reveals two strong and three moderate reflections shown by 5b(Lig) in the 2θ range of 30–80° (Figure 6a), whereas 6d showed five strong and three moderate reflections in the 2θ range of 18–80° (Figure 6b) corresponding to their inter-planar distances d = 7.03 and 2.82 A°, respectively.
Figure 6.
X-ray Diffraction pattern of (a): 4-[(4-bromo-3-methylphenyl)diazenyl]-2-[(4-(4-chlorophenyl)thiazol-2-ylimino)methyl]phenol 5b(Lig): and (b): bis[4-{(4-bromo-3-methylphenyl)diazenyl}-2-{(4-(4-chlorophenyl)thiazol-2-ylimino)methyl}phenoxy]cobalt (6d).
SEM analysis of 5b(Lig) and its cobalt complex 6d suggested that the particle size of 6d significantly differed from the particle size of 5b(Lig) due to the presence of an auxiliary molecule. The particle diameter of 5b(Lig) was 355.2 nm at an external angle of 360° (Figure 7a), whereas it was 366 nm at an external angle of 355.2° for its cobalt complex (Figure 7b).
Figure 7.
SEM micrograph of (a): 4-[(4-bromo-3-methylphenyl)diazenyl]-2-[(4-(4-chlorophenyl)thiazol-2-ylimino)methyl]phenol 5b(Lig): and (b): bis[4-{(4-bromo-3-methylphenyl)diazenyl}-2-{(4-(4-chlorophenyl)thiazol-2-ylimino)methyl}phenoxy]cobalt (6d).
The magnetic susceptibilities of the Co2+ complex were 5.09 and 5.11 BM, indicating an octahedral symmetry.13 The Ni2+ complex showed magnetic susceptibilities of 2.91 and 2.99 BM, whereas the Cu2+ complex showed magnetic susceptibilities of 2.01 and 1.93 BM, suggesting that they have an octahedral geometry. Compounds 5a(Lig) and 5b(Lig) act as bidentate ligands by chelating with transitional metal ions through the azomethine bearing nitrogen atom. Based on magnetic susceptibilities, the presumed structures of the complexes are shown in Scheme I.
All the compounds showed good antimicrobial activities (Table 3). Compound 4e, Schiff base 5a(Lig), and metal complexes 6a, 6b, and 6d showed significant antimicrobial activities against E. coli (res), K. pneumoniae, and S. aureus (res) in comparison to both the standards, amoxicillin (RA) and ampicillin (RA1). The cobalt–conjugated complex 6a showed significant (P < 0.05) antimicrobial activities against E. coli (res), K. pneumoniae, and S. aureus (res) with the highest mean zones of inhibition (16.67 ± 2.34, 16.17 ± 1.94, and 20.83 ± 1.84 mm, respectively). Similarly, the cobalt–conjugated complex 6d showed significant (P < 0.05) antimicrobial activities against E. coli (res), K. pneumoniae, and S. aureus (res) with mean zones of inhibition as 15.5 ± 3.21, 16.17 ± 2.99, and 16.83 ± 2.71 mm, respectively in comparison to both amoxicillin (RA) and ampicillin (RA1). The antibiograms of the synthesized molecules are shown in Figure 8. The antimicrobial activities of the synthesized molecules against E. coli (res), S. aureus (res), C. neoformans, and C. albicans in comparison to that of amoxicillin (RA) are graphically presented in Figure 9.
Table 3.
Antimicrobial activities of novel metal complexes of 2-amino-4-substituted phenylthiazole Schiff bases against different microbial strains (mean ± S.D.).
| Compounds |
Bacterial strain |
Fungal strain |
|||
|---|---|---|---|---|---|
| E. coli (res) | K. pneumoniae | S. aureus (res) | C. albicans | C. neoformans | |
| 4e | 15.83 ± 1.47∗ | 15.17 ± 2.14 | 16.17 ± 2.04∗ | 10.17 ± 1.84 | 12.5 ± 1.52 |
| 5a(Lig) | 13.67 ± 2.34 | 12.67 ± 2.25 | 15.83 ± 2.79∗ | 12.17 ± 2.23 | 13.33 ± 1.37 |
| 5b(Lig) | 15.33 ± 2.07 | 13.5 ± 2.43 | 11.5 ± 1.87 | 12 ± 1.27 | 12.33 ± 1.75 |
| 6a | 16.67 ± 2.34∗ | 16.17 ± 1.94∗ | 20.83 ± 1.84∗ | 12.83 ± 2.23 | 13.67 ± 2.94 |
| 6b | 15.83 ± 1.84∗ | 16 ± 2.37∗ | 18.33 ± 1.75∗ | 13.33 ± 2.34 | 12.67 ± 1.51 |
| 6c | – | 14.67 ± 3.14 | 14.83 ± 1.84 | 11.33 ± 1.51 | 15.83 ± 1.84 |
| 6d | 15.5 ± 3.21∗ | 16.17 ± 2.99∗ | 16.83 ± 2.71∗ | 12.67 ± 2.34 | 12.17 ± 2.71 |
| 6e | 14.83 ± 2.56 | 15.83 ± 2.23 | 14.5 ± 1.52 | 11.17 ± 6.21 | 10.5 ± 1.52 |
| 6f | – | 16.67 ± 1.37∗ | 15.17 ± 1.94 | 12.5 ± 1.52 | 10.33 ± 1.75 |
| RA | 12.67 ± 1.51 | 15.33 ± 1.97 | 13 ± 1.67 | 19.33 ± 4.68 | 24.17 ± 1.94 |
| RA1 | – | 15.09 ± 1.13 | – | ||
Results expressed in Mean ± S.D. (n = 6), The data were analysed by One Way ANOVA followed by Dunnett's Post Hoc test, (statistical significance at *p < 0.05 in comparison to reference antibiotic (RA): amoxicillin (antibacterial), fluconazole (antifungal) and RA1: ampicillin (antibacterial); -, no zone of inhibition.
Figure 8.
Antibiogram pattern of 2-amino-4-substituted phenylthiazole based Schiff base bearing metal complexes against S. aureus (res) (a) and C. neoformans (b).
Figure 9.
Error gram showing the statistical interpretation of antimicrobial activity of 2-amino-4-substituted phenylthiazole based Schiff base bearing metal complexes against (a) E. coli (res), (b) S. aureus (res), (c) C. albicans, and (d) C. neoformans.
Molecules 4e, 5a(Lig), 5b(Lig) 6a, 6b, and 6d inhibited the growth of E. coli (res), K. pneumoniae, and S. aureus (res) at an MIC of 31.25 μg/mL (Table 4). All the complexes showed potential antimicrobial activities against K. pneumoniae at 31.25 μg/mL, whereas Schiff base ligands 5a(Lig) and 5b(Lig) were active only at 125 μgmL−1.
Table 4.
Minimum inhibitory concentration (MIC, μg/mL) of novel metal complexes of 2-amino-4-substituted phenylthiazole Schiff bases against different microbial strains.
| Compounds |
Bacterial strain |
Fungal strain |
|||
|---|---|---|---|---|---|
| E. coli (res) | K. pneumoniae | S. aureus (res) | C. albicans | C. neoformans | |
| 4e | 31.25 | 31.25 | 31.25 | 500 | 250 |
| 5a(Lig) | 125 | 125 | 31.25 | 250 | 250 |
| 5b(Lig) | 31.25 | 125 | 250 | 250 | 250 |
| 6a | 31.25 | 31.25 | 31.25 | 31.25 | 125 |
| 6b | 31.25 | 31.25 | 31.25 | 125 | 250 |
| 6c | – | 62.5 | 62.5 | 250 | 31.25 |
| 6d | 31.25 | 31.25 | 31.25 | 250 | 250 |
| 6e | 62.5 | 31.25 | 62.5 | 250 | 250 |
| 6f | – | 31.25 | 31.25 | 125 | 250 |
–, no zone of inhibition; E. coli(res), Escherichia coli resistant; S. aureus(res), Staphylococcus aureus resistant; C. albicans, Candida albicans; C. neoformans, Cryptococcus neoformans.
The results of the oral acute toxicity study revealed no mortality, toxicity, and gross behavioural changes in the experimental rats. All the compounds are found to be safe up to a dose of 2000 mg/kg body weight.
The radical scavenging activities of the newly synthesized molecules are reported in Table 5. All the test compounds (4e-6f) showed 50% inhibition at concentrations of 72 ± 1.4, 56 ± 0.78, 52 ± 1.13, 36 ± 0.56, 48 ± 0.89, 40 ± 0.59, 38 ± 1.4, 46 ± 0.98, 37.3 ± 0.02, and 31 ± 0.70 μg/mL, respectively. Molecules 6a, 6c, and 6d showed significant radical scavenging activities (>61%) at a concentration of 50 μg/mL in comparison to standard BHT. However, the cobalt-conjugated complexes 6a and 6d showed significantly low IC50 values of 36 and 38 μg/mL, respectively. The antioxidant activities of the synthesized molecules showing % inhibition at different concentrations are graphically presented in Figure 10.
Table 5.
DPPH radical scavenging activities of novel metal complexes of 2-amino-4-substituted phenylthiazole Schiff bases.
| Conc. (μg/mL) |
10 |
30 |
50 |
70 |
IC50 |
|---|---|---|---|---|---|
| Compound | % Inhibition | ||||
| 4e | 17.96 ± 0.03 | 29.54 ± 0.05 | 33.64 ± 0.09 | 56.39 ± 0.08 | 72 ± 1.4 |
| 5a(Lig) | 27.99 ± 0.04 | 39.34 ± 0.02 | 49.09 ± 0.07 | 63.9 ± 0.05 | 56 ± 0.78 |
| 5b(Lig) | 19.26 ± 0.04 | 38.53 ± 0.09 | 48.33 ± 0.07 | 79.89 ± 0.06 | 52 ± 1.13 |
| 6a | 29.72 ± 0.07 | 43.32 ± 0.11 | 63.44 ± 0.07* | 83.02 ± 0.06 | 36 ± 0.56 |
| 6b | 28.43 ± 0.07 | 41.83 ± 0.02 | 51.41 ± 0.05 | 79.97 ± 0.06 | 48 ± 0.89 |
| 6c | 25.43 ± 0.08 | 39.65 ± 0.09 | 61.41 ± 0.04* | 83.11 ± 0.04 | 40 ± 0.59 |
| 6d | 36.53 ± 0.02* | 43.03 ± 0.04 | 61.97 ± 0.08* | 73.78 ± 0.07 | 38 ± 1.4 |
| 6e | 31.24 ± 0.06 | 41.31 ± 0.02 | 51.77 ± 0.08 | 79.89 ± 0.04 | 46 ± 0.98 |
| 6f | 39.73 ± 0.02* | 53.38 ± 0.03* | 63.38 ± 0.01* | 79.53 ± 0.02 | 37.3 ± 0.02 |
| BHT | 32.32 ± 0.01 | 49.54 ± 0.01 | 59.99 ± 0.10 | 87.74 ± 0.06 | 31 ± 0.70 |
All values are expressed as Mean ± S.D., (n = 3), The data were analysed by one-way ANOVA followed by Dunnett's post hoc test, (statistical significance at *p < 0.05 in comparison to standard).
Figure 10.
Antioxidant activities of metal complexes of 2-amino-4-substituted phenylthiazole Schiff bases and their graphical presentation.
Discussion
Weak absorption bands at ν 1481-1477 cm−1 in the FT/IR spectra of 5a(Lig) and 5b(Lig) confirm the existence of an —N N— group; the azomethine group preferentially forms a coordination bond with the metal ion, whereas the azo group is left free, as indicted by an unaltered —N N— band.8 The absence of a carbonyl stretching band of 4e at ν 1660 cm−1 in the spectra of 5a(Lig) and 5b(Lig) confirmed the formation of an azomethine moiety. The spectra of the metal complexes 6(a–f) derived from 5a and 5b showed moderately altered frequencies for the azomethine group in the range of ν 1614–1609 cm−1. Broad medium-intensity bands at 3155 and 3159 cm−1 observed in the spectra of Schiff bases 5a(Lig) and 5b(Lig), respectively, indicate intra-molecular hydrogen bonding between hydroxyl groups and the azomethine nitrogen.
Singlet peaks due to the phenolic —OH proton of salicylaldehyde were observed in the NMR spectra of Schiff bases 5a(Lig) and 5b(Lig) at δ 11.29 and 11.33 ppm, respectively; they were absent in the spectra of all the metal complexes 6(a–f) due to the deprotonation of the hydroxyl proton by the interaction with metal chlorides. Because the nitrogen atom of the —CH N— group formed coordinate bonds with metal ions, all the metal complexes 6(a–f) showed sharp singlets in the range of δ 8.17–8.23 ppm corresponding to the azomethine proton.
XRD analysis of cobalt complex (6d) revealed three moderate reflections at 20, 27, and 40°, which were not shown by 5b(Lig), verifying the conjugation of the metal (cobalt) with the Schiff base.
Complexes 6a and 6d exhibited better antibacterial activities against E. coli (res), K. pneumoniae, and S. aureus (res) than their respective Schiff bases due to the insertion of the Co2+ ion to the intermediate Schiff bases 5a(Lig) and 5b(Lig), respectively. The coordination of metals with ligands reduces the polarity and enhances the lipophilicity of the resulting molecules, increasing their permeation across the lipoidal cell membranes of microorganisms, causing increased inhibition of microbial growth.14
The variation in the DPPH scavenging capacity of the different molecules may be attributed to the substitution of the various metals to the Schiff base ligands, which increases the capacity to stabilize unpaired electrons, thereby scavenging free radicals.11 Our findings are consistent with previous reports that metal complexes of Schiff bases show improved antimicrobial and antioxidant activities. This study is a continuation of our earlier reported study.6
Conclusions
We previously showed that the complex bis[2-{(E)-(4-methoxyphenylimino)methyl}-4-{(E)-(3-nitrophenyl)diazenylphenoxy}cobalt], 5a(Co Lig2), exhibits significant antimicrobial activity.6 In the present study, we consistently observed that the complexes bis[{4-((4-bromo-3-methylphenyl)diazenyl)-2-((4-phenylthiazol-2-limino)methyl)phenoxy}]cobalt, 6a, and bis[4-{(4-bromo-3-methylphenyl)diazenyl}-2-{(4-(4-chlorophenyl)thiazol-2-ylimino)methyl}phenoxy]cobalt, 6d, exhibit significant biological activities. We reported earlier that the complex 5a(Co Lig2) exhibited significant antibacterial activity against K. pneumoniae. In the present study, the complexes 6a and 6d exhibited significant antibacterial activities against drug-resistant pathogens, E. coli (res), K. pneumonia, and S. aureus (res). In the previous study, all the complexes showed significant antifungal activities; however, none of the complexes in the present study exhibited a significant antifungal activity. Comparing the antimicrobial activities of the complexes in our earlier report with those of the complexes in the present study, we found that complexes bearing the 3-nitrophenylazosalicylaldehyde nucleus exhibit excellent antifungal activities, whereas those with the 4-bromo-3-methylphenylazosalicylaldehyde nucleus showed significant antibacterial activities against drug-resistant bacterial strains, despite both the Schiff base complexes bearing the azosalicylaldehyde nucleus. However, the molecules described in the present study also showed significant antioxidant activities. Based on our findings on the biological activities of previously and recently synthesized complexes, it can be concluded that chelating the transitional metal Co2+ with Schiff base ligands bearing an azosalicylaldehyde nucleus enhances their biological activities. Because the novel synthesized metal complexes of 2-amino-4-substituted phenylthiazole Schiff bases showed potential antimicrobial activities against drug-resistant pathogens and exhibited improved radical scavenging activities, they may be effective in the treatment of infections.
Conflict of interest
The authors have no conflict of interest.
Author contribution
The concept of the work was fabricated by SKP. The experimental work and the drafting of the manuscript was performed by JS. Both the authors equally contributed to this research.
Acknowledgements
The authors are thankful to the Principal and Chairman of Sri Jayadev College of Pharmaceutical Sciences and Dean of School of Pharmaceutical Sciences, Siksha ‘O’ Anusandhan University Bhubaneswar, India.
Footnotes
Peer review under responsibility of Taibah University.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jtumed.2017.10.007.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Sarigul M., Deveci P., Kose M., Arslan U., Dagi H.T., Kurtoglu M. New tridentate azo–azomethines and their copper (II) complexes: synthesis, solvent effect on tautomerism, electrochemical and biological studies. Mol Struc. 2015;1096:64–73. [Google Scholar]
- 2.Ejidike I.P., Ajibade P.A. Synthesis and in vitro anticancer, antibacterial, and antioxidant studies of unsymmetrical Schiff base derivatives of 4-[(1E)-N-(2-aminoethyl)ethanimidoyl]benzene-1,3-diol. Res Chem Intermed. 2016;42(8):6543–6555. [Google Scholar]
- 3.Pahlavani E., Kargar H., Rad N.S. A study on antitubercular and antimicrobial activity of isoniazid Derivative. Zahedan J Res Med Sci. 2015;17(7):1–4. [Google Scholar]
- 4.Ejiah F.N., Fasina T.M., Familoni O.B., Ogunsola F.T. Substituent effect on spectral and antimicrobial activity of Schiff bases derived from aminobenzoic acids. Adv Biol Chem. 2013;3:475–479. [Google Scholar]
- 5.Kulandasamy R., Adhikari A.V., Stables J.P. Synthesis and anticonvulsant activity of some new bishydrazones derived from3,4-dipropyloxythiophene. Eur J Med Chem. 2009;44:3672–3679. doi: 10.1016/j.ejmech.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Sahoo J., Parween G., Sahoo S., Mekap S.K., Sahoo S., Paidesetty S.K. Synthesis, spectral characterization, in silico and in vitro antimicrobial investigations of some Schiff base metal complexes derived from azo salicylaldehyde analogues. Ind J Chem. 2016;55B:1267–1276. [Google Scholar]
- 7.Rajavel P., Senthil M., Anitha C. Synthesis, physical characterization and biological activity of some Schiff bases complexes. E J Chem. 2008;5(3):620–626. [Google Scholar]
- 8.Ahmadi R.A., Amani S. Synthesis, spectroscopy, thermal analysis, magnetic properties and biological activity studies of Cu(II) and Co(II) complexes with Schiff base dye ligands. Molecules. 2012;17:6434–6448. doi: 10.3390/molecules17066434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joseph J., Nagashri K., Ayisha Bibin Rani G. Synthesis, characterization and antimicrobial activities of copper complexes derived from 4-aminoantipyrine derivatives. J Saudi Chem Soc. 2013;17(3):285–294. [Google Scholar]
- 10.Abu-Dief A.M., Mohamed I.M.A. A review on versatile applications of transition metal complexes incorporating Schiff bases. Benisuef Univ J Basic Appl Sci. 2015;4(11):119–133. doi: 10.1016/j.bjbas.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tadele K.T. Antioxidant activity of Schiff bases and their metal complexes: a recent review. J Pharm Med Res. 2017;3(1):73–77. [Google Scholar]
- 12.Desai N.C., Bhatt N., Hardik S., Trivedi A. Synthesis, antimicrobial and cytotoxic activities of some novel thiazole clubbed 1,3,4-oxadiazoles. Eur J Med Chem. 2013;67:54–59. doi: 10.1016/j.ejmech.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grare M., Mourer M., Fontanay S., Regnouf-de-Vains J.B., Finance C., Duval R.E. In vitro activity of para-guanidinoethylcalix [4] arene against susceptible and antibiotic-resistant Gram negative and Gram-positive bacteria. J Antimicrob Chemother. 2007;60:575–581. doi: 10.1093/jac/dkm244. [DOI] [PubMed] [Google Scholar]
- 14.Sahoo J., Paidesetty S.K. Antimicrobial activity of novel synthesized coumarin based transitional metal complexes. J Taibah Univ Med Sci. 2017;12(2):115–124. doi: 10.1016/j.jtumed.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aslan H.G., Ozcan S., Karacan N. Synthesis, characterization and antimicrobial activity of salicylaldehyde benzenesulfonylhydrazone (Hsalbsmh) and its Nickel(II), Palladium(II), Platinum(II), Copper(II), Cobalt(II) complexes. Inorg Chem Commun. 2011;14(9):1550–1553. [Google Scholar]
- 16.Sriram D., Yogeeswari P., Myneedu N.S., Saraswat V. Abacavir prodrugs: microwave-assisted synthesis and their evaluation of anti-HIV activities. Bioorg Med Chem Lett. 2006;16(8):2127–2129. doi: 10.1016/j.bmcl.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 17.El-Sherif A.A., Eldebss T.M.A. Synthesis, spectral characterization, solution equilibria, in vitro antibacterial and cytotoxic activities of Cu(II), Ni(II), Mn(II), Co(II) and Zn(II) complexes with Schiff base derived from 5-bromosalicylaldehyde and 2-aminomethylthiophene. Spectrochim Acta. 2011;79A:1803–1814. doi: 10.1016/j.saa.2011.05.062. [DOI] [PubMed] [Google Scholar]
- 18.Kalaivani P., Prabhakaran R., Poornima P., Dallemer F., Vijayalakshmi K., Padma V.V., et al. Versatile coordination behavior of salicylaldehydethiosemicarbazone in ruthenium(II) carbonyl Complexes: synthesis, spectral, X-ray, electrochemistry, DNA binding, cytotoxicity, and cellular uptake studies. Organometallics. 2012;31(23):8323–8332. [Google Scholar]
- 19.Caro A.A., Commissariat A., Dunn C., Kim H., Gracia S.L., Smith A., et al. Prooxidant and antioxidant properties of salicylaldehyde isonicotinoyl hydrazone iron chelators in HepG2 cells. Biochim Biophys Acta. 2015;1850(11):2256–2264. doi: 10.1016/j.bbagen.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Refat S.M., Abdel Y.E.S., Majid A.A. Cu(II), Co(II) and Ni(II) complexes of new Schiff base ligand: synthesis, thermal and spectroscopic characterizations. J Mol Str. 2013;1038:62–72. [Google Scholar]
- 21.Sahoo J., Paidesetty S.K. Biological evaluation and spectral characterization of 4-hydroxy coumarin analogues. J Taibah Univ Med Sci. 2015;10(3):306–319. [Google Scholar]
- 22.Sahu L., Jenaa S., Swain S.S., Sahoo S., Chand P.K. Agrobacterium rhizogenes-mediated transformation of a multi-medicinal herb, Boerhaavia diffusa L.: optimization of the process and anti-microbial activity against bacterial pathogens causing urinary tract infections. Front Life Sci. 2014:1–13. [Google Scholar]
- 23.McFarland J. Standardization of bacterial culture for disc diffusion assay. J Am Med Assoc. 1987;49:1176–1178. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.