Abstract
Objectives
The World Health Organization (WHO) has announced a pandemic alert following successive waves of H1N1 epidemics that have swept the world. KSA became vulnerable to influenza virus due to Hajj and Umrah pilgrimages. Antibodies have been tested to inhibit the attachment and spread of influenza viruses to epithelial cells. This study aimed to assess the immunological indices of the influenza vaccine, VAXIGRIP, in triggering humoural immunity in volunteers.
Methods
Sera from pre- and 14 days post-vaccinated subjects were analysed for haemagglutinin (HA)-specific anti-H1 and anti-H3 antibodies using ELISA. Expansion of CD19+ B cells was quantified using FACSCalibur.
Results
The VAXIGRIP vaccine induced specific anti-H1 and anti-H3 HA IgG antibodies against H1N1 and H3N2 influenza viruses, respectively. There was a significant increase in B cell numbers post-vaccination that directly matched the anti-H1 antibody titre. The results suggest that B cells are likely to be primed by the same antigenic strain derived from both H1N1 and H3N2 viruses, which were likely to be included in the vaccine.
Conclusion
Influenza vaccine triggered a humoural immune response to surface HA proteins in vaccinated subjects. To our knowledge, this is the first report corroborating the immunogenicity of the influenza vaccine in KSA volunteers. These results may be beneficial to the ministry of health and the Saudi FDA in terms of weighing the role of this vaccine in inducing protective immunity.
Keywords: CD19+ B cells, Flu vaccine, Haemagglutinin, IgG antibody, Vaxigrip
الملخص
أهداف البحث
أعلنت منظمة الصحة العالمية حالة التأهب الشاملة بعد موجات متتالية من وباء الإنفلونزا الذي اجتاح العالم. وأصبحت المملكة العربية السعودية عرضة لفيروس الإنفلونزا بسبب موسم الحج والعمرة. تم اختبار الأجسام المضادة لمنع اتصال وانتشار فيروسات الإنفلونزا للخلايا الظهارية. تهدف هذه الدراسة لتقييم المؤشرات المناعية للقاح الإنفلونزا ”فاكسيجريب“ لإثارة المناعة الخِلطية لدى المتطوعين.
طرق البحث
تم تحليل أمصال الدم قبل وبعد ١٤ يوما من تلقيح المشاركين للتأكد من وجود أجسام مضادة بطريقة الإليزا. وتم عد نمو الخلايا المناعية البائية CD19+ عن طريق تقنية التدفق الخلوي.
النتائج
أحدث لقاح الإنفلونزا قدرة على إثارة الجهاز المناعي ج لإنتاج أجسام مضادة لـ H1وكذلك H3 ضد فيروس الإنفلونزاH1N1 وH3N2 على التوالي. كان هناك زيادة ملحوظة من خلايا المناعة البائية بعد اللقاح والتي تتطابق مباشرة مع معدل الأجسام المضادة H1. وأظهرت النتائج أن الخلايا المناعة البائية من المحتمل أن تعمل بنفس سلالة المضاد المستمدة من كلا من فيروساتH1N1 وH3N2 والتي من المحتمل أن تكون متضمَنة في اللقاح.
الاستنتاجات
يسبب لقاح الإنفلونزا إستجابة مناعية خِلطية للبروتين السطحي لدى الأشخاص الملقحين. ويمكن أن يكون هذا التقرير الأول الذي يؤيد القدرة المناعية للقاح الإنفلونزا عند المتطوعين في السعودية. ومن الممكن أن تكون هذه النتائج مفيدة لوزارة الصحة وإدارة الغذاء والدواء السعودية من حيث أهمية دور هذا اللقاح في إحداث مناعة وقائية.
الكلمات المفتاحية: CD19+ الخلايا البائية, لقاح الإنفلونزا, هيماجلوتنين, الأجسام المضادة ج, فاكسيجريب
Introduction
Influenza viruses are accountable for influenza outbreaks that cause noteworthy burden and significant morbidity and mortality worldwide.1 The influenza virus causes annual epidemics and occasional pandemics that have threatened the lives of millions of people all over the world. This virus circulated in humans until 1957 when a new influenza pandemic, the ‘Asian Flu’, appeared and the influenza A/H2N2 virus substituted the H1N1 virus.2 A decade later, in 1968, the ‘Hong Kong’ influenza pandemic, caused by the influenza A/H3N2 subtype, emerged. In 1977, the influenza A virus of the H1N1 subtype resurfaced without causing a major pandemic. However, in 2009, a new influenza, A/H1N1 virus of swine-origin, caused the first influenza pandemic of the 21st century according to the World Health Organization (WHO).3, 4, 5, 6
The pandemic H1N1 influenza A virus (2009 H1N1) was identified as the cause of outbreaks of respiratory infection, mainly in younger age groups. The virus spread to over 214 countries, with more than 18,000 deaths reported worldwide. In the United States, the 2009 H1N1 virus infected an estimated 59 million people, resulting in 12,000 deaths5 (Accessed on 19.10.2016).
KSA also witnessed the H1N1 pandemic, with 15,850 laboratory-confirmed cases and 124 deaths in 2009 according to the Saudi Ministry of Health (MOH).7
Cough, fever, headache, runny nose and muscle pain were the most common symptoms. Common presenting symptoms were different based on the patient's setting (i.e., inpatients, outpatients, school outbreak, community cases, and others). Nevertheless, cough and fever were the most commonly reported symptoms in all settings.8
As a host to Umrah and Hajj pilgrims and expatriate workers in different sectors, KSA is threatened with the possibility of an influenza A (H1N1) epidemic. Thus, effective preventive measures are urgently needed to control the spread of the virus.
Prevention with vaccines would afford protection in the face of any unforeseen threat. Surface haemagglutinin (HA) is a major virulence factor crucial for virus binding to the host cell membrane and is essential for the induction of host protective immunity. HA-specific antibodies play key roles in protection against influenza.9, 10, 11, 12
The effectiveness of the vaccine is dependent upon enhancing the immune system against the serotypes comprised within it. As influenza viruses experience periodic alterations in their antigens, the vaccine is revised annually to prevent susceptibility.1
An injectable flu vaccine (VAXIGRIP) is currently being administered in KSA. However, its efficacy has not yet been studied. This prompted the investigator to examine the ability of VAXIGRIP to induce a specific antibody response by measuring the HA-specific antibody levels targeting influenza viruses. Further, B cell numbers were also analysed in the same volunteers before and after vaccination using flow cytometry. The results could help form a scientific opinion regarding the efficacy of the flu vaccine for the MOH and other authorities, such as the Saudi Food and Drug Administration (FDA).
Materials and Methods
Volunteers, flu vaccination and ethics statement
All volunteers were staff members of Taibah University (male section). They were academics and employees, aged 25–48 years. Volunteers went to the medical centre for an elective seasonal influenza vaccine. The Infection Control section of the medical centre performed the vaccination and extraction of blood samples from the volunteers, following informed consent. The study was conducted during the winter season (December 2015), and the timeline was four weeks. The flu vaccine (VAXIGRIP) is an inactivated influenza vaccine of trivalent types A and B (split virion) in suspension for injection intramuscularly or deep subcutaneously at 0.5 ml (Sanofi Pasteur, France). A total of 75 (90%) volunteers out of 83 completed the study. Ethical approval for the study protocol was obtained from Taibah University – College of Applied Medical Sciences – Ethical Committee (MLT 201501).
Collection of blood samples
Venous blood (5–7 ml) was collected pre- and 14 days post-vaccination from the volunteers (75 subjects). From one fraction of the blood, serum was separated and kept at −80 °C until used to perform the ELISA. The second blood to stain and numerate B lymphocytes portion was collected in EDTA tubes and processed for surface phenotyping of lymphocytes by flow cytometry.
Measurement of HA-specific antibody levels using ELISA
HA-specific IgG antibodies were analysed in the serum of volunteers using ELISA13, 14 with recombinant HA proteins of the influenza virus obtained from ATTC, USA. In brief, 96 well ELISA plates were coated with recombinant HA and then incubated overnight at 4 °C. After being washed, the plates were blocked, followed by incubation of the serum at optimal dilutions for 1.5 h. Alkaline phosphatase conjugated anti-human IgG (Sigma) was then added for 1.5 h. After the plates were washed, p-nitrophenyl phosphate (pNPP) was applied, and optical density (OD) was measured at 405 nm using an ELISA plate reader.
Flow cytometric estimation of B cells
Flow cytometry was performed according the manufacturer's standard protocol as follows. The whole blood collected in EDTA tubes was incubated with 5 μL of conjugated anti-CD19 antibody for 15 min in the dark, followed by FACS-lyse buffer for a further 15 min. After centrifugation (500 ×g, 5 min), the supernatant was aspirated, and the cells were harvested and washed, as described above. Following fixation, the cells were re-suspended in PBS, acquired using the flow cytometer, and analysed using WinMDI software.
Statistical analysis
Differences between antibody titres pre- and post-vaccination were analysed by analysis of variance and Student's t test. Association between two factors was analysed by Pearson's correlation. A P value of <0.05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism 5.0 software, USA.
Results
The influenza vaccine (VAXIGRIP) induces specific anti-H1 HA IgG antibodies against H1N1
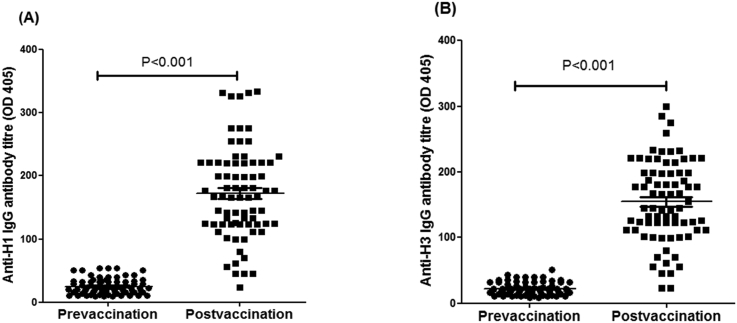
Serum was separated from pre- and 14 days post-vaccination subjects administered VAXIGRIP, and antibody levels were assayed using ELISA. The vast majority of vaccinated subjects (97%) were found to have developed significant levels of anti-HA-specific IgG antibody (P < 0.001) against the H1N1 virus (Figure 1A). Thus, the VAXIGRIP appears to lead to the development of antibodies fourteen days post vaccination. These antibodies are against the most virulent influenza surface protein, HA which plays a critical role in viral attachment to the sialic acid receptor on epithelial cells. Additionally, there was no age association among participants in terms of increasing immune response with age (data not shown).
Figure 1.
The influenza vaccine (VAXIGRIP) induces specific anti-H1 and anti-H3 HA IgG antibodies against H1N1 (A) and H3N2 (B). Significant levels of anti HA-specific IgG antibodies (P < 0.001) against H1N1 and H3N2 viruses were detected. Consequently, the vaccine appears to promote the development of antibodies when pre- and post-vaccination titrations tested using ELISA were compared.
The influenza vaccine (VAXIGRIP) induces specific anti-H3 of HA IgG antibodies against H3N2
In a parallel ELISA, significant levels of anti-H3 of HA IgG antibodies to influenza H3N2 were detected in serum samples of pre- and post-vaccinated subjects (P < 0.001) (Figure 1B). The results confirm that the VAXIGRIP vaccine induces a humoural immune response against the influenza surface protein, H3, which limits viral attachment and subsequent infection of the respiratory tract epithelial cells. Hence, the VAXIGRIP vaccine stimulates antibody production against the viral surface protein, HA, included in the vaccine.
The VAXIGRIP vaccine triggers the proliferation of B cells
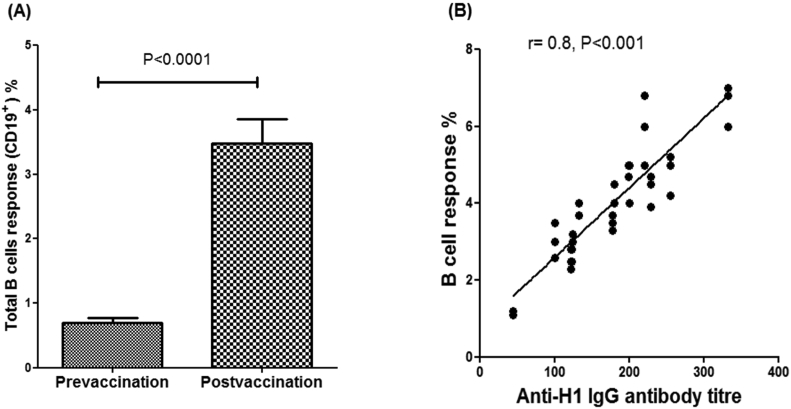
HA-specific B-lymphocytes were quantified in pre- and post-vaccination samples using FACS. Interestingly, the flu vaccine was found to stimulate one of the most important components of the humoural immune response, a significant increase in the total number of B cells (P < 0.0001) post-vaccination compared to the same paired subjects pre-vaccination (Figure 2A). Moreover, there was a significant positive correlation between B cell numbers and the anti-H1 antibody titre (P < 0.001) in the post-vaccination subjects (Figure 2B).
Figure 2.
The VAXIGRIP vaccine triggers the proliferation of total B cells. B-lymphocytes were quantified pre- and post-vaccination using FACS. Interestingly, the flu vaccine was found to stimulate a significant increase in the total number of B cells post-vaccination compared with pre-vaccination subjects (P < 0.0001). There was a significant positive correlation between B cell numbers and anti-H1 IgG antibody titres (P < 0.001) in the post-vaccination subjects.
Discussion and conclusion
Influenza viruses are accountable for influenza outbreaks that caused major global morbidity and mortality over the last several decades. The H1N1 strain of influenza viruses stirred a worldwide pandemic in 2009, infecting approximately 11–21% of the world's population and causing significant morbidity and mortality.15
This study showed a significant HA-specific IgG antibody response to the H1N1 virus in serum from individuals who were previously vaccinated with the VAXIGRIP flu vaccine. Moreover, the results showed an increased activation of B cells resulting from the VAXIGRIP flu vaccine, with the production of HA-specific IgG antibodies against not only H1N1 but also the H3N2 virus.
There was also a good correlation between the numbers of activated B cells and HA-specific antibodies in response to the VAXIGRIP vaccine, as shown in post-vaccination assays (Figure 2B).
The results suggest that B cells were likely to be primed by the same antigenic strains derived from both H1N1 and H3N2 viruses, which are likely included in the vaccine.
Additionally, the finding that the VAXIGRIP-activated B cell response was cross-reactive to H1N1 and H3N2 HAs is consistent with earlier studies valuing the cross-reactivity of serum antibodies in patients infected with the pH1N1 virus.9, 11
To our knowledge, this is the first report to validate the immunogenicity of the VAXIGRIP flu vaccine in KSA, targeting one of the most important components of the immune system in response to influenza viruses.
When compared with other countries, KSA encounters exclusive and challenging conditions because of the extraordinary annual heavy seasons of Hajj and Umrah pilgrims. Millions of people gather at the holy cities of Makkah and Almadinah Almunawwarah for a short of period of time each year, where influenza outbreaks are ordinarily encountered. Such challenges require the strict implementation of stringent vaccination strategies.1, 16
Despite many advances in viral therapeutics, vaccination is still the most cost-effective way to defend against the distressful clinical manifestations of influenza infections. HA-specific antibodies represent a vital and promising role in protection against influenza infections.17 Influenza vaccination triggers a humoural immune response to the surface HA protein in vaccinated subjects, as shown in this study.
Pooled estimates from observational studies indicate that the influenza vaccine is effective against laboratory-validated influenza in Hajj pilgrims.18
The compulsory vaccination is most necessary for the high-risk domestic pilgrims and health-care workers during the winter season (when a vaccine is available). Continuous surveillance of influenza and evaluation of the uptake and effectiveness of vaccination in Hajj pilgrims is required to inform a practically feasible policy.19
This is the first report corroborating the immunogenicity of the influenza vaccine in volunteers from KSA, where the results may be beneficial to both the MOH and Saudi FDA with regards to the efficacy of this vaccine. Further studies on T cells, secretory IgA and memory response seem logical and would require further investigations. Additionally, study of the functionality of secreted antibodies is important in terms of capability to neutralize the virus. A previous study showed that antibodies secreted in response to different influenza viruses have neutralized the viruses.14 Additionally, antibodies found in the serum of previously infected individuals with 1918 H1N1 were found to have the ability to neutralize the pandemic strain of the H1N1 influenza virus that appeared in 2009.20
Activation of B lymphocytes via flu vaccine will be important to induce and/or enhance the protective humoral response. The ability of flu vaccines to stimulate antibody responses will potentially warrant prevention or/and limitation of viral transmission among pilgrims. This study can provide supporting evidence for officials in KSA to carry out new regulations and encourage campaigns to support the Ministry of Health in making vaccination obligatory to pilgrims before performing Hajj and Umrah.
Recommendations
These results affect the health of millions of pilgrims as well as visitors to KSA and need to be considered on national and international levels. Due to the very small amounts of influenza vaccine effectiveness studies, I urge all of the relevant authorities in KSA, such as the Ministry of Hajj and the Ministry of Health, to support such an important subject. Additionally, further studies should be conducted to test the immunogenicity of the flu vaccine in inducing other immune components such as the T cell response and innate immunity. Finally, vaccine recipients must be followed up for any potential side effects following vaccination to assure that the vaccine is safe.
Conflict of interest
The author has no conflict of interest to declare.
Acknowledgments
I would like to acknowledge the following people from Taibah University Medical Centre for collaboration in giving the flu vaccines as well as collecting blood from volunteers: Mr Rami Aljuhani, Mr Mohammed Alsarrani and Mr Basim Alruhaily. In addition, I would like to thank all of the volunteers who generously contributed to the study.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Zeitouni M.O., Al Barrak A.M., Al-Moamary M.S., Alharbi N.S., Idrees M.M., Al Shimemeri A.A. The Saudi Thoracic Society guidelines for influenza vaccinations. Ann Thorac Med. 2015;10(4):223–230. doi: 10.4103/1817-1737.167065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kreijtz J.H., Fouchier R.A., Rimmelzwaan G.F. Immune responses to influenza virus infection. Virus Res. 2011;162(1–2):19–30. doi: 10.1016/j.virusres.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 3.Johnson N.P., Mueller J. Updating the accounts: global mortality of the 1918-1920 “Spanish” influenza pandemic. Bull Hist Med. 2002;76(1):105–115. doi: 10.1353/bhm.2002.0022. [DOI] [PubMed] [Google Scholar]
- 4.Simmons C.P., Bernasconi N.L., Suguitan A.L., Mills K., Ward J.M., Chau N.V. Prophylactic and therapeutic efficacy of human monoclonal antibodies against H5N1 influenza. PLoS Med. 2007;4(5):e178. doi: 10.1371/journal.pmed.0040178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO; [cited 2016 19/10]. Available from: http://www.who.int/foodsafety/fs_management/No_02_influenza_Apr09_en_rev1.pdf?ua=1.
- 6.Stohr K. Influenza–WHO cares. Lancet Infect Dis. 2002;2(9):517. doi: 10.1016/s1473-3099(02)00366-3. [DOI] [PubMed] [Google Scholar]
- 7.AlMazroa M.A., Memish Z.A., AlWadey A.M. Pandemic influenza A (H1N1) in Saudi Arabia: description of the first one hundred cases. Ann Saudi Med. 2010;30(1):11–14. doi: 10.4103/0256-4947.59366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khandaker G., Dierig A., Rashid H., King C., Heron L., Booy R. Systematic review of clinical and epidemiological features of the pandemic influenza A (H1N1) 2009. Influenza Other Respir Viruses. 2011;5(3):148–156. doi: 10.1111/j.1750-2659.2011.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corti D., Suguitan A.L., Jr., Pinna D., Silacci C., Fernandez-Rodriguez B.M., Vanzetta F. Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J Clin Invest. 2010;120(5):1663–1673. doi: 10.1172/JCI41902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hai R., Krammer F., Tan G.S., Pica N., Eggink D., Maamary J. Influenza viruses expressing chimeric hemagglutinins: globular head and stalk domains derived from different subtypes. J Virol. 2012;86(10):5774–5781. doi: 10.1128/JVI.00137-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pica N., Hai R., Krammer F., Wang T.T., Maamary J., Eggink D. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc Natl Acad Sci U S A. 2012;109(7):2573–2578. doi: 10.1073/pnas.1200039109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wrammert J., Smith K., Miller J., Langley W.A., Kokko K., Larsen C. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453(7195):667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed M.S., Jacques L.C., Mahallawi W., Ferrara F., Temperton N., Upile N. Cross-reactive immunity against influenza viruses in children and adults following 2009 pandemic H1N1 infection. Antivir Res. 2015;114:106–112. doi: 10.1016/j.antiviral.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Mahallawi W.H., Kasbekar A.V., McCormick M.S., Hoschler K., Temperton N., Leong S.C. Infection with 2009 H1N1 influenza virus primes for immunological memory in human nose-associated lymphoid tissue, offering cross-reactive immunity to H1N1 and avian H5N1 viruses. J Virol. 2013;87(10):5331–5339. doi: 10.1128/JVI.03547-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly H., Peck H.A., Laurie K.L., Wu P., Nishiura H., Cowling B.J. The age-specific cumulative incidence of infection with pandemic influenza H1N1 2009 was similar in various countries prior to vaccination. PLoS One. 2011;6(8):e21828. doi: 10.1371/journal.pone.0021828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balkhy H.H., Memish Z.A., Bafaqeer S., Almuneef M.A. Influenza a common viral infection among Hajj pilgrims: time for routine surveillance and vaccination. J Travel Med. 2004;11(2):82–86. doi: 10.2310/7060.2004.17027. [DOI] [PubMed] [Google Scholar]
- 17.Ekiert D.C., Bhabha G., Elsliger M.A., Friesen R.H., Jongeneelen M., Throsby M. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324(5924):246–251. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alqahtani A.S., Rashid H., Heywood A.E. Vaccinations against respiratory tract infections at Hajj. Clin Microbiol Infect. 2015;21(2):115–127. doi: 10.1016/j.cmi.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 19.Alfelali M., Alqahtani A.S., Barasheed O., Booy R., Rashid H. Mandating influenza vaccine for Hajj pilgrims. Lancet Infect Dis. 2016;16(6):633–634. doi: 10.1016/S1473-3099(16)30064-0. [DOI] [PubMed] [Google Scholar]
- 20.Krause J.C., Tumpey T.M., Huffman C.J., McGraw P.A., Pearce M.B., Tsibane T. Naturally occurring human monoclonal antibodies neutralize both 1918 and 2009 pandemic influenza A (H1N1) viruses. J Virol. 2010;84(6):3127–3130. doi: 10.1128/JVI.02184-09. [DOI] [PMC free article] [PubMed] [Google Scholar]