Abstract
Objectives
Many cross-sectional and prospective studies have shown that type 2 diabetes mellitus is a probable cause of non-alcoholic fatty liver disease (NAFLD) with fibrosis and cirrhosis. This research aimed to examine the plasma amino transaminase levels as biomarkers of NAFLD and their association with apoptosis markers (Fas and FasL) as well as the lipid profile in type II diabetic patients.
Methods
This cross-sectional comparative study included 120 type II diabetic and 100 non-diabetic patients, and their defined biomarkers were studied.
Results
The results showed that the mean ALT levels, FasL and triglyceride/high density lipoprotein (TG/HDL) ratio were significantly higher in patients with type II diabetics. According to the Atherogenic Index of Plasma (Log TG/HDL), approximately 45% of diabetic patients had a high risk and 11% had an intermediate risk of developing cardiovascular disease. Alanine aminotransferase (ALT) was significantly and positively correlated with FasL, TG, glucose levels and body mass index (BMI) in diabetic patients. Moreover, TG was positively correlated with blood glucose levels and BMI, whereas HDL was negatively correlated with FasL and ALT.
Conclusion
The results of this study showed that in diabetic patients, elevated ALT levels and FasL may play a role in the risk of developing liver disease and could be used as a distinct marker of NAFLD, indicating liver injury. Moreover, atherogenic dyslipidaemia is a prominent feature in type II diabetes mellitus. Low HDL-c is closely associated with hypertriglyceridemia with an increased risk of cardiovascular disease and NAFLD in diabetics.
Keywords: Aminotransaminases, Apoptosis, Hyperglycaemia, Lipid profile, Type II diabetes mellitus
الملخص
أهداف البحث
أظهرت العديد من الدراسات المقطعية والمستقبلية علاقة داء السكري من النوع الثاني كمسبب محتمل للإصابة بمرض الكبد الدهني غير الكحولي المصحوب بالتليف والتشمع. يهدف البحث إلى دراسة مستويات الترانساميناسات الأمينية في البلازما كمؤشرات حيوية في مرض الكبد الدهني غير الكحولي وعلاقتها بكل من مؤشرات موت الخلايا المبرمج ( فاس و فاس لجن ) ومستوى الدهون في الدم في مرضى داء السكري من النوع الثاني.
طرق البحث
شملت هذه الدراسة المقطعية المقارنة ١٢٠ من مرضى داء السكري من النوع الثاني و١٠٠ مريض لا يعانون من داء السكري٬ وتمت دراسة مؤشراتهم الحيوية المُعرَّفة.
النتائج
أظهرت النتائج بأن متوسط مستويات ألانين أماينوترانسفريز والفاس لجن ونسبة الدهون الثلاثية إلى البروتين الدهني عالي الكثافة٬ كان أعلى بدرجة واضحة في مرضى داء السكري من النوع الثاني. واعتمادا على مؤشر التصلب في البلازما (لوغارثم نسبة الدهون الثلاثية إلى البروتين الدهني عالي الكثافة)٬ فإن ما يقارب ٤٥٪ من مرضى داء السكري كانوا في خطورة عالية، و١١٪ كانوا في خطورة متوسطة للإصابة بأمراض القلب والأوعية الدموية. كان مستوى ألانين أماينوترانسفريز مرتبط بشكل واضح وإيجابي مع مستويات كل من الفاس لجن والدهون الثلاثية والجلوكوز ومؤشر كتلة الجسم في مرضى داء السكري. إضافة إلى ذلك فإن مستوى الدهون الثلاثية كان مرتبطا بصورة إيجابية مع كل من مستوى الجلوكوز ومؤشر كتلة الجسم في حين ارتبط البروتين الدهني عالي الكثافة بصورة سلبية مع مستوى كل من الفاس لجن وألانين أماينوترانسفريز.
الاستنتاجات
أوضحت نتائج هذه الدراسة بأنه في مرضى داء السكري فإن ارتفاع مستويات ألانين أماينوترانسفريز والفاس لجن قد يلعبان دورا في خطورة الإصابة بأمراض الكبد ويمكن استخدامهما كمؤشر مستقل لمرض الكبد الدهني غير الكحولي مشيرا إلى إصابة الكبد. إضافة إلى ذلك فإن اضطراب دهون الدم المسببة لتصلب الشرايين هو خاصية بارزة في داء السكري من النوع الثاني. يرتبط انخفاض مستوى البروتين الدهني عالي الكثافة وارتفاع مستوى الدهون الثلاثية ارتباطا وثيقا بزيادة خطورة الإصابة بكل من أمراض القلب والأوعية الدموية ومرض الكبد الدهني غير الكحولي عند مرضى داء السكري.
الكلمات المفتاحية: الترانساميناسات الأمينية, موت الخلايا المبرمج, ارتفاع السكر في الدم, مستوى الدهون في الدم, داء السكري من النوع الثاني
Introduction
Over the next 10–20 years, the expansion of diabetes is estimated to reach pandemic proportions. According to the World Health Organization, KSA is considered to have the seventh highest rate of diabetes in the world and the second highest in the Middle East.1
The damaging effect of diabetes mellitus on the retinal, renal, nervous and cardiovascular systems is known, but its influence on the liver has not been firmly established.2 Recently, it has been recognized that hepatic dysfunction in obesity is associated with oxidative stress and steatosis accompanying insulin resistance. The resulting consequence is non-alcoholic fatty liver disease, a familiar spectrum of disease extending from simple steatosis (fatty infiltration) to inflammatory steatohepatitis to possible long-term injury (fibrosis and cirrhosis) and subsequent liver failure.3
Type 2 diabetes is a risk factor for the development of non-alcoholic fatty liver disease (NAFLD) with fibrosis and cirrhosis. It is important to note that recognizing type 2 diabetic patients who are at risk of acquiring cirrhosis and hepatocellular carcinoma is very difficult. ALT is the liver enzyme that is most associated with liver fat accumulation,4 and therefore, ALT has been applied as an indicator of NAFLD. Some cross-sectional studies have noticed that ALT is connected to multiple characteristics of metabolic syndrome. Currently, ALT is frequently applied in epidemiological studies as a substitute indicator for NAFLD.5 Regularly accessible laboratory analysis has been established to be insufficient, and a variety of scoring systems constructed on clinical and laboratory considerations have been suggested, but have not been confirmed as satisfactorily consistent in assessing individual patients.6 It is well documented that the presence of insulin metabolic syndrome (diabetes mellitus, obesity, dyslipidaemia and hypertension) is associated with a possibly progressive, severe liver disease. Visceral adipose tissue, which releases adipokines is a predictor of altered liver function and insulin resistance. Free fatty acid (FFA) lipolysis from visceral adipocytes pass through the liver, and because of insulin resistance, the oxidative capacity is reduced, which leads to overburdened hepatocytes with fat and hepatic triglyceride accumulation.7 Necrosis or apoptosis are damaged hepatocyte responses. The physiological way to remove injured cells is apoptosis through the Fas–FasL mechanism.8
The present study aims to examine the plasma level of the aminotransaminases (ALT and AST) as markers of non-alcoholic fatty liver disease (NAFLD) and the levels of both Fas & FasL as markers of apoptosis as well as their association with type II diabetic Saudi female patients compared to healthy females.
Materials and Methods
One-hundred-twenty patients with type 2 diabetes mellitus matched for age and sex with one-hundred normal control subjects enrolled from Prince Maged Ben Abdel Aziz diabetic centre – Almadinah Almunawwarah, S.A. were studied in 2015. The exclusion criteria for both diabetic and control subjects were the following:
-
•
Hypertension (defined as blood pressure greater than 150/90 mmHg),
-
•
Hypercholesterolaemia (defined as LDL-cholesterol 75 percentile for age and sex),
-
•
Tobacco use within the past 5 years,
-
•
Current use of insulin, anti-oxidants or hormone replacement therapy
-
•
Laboratory evidence of renal, hepatic, or haematological abnormalities.
-
•
Alcohol use
Consent was obtained from all patients before the research was conducted.
The two groups were subjected to the following laboratory investigations:
-
1)
Estimation of glycated haemoglobin A1C (HbA1C)%
-
2)
Serum glucose concentration determination.
-
3)
Serum liver enzyme ALT and AST concentration determination.
-
4)
Levels of Fas and FasL determination.
-
5)
Serum lipid profile [Low-density lipoprotein (LDL), High-density lipoprotein (HDL), Triglycerides (TG) and total cholesterol] concentration determination.
-
6)
Serum kidney function tests and electrolytes.
Blood collection
Five-millilitre blood samples were collected in plain and EDTA tubes, and whole blood from the EDTA tubes was used for HbA1c% estimation. Then, serum separation was performed for each sample using a centrifuge at 3000 rpm for 5 min to estimate the liver enzymes ALT and AST as well as the lipid profile [LDL, HDL, TG and cholesterol] on the same day of sampling. Additionally, estimation of fasting blood glucose was conducted, as well as kidney function tests and Major minerals. All of the tests were performed using the clinical chemistry automated machine Dimension X Pand, Siemens Healthcare Diagnostics Ltd. (Frimley, Camberley, UK). Kits, sampling, reagent delivery, mixing, processing and printing of results were automatically performed by the Dimension® System. The rest of the samples were kept at −20 for Fas and FasL analyses. Fas and FasL were determined using Fas enzyme-linked immunosorbent assay (ELISA) kit (Sigma–Aldrich, Saint Louis, U.S.A.), with a minimum detectable dose of 5 and 2 pg/ml in serum, respectively. All procedures were performed at room temperature according to the manufacturer's instructions. Each sample and standard protein were assayed in duplicate. The optical density at 450 nm for FasL and Fas was measured with a spectrophotometric microtiter plate reader (Labsystems iEMS Reader, Helsinki, Finland). A standard curve obtained with FasL or Fas samples provided with the kit was used to determine FasL and Fas in each sample.
Statistical analysis
All of the obtained data were subjected to statistical analysis using SPSS for Windows (version 20, SPSS, Chicago, IL, USA). Data are presented as the means ± standard deviation for continuous variables. Student's t test was used for comparison of the baseline continuous characteristics between the two groups (control and type 2 diabetes). The correlations were found using the Spearman correlation. Differences between groups, control group, controlled diabetics and uncontrolled diabetics, were tested using one way analysis of variance (ANOVA) test; p < 0.05 was considered to be significant. The studied population was divided into six BMI ranges according to the WHO categorization (<18.5, 18.5–25, 25–30, 30–35, 35–40, and >40 kg/m2).
Results
A total of 120 female diabetic patients with a mean age of 53.72 ± 9.82 years old and mean duration of disease of 7.41 ± 6.01 years were included in the final analysis. They were compared to 100 healthy control subjects with a mean age of 47.25 ± 10.15, p = 0.36. The prevalence of metabolic syndrome was 23.5% among diabetic patients, which was determined by using the National Cholesterol Education Program, Adult Treatment Panel III (2002 panel guidelines), in which metabolic syndrome was defined by the presence of three or more of the following components: abdominal obesity, hypertriglyceridemia, low HDL-C, high blood pressure, and high fasting glucose. The results of this study showed that the ALT levels were significantly higher in type II diabetics (39.35 ± 12.02) compared to the non-diabetic healthy control group (34.38 ± 7.3), p < 0.001 (Table 1). Our results revealed a significant difference in the levels of FasL between patients and the control group (179.36 ± 20.34 vs. 153.90 ± 21.58) (p < 0.01). The results also showed that the TG concentration significantly increased in the type II diabetic group (1.82 ± 1.06) compared with the control group (0.71 ± 0.42), p < 0.001. The serum high-density lipoprotein (HDL) levels decreased significantly in type II diabetics compared to the control group (1.16 ± 0.64 vs. 1.55 ± 0.39), (p < 0.001) (Table 1, Table 2). The TG/HDL ratio significantly increased in diabetic patients (1.84 ± 1.54) compared to the control group (0.52 ± 0.47) (p < 0.001) (Table 1). The Atherogenic Index of Plasma (AIP = Log TG/HDL) predicts cardiovascular risk, for which AIP < 0.11 indicates low risk, AIP (0.11–0.21) indicates intermediate risk and AIP > 0.21 indicates increased risk. In this study, 45% of our diabetic patients had and increased risk and 11% had an intermediate risk. Na was significantly lower in diabetic patients compared to controls (139.6 ± 4.3 vs. 142.71 ± 3.42, p < 0.001) (Table 1). Correction for the hyperglycaemia effect on Na levels was applied. Thus, to obtain the “true” sodium level in cases of extreme hyperglycaemia, the addition of 2.4 mmol/L to the measured concentration for every 100 mg/dl increment in plasma glucose above normal levels was required.9 There were no significant difference in the levels of Fas, AST, Cl, K, Ca, BUN, creatinine and uric acid in type II diabetics compared to the non-diabetic healthy control group (Table 1, Table 2).
Table 1.
The mean concentrations ± standard deviation of Anthropometric data and biochemical data as lipid profile (LDL, HDL, cholesterol and triglyceride, mmol/L, TG/HDL ratio), aminotransferase (ALT and AST, U/L), apoptosis marker (Fas & FasL pg/ml), glycaemic status (fasting glucose mmol/L & glycated Hb%), kidney function tests and Minerals in the control group and type II diabetic patients.
| Variables | Control n = 100 Mean ± SD |
Patients n = 120 Mean ± SD |
p values |
|---|---|---|---|
| BMI kg/m2 | 23.01 ± 5.19 | 32.9 ± 6.52 | p < 0.01 |
| Duration of the disease/years | – | 7.41 ± 6.01 | – |
| Age/years | 47.25 ± 10.15 | 53.72 ± 9.82 | p = 0.360 |
| LDL mmol/L | 2.66 ± 0.63 | 2.58 ± 0.90 | p = 0.823 |
| HDL mmol/L | 1.55 ± 0.39 | 1.16 ± 0.64 | p < 0.001 |
| Cholesterol mmol/L | 4.58 ± 0.84 | 4.57 ± 1.15 | p = 0.541 |
| Triglycerides mmol/L | 0.71 ± 0.42 | 1.82 ± 1.06 | p < 0.001 |
| TG/HDL-atherogenic dyslipidaemia | 0.52 ± 0.47 | 1.84 ± 1.54 | p < 0.001 |
| Glucose mmol/L | 5.16 ± 0.41 | 9.77 ± 4.03 | p < 0.001 |
| ALT U/L | 34.38 ± 7.3 | 39.35 ± 12.02 | p < 0.001 |
| AST U/L | 20.78 ± 5.56 | 22.51 ± 9.67 | p = 0.154 |
| Glycated Hb % | – | 8.82 ± 1.95 | – |
| Fasting glucose | 5.23 ± 0.73 | 9.66 ± 3.8 | p < 0.001 |
| Na (mmol/L) | 142.71 ± 3.42 | 139.6 ± 4.30 | p < 0.001 |
| Cl (mmol/L) | 103.53 ± 2.22 | 101.77 ± 11.7 | p = 0.065 |
| K (mmol/L) | 4.2 ± 0.38 | 4.15 ± 0.42 | p = 0.299 |
| Ca (mmol/L) | 2.25 ± 0.09 | 2.27 ± 0.100 | p = 0.592 |
| BUN (mmol/L) | 3.79 ± 0.84 | 4.69 ± 3.6 | p = 0.160 |
| Creatinine (μmol/L) | 69.87 ± 10.54 | 72.53 ± 26.70 | p = 0.193 |
| Uric acid (μmol/L) | 231.28 ± 51.43 | 238.29 ± 104.58 | p = 0.59 |
| Fas (pg/ml) | 145.14 ± 23.79 (n = 35) | 149.80 ± 24.41 (n = 40) | p = 0.508 |
| FasL (pg/ml) | 153.90 ± 21.58 (n = 35) | 179.36 ± 20.34 (n = 40) | P < 0.01 |
p < 0.05 consider significance.
Table 2.
The difference in the mean between the three studied groups: Control group, Controlled diabetics and uncontrolled diabetics in BMI, ALT, glucose, triglycerides, HDL, Na and FasL. Using ANOVA test, the mean difference between control and diabetic groups,*p < 0.05, **p < 0.001 and between diabetics groups, ■p < 0.05, ■■p < 0.01.
| Biochemical parameters | Control group n = 100 (mean ± SD) |
Controlled diabetics n = 18 (mean ± SD) |
Uncontrolled diabetics n = 102 (mean ± SD) |
|---|---|---|---|
| BMI | 23.01 ± 5.19 | 30.36 ± 8.37** | 31.37 ± 6.4** |
| ALT | 34.38 ± 7.3 | 35.94 ± 7.11■■ | 39.96 ± 12.63■■** |
| Glucose | 5.23 ± 0.73 | 5.56 ± 0.75■■ | 10.52 ± 3.91■■** |
| Triglycerides | 0.71 ± 0.42 | 1.33 ± 0.54■* | 1.92 ± 1.11■** |
| HDL | 1.56 ± 0.40 | 1.13 ± 0.34** | 1.16 ± 0.68** |
| Na | 142.71 ± 3.42 | 140.42 ± 1.62 | 139.48 ± 4.60** |
| FasL | 153.90 ± 21.58 (n = 35) | 177.16 ± 20.49 (n = 6)** | 189.26 ± 17.88 (n = 34)** |
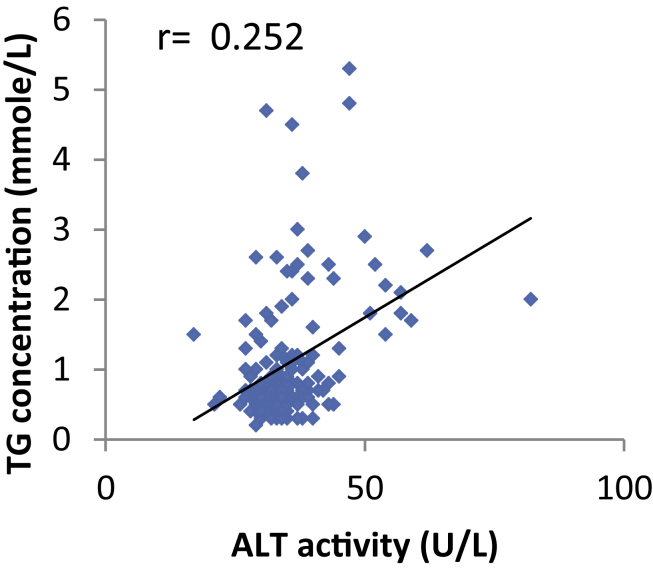
Regarding the correlation between variables, the results show that in type II diabetic patients, there was a significant positive correlation found between ALT and FasL, TG, glucose levels, BMI. A significant negative correlation was found between HDL and FasL, ALT, TG, glucose levels, and BMI. Additionally, a significant negative correlation was found in diabetic patients between Na and ALT, TG, glucose levels, and glycated Hb% (Figure 1, Figure 2).
Figure 1.
The correlation between Alanine aminotransferase (ALT) and FasL in diabetic patients was a significant positive correlation (r = 0.385∗∗, p = 0.006).
Figure 2.
The correlation between TG concentration and ALT activity (U/L) in diabetic patients was a significant positive correlation (r = 0.252, p < 0.001).
Analysis of the biochemical parameters using ANOVA revealed a significant difference between the means of the three groups, control subjects, controlled diabetics and uncontrolled diabetics, for ALT (p < 0.001) and triglycerides (P < 0.05); between the control and diabetic groups for HDL (p < 0.001); and between the control subjects and uncontrolled diabetic group as well as between the two diabetic groups for glucose (p < 0.001). The FasL levels were different in both diabetics and control subjects (p < 0.001). Additionally, the Na levels were different in uncontrolled diabetics and control subjects (p < 0.001). The results show significant differences in the ALT, TG, HDL, FasL, Na and glucose mean levels in the three studied groups. In multivariate analysis, outpatients who were older, had low high density lipoprotein and showed high blood glucose levels displayed the most significant clinical explanatory variables associated with abnormal ALT.
There was a significant difference between patients with abnormal ALT and normal ALT in glucose concentration, HDL and Age, Wilk's λ = 0.883, F (3, 107) = 4.714, p = 0.004 partial η2 = 0.117. A separate ANOVA was conducted for each dependent variable, with each ANOVA evaluated at an alpha level of 0.025. There was a significant difference between patients with abnormal ALT and normal ALT as a function of age, F (1–109) = 7.857, mean-square error (MSE) = 84.914, p < 0.006, partial η2 = 0.067 (6.7%), and observed Power = 0.793. Patients with elevated ALT (Mean = 55.54 years) scored higher than normal ALT patients (Mean = 50.51 years). There was no significant difference between abnormal ALT patients and normal ALT patients in glucose levels, F (1–109) = 4.250, MSE = 16.052, p < 0.042, Partial η2 = 0.036 (3.6%), observed Power = 0.533. Similarly, the ALT levels in diabetic patient differed insignificantly as a function of the HDL levels, F (1–109) = 5.056, MSE = 0.085, p < 0.027, Partial η2 = 0.044 (4.4%), and Observed Power = 0.606.
Regarding BMI in the diabetic group, the mean value of body mass index (BMI) was 32.9 ± 6.52 kg/m2. More than 50% of patients were observed to be obese, with a BMI over 30% in the uncontrolled diabetics, and approximately 42% of controlled diabetics had a BMI over 30%.
Discussion
Elevated ALT and AST were observed in 4.5% and 5.3% of our diabetic patients, respectively. However, the mean values were within the normal range. At least 50% of patients with type 2 diabetes had NAFLD. This result is in agreement with that of the study by Erbey and his co-worker, which reported that the prevalence of elevated ALT levels among U.S. type 2 diabetics was 7.8%, and this prevalence was higher among obese (BMI > 25 kg/m2) compared to non-obese diabetics (10.6% vs. 6.6%).10 In support of our finding regarding ALT elevation in type II diabetic female patients, the study by Chen et al. found that 3245 adults suffering from NAFLD had elevated ALT, obesity, diabetes mellitus, hypercholesterolemia.11 Previous papers have revealed that the prevalence of NAFLD and its development to fibrosis and cirrhosis rises with age.12 Our results are in accordance with those of another study that reported that elevated ALT levels were significantly related to a BMI >25 kg/m2 among type 2 diabetics in a study conducted in India. A positive correlation was also reported between glycaemic control (FBS and PPBS) and the duration of diabetes mellitus with ALT levels.13
Our results revealed that there was a significant increase in FasL in diabetic patients, which indicated an increase in the rate of apoptosis, similar to another study that revealed increasing β-cell apoptosis in type II diabetes mellitus.14 It is known that an increasing rate of apoptosis in β-cells is a trigger of type II diabetes mellitus.15 Additionally, in diabetes, reactive oxygen species stimulate the expression of FasL on hepatocytes, which bind to its receptor (Fas) on the neighbouring hepatocyte and accelerate apoptosis.16 The association of a microsatellite in FASL to type II diabetes and of the FAS-670G > A genotype to insulin resistance was found in another study.15 The non-significant increase in the levels of Fas in our results could be explained by the fact that the Fas receptor is not expressed normally by Islet β-cells and the FasL is expressed normally.17 Moreover, the expression of the Fas receptor is an initial measurable indicator of apoptosis compared to FasL, and most of the patients in our research had diabetes for a long duration and a high BMI.
A positive correlation existed between FasL and ALT in our study, as was previously reported by another study in which the correlation between ALT and the stimulation of FasL intrahepatic mRNA was observed.18
The prevalence of hypertriglyceridemia in our study in type 2 diabetic patients was 36% and of low HDL was 41%. A similar finding was reported for most diabetic patients with NAFLD. A net retaining of lipids within hepatocytes, generally in the form of triglycerides, is a requirement for the occurrence of NAFLD. The abnormal blood lipid component is most often associated with obesity and insulin resistance.19 In our investigation, the Triglyceride: HDL ratio (atherogenic dyslipidaemia) significantly increased in diabetic patients compared with the control group. Forty-five percent of our diabetic patients had an increased risk, and 11% had an intermediate risk of cardiovascular disease according to the Atherogenic Index of Plasma (Log TG/HDL), which predicts cardiovascular risk. The atherogenic lipid profile is often found in patients with type 2 diabetes, which leads to significant increases in the risk of coronary heart disease (CHD) in diabetic patients compared with people without diabetes. The greatest difference between people with and without diabetes in lipid profile occurs in triglycerides and high-density lipoprotein cholesterol (HDL). HDL tends to be lower in patients with diabetes, whereas triglycerides markedly increase. However, the atherogenic form of LDL is more likely to be found in patients with type 2 diabetes than people without diabetes. Additionally, low HDL levels restrict reverse cholesterol transport, which is usually associated with diabetes and may also be associated with increased lipid oxidation.20
Regarding the hyponatremia noticed in this study, diabetes is considered to be a disorder with an increased electrolyte imbalance because of the influences of impaired renal function, acid-base disorders, malabsorption syndromes and multidrug treatments (which often exists in diabetics).21 Osmotic diuresis frequently occurs in uncontrolled DM and may additionally cause hypovolemic-hyponatremia. Forced urinary electrolyte losses and aggravation of renal sodium loss occurs in diabetic ketoacidosis as a result of ketone bodies (b-hydroxybutyrate and acetoacetate).22 The changed metabolism of vasopressin, communication between vasopressin and insulin (both hormones work in the renal collecting duct), and reabsorption of more hypotonic fluid due to delayed stomach emptying have been suggested as likely important mechanisms of this association.23
Conclusion
ALT and FasL were the variables that were most strongly associated with diabetes mellitus. NAFLD is the most common cause of a mild elevation of serum ALT and also the most prevalent liver disease in type 2 diabetes. The Triglyceride:HDL ratio (atherogenic dyslipidaemia) significantly increased in diabetic patients. This ratio was most related to diabetic patients with older age, long duration of the disease and increased glucose levels. These data support a possible contribution of hyperglycaemia (glycaemic status) in type 2 diabetics to the pathogenesis of hypertriglyceridemia and atherogenic dyslipidaemia. Additionally, he ALT and FasL levels play a role in the risk of developing liver disease and can be used as markers for NAFLD.
Therapy to inhibit apoptosis may be important in treating type II diabetes, especially its complications.
Recommendations
Based on this research, it is recommended to determine the levels of ALT and FasL in type 2 diabetes patients because they are good indicators of NAFLD. Developing a treatment to increase B-cell apoptosis in patients with type 2 diabetes may slow progression to NAFLD.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Authors' contributions
DH conceived and designed the study, is responsible for the practical part, provided research materials and helped to write the scientific publication. DL analysed and interpreted data, provided the samples for the research, and helped to write the scientific publication. DAA coordinated the plan of the project and participated in collecting the scientific material. DA participated in collecting the scientific material and helped to write the scientific publication. All of the authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Acknowledgments
This work was supported by the project N°. 4012/1435 from Deanship of Scientific-Research; Taibah-University Almadinah Almunawwarah, KSA. Additionally, we thank the staff of the Prince Maged Ben Abdl Aziz diabetic centre – Almadinah Almunawwarah, KSA for their support of this study.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.IDF Diabetes Atlas, Sixth Edition. Online version of IDF Diabetes Atlas; 2013. Available: http://www.idf.org/diabetesatlas. ISBN: 2-930229-85-3.
- 2.Orasanu G., Plutzky J. The pathologic continuum of diabetic vascular disease. J Am Coll Cardiol. 2009;53:S35–S42. doi: 10.1016/j.jacc.2008.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ong J.P., Pitts A., Younossi Z.M. Increased overall mortality and liver-related mortality in nonalcoholic fatty liver disease. J Hepatol. 2008;49:608–612. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Westerbacka J., Corner A., Tiikkainen M. Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: implications for sex differences in markers of cardiovascular risk. Diabetologia. 2004;47:1360–1369. doi: 10.1007/s00125-004-1460-1. [DOI] [PubMed] [Google Scholar]
- 5.Hanley A.J., Williams K., Festa A. Elevations in markers of liver injury and risk of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2004;53:2623–2632. doi: 10.2337/diabetes.53.10.2623. [DOI] [PubMed] [Google Scholar]
- 6.Wieckowska A., McCullough A.J., Feldstein A.E. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. J Hepatol. 2007;46:582–589. doi: 10.1002/hep.21768. [DOI] [PubMed] [Google Scholar]
- 7.Marchesini G., Marzocchi R. Metabolic syndrome and NASH. Clin Liver Dis. 2007;11:105–117. doi: 10.1016/j.cld.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Guicciardi Maria Eugenia, Malhi Harmeet, Mott Justin L., Gores Gregory J. Apoptosis and Necrosis in the liver. Compr Physiol. 2013;3(2):1–62. doi: 10.1002/cphy.c120020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hillier T.A., Abbott R.D., Barrett E.J. Hyponatremia: evaluating the correction factor for hyperglycemia. Am J Med. 1999;106:399–403. doi: 10.1016/s0002-9343(99)00055-8. [DOI] [PubMed] [Google Scholar]
- 10.Erbey J.R., Silberman C., Lydick E. Prevalence of abnormal serum alanine aminotransferase levels in obese patients and patients with type 2 diabetes. Am J Med. 2000;109:588–590. doi: 10.1016/s0002-9343(00)00602-1. [DOI] [PubMed] [Google Scholar]
- 11.Chen C.H., Huang M.H., Yang J.C., Nien C.K., Yang C.C., Yeh Y.H. Prevalence and etiology of elevated serum alanine aminotransferase level in an adult population in Taiwan. J Gastroenterol Hepatol. 2007;22:14829. doi: 10.1111/j.1440-1746.2006.04615.x. [DOI] [PubMed] [Google Scholar]
- 12.Williamson R.M., Price J.F., Hayes P.C., Glancy S., Frier B.M., Johnston G.I., Reynolds R.M., Strachan M.W.J. Prevalence and markers of advanced liver disease in type 2 diabetes. Q J Med. 2012;105:425–432. doi: 10.1093/qjmed/hcr233. [DOI] [PubMed] [Google Scholar]
- 13.Jayarama N., Sudha R. A study of non-alcoholic fatty liver disease (NAFLD) in type 2 diabetes mellitus in a tertiary care centre, Southern India. J Clin Diagn Res. 2012;3670 [Google Scholar]
- 14.Butler Alexandra E., Janson Juliette, Bonner-Weir Susan, Ritzel Robert, Rizza Robert A., Butler Peter C. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 15.Nolsøe R.L., Hamid Y.H., Pociot F., Paulsen S., Andersen K.M., Borch-Johnsen K., Drivsholm T., Hansen T., Pedersen O., Mandrup-Poulsen T. Association of a microsatellite in FASL to type II diabetes and of the FAS-670G>A genotype to insulin resistance was found in another study. Genes Immun. 2006;7(4):316–321. doi: 10.1038/sj.gene.6364300. [DOI] [PubMed] [Google Scholar]
- 16.Feldstein A.E., Canbay A., Angulo P., Taniai M., Burgart L.J., Lindor K.D., Gores G.J. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 17.Lupi R.F., Dotta L., Marselli S., Masini Del Guerra M., Santangelo C. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic evidence that beta-cell death is caspase mediated, partially dependent on ceramide pathway and Bcl-2 regulated. Diabetes. 2002;51:1437–1442. doi: 10.2337/diabetes.51.5.1437. [DOI] [PubMed] [Google Scholar]
- 18.Lee J.Y., Chae D.W., Kim S.M., Nam E.S., Jang M.K., Lee J.H., Kim H.Y., Yoo J.Y. Expression of FasL and perforin/granzyme B mRNA in chronic hepatitis B virus infection. J Viral Hepat. 2004;112:130–135. doi: 10.1046/j.1365-2893.2003.00486.x. [DOI] [PubMed] [Google Scholar]
- 19.El-Koofy N.M., Anwar G.M., El-Raziky M.S., El-Hennawy A.M., El-Mougy F.M., El-Karaksy H.M. The association of metabolic syndrome, insulin resistance and non-alcoholic fatty liver disease in overweight/obese children. Saudi J Gastroenterol. 2012;18:44–49. doi: 10.4103/1319-3767.91738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Askari F., Rashidkhani B., Hekmatdoost A. Cinnamon may have therapeutic benefits on lipid profile, liver enzymes, insulin resistance, and high-sensitivity C-reactive protein in nonalcoholic fatty liver disease patients. Nutr Res. 2013;34(2):143–148. doi: 10.1016/j.nutres.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Elisaf M.S., Tsatsoulis A.A., Katopodis K.P., Siamopoulos K.C. Acid-base and electrolyte disturbances in patients with diabetic ketoacidosis. Diabetes Res Clin Pract. 1996;34:23–27. doi: 10.1016/s0168-8227(96)01332-0. [DOI] [PubMed] [Google Scholar]
- 22.Liamis G., Milionis H.J., Elisaf M. Hyponatremia in patients with infectious diseases. J Infect. 2011;63:327–335. doi: 10.1016/j.jinf.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Bankir L., Bardoux P., Ahloulay M. Vasopressin and diabetes mellitus. Nephron. 2001;87:8–18. doi: 10.1159/000045879. [DOI] [PubMed] [Google Scholar]