Abstract
Objectives
This study aimed to investigate the association between the IL6 −174 G/C gene polymorphism and the risk of osteoporosis by performing a meta-analysis.
Methods
Published literature from PubMed and Embase databases was searched for eligible publications. The following information was extracted from each study: Name of first author, year of publication, country of origin, sample size of cases and controls, and size of each allele. The combined odds ratio (ORs) and 95% confidence intervals (95%CIs) for the association between the IL6 −174 G/C gene polymorphism and the risk of osteoporosis were assessed using a random or fixed effects model. A comprehensive meta-analysis (CMA) 2.0 was used to analyse the data.
Results
Twelve studies (4923 cases/3431 controls) were included in this meta-analysis. The results indicated that IL6 −174 G/C gene polymorphism was associated with an increased (G vs C, OR 95%CI = 1.29 [1.03–1.62], p = 0.029) and decreased risk of osteoporosis (C vs G, OR 95%CI = 0.77 [0.62–0.97], p = 0.029; CC vs GG + GC, OR 95%CI = 0.58 [0.39–0.88], p = 0.010).
Conclusion
The IL6 −174 G/C gene polymorphism was shown to be positively correlated with osteoporosis risk.
Keywords: Genetic polymorphism, IL6 −174 G/C gene polymorphism, Osteoporosis
الملخص
أهداف البحث
تهدف هذه الدراسة للتحقيق في العلاقة بين تعدد الأشكال الجينية للجين IL 6-174 G/C وخطر هشاشة العظام باستخدام التحليل التلوي.
طرق البحث
تم البحث عن المقالات المنشورة في قاعدة المعلوماتPubMed و EMBASE المؤهلة للنشر. واُستخرجت المعلومات التالية من كل بحث: اسم الباحث الأول، سنة النشر، بلد المنشأ، حجم عينة الحالات والضوابط٬ وحجم كل أليل. كما تم تقييم نسبة الأرجحية المشتركة وفترة الثقة ٩٥٪ للعلاقة بين تعدد الأشكال الجينية للجين IL 6-174 G/C مع خطر هشاشة العظام باستخدام نموذج الأثر العشوائي أو الثابت. واستخدام التحليل التلوي الشامل لتحليل البيانات.
النتائج
شملت الدراسة ١٢ بحثا (٤٩٢٣حالة ⁄ ٣٤٣١ ضابطة) في التحليل التلوي. وأظهرت النتائج أن الجين IL 6-174 G/C متعدد الأشكال الجينية كان مرتبطا بزيادة (G vs C) وانخفاض خطورة حدوث هشاشة العظام (G vs C).
الاستنتاجات
أظهر الجين متعدد الأشكال IL 6-174 G/Cارتباطا إيجابيا مع خطورة حدوث هشاشة العظام.
الكلمات المفتاحية: IL 6-174 G/C, هشاشة العظام, تعدد الأشكال الجيني
Introduction
Osteoporosis, an important public health problem, is a very common multifactorial progressive skeletal or metabolic bone disorder in the elderly that is characterized by low bone density and microarchitectural deterioration of bony tissue or bone mineral density (BMD) more than 2.5 SDs below the young normal mean.1, 2, 3 It results in loss of bone mass, decreased bone strength,4 increased bone fragility, and increased risk of developing spontaneous and traumatic fractures.5, 6 Osteoporosis is considered a silent disease because it progresses without symptoms until a fracture occurs.6 Osteoporosis affects 200 million individuals worldwide,2 2.1 million females in England and Wales,3 13 million in Japan,4 3.5 million in Italy,5 and 26 million in India.6 The frequency of osteoporosis varies among studies. In the United States, the frequency of osteoporosis was 4% of 50- to 59-year-old Caucasian women and 52% of women aged 80 years or more,1 whereas in India, the frequency of osteoporosis was 20% of women and 10–15% of men.6 Osteoporosis is estimated to cause 1.5 million fractures annually in the United States, including 300,000 hip fractures, approximately 700,000 vertebral fractures, 250,000 wrist fractures, and more than 300,000 fractures at other sites.7 The mortality associated with osteoporotic fractures ranges from 15 to 30%, a rate similar to that of breast cancer and stroke.5 The annual cost expenditure for osteoporotic fractures is approximately £1.7 billion in England and Wales,1 £2100 million in England,3 over $14 billion in the United States in 20031 and $16 billion in 2008,8 and it is predicted to reach approximately $25.3 billion in 2025.9 Osteoporosis is a common disease with a strong genetic component.10 Stewart and Ralston11 revealed that genetic factors play an important role in regulating bone mineral density and other determinants of osteoporotic fracture risk. IL6 is one of the candidate genes that regulate bone density because IL6 has some effect on the stimulation of osteoclast resorption and has been implicated in the pathogenesis of bone loss associated with oestrogen deficiency.12
The IL6 gene is located at chromosome 7p21 and 5 in the human genome. The gene contains four introns and five exons.13 IL6 is a cytokine involved not only in inflammation and infection responses but also in the regulation of metabolic, regenerative, and neural processes.14 Overexpression of IL6 has been implicated in the pathology of a number of diseases, including osteoporosis.13 Several studies have reported the role of IL6 in the pathogenesis of osteoporosis. Bellido et al.15 revealed that IL6 mediates the upregulation of osteoclastogenesis. Kudo et al.16 reported that IL6, which is thought to play a role in several osteolytic bone disorders, is directly capable of inducing osteoclast formation by a receptor activator of nuclear factor-kappa B ligand (RANKL)-independent mechanism. Yoshitake et al.17 reported that IL6 directly acts on osteoclast progenitors and suppresses their differentiation by regulating the transcription of specific genes related to mitogen-activated protein kinase (MAPK) phosphatases and the ubiquitin pathway. Scheidt-Nave et al.18 found that serum IL6 was elevated in postmenopausal osteoporosis. Gaber et al.19 showed that the G/C promoter polymorphism at −174 of the IL6 gene had an effect on basal IL6 levels, and the C allele of the IL6 −174 G/C gene was associated with reduced gene expression and reduced plasma levels of IL6.
Several polymorphism studies regarding the association between the IL6 −174 G/C gene polymorphism and the risk of osteoporosis have shown inconsistent results. The studies conducted by Magana et al.,20 Mendez et al.,21 and Ferrari et al.22 showed that the IL6 −174 G/C gene polymorphism was associated with an increased risk of osteoporosis. In contrast, the studies conducted by Czerny et al.,23 Garnero et al.,24 Deveci et al.,25 Maedeb26, 26 Nordstrom et al.,27 Lee et al.,28 Korvala et al.,29 Dincel et al.,30 and Moffet et al.31 showed that the IL6 −174 G/C gene polymorphism had no significant association with an increased risk of osteoporosis. A meta-analysis study is the solution to determine the actual association in the many studies.
This study aimed to investigate the association between the IL6 −174 G/C gene polymorphism and the risk of osteoporosis by performing a meta-analysis. The results of this study are expected to be useful for the future treatment and prevention of osteoporosis. In addition, this study is expected to be useful as a comparison to other studies of the IL6 −174 G/C gene polymorphism and osteoporosis.
Materials and Methods
Study design
A meta-analysis was conducted to assess the association between the IL6 −174 G/C gene polymorphism and the risk of osteoporosis. To achieve this goal, several studies regarding this association were collected to calculate a combined OR 95%CI, and the data were assessed using a fixed or random effects model. An article search was conducted in PubMed and Embase. The study was conducted from January to April 2016.
Study procedures
The procedures of this study were to (1) identify the potentially relevant studies in PubMed and Embase up to March 20th, 2016; (2) determine the eligibility of each study using an exclusion process involving the following several steps: (a) reading the title and abstract, (b) ensuring that the study design complied with the inclusion criteria, and (c) ensuring that the study provided sufficient data to calculate OR 95%CI; (3) collect the abstract and full-text data from the studies; (4) collect the data for calculating OR 95%CI; and (5) analyse the data statistically.
Eligibility criteria and data extraction
The eligibility criteria consisted of predefined inclusion and exclusion criteria. Studies were included in the analysis if they met the following inclusion criteria: (1) case-control; (2) cohort; (3) cross-sectional studies; (4) randomized-controlled trials (RCTs); (5) controlled before-and-after studies; (6) cross-over studies; (7) evaluation of the association between the IL6 −174 G/C gene polymorphism and the risk of osteoporosis; and (8) sufficient data for calculation of OR 95%CI. Some of the required data were extracted from each study for calculating OR 95%CI. The following information was extracted from each study: (1) name of first author; (2) year of publication; (3) country of origin; (4) sample size of cases and controls; and (5) size of each allele.
Search strategy and literature
PubMed and Embase were searched with no language restrictions using specified search terms to identify studies published up to March 20th, 2016. The search strategy involved the use of a combination of the following key words: (IL 6 −174 G/C gene) and (variant or variation or polymorphism) and (osteoporosis). The publication languages were restricted to English. The reference lists of retrieved articles were hand-searched. If more than one article was published using the same study data, only the study with the largest sample size was included. We used a scoring system to evaluate the quality of the studies. We used a quality assessment score modified from previous meta-analysis for observational studies. The total scores ranged from 0 (worst) to 9 (best). A study was considered low quality if the score was <6 and high quality if the score was ≥6.32
Study variables
-
1.
Interleukin 6 −174 G/C
Interleukin-6 is a cytokine involved not only in inflammation and infection responses but also in the regulation of metabolic, regenerative, and neural processes.14 The measurement results of this variable were the G and C alleles. Data were obtained by a search strategy. A nominal scale was used to assess this variable.
-
2.
Risk of osteoporosis
Osteoporosis is a very common multifactorial progressive skeletal or metabolic bone disorder in the elderly that is characterized by low bone density and microarchitectural deterioration of bony tissue or a bone mineral density (BMD) more than 2.5 SDs below the young normal mean.1, 2, 3 The measurement results of this variable were an increased or decreased risk of osteoporosis. The data were obtained by a search strategy. A nominal scale was used to assess this variable.
Statistical analysis
The correlation between the IL6 −174 G/C gene polymorphism and the risk of osteoporosis was estimated by calculating pooled ORs and 95%CIs. The significance of pooled ORs was determined by Z tests (p < 0.05 was considered statistically significant). A Q test was performed to evaluate whether heterogeneity existed. A random effects model was used to calculate OR 95%CI if heterogeneity existed (p < 0.10). A fixed effect model was used to calculate OR 95%CI if no heterogeneity existed. Publication bias was assessed by Egger's test (p < 0.05 was considered statistically significant). Subgroup analyses based on continent (Europe, America, and Asia), gender (female, male, and mixed), and sample size (small < 400, large ≥ 400 samples) were also performed. A comprehensive meta-analysis (CMA) 2.0 was used to analyse the data.
Results
Characteristics of the studies
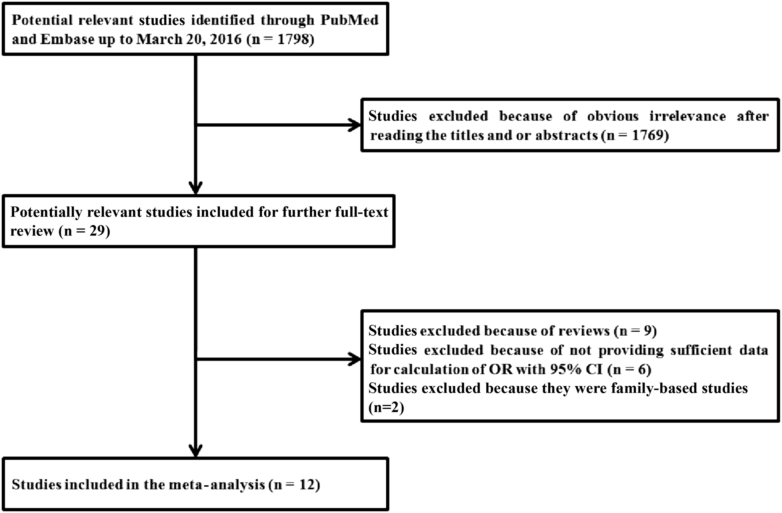
A total of 1798 potentially relevant papers were identified based on the search strategy. Of these, 1769 papers were excluded because of obvious irrelevance by reading their titles and abstracts. After the full texts were read, six papers were excluded because they did not provide sufficient data for calculation of OR with 95%CI; two papers were excluded because they were family-based studies. In addition, nine reviews were excluded. A flow chart demonstrating the inclusion or exclusion of studies is displayed in Figure 1. A total of 12 studies were included in the meta-analysis. Seven studies were from Europe, four studies were from America, and one study was from Asia. Table 1 describes the characteristics of the studies included in the meta-analysis.
Figure 1.
Selection of articles for inclusion in the meta-analysis.
Table 1.
Characteristics of studies included in the meta-analysis.
| Author & Year | Country | Continent | Mean age ± SD | Gender | SS | NS |
Ost genotype |
Cont genotype |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ost | Cont | GG | GC | CC | GG | GC | CC | ||||||
| Czerny et al., 2010 | Poland | Europe | 63.3 ± 5.1 | Female | L | 226 | 224 | 67 | 126 | 33 | 76 | 103 | 45 |
| Magana et al., 2008 | Mexico | America | 34.3 ± 10.2 | Female | S | 70 | 70 | 56 | 13 | 1 | 42 | 25 | 3 |
| Garnero et al., 2002 | France | Europe | 64.6 ± 8.8 | Female | L | 372 | 255 | 134 | 180 | 58 | 93 | 126 | 36 |
| Deveci et al., 2012 | Turkey | Europe | 57.0 ± 7.0 | Female | S | 201 | 155 | 127 | 50 | 24 | 93 | 31 | 31 |
| Mendez et al., 2013 | Mexico | America | 58.3 ± 6.8 | Female | S | 180 | 180 | 138 | 37 | 5 | 53 | 95 | 32 |
| Maedeb 2009 | France | Europe | 70.0 ± 7.4 | Female | S | 92 | 69 | 34 | 47 | 11 | 30 | 30 | 9 |
| Nordstrom et al., 2004 | Sweden | Europe | 75.0 ± 0.0 | Female | L | 232 | 544 | 68 | 121 | 43 | 167 | 246 | 131 |
| Lee et al., 2010 | South Korea | Asia | 12.5 ± 1.4 | Female | S | 198 | 120 | 197 | 1 | 0 | 119 | 1 | 0 |
| Korvala et al., 2010 | Finland | Europe | 20.3 ± 1.6 | Male | S | 72 | 120 | 15 | 42 | 15 | 35 | 56 | 29 |
| Dincel et al., 2008 | Turkey | Europe | 74.5 ± 8.9 | Mixed | S | 20 | 17 | 0 | 10 | 10 | 0 | 7 | 10 |
| Ferrari et al., 2004 | USA | America | 60.1 ± 9.5 | Mixed | L | 626 | 935 | 206 | 390 | 30 | 360 | 390 | 185 |
| Moffett et al., 2004 | USA | America | 73.0 ± 5.0 | Female | L | 2634 | 742 | 869 | 1272 | 493 | 245 | 354 | 143 |
Notes USA = United States of America, SD = standard deviation, SS = sample size, L = large (≥400 samples), S = small (<400 samples), NS = number of samples, Ost = osteoporosis, cont = control.
Quantitative data synthesis
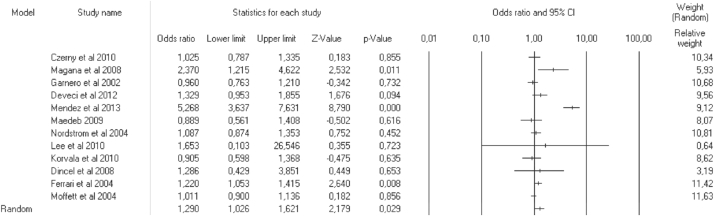
A total of 4923 cases and 3431 controls were identified. Overall, the results showed that allele G vs C, C vs G, and CC vs GG + GC had significant associations with the risk of osteoporosis. In contrast, GG vs GC + CC and GC vs GG + CC GC had no significant associations with the risk of osteoporosis. The results indicated that the IL6 −174 G/C gene polymorphism was associated with an increased (G vs C, OR 95%CI = 1.29 [1.03–1.62], p = 0.029) and a decreased risk of osteoporosis (C vs G, OR 95%CI = 0.77 [0.62–0.97], p = 0.029; CC vs GG + GC, OR 95%CI = 0.58 [0.39–0.88], p = 0.010). A forest plot showing the correlation between the IL6 −174 G/C gene polymorphism and the risk of osteoporosis is shown in Figure 2 for G vs C. A summary of ORs and 95%CIs regarding the correlation between the IL6 −174 G/C gene polymorphism and the risk of osteoporosis is described in Table 2. In the subgroup analysis, the IL6 −174 G/C gene polymorphism was associated with the risk of osteoporosis in two genetic models of the European continent subgroup (GC vs GG + CC p = 0.005; CC vs GG + GC p = 0.017), two genetic models of the American continent subgroup (G vs C p = 0.019; C vs G p = 0.019), three genetic models of the female subgroup (G vs C p = 0.047; C vs G p = 0.047; CC vs GG + GC p = 0.028), four genetic models of the mixed gender subgroup (G vs C p = 0.007; GG vs GC + CC p = 0.024; GC vs GG + CC p < 0.001; C vs G p = 0.007), and one genetic model of the small-sample-size subgroup (CC vs GG + GC p = 0.024). In contrast, the IL6 −174 G/C gene polymorphism had no significant association with the risk of osteoporosis in three genetic models of the European continent subgroup (G vs C p = 0.497; GG vs GC + CC p = 0.333; C vs G p = 0.497), three genetic models of the American continent subgroup (GG vs GC + C p = 0.100; GC vs GG + CC p = 0.433; CC vs GG + GC p = 0.054), all genetic models of the Asia continent subgroup (G vs C p = 0.723; GG vs GC + CC p = 0.722; GC vs GG + CC p = 0.722; C vs G p = 0.723; CC vs GG + GC p = NA), two genetic models of the female subgroup (GG vs GC + CC p = 0.163; GC vs GG + CC p = 0.535), all genetic models of the male subgroup (G vs C p = 0.635; GG vs GC + CC p = 0.205; GC vs GG + CC p = 0.118; C vs G p = 0.635; CC vs GG + GC p = 0.595), one genetic model of the mixed gender subgroup (CC vs GG + GC p = 0.056), four genetic models of the small-sample-size subgroup (G vs C p = 0.129; GG vs GC + CC p = 0.291; GC vs GG + CC p = 0.591; C vs G p = 0.129), and all genetic models of the large-sample-size subgroup (G vs C p = 0.094; GG vs GC + CC p = 0.102; GC vs GG + CC p = 0.108; C vs G p = 0.094; CC vs GG + GC p = 0.131).
Figure 2.
Meta-analysis of the association between the IL6 −174 G/C gene polymorphism and the risk of osteoporosis (G vs C).
Table 2.
Summary of ORs and 95%CIs in the association between the IL6 −174 G/C gene polymorphism and the risk of osteoporosis.
| No. | Genetic model | Parameter | All | Continent |
Gender |
Sample size |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Europe | America | Asia | Female | Male | Mixed | S | L | ||||
| 1 | G vs C | OR | 1.29 | 1.04 | 1.90 | 1.65 | 1.37 | 0.90 | 1.22 | 1.62 | 1.07 |
| 95%CI | 1.03–1.62 | 0.93–1.17 | 1.11–3.23 | 0.10–26.54 | 1.00–1.87 | 0.60–1.37 | 1.05–1.41 | 0.87–3.03 | 1.00–1.15 | ||
| p | 0.029 | 0.497 | 0.019 | 0.723 | 0.047 | 0.635 | 0.007 | 0.129 | 0.094 | ||
| pH | <0.001 | 0.711 | <0.001 | 1.000 | <0.001 | 1.000 | <0.001 | <0.001 | 0.296 | ||
| pE | 0.337 | <0.001 | 0.511 | <0.001 | 0.415 | <0.001 | <0.001 | 0.741 | 0.044 | ||
| 2 | GG vs GC + CC | OR | 1.20 | 0.92 | 1.93 | 1.65 | 1.36 | 0.64 | 0.78 | 1.65 | 0.91 |
| 95%CI | 0.84–1.70 | 0.77–1.09 | 0.88–4.24 | 0.10–26.71 | 0.88–2.10 | 0.32–1.28 | 0.63–0.97 | 0.65–4.18 | 0.81–1.02 | ||
| p | 0.313 | 0.333 | 0.100 | 0.722 | 0.163 | 0.205 | 0.024 | 0.291 | 0.102 | ||
| pH | <0.001 | 0.722 | <0.001 | 1.000 | <0.001 | 1.000 | 1.000 | <0.001 | 0.478 | ||
| pE | 0.517 | <0.001 | 0.768 | <0.001 | 0.588 | <0.001 | <0.001 | 1.056 | <0.001 | ||
| 3 | GC vs GG + CC | OR | 1.06 | 1.26 | 0.72 | 1.60 | 0.89 | 1.60 | 2.28 | 0.82 | 1.35 |
| 95%CI | 0.74–1.50 | 1.07–1.49 | 0.37–1.64 | 0.04–9.75 | 0.63–1.27 | 0.89–1.89 | 1.86–2.80 | 0.40–1.69 | 0.94–1.95 | ||
| p | 0.762 | 0.005 | 0.433 | 0.722 | 0.535 | 0.118 | <0.001 | 0.591 | 0.108 | ||
| pH | <0.001 | 0.627 | <0.001 | 1.000 | <0.001 | 1.000 | 0.476 | <0.001 | <0.001 | ||
| pE | 0.534 | <0.001 | 0.808 | <0.001 | 0.456 | <0.001 | <0.001 | 0.855 | 0.392 | ||
| 4 | C vs G | OR | 0.77 | 0.96 | 0.53 | 0.60 | 0.73 | 1.10 | 0.82 | 0.62 | 0.94 |
| 95%CI | 0.62–0.97 | 0.86–1.08 | 0.31–1.90 | 0.04–9.72 | 0.53–0.10 | 0.73–1.67 | 0.70–0.95 | 0.33–1.15 | 0.87–1.01 | ||
| p | 0.029 | 0.497 | 0.019 | 0.723 | 0.047 | 0.635 | 0.007 | 0.129 | 0.094 | ||
| pH | <0.001 | 0.711 | <0.001 | 1.000 | <0.001 | 1.000 | 0.926 | <0.001 | 0.296 | ||
| pE | 0.337 | <0.001 | 0.511 | <0.001 | 0.415 | <0.001 | <0.001 | 0.741 | 0.044 | ||
| 5 | CC vs GG + GC | OR | 0.58 | 0.77 | 0.31 | NA | 0.69 | 0.83 | 0.32 | 0.51 | 0.64 |
| 95%CI | 0.39–0.88 | 0.63–0.95 | 0.09–1.02 | NA | 0.49–0.96 | 0.41–1.67 | 0.10–1.03 | 0.29–0.92 | 0.36–1.14 | ||
| p | 0.010 | 0.017 | 0.054 | NA | 0.028 | 0.595 | 0.056 | 0.024 | 0.131 | ||
| pH | <0.001 | 0.589 | <0.001 | NA | 0.003 | 1.000 | 0.077 | 0.051 | <0.001 | ||
| pE | 0.584 | <0.001 | 1.087 | NA | 0.355 | <0.001 | 0.719 | 0.512 | 0.622 | ||
Notes OR = odds ratio, CI = confidence interval, p = p value based on a between-study Z test, pH = p value based on Q test for heterogeneity between studies, pE = p value based on Egger's test between studies, NA = not available, L = large (≥400 samples), S = small (<400 samples).
Source of heterogeneity
Evidence for heterogeneity (p < 0.10) between studies was found in all multiplicative models (G vs C pH < 0.001; GG vs GC + CC pH < 0.001; GC vs GG + CC pH < 0.001; C vs G pH < 0.001; CC vs GG + GC pH < 0.001). Therefore, the data in this study were assessed using a random effects model. A summary of the evidence of heterogeneity regarding the correlation between the IL6 −174 G/C gene polymorphism and the risk of osteoporosis is described in Table 2. In the subgroup analysis, evidence for heterogeneity was found in the American continent subgroup, female subgroup, mixed gender subgroup, small-sample-size subgroup, and large-sample-size subgroup. Therefore, a random effects model was used to calculate OR 95%CI in these subgroups. In contrast, no evidence of heterogeneity was found in the European continent subgroup, Asian continent subgroup, male subgroup, mixed gender subgroup, and large-sample-size subgroup. Therefore, a fixed effect model was used to calculate OR 95%CI in these subgroups.
Potential publication bias
Using Egger's test, no publication bias could be detected (G vs C pE = 0.337; GG vs GC + CC pE = 0.517; GC vs GG + CC pE = 0.534; C vs G pE = 0.337; CC vs GG + GC pE = 0.584). A summary of Egger's test regarding the correlation between the IL6 −174 G/C gene polymorphism and the risk of osteoporosis is described in Table 2. In the subgroup analysis, no publication bias was detected in the American continent subgroup (G vs C pE = 0.511; GG vs GC + CC pE = 0.768; GC vs GG + CC pE = 0.808; C vs G pE = 0.511; CC vs GG + GC pE = 1.087), female subgroup (G vs C pE = 0.415; GG vs GC + CC pE = 0.588; GC vs GG + CC pE = 0.456; C vs G pE = 0.415; CC vs GG + GC pE = 0.355), mixed gender subgroup (CC vs GG + GC pE = 0.719), small-sample-size subgroup (G vs C pE = 0.741; GG vs GC + CC pE = 1.056; GC vs GG + CC pE = 0.855; C vs G pE = 0.741; CC vs GG + GC pE = 0.512), and large-sample-size subgroup (GC vs GG + CC pE = 0.392; CC vs GG + GC pE = 0.622). In contrast, publication bias was detected in the European continent subgroup (G vs C pE < 0.001; GG vs GC + C pE < 0.001; GC vs GG + CC pE < 0.001; C vs G pE < 0.001; CC vs GG + GC pE < 0.001), Asian continent subgroup (G vs C pE < 0.001; GG vs GC + C pE < 0.001; GC vs GG + CC pE < 0.001; C vs G pE < 0.001), male subgroup (G vs C pE < 0.001; GG vs GC + C pE < 0.001; GC vs GG + CC pE < 0.001; C vs G pE < 0.001; CC vs GG + GC pE < 0.001), mixed gender subgroup (G vs C pE < 0.001; GG vs GC + C pE < 0.001; GC vs GG + CC pE < 0.001; C vs G pE < 0.001), and large-sample-size subgroup (G vs C pE = 0.044; C vs G pE = 0.044).
Discussion
IL6 is a cytokine involved not only in inflammation and infection responses but also in the regulation of metabolic, regenerative, and neural processes.14 Overexpression of IL6 has been implicated in the pathology of a number of diseases including osteoporosis.13 Several studies have reported the role of IL6 in the pathogenesis of osteoporosis. Scheidt-Nave et al.18 found that serum IL6 was elevated in postmenopausal osteoporosis. Gaber et al.19 showed that G/C promoter polymorphism at −174 of the IL-6 gene affected basal IL6 levels, and the C allele of the IL6 −174 G/C gene was associated with reduced gene expression and reduced plasma levels of IL6. Because of the effects of IL6 on the inflammatory response, a series of studies have focused on the contribution of polymorphisms within IL6 cluster genes to the osteoporosis risk. However, the results have been contradictory. This study reported the association between the IL6 −174 G/C gene polymorphism and the risk of osteoporosis, although the meta-analysis was limited due to the size and heterogeneity of the studies.
The results indicated that the IL6 −174 G/C gene polymorphism was associated with an increased (G vs C, OR 95%CI = 1.29 [1.03–1.62], p = 0.029) and decreased risk of osteoporosis (C vs G, OR 95%CI = 0.77 [0.62–0.97], p = 0.029; CC vs GG + GC, OR 95%CI = 0.58 [0.39–0.88], p = 0.010). A summary of the ORs 95%CIs, correlation, heterogeneity, and Egger's test regarding the correlation between the IL6 −174 G/C gene polymorphism and the risk of osteoporosis is described in Table 2, and the study characteristics are described in Table 1. A forest plot showing the correlation between the IL6 −174 G/C gene polymorphism and the risk of osteoporosis is described in Figure 2 for G vs C. Previous similar meta-analyses have reported the correlation between IL6 gene polymorphism and the risk of osteoporosis and fracture. Wang et al.33 studied the association between the IL6 −634 C/G and −174 G/C polymorphisms with bone mineral density (BMD). They showed that the −634 C/G and −174 G/C polymorphisms had modest effects on BMD. However, they did not include an analysis of each genetic model and did not focus on the risk of osteoporosis; they only correlated the polymorphisms to BMD score. Our study only focused on the risk of osteoporosis and provided the results of an analysis of several genetic models. Therefore, these results are expected to be complementary to several gene studies regarding the risk of osteoporosis. Another study by Wang et al.34 evaluated the association between the IL6 −174 G/C gene polymorphism and the risk of fracture. They showed that the IL6 −174 C/G gene polymorphism was associated with an increased risk of wrist and osteoporotic fracture (OR 95%CI = 1.60 [1.12–2.28]). A study by Zhang et al.35 analysed the association between IL6 −174 G/C and fracture risk. They found that the IL6 −174 G/C polymorphism contributed to the development of fracture (OR 95%CI = 1.32 [1.10–1.58]). However, the study characteristics were not included, and only six studies were included in the Wang et al.34 and Zhang et al.35 studies. Thus, a larger sample size was needed to determine a better association. Our study used a larger sample size. Therefore, the results of our study are expected to provide superior results. Furthermore, in the subgroup analysis, the IL6 −174 G/C gene polymorphism was associated with the risk of osteoporosis in two genetic models of the European continent subgroup (GC vs GG + CC; CC vs GG + GC), two genetic models of the American continent subgroup (G vs C; C vs G), three genetic models of the female subgroup (G vs C; C vs G; CC vs GG + GC), four genetic models of the mixed gender subgroup (G vs C; GG vs GC + CC; GC vs GG + CC; C vs G), and one genetic model of the small-sample-size subgroup (CC vs GG + GC). In contrast, the IL6 −174 G/C gene polymorphism had no significant association with the risk of osteoporosis in three genetic models of the European continent subgroup (G vs C; GG vs GC + CC; C vs G), three genetic models of the American continent subgroup (GG vs GC + C; GC vs GG + CC; CC vs GG + GC), all genetic models of the Asian continent subgroup, two genetic models of the female subgroup (GG vs GC + CC; GC vs GG + CC), all genetic models of the male subgroup, one genetic model of the mixed gender subgroup (CC vs GG + GC), four genetic model of the small-sample-size subgroup (G vs C; GG vs GC + CC; GC vs GG + CC; C vs G), and all genetic models of the large-sample-size subgroup. However, these results should be interpreted with caution considering that the relatively small sample size or multiple testing could have resulted in false-positive findings.
These results also indicated that the G allele of the IL6 −174 G/C gene was correlated with susceptibility to osteoporosis, whereas the C allele was correlated with a reduced risk of osteoporosis. See Table 2 for a detailed summary of the ORs 95%CIs regarding the correlation between the IL6 −174 G/C gene polymorphism and the risk of osteoporosis. Theoretically, these results are clearly unexplainable. However, several studies have supported these results. Scheidt-Nave et al.18 conducted a study regarding the role of serum IL6 as a predictor of bone loss in 137 postmenopausal German women. They found that serum IL6 was a predictor of postmenopausal bone loss and that the effect appears to be most relevant through the first postmenopausal decade. A study by Ershler and Keller36 revealed that after menopause, IL6 levels are elevated, even in the absence of infection, trauma, or stress, and this condition was associated with low BMD levels. Li et al.37 conducted a study regarding the contribution of IL6 to the osteogenesis of osteoporotic mice. They showed that IL6 over-secretion impaired osteogenesis in osteoporotic mice. They also showed that in vivo administration of IL6 neutralizing antibody was helpful to rescue the osteoporotic phenotype in the mouse vertebral body. De Benedetti et al.38 conducted a study regarding in vivo neutralization of IL6 by human IL-6 (hIL-6) receptor antagonist in osteoporotic mice. They found that immunization with hIL-6 receptor antagonist could represent a novel and simple therapeutic approach for the specific neutralization of IL6. A study by Theoharides et al.39 also found that serum IL6 was elevated in osteoporosis patients and was correlated with severity of symptoms. Based on these studies, it can be concluded that serum IL6 levels have a correlation with osteoporosis. Serum IL6 levels are determined by −174 G/C promoter polymorphism. No study has reported the association of serum IL levels with −174 G/C promoter polymorphism in osteoporotic models. However, several studies have reported this association in other disease models. Gaber et al.19 conducted a study regarding the correlation between serum IL6 and −174 G/C promoter polymorphism in rheumatoid arthritis patients. They found that the G allele of the IL6 −174 gene had a significant positive correlation with serum IL6 levels, whereas the C allele had a significant negative correlation with serum IL6 levels. Belluco et al.40 conducted a study regarding the association between serum IL6 levels and the −174 G/C gene polymorphism in patients with colorectal cancer. They found that C− subjects produced higher IL6 levels than did C+ subjects. Tonet et al.41 conducted a study regarding the correlation between serum IL6 levels and the −174 G/C gene polymorphism in patients with cardiovascular disease. They found that serum IL6 levels were markedly lower in the C allele, whereas the G allele displayed a trend towards higher levels of circulating IL6. Fishman et al.42 conducted a study regarding the association between serum IL6 levels and the −174 G/C gene polymorphism in patients with systemic-onset juvenile chronic arthritis. They found that the C allele was associated with significantly lower levels of plasma IL6. Burzotta et al.43 conducted a study regarding the association between serum IL6 levels and the −174 G/C gene polymorphism in patients with cardiovascular disease. They showed that the G allele was associated with higher levels of serum IL6. Therefore, the results of this study showing that the G allele of the IL6 −174 G/C gene polymorphism correlated with a susceptibility to osteoporosis are understandable because the G allele of the IL6 −174 G/C gene polymorphism has an association with increased levels of serum IL6.
Osteoporosis is a very common multifactorial progressive skeletal or metabolic bone disorder in the elderly characterized by low bone density and microarchitectural deterioration of bony tissue or BMD more than 2.5 SDs below the young normal mean.1, 2, 3 The role of IL6 in the osteoporosis process is complex. The balance between bone resorption caused by osteoclasts and bone formation caused by osteoblasts plays an important role in bone metabolism. The lower serum oestrogen levels in postmenopausal women cause an imbalance between osteoblasts and osteoclasts.44 IL6, a multifunctional cytokine involved in osteoclast differentiation, is secreted by osteoblasts and appears to be a key molecule in the osteoporotic process.45 The expression of the IL6 gene in osteoblasts and bone marrow stromal cells is down-regulated by oestrogen.44 IL6 is widely recognized as a potent stimulator of osteoclast-driven bone absorption in the context of chronic inflammation and oestrogen deficiency.46 The mechanism of IL6 in the process of osteoporosis is twofold, occurring both inside and outside the osteoclast cells. In osteoclast cells, IL6 has an important role in bone resorption by activating immature osteoclasts. Under normal conditions, oestrogen inhibits the IL6 promoter in the absence of a functional oestrogen receptor (ER) binding site. This process is mediated by nuclear factor – kappa B (NF-kB) and CCAAT-enhancer-binding proteins (C/EBP) β.44 The ER impairs IL6 induction by preventing c-rel and, to a lesser extent, RelA proteins from binding to the NF-kB site of the IL6 promoter.47 ER directly interacts with the nuclear factor interleukin-6 (NF-IL6) and NF-kB and inhibits their ability to bind deoxyribonucleic acid (DNA), which might be the molecular basis for repression of IL6 gene expression by oestrogens.48 The physical and functional interaction depends on the DNA binding domain and region D of the ER and on the Rel homology domain of NF-kB and the basic leucine zipper (bZIP) region of C/EBP β. The cross-coupling among ER, NF-kB, and C/EBP β also results in reduced activity of promoters with the ER binding sites.44 This leads to a balance between osteoblast and osteoclast activity. However, in pathological states or oestrogen deficiency, IL6 acts to activate immature osteoclasts without restriction, mediated by NF-kB, C/EBP β, and nuclear factors of activated T-cells cytoplasmic 1 (NFATc1).44 This leads to an imbalance between osteoblast and osteoclast activity, where osteoclast activity is more dominant than osteoblast activity, causing bone loss and osteoporosis. Outside osteoclast cells, after release from osteoblast-lineage cells,49 IL6 first binds to IL6R and forms a complex with gp130 to stimulate intracellular signalling machinery. IL6 then stimulates osteoblastic downstream production of signalling molecules, especially RANKL, which subsequently enhances osteoclast formation and activity.46 IL6 induces the expression of RANKL on the surface of osteoblasts. RANKL then interacts with receptor activator of nuclear factor – kappa B (RANK) expressed on osteoclast progenitors, inducing osteoclast differentiation via the RANK signalling pathway, which involves NF-κB, c-Jun N-terminal kinase (JNK), and p38. IL6 also directly acts on osteoclast progenitors to suppress their differentiation via an inhibition of RANK signalling pathways. IL6 specifically suppresses RANK-mediated I kappa B (IκB) degradation and JNK activation. IL6 and RANKL up-regulate the transcription of MKP1 and MKP7, which encode enzymes that dephosphorylate JNK, and down-regulate the transcription of sentrin-specific protease 2 (Senp2) and cullin 4A (Cul4A), which are related to the ubiquitin pathway.17 Furthermore, the activity of IL6 on osteoclasts frequently interplays with IL1 and tumour necrosis factor (TNF), and IL6 increases the stimulatory effect of IL1 and TNF on bone resorption by increasing the osteoclastic progenitor pool.46 These mechanisms are thought to underlie the results of this study that showed a correlation between the IL6 −174 G/C gene polymorphism and the risk of osteoporosis.
There were several limitations to the meta-analysis. First, our analysis was primarily based on unadjusted effect estimates. Therefore, the potential covariates, including age, gender, menopausal factors, history of trauma, and smoking habits, were not controlled for. Second, the possibility of a false negative exists due to the small size of the studies, even when combined. Thus, further studies with larger sample sizes are required to investigate the associations.
Conclusions and suggestions
In summary, our meta-analysis suggested that the G allele of the IL6 −174 G/C gene polymorphism was associated with an increased risk of osteoporosis, whereas the C allele of the IL6 −174 G/C gene polymorphism was associated with a decreased risk of osteoporosis. Further studies considering gene–environment interactions should be conducted to investigate the associations between the IL6 −174 G/C gene polymorphism and the risk of osteoporosis.
Conflicts of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Authors' contributions
Conceived and designed the experiments = Jonny Karunia Fajar (JKF), Azharuddin Azharuddin (AA). Performed the experiments = JKF. Analysed the data = JKF. Contributed reagents/materials/analysis tools = JKF. Wrote the manuscript = JKF, AA. Reference collection and data management = JKF, AA. Statistical analyses and paper writing = JKF. Study design = JKF, AA.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Christodoulou C., Cooper C. What is osteoporosis? Postgrad Med J. 2003;79:133–138. doi: 10.1136/pmj.79.929.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin J.T., Lane J.M. Osteoporosis: a review. Clin Orthop Relat Res. 2004;425:126–134. [PubMed] [Google Scholar]
- 3.Stevenson M., Jones M.L., De Nigris E., Brewer N., Davis S., Oakley J. A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis. Health Technol Assess. 2005;9(22):1–160. doi: 10.3310/hta9220. [DOI] [PubMed] [Google Scholar]
- 4.Imai K. Alendronate sodium hydrate (oral jelly) for the treatment of osteoporosis: review of a novel, easy to swallow formulation. Clin Interventions Aging. 2013;8:681–688. doi: 10.2147/CIA.S37199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernabei R., Martone A.M., Ortolani E., Landi F., Marzetti E. Screening, diagnosis and treatment of osteoporosis: a brief review. Clin Cases Min Bone Metab. 2014;11(3):201–207. [PMC free article] [PubMed] [Google Scholar]
- 6.Khajuria D.K., Razdan R., Mahapatra D.R. Drugs for the management of osteoporosis: a review. Rev Bras Reumatol. 2011;51(4):365–382. [PubMed] [Google Scholar]
- 7.Masi L. Epidemiology of osteoporosis. Clin Cases Min Bone Metab. 2008;5(1):11–13. [PMC free article] [PubMed] [Google Scholar]
- 8.Blume S.W., Curtis J.R. Medical costs of osteoporosis in the elderly Medicare population. Osteoporos Int. 2011;22(6):1835–1844. doi: 10.1007/s00198-010-1419-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dempster D.W. Osteoporosis and the burden of osteoporosis-related fractures. Am J Manag Care. 2011;17(6):S164–S169. [PubMed] [Google Scholar]
- 10.Huang Q.Y., Kung A.W. Genetics of osteoporosis. Mol Genet Metab. 2006;88(4):295–306. doi: 10.1016/j.ymgme.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Stewart T.L., Ralston S.H. Role of genetic factors in the pathogenesis of osteoporosis. J Endocrinol. 2000;166(2):235–245. doi: 10.1677/joe.0.1660235. [DOI] [PubMed] [Google Scholar]
- 12.Weerakulwattana L., Tirawanchai N., Bunyaratavej N. Analysis of polymorphism of the interleukin-6 gene in Thai subjects with osteoporosis. J Med Assoc Thai. 2001;84(2):S547–S552. [PubMed] [Google Scholar]
- 13.Simpson R.J., Hammacher A., Smith D.K., Matthews J.M., Ward L.D. Interleukin-6: structure-function relationships. Protein Sci. 1997;6(5):929–955. doi: 10.1002/pro.5560060501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(2011):878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 15.Bellido T., Jilka R.L., Boyce B.F., Girasole G., Broxmeyer H., Dalrymple S.A., Murray R., Manolagas S.C. Regulation of interleukin-6, osteoclastogenesis, and bone mass by androgens. The role of the androgen receptor. J Clin Invest. 1995;95(6):2886–2895. doi: 10.1172/JCI117995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kudo O., Sabokbar A., Pocock A., Itonaga I., Fujikawa Y., Athanasou N.A. Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone. 2003;32(1):1–7. doi: 10.1016/s8756-3282(02)00915-8. [DOI] [PubMed] [Google Scholar]
- 17.Yoshitake F., Itoh S., Narita H., Ishihara K., Ebisu S. Interleukin-6 directly inhibits osteoclast differentiation by suppressing receptor activator of NF-kappaB signaling pathways. J Biol Chem. 2008;283(17):11535–11540. doi: 10.1074/jbc.M607999200. [DOI] [PubMed] [Google Scholar]
- 18.Scheidt-Nave C., Bismar H., Leidig-Bruckner G., Woitge H., Seibel M.J., Ziegler R., Pfeilschifter J. Serum interleukin 6 is a major predictor of bone loss in women specific to the first decade past menopause. J Clin Endocrinol Metab. 2001;86(5):2032–2042. doi: 10.1210/jcem.86.5.7445. [DOI] [PubMed] [Google Scholar]
- 19.Gaber W., Azkalany G.S., Gheita T.A., Mohey A., Sabry R. Clinical significance of serum interleukin-6 and −174 G/C promoter polymorphism in Rheumatoid arthritis patients. Egypt Rheumatol. 2013;35(2):107–113. [Google Scholar]
- 20.Magana J.J., Gomez R., Cisneros B., Casas L., Valdés-Floresa M. Association of interleukin-6 gene polymorphisms with bone mineral density in mexican women. Arch Med Res. 2008;39(2008):618–624. doi: 10.1016/j.arcmed.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Méndez J.P., Rojano-Mejía D., Coral-Vázquez R.M., Coronel A., Pedraza J., Casas M.J., Soriano R., García-García E., Vilchis F., Canto P. Impact of genetic variants of IL-6, IL6R, LRP5, ESR1 and SP7 genes on bone mineral density in postmenopausal Mexican-Mestizo women with obesity. Gene. 2013;528(2013):216–220. doi: 10.1016/j.gene.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Ferrari S.L., Karasik D., Liu J., Karamohamed S., Herbert A.G., Cupples L.A., Kiel D.P. Interactions of interleukin-6 promoter polymorphisms with dietary and lifestyle factors and their association with bone mass in men and women from the Framingham osteoporosis study. J Bone Min Res. 2004;19:552–559. doi: 10.1359/JBMR.040103. [DOI] [PubMed] [Google Scholar]
- 23.Czerny B., Kaminski A., Kurzawski M., Kotrych D., Safranow K., Dziedziejko V., Bohatyrewicz A., Pawlik A. The association of IL-1b, IL-2, and IL-6 gene polymorphisms with bone mineral density and osteoporosis in postmenopausal women. Eur J Obstet Gynecol Reprod Biol. 2010;149(2010):82–85. doi: 10.1016/j.ejogrb.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Garnero P., Borel O., Sornay-Rendu E., Duboeuf F., Jeffery R., Woo P., Delmas P.D. Association between a functional interleukin-6 gene polymorphism and peak bone mineral density and postmenopausal bone loss in women: the OFELY study. Bone. 2002;31(1):43–50. doi: 10.1016/s8756-3282(02)00810-4. [DOI] [PubMed] [Google Scholar]
- 25.Deveci D., Ozkan Z.S., Yuce H. Is there any relation between IL-6 gene 174 G>C polymorphism and postmenopausal osteoporosis? Eur J Obstet Gynecol Reprod Biol. 2012;164(2012):98–101. doi: 10.1016/j.ejogrb.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 26.Meadeb J. Gene polymorphisms and osteoporotic fractures: a study in postmenopausal French women. Joint Bone Spine. 2009;76(2009):312–320. doi: 10.1016/j.jbspin.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Nordstrom A., Gerdhem P., Brandstrom H., Stiger F., Lerner U.H., Lorentzon M., Obrant K., Nordstrom P., Akesson K. Interleukin-6 promoter polymorphism is associated with bone quality assessed by calcaneus ultrasound and previous fractures in a cohort of 75-year-old women. Osteoporos Int. 2004;15:820–826. doi: 10.1007/s00198-004-1610-9. [DOI] [PubMed] [Google Scholar]
- 28.Lee J.S., Suh K.T., Eun I.S. Polymorphism in interleukin-6 gene is associated with bone mineral density in patients with adolescent idiopathic scoliosis. J Bone Joint Surg [Br] 2010;92-B:1118–1122. doi: 10.1302/0301-620X.92B8.23676. [DOI] [PubMed] [Google Scholar]
- 29.Korvala J., Hartikka H., Pihlajamäki H., Solovieva S., Ruohola J., Sahi T., Barral S., Ott J., Ala-Kokko L., Männikkö M. Genetic predisposition for femoral neck stressfractures in military conscripts. BMC Genet. 2010;11:95. doi: 10.1186/1471-2156-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dinçel E., Sepici-Dinçel A., Sepici V., Özsoy H., Sepici B. Hip fracture risk and different gene polymorphisms in the Turkish population. Clinics. 2008;63(5):645–650. doi: 10.1590/S1807-59322008000500013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moffett S.P., Zmuda J.M., Cauley J.A., Stone K.L., Nevitt M.C., Ensrud K.E., Hillier T.A., Hochberg M.C., Joslyn G., Morin P., Cummings SR for the SOF Research Group Association of the G-174C variant in the interleukin-6 promoter region with bone loss and fracture risk in older women. J Bone Min Res. 2004;19:1612–1618. doi: 10.1359/JBMR.040707. [DOI] [PubMed] [Google Scholar]
- 32.Thakkinstian A., D'Este C., Eisman J., Nguyen T., Attia J. Meta-analysis of molecular association studies: vitamin D receptor gene polymorphisms and BMD as a case study. J Bone Min Res. 2004;19(3):419–428. doi: 10.1359/JBMR.0301265. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z., Yang Y., He M., Wang R., Ma J., Zhang Y., Zhao L., Yu K. Association between interleukin-6 gene polymorphisms and bone mineral density: a meta-analysis. Genet Test Mol Biomarkers. 2013;17(12):898–909. doi: 10.1089/gtmb.2013.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C., Ge J., Ni S. Effect of interleukin-6 polymorphism on fracture risk. Int J Clin Exp Med. 2015;8(6):9599–9602. [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Z., He N., Zhang T. IL6–174G/C polymorphism and fracture risk. Int J Clin Exp Med. 2014;7(10):3795–3799. [PMC free article] [PubMed] [Google Scholar]
- 36.Ershler W.B., Keller E.T. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 37.Li X., Zhou Z.Y., Zhang Y.Y., Yang H.L. IL-6 contributes to the defective osteogenesis of bone marrow stromal cells from the vertebral body of the glucocorticoid-induced osteoporotic mouse. PLoS One. 2016;11(4):e0154677. doi: 10.1371/journal.pone.0154677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Benedetti F., Pignatti P., Vivarelli M., Meazza C., Ciliberto G., Savino R., Martini A. In vivo neutralization of human IL-6 (hIL-6) achieved by immunization of hIL-6-transgenic mice with a hIL-6 receptor antagonist. J Immunol. 2001;166:4334–4340. doi: 10.4049/jimmunol.166.7.4334. [DOI] [PubMed] [Google Scholar]
- 39.Theoharides T.C., Boucher W., Spear K. Serum interleukin-6 reflects disease severity and osteoporosis in mastocytosis patients. Int Arch Allergy Immunol. 2002;128(4):344–350. doi: 10.1159/000063858. [DOI] [PubMed] [Google Scholar]
- 40.Belluco C., Olivieri F., Bonafè M., Giovagnetti S., Mammano E., Scalerta R., Ambrosi A., Franceschi C., Nitti D., Lise M. −174G>C Polymorphism of interleukin 6 gene promoter affects interleukin 6 serum level in patients with colorectal cancer. Clin Cancer Res. 2003;9(6):2173–2176. [PubMed] [Google Scholar]
- 41.Tonet A.C., Karnikowski M., Moraes C.F., Gomes L., Karnikowski M.G.O., Córdova C., Nóbrega O.T. Association between the -174 G/C promoter polymorphism of the interleukin-6 gene and cardiovascular disease risk factors in Brazilian older women. Braz J Med Biol Res. 2008;41(1):47–53. doi: 10.1590/s0100-879x2006005000190. [DOI] [PubMed] [Google Scholar]
- 42.Fishman D., Faulds G., Jeffery R., Mohamed-Ali V., Yudkin J.S., Humphries S., Woo P. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102(7):1369–1376. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burzotta F., Iacoviello L., Di Castelnuovo A., Glieca F., Luciani N., Zamparelli R., Schiavello R., Donati M.B., Maseri A., Possati G.F., Andreotti F. Relation of the −174 G/C polymorphism of interleukin-6 to interleukin-6 plasma levels and to length of hospitalization after surgical coronary revascularization. Am J Cardiol. 2001;88(10):1125–1128. doi: 10.1016/s0002-9149(01)02046-x. [DOI] [PubMed] [Google Scholar]
- 44.Stein B., Yang M.X. Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta. Mol Cell Biol. 1995;15(9):4971–4979. doi: 10.1128/mcb.15.9.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galien R., Evans H.F., Garcia T. Involvement of CCAAT/enhancer-binding protein and nuclear factor-kappa B binding sites in interleukin-6 promoter inhibition by estrogens. Mol Endocrinol. 1996;10(6):713–722. doi: 10.1210/mend.10.6.8776731. [DOI] [PubMed] [Google Scholar]
- 46.Zhao R. Immune regulation of osteoclast function in postmenopausal osteoporosis: a critical interdisciplinary perspective. Int J Med Sci. 2012;9(9):825–832. doi: 10.7150/ijms.5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galien R., Garcia T. Estrogen receptor impairs interleukin-6 expression by preventing protein binding on the NF-kappaB site. Nucleic Acids Res. 1997;25(12):2424–2429. doi: 10.1093/nar/25.12.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ray P., Ghosh S.K., Zhang D., Ray A. Repression of interleukin-6 gene expression by 17β-estradiol: inhibition of the DNA-binding activity of the transcription factors NF-IL6 and NF-κB by the estrogen receptor. FEBS Lett. 1997;409(1):79–85. doi: 10.1016/s0014-5793(97)00487-0. [DOI] [PubMed] [Google Scholar]
- 49.Sugiyama T. Involvement of interleukin-6 and prostaglandin E2 in periarticular osteoporosis of postmenopausal women with rheumatoid arthritis. J Bone Min Metab. 2001;19(2):89–96. doi: 10.1007/s007740170046. [DOI] [PubMed] [Google Scholar]