Abstract
Objectives
In this study, we aimed to determine the antioxidant activities of two varieties of sesame (Sesamum indicum L.) seeds using 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS), and ferric reducing antioxidant power (FRAP) method, and to investigate the correlation between total phenolic as well as flavonoid contents and antioxidant activities of the extracts.
Methods
The antioxidant activities were determined using DPPH, ABTS, and FRAP assays, and the total phenolic content (TPC) and total flavonoid content (TFC) were measured by ultraviolet (UV)-Vis spectrophotometry. The correlation between total phenolic and flavonoid contents and DPPH IC50, ABTS IC50, and FRAP EC50 values was analyzed by Pearson's method.
Results
The IC50 DPPH values of all sesame seed extracts were in the range of 8.88–44.21 μg/mL and IC50 ABTS values were in the range of 24.91–141.19 μg/mL. EC50 FRAP value ranged from 222.40 to 872.57 μg/mL. The highest TPC of 1.57 g gallic acid equivalent (GAE)/100 g was observed in ethanolic extract of black sesame seed, while the highest TFC of 4.29 g quercetin equivalent (QE)/100 g was observed in ethyl acetate extract of white sesame seeds. The TPC in black sesame seed extract was significantly negative correlated with IC50 ABTS value (r = −0.828, p < 0.01) and EC50 FRAP value (r = −0.976, p < 0.01).
Conclusions
All sesame seed extracts were categorized as very strong antioxidants by DPPH assay. Phenolic compounds in black sesame seeds were found to be the major contributors to antioxidant activities by using ABTS and FRAP methods. White and black sesame seeds have the potential to be developed as sources of natural antioxidants.
Keywords: ABTS, Antioxidant, Black sesame seed, DPPH, FRAP, White sesame seed
الملخص
أهداف البحث
يهدف هذا البحث إلى تحديد الأنشطة المضادة للأكسدة لنوعين من بذور السمسم (سيساموم إنديكوم) باستخدام ٢،٢- ثنائي فينايل-١-بيكريلهايدرازايل و٢،٢'-أزينو- بيس (حمض ٣- إبثايلبنزثايازولين-٦- سولفونيك) وطرق قياس تضاد الأكسدة الناتجة عن اختزال الحديديك ومطابقة المحتوى الفينولي والفلافونويدي الكلي مع أنشطتها المضادة للأكسدة.
طرق البحث
تم تحديد الأنشطة المضادة للأكسدة باستخدام ٢،٢- ثنائي فينايل-١-بيكريلهايدرازايل و٢،٢'-أزينو- بيس (حمض ٣- إبثايلبنزثايازولين-٦- سولفونيك) وطرق قياس تضاد الأكسدة الناتجة عن اختزال الحديديك بواسطة المقياس الطيفي للأشعة فوق البنفسجية. كما تم تحليل مطابقة المحتوى الفينولي والفلافونويدي الكلي باستخدام ٢،٢- ثنائي فينايل-١-بيكريلهيدرازايل و ٢،٢'-أزينو- بيس (حمض ٣- إبثايلبنزثايازولين-٦- سولفونيك) بعد تقليل الاستجابة إلى النصف واستخدام تضاد الأكسدة الناتجة عن اختزال الحديديك لقياس تركيز الدواء الذي يعطي استجابة نصف الاستجابة القصوى بواسطة طريقة بيرسون.
النتائج
كانت القيم الناتجة لجميع مستخلصات بذور السمسم باستخدام ٢،٢- ثنائي فينايل-١-بيكريلهايدرازايل بعد تقليل الاستجابة إلى النصف في نطاق ٨.٨٨ - ٢١.٤٤ ميكروغرام / مل، وباستخدام ٢،٢'-أزينو- بيس (حمض ٣- إبثايلبنزثايازولين-٦- سولفونيك) في نطاق ٩١.٢٤ - ١٩.١٤١ ميكروغرام / مل، وتركيز الدواء الذي يعطي استجابة نصف القصوى باستخدام تضاد الأكسدة الناتجة عن اختزال الحديديك ٤٠.٢٢٢ - ٥٧.٨٧٢ ميكروغرام / مل. كان أعلى محتوى للفينول ٥٧.١غرام من معادل حمض القاليك / ١٠٠غرام وكان قد تم الكشف عليه باستخدام المستخلص الإثانولي لبذور السمسم الأسود في حين كان أعلى محتوى للفلافينويد ٢٩.٤ غرام من معادل كويرسيتين/ ١٠٠غرام وكان قد تم الكشف عليه باستخدام المستخلص الإيثايلي الأسيتيتي لبذور السمسم الأبيض. وكان أعلى محتوى للفينول في مستخلص بذور السمسم الأسود قد أظهر علاقة سلبية ذات قيمة مع كل من ٢،٢'-أزينو- بيس (حمض ٣- إبثايلبنزثايازولين-٦- سولفونيك) بعد تقليل الاستجابة إلى النصف وتضاد الأكسدة الناتجة عن اختزال الحديديك لقياس تركيز الدواء الذي يعطي استجابة النصف.
الاستنتاجات
تم تصنيف جميع مستخلصات بذور السمسم على أنها مضادات أكسدة قوية جدا باستخدام طريقة ٢،٢ - ثنائي فينايل-١-بيكريلهايدرازايل. وكانت المركبات الفينولية في بذور السمسم الأسود المساهم الرئيس في الأنشطة المضادة للأكسدة باستخدام أسلوب٢،٢'-أزينو- بيس (حمض ٣- إبثايلبنزثايازولين-٦- سولفونيك) وأسلوب تضاد الأكسدة الناتجة عن اختزال الحديديك.
الكلمات المفتاحية: مضاد الأكسدة, السمسم, بذرة, أبيض, أسود
Introduction
Many degenerative diseases are caused by the excessive production of free radicals. These free radicals can be scavenged by antioxidants. Many plants, including fruits and vegetables, are natural antioxidants owing to the presence of phenolic and flavonoid compounds that exert antioxidant capacity.1, 2, 3 Phenolic compounds such as flavonoids have many benefits including antioxidant, antibacterial, and antidiabetic activities.4, 5, 6
Methods such as 2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric reducing antioxidant power (FRAP), and 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) have been used to determine antioxidant activity in many plant extracts.2, 3, 7 Previous studies7, 8, 9, 10, 11 showed that DPPH, ABTS, and FRAP can be used to determine the antioxidant potential of fruits, vegetables, beverages, and food.
Two varieties of sesame (Sesamum indicum L.) seeds are widely consumed. Previous studies demonstrated that sesame seeds contain flavonoids and other phenolic compounds that can act as antioxidants.12, 13 Bopitiya and Madhujith14 reported that the methanolic extract of sesame oil exhibited antioxidant activity. Zhou et al. reported the antioxidant activities of six sesame seed varieties from Taiyuan-China by using oxygen radical absorbance capacity (ORAC) method.13
Sesame seeds contain various compounds, including nonpolar, semipolar, and polar compounds. The different polarities of compounds in sesame seeds may render different antioxidant potentials. To date, no study has reported the antioxidant potential of two varieties of sesame (S. indicum L.) seeds extracted with different polarity solvents.
In the present study, we aimed to determine the antioxidant potential of extracts of varying polarities (n-hexane, ethyl acetate, and ethanol) from two varieties of sesame seeds grown in East Java-Indonesia by using DPPH, ABTS, and FRAP assays. In addition, we aimed to investigate the correlation between total phenolic and flavonoid contents and the antioxidant activities of the extracts. We hope white and black sesame seeds has potential as a source of natural antioxidants.
Materials and Methods
Materials
DPPH, ABTS diammonium salt, 2,4,6-tripyridyl-S-triazine (TPTZ), gallic acid, and quercetin were purchased from Sigma-Aldrich (MO, USA).
Sample preparation
Sesame seeds were purchased from a local market in Bandung, East Java-Indonesia. The two varieties of sesame seeds used in this research are WS seeds and BS seeds. These seeds were ground into a powder for further experiments.
Extraction
Each sample was extracted using solvents with different polarities by reflux. Powdered samples (300 g) were extracted in triplicate with n-hexane. The remaining residue was then extracted in triplicate with ethyl acetate. The subsequent residue was extracted in triplicate with ethanol. Thus, there were six extracts: two n-hexane extracts (namely WS1 and BS1), two ethyl acetate extracts (WS2 and BS2), and two ethanolic extracts (WS3 and BS3).
Determination of antioxidant activity by DPPH assay
The antioxidant activity of extracts was determined by DPPH assay using Blois's method with some modifications.15 Each extract was prepared in various concentrations. The extract (2 mL) was added to 2 mL of DPPH solution (50 μg/mL) to initiate the reaction for obtaining a calibration curve. The absorbance at 515 nm was measured after incubation for 30 min by using an ultraviolet (UV)–Vis spectrophotometer (Beckman Coulter DU 720). DPPH (50 μg/mL) was used as the control, ascorbic acid as the standard, and methanol as the blank. Analysis was conducted in triplicate for the standard and each extract. The antioxidant activity was revealed as IC50 of DPPH scavenging activity by observing the 50% inhibitory concentration for each extract using the calibration curve.
Determination of antioxidant activity by ABTS assay
ABTS solution was prepared by the modification of a previous method.16 Solution of ABTS diammonium salt (7.6 mM) and potassium persulfate (2.5 mM) each in aqua dest was prepared and left in a dark room for 12 h. The two solutions were mixed and incubated for 30 min, left in the refrigerator for 24 h, and then diluted in ethanol. Each extract was prepared in various concentrations. The extract (2 mL) was added to 2 mL of ABTS (50 μg/mL). The absorbance was read at 734 nm using the UV–Vis spectrophotometer Beckman Coulter DU 720. Ethanol (95%) was used as the blank, ABTS (50 μg/mL) as the control, and ascorbic acid as the standard. Antioxidant activity was demonstrated as IC50 of ABTS scavenging activity by calculating the 50% inhibitory concentration for each extract using the calibration curve.
Determination of antioxidant capacity by FRAP assay
FRAP assay to determine the antioxidant capacity of extracts was performed according to the method by Benzi and Strain,17 with minor modifications. FRAP solution was prepared in acetate buffer (pH 3.6). Various concentrations of each extract were prepared. The extract (2 mL) was added to 2 mL of FRAP (50 μg/mL). After incubation for 30 min, the absorbance was read at 593 nm. Ascorbic acid was used as the standard, FRAP (50 μg/mL) as the control, and acetate buffer as the blank. The antioxidant capacity was presented as EC50 of FRAP capacity by determining the 50% exhibitory concentration using the calibration curve.
Determination of total phenolic content (TPC)
Folin-Ciolcalteu's reagent was used to determine the TPC.18 The absorbance was read at 765 nm. Gallic acid solution (50–160 μg/mL) was used to obtain the calibration curve. TPC was expressed as the percentage of gallic acid equivalent (GAE) per 100 g extract (g GAE/100 g).
Determination of total flavonoid content (TFC)
A modification of Chang's method was used to determine TFC.19 The absorbance was read at 415 nm. Quercetin solution (50–125 μg/mL) was used to obtain a calibration curve. TFC was expressed as the percentage of quercetin equivalent (QE) per 100 g extract (g QE/100 g).
Statistical analysis
Each sample analysis was performed in triplicate. All of the presented results are the means (±standard deviation) of at least three independent experiments. Statistical analysis was performed by SPSS 16 for Windows. Statistical significance was observed using independent samples t-test (p < 0.05). Correlation between total phenolic and flavonoid contents and the antioxidant activities of the extracts, as well as the correlation between the three assays were analyzed using the Pearson's method.
Results
The density of the extract did not observe in 100% concentrated extract. It is difficult to put 100% concentrated extract into pycnometer. Therefore the density of each extract was presented in diluted extract and prepared in 1% extract (Table 1).
Table 1.
Density of various extracts of sesame seeds.
| Sample | Density 1% extract (g/mL) |
||
|---|---|---|---|
| n-hexane extract | Ethyl acetate extract | Ethanol extract | |
| White sesame | 0.7693 | 1.0317 | 0.9237 |
| Black sesame | 0.7699 | 1.0310 | 0.9275 |
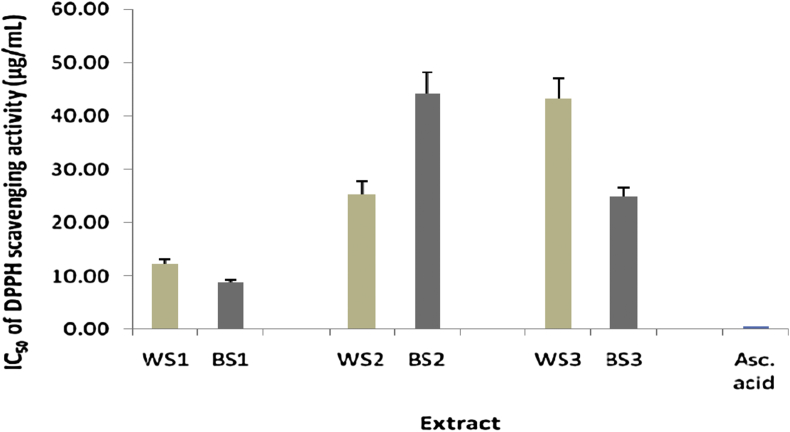
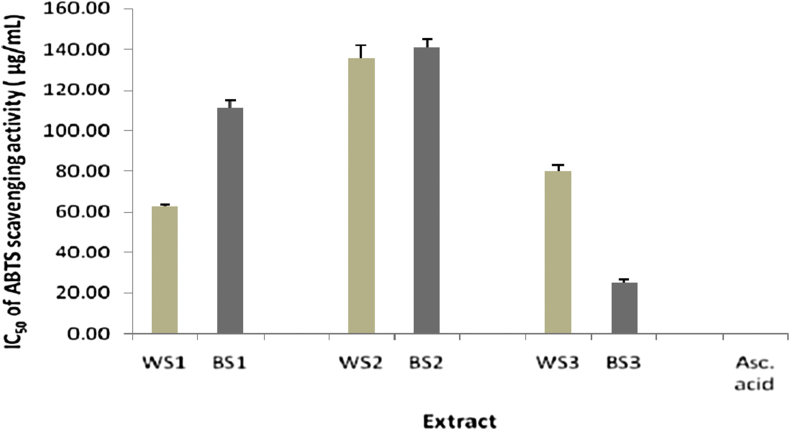
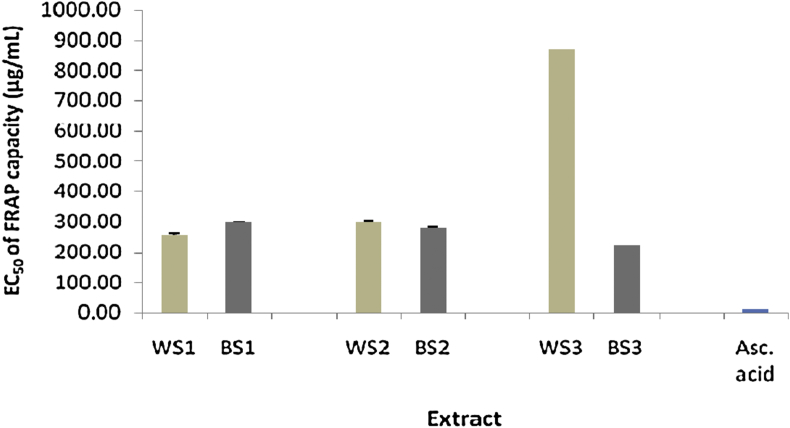
The antioxidant activity of different extracts of the two varieties of sesame seeds was determined by calculating the IC50 of DPPH and ABTS scavenging activities. Meanwhile, in FRAP assay, the antioxidant activity was determined by calculating the EC50 of FRAP capacity for each extract. The lowest IC50 or EC50 value corresponds to the highest antioxidant activity. DPPH IC50, ABTS IC50, and FRAP EC50 values were compared to the IC50 of ascorbic acid (standard). The IC50 of DPPH and ABTS scavenging activities of different extracts of the two varieties of sesame seeds was in the range of 8.88–44.21 and 24.91–141.19 μg/mL, respectively, while the EC50 of FRAP was in the range of 222.40–872.57 μg/mL (Figure 1, Figure 2, Figure 3).
Figure 1.
IC50 of DPPH scavenging activity in various extracts of sesame seeds (n = 3, all extracts were significantly different compared to ascorbic acid, p < 0.05).
Figure 2.
IC50 of ABTS scavenging activity in various extracts of sesame seeds (n = 3, all extracts were significantly different compared to ascorbic acid, p < 0.05).
Figure 3.
EC50 of FRAP capacity in various extracts of sesame seeds (n = 3, all extracts were significantly different compared to ascorbic acid, p < 0.05).
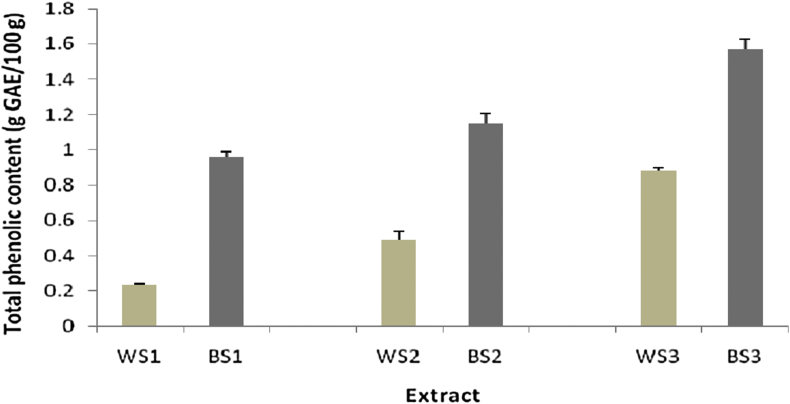
The TPC in different extracts of the two varieties of sesame seeds was expressed in terms of GAE and was in the range of 0.23–1.57 g GAE/100 g. BS3 had the highest TPC (1.57 g GAE/100 g), while WS1 had the lowest (0.23 g GAE/100 g; Figure 4).
Figure 4.
Total phenolic content in various extracts of sesame seeds (n = 3).
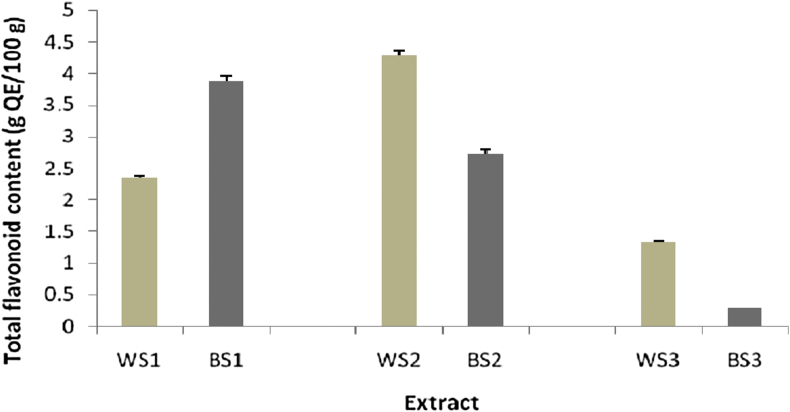
The TFC in different extracts of the two varieties of sesame seeds was expressed in terms of QE, and the values ranged from 0.29 to 4.29 g QE/100 g. The highest TFC was shown by WS2 (4.29 g QE/100 g), while the lowest TFC was observed in BS3 (0.29 g QE/100 g; Figure 5).
Figure 5.
Total flavonoid content in various extracts of sesame seeds (n = 3).
The TPC in black sesame seed extract was significantly negatively correlated with IC50 ABTS (r = −0.828, p < 0.01) and EC50 FRAP (r = −0.976, p < 0.01). The TFC in WS seed extract showed significant negative correlation with EC50 FRAP (r = −0.72; p < 0.05) (Table 2). Significant positive correlations were observed between IC50 DPPH and EC50 FRAP (r = 0.921, p < 0.01) for WS extracts, and IC50 ABTS and EC50 FRAP (r = 0.898, p < 0.01) for BS extracts (Table 2).
Table 2.
Pearson's correlation coefficient of TPC and TFC in various extracts with IC50 of DPPH, IC50 of ABTS, and EC50 of FRAP.
| Antioxidant parameter | Pearson's correlation coefficient (r) |
|||||
|---|---|---|---|---|---|---|
| TPC | TFC | EC50 FRAP WS | EC50 FRAP BS | IC50 ABTS WS | IC50 ABTS BS | |
| IC50 DPPH WS | 0.990** | −0.423 ns | 0.921** | |||
| IC50 DPPH BS | 0.26 ns | −0.252 ns | −0.144 ns | |||
| IC50 ABTS WS | 0.097 ns | 0.834** | ||||
| IC50 ABTS BS | −0.828** | 0.843** | ||||
| EC50 FRAP WS | 0.945** | −0.720* | −0.229 ns | |||
| EC50 FRAP BS | −0.976** | 0.993** | 0.898** | |||
WS = white sesame seed, BS = black sesame seed, ns = not significant, * = significant at p < 0.05, ** = significant at p < 0.01.
Discussion
The components in each crude drug were separated using three solvents of different polarities. N-hexane solvent was using to separate nonpolar compounds. The residue of crude drug was extracted in triplicate with ethyl acetate solvent to separate most of the semi polar compounds. The polar compounds in the crude drug residue were separated with ethanol solvent.
The phytochemical contents and antioxidant activities among the extracts can be compared if the density of the extracts is similar. High-density extracts may show higher phytochemical content and higher activity than low-density extracts. Therefore, all the extracts (six extracts) used in the present study were prepared at similar density.
The major antioxidant assays can be divided into two categories: single electron transfer (SET)-based assay and hydrogen atom transfer (HAT)-based assay.20 In SET-based methods, the ability of antioxidants to transfer one electron to reduce any oxidant is measured, while HAT-based methods measure the ability of antioxidants to quench radicals by hydrogen donation. SET and HAT mechanisms usually occur together. The predominant mechanism is influenced by the ionization potential (ΔIP), bond dissociation energy (BDE), redox potential, pH, and solvent.20 The predominant mechanism is HAT for compounds with ΔIP <−36 kcal/mol, whereas the predominant mechanism is SET for compounds with ΔIP >−45 kcal/mol.
Antioxidants scavenge DPPH free radicals through HAT to form a stable DPPH product. DPPH free radical absorption was observed at 516 nm. The decrease in absorbance of DPPH is related to the antioxidant potential of a sample. The concentration of a sample or standard that can inhibit 50% of DPPH radical activity is termed as the IC50 of DPPH scavenging activity.
A standard compound is important for determining the validity of antioxidant assays. A method is considered valid if the standard gives positive results. Antioxidant activities can be expressed as the percentage of DPPH scavenging activity and compared to the percentage of DPPH scavenging activity of standards, such as ascorbic acid, butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), alpha (α)-tocopherol, and Trolox. Ascorbic acid did not achieve 100% DPPH scavenging activity, which was indicated by the residual yellow color in the solution even after the antioxidant transferred hydrogen atom to DPPH.21, 22 The percentage of DPPH scavenging activity does not indicate the actual antioxidant activity because higher concentration of samples does not always show a higher percentage of DPPH scavenging activity. A linear result is observed only at certain concentrations. A previous study23 reported that 8 mg/mL of methanol peel extract of pineapple waste collected from Egypt had the highest percentage of DPPH scavenging activity when compared to 2, 4, 6, and 10 mg/mL of the extract. Similar results were shown in another study, which revealed that the percentage of DPPH scavenging activity of 25, 50, 100, 200, and 400 μg/mL of methanol peel extract of pineapple collected from Nigeria ranged from 95.52 to 95.74%.24 The percentage of DPPH scavenging activity of 100 μg/mL of methanol peel extract of pineapple (95.74%) was higher than that of 200 μg/mL (95.17%) and 400 μg/mL (94.96%) methanol peel extract, respectively. These findings can be observed in extracts or samples comprising more than one compound. In an extract containing many compounds, some of them may exhibit antioxidant activities, while other compounds may demonstrate antagonistic effects on the compounds exhibiting antioxidant potential. A compound exhibits antagonistic effects on antioxidant property when it reaches the minimum effective concentration. Compounds exhibiting antagonistic effects on the antioxidant compounds might reach their effective minimum concentration in 200 μg/mL of methanol peel extract, resulting in the decrease in percentage of DPPH scavenging activity. These findings can provide the explanation for the lower activity of high concentration extracts than extracts with low concentration.
A previous study12 investigated the antioxidant capacities of 35 sesame seed cultivars collected from Morocco and revealed that the ethanolic seed extract of souk sebt ouled slimane2 sesame cultivar showed the highest percentage of DPPH scavenging activity (63.33%) than other cultivars. Another study reported that 1 mg/mL of ethanolic extract of WS seed exhibited a higher percentage of DPPH scavenging activity (61.16%) than 1 mg/mL ethanolic extract of BS seed (56.73%).25
Bopitiya and Madhujith14 revealed that the methanolic extract of sesame oil had an IC50 DPPH of 26 μg/mL, which was similar to the IC50 DPPH of α-tocopherol (31 μg/mL). Xu et al.26 showed that the ethanolic extract of brown pigment of BS seed had the highest antioxidant activity by DPPH assay, and it showed a lower IC50 DPPH (13.3 μg/mL) than n-hexane extract (78.3 μg/mL) and supercritical (SC)-CO2 extract (114 μg/mL). The present study demonstrated that the IC50 DPPH of all extracts ranged from 8.88 to 44.21 μg/mL. A sample was categorized as a very strong antioxidant if it had an IC50 lower than 50 μg/mL.15
As ABTS is not soluble in polar solvents, ABTS diammonium salt, which is soluble in polar solvents, should be used for determining antioxidant activity by ABTS assay. ABTS method is the same as Trolox equivalency antioxidant capacity (TEAC) method. In TEAC, the antioxidant capacity is expressed as Trolox equivalent. A sample with higher Trolox equivalent value has higher antioxidant capacity. ABTS27 assay was modified, and the antioxidant activities presented as IC50 of ABTS were compared to that of ascorbic acid (standard). ABTS reagent reacts with potassium persulfate to form ABTS free radical and gives a blue color at 734 nm. The ability of an antioxidant to scavenge ABTS free radicals is associated with the decrease in absorbance of the free radical.
BS3 demonstrated the highest antioxidant activity in ABTS assay (IC50 24.91 μg/mL), while ascorbic acid (standard) exhibited an IC50 ABTS of 0.521 μg/mL. It can be stated that the antioxidant activity of BS3 was around fifty-fold higher than that of ascorbic acid. In a previous research, it was reported that a methanol extract of sesame oil 2% (w/v) showed 58% ABTS scavenging activity, while α-tocopherol standard exhibited 46% activity.14
Free radicals are produced in human body through reactions catalyzed by Fe (III). In FRAP method, antioxidants reduce Fe (III) to Fe (II), which forms a complex with TPTZ in acetate buffer (pH 3.6). The Fe (II)-TPTZ complex is blue colored and gives characteristic absorption at 593 nm. Antioxidant compounds can reduce Fe (III) to Fe (II) if their reduction potential is lower than that of Fe (III)/Fe (II) (0.77 V). The increase in absorbance of Fe (II)-TPTZ corresponds to the antioxidant capacity. The concentration of a sample or standard that can exhibit 50% of FRAP capacity is EC50 of FRAP capacity.
Sani et al.28 reported that 1 mL of n-hexane extracts of brown sesame and WS seed oil collected from Nigeria exhibited 70.7 and 96.8% FRAP capacity, respectively. Another study reported that 9 mg/mL of methanol extract of BS seed exhibited 98.55% FRAP capacity.29 Vishwanath et al.25 reported that 25 mg/mL of ethanolic extract of WS seed exhibited higher reducing power than ethanolic extract of BS seed. These results were different from that observed in the present study, which presented the antioxidant capacity as EC50 of FRAP. The EC50 FRAP of BS3, WS3, and ascorbic acid was 222.40, 872.57, and 12.01 μg/mL, respectively. It was previously reported that the BS variety B2 had the highest total ORAC value (132.33 μmol TE/g) among all sesame varieties.13
Nigam et al.29 reported that the TPC in methanol extract of BS seed collected from Agra-India was 1.948 g GAE/100 g dry weight. This result is in agreement with the results of the present study, where the TPC in BS3 from East Java-Indonesia was 1.57 g GAE/100 g extract and that in WS3 was 0.89 g/100 g extract. Bopitiya and Madhujith14 previously reported that the TPC in methanol extract of sesame seed oil was 2.6 g GAE/100 g extract. Other studies reported that the TPC in ethanol extract of WS seed and BS seed was 0.288 and 0.138 g/100 g, respectively,25 while that in ethanol extract from 35 sesame seed cultivars ranged from 0.375 to 0.392 g GAE/100 g extract.12 Zhou et al. demonstrated that the TPC in BS seed (4.54–7.32 g GAE/kg) was higher than that in WS seed varieties (3.56–4.04 g GAE/kg).13 We observed similar results in the present study, wherein the TPC in BS1 was higher than that in WS1, BS2 was higher than that in WS2, and BS3 was higher than that in WS3.
The TPC in BS2 (1.15 g GAE/100 g) was higher than that in WS2 (0.48 g GAE/100 g). In ABTS assay, BS2 had similar antioxidant activity (IC50 141.19 μg/mL) to WS2 (IC50 136.08 μg/mL). This could be because most of the phenolic compounds in WS2 had high antioxidant activity.
Sani et al.28 reported that the TFC in n-hexane extract of WS seed oil from Nigeria (480 mg/g) was higher than that in brown sesame seed (360 mg/g). The TFC in ethanol extract of 35 sesame seed cultivars ranged from 0.13 to 0.15 g QE/100 g extract.12 The above findings are in agreement with the results of the present study in which the TFC in BS3 was 0.29 g QE/100 g extract. Vishwanath et al.25 reported that the TFC in ethanol extract of WS seed (0.12 mg CE/g extract) was higher than that in BS seed extract (0.05 mg CE/g extract). This result was contradictory to that reported by Zhou et al. who reported that the TFC in BS varieties showed no significant differences with WS varieties.13
The TFC in BS3 (0.29 g QE/100 g extract) was lower than that in WS3 (1.33 g QE/100 g extract); however, the antioxidant activity of BS3 (IC50 24.81 μg/mL) was higher than that of WS3 (IC50 43.18 μg/mL) in DPPH assay. A possible reason might be that BS3 had the highest amount of flavonoid compounds with ortho di-OH in C3′–C4′, double bond in C2–C3, —OH in C3, and oxo function in C4.30
The increase in TFC and TPC may be related to the increase in antioxidant activities, indicated by lower IC50 DPPH, IC50 ABTS, and EC50 FRAP. Therefore, TPC and TFC were significantly negatively correlated with IC50 DPPH or IC50 ABTS or EC50 FRAP.31 Thaipong et al.32 reported that Pearson's correlation coefficient was significantly negative if −0.61 ≤ r ≤ −0.97 and significantly positive if 0.61 ≤ r ≤ 0.97.
Table 2 shows that the TPC in all BS extracts (n-hexane, ethyl acetate, and ethanol) exhibited significant and negative correlation with their IC50 ABTS and EC50 FRAP. From the results of ABTS and FRAP assays, it can be suggested that phenolic compounds in BS were the major contributors to their antioxidant activities.
In a previous study,12 the coefficient of correlation between TPC, TFC, and percentage of DPPH scavenging activities was analyzed in 35 sesame cultivar extracts. A significant and positive correlation indicated a good correlation, suggesting that TPC or TFC or both may have contributed to the increase in percentage of DPPH scavenging activity. Thus, TPC and TFC in all the 35 cultivars exhibited significant and positive correlation with the percentage of DPPH scavenging activities (R2 = 0.8832, R2 = 0.8504, p < 0.01, respectively). Similar results were observed by Bopitiya and Madhujith14 who reported that the quantity of sesame oil extract exhibited a significantly positive correlation with the percentage of DPPH and ABTS scavenging activities (R2 = 0.972, R2 = 0.892, respectively).
The limitations of the present study exposed that the higher TPC and TFC in extracts of two varieties of sesame seed did not always correlated with the higher antioxidant activity by DPPH, ABTS and FRAP methods. Therefore it need a future study to identify phenolic and flavonoid compounds in sesame seeds extracts which have antioxidant activity by DPPH, ABTS and FRAP methods.
Conclusion
The extracts of the two varieties of sesame seeds (black and white) with different polarities can be categorized as very strong antioxidants using the DPPH assay. Phenolic compounds in BS seed extracts were found to be the major contributors to the antioxidant activity by using ABTS and FRAP methods. White and black sesame seeds have the potential to be developed as sources of natural antioxidants.
Recommendation
Based on the results of the present study, the use of white and black sesame (S. indicum L.) seeds as a diet supplement is recommended.
Authors' contributions
SH conducted antioxidant studies and statistical analysis interpretation. KR supervised the work. IF designed the study, wrote the article, and carried out careful revision of this article. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgement
The authors are grateful to the authorities of School of Pharmacy-Bandung Institute of Technology for providing the necessary facilities to perform this research.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Othman A., Mukhtar N.J., Ismail N.S., Chang S.K. Phenolics, flavonoids content and antioxidant activities of 4 Malaysian herbal plants. Int Food Res J. 2014;21(2):759–766. [Google Scholar]
- 2.Yadav B.S., Yadav R., Yadav R.B., Garg M. Antioxidant activity of various extracts of selected gourd vegetables. J Food Sci Technol. 2016;53(4):1823–1833. doi: 10.1007/s13197-015-1886-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tharasena B., Lawan S. Phenolics, flavonoids and antioxidant activity of vegetables as Thai side dish. APCBEE Procedia. 2014;8:99–104. [Google Scholar]
- 4.Kasali F.M., Wendo F.M., Muyisa S.K., Kadima J.N. Comparative hypoglycemic activity of flavonoids and tannins fractions of Stachytarpheta indica (L.) Vahl leaves extracts in guinea-pigs and rabbits. Int J Pharm Pharm Res Human. 2016;5(2):48–57. [Google Scholar]
- 5.Parashar S., Sharma H., Garg M. Antimicrobial and Antioxidant activities of fruits and vegetable peels: a review. J Pharmacogn Phytochem. 2014;3(1):160–164. [Google Scholar]
- 6.Zou Z., Xi W., Hu Y., Nie C., Zhou Z. Antioxidant activity of Citrus fruits. Food Chem. 2016;196:885–896. doi: 10.1016/j.foodchem.2015.09.072. [DOI] [PubMed] [Google Scholar]
- 7.Somawathi K.M., Rizliya V., Wijesinghe D.G.N.G., Madhujith W.M.T. Antioxidant activity and total phenolic content of different skin coloured brinjal (Solanum melongena) Trop Agric Res. 2014;26(1):152–161. [Google Scholar]
- 8.Moukette B.M., Moor V.J.A., Nya C.P.B., Nanfack P., Nzufo F.T., Kenfack M.A., Ngogang J.Y., Constant Anatole Pieme C.A. Antioxidant and synergistic antidiabetic activities of a three-plant preparation used in Cameroon folk medicine. Int Sch Res Notices. 2017;2017:1–7. doi: 10.1155/2017/9501675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venkatachalam K., Rangasamy R., Krishnan V. Total antioxidant activity and radical scavenging capacity of selected fruits and vegetables from South India. Int Food Res J. 2014;21(3):1039–1043. [Google Scholar]
- 10.Raman S.T., Ganeshan A.K.P.G., Chen C., Jin C., Li S.H., Chen H.J., Gui Z. In vitro and in vivo antioxidant activity of flavonoid extracted from mulberry fruit (Morus alba L.) Pharmacogn Mag. 2016;12(46):128–133. doi: 10.4103/0973-1296.177910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iamjuda K., Srimata S., Sangwanangkulb P., Waseec S., Thaipong K. Antioxidant properties and fruit quality of selected papaya breeding lines. Sci Asia. 2016;42:332–339. [Google Scholar]
- 12.Rizki H., Kzaiber F., Elharfi M., Latrache H., Zahir H., Hanine H. Physicochemical characterization and in vitro antioxidant capacity of 35 cultivars of sesame (Sesamum indicum L.) from different areas in Morocco. Int J Sci Res. 2014;3:2306–2311. [Google Scholar]
- 13.Zhou L., Lin X., Abbasi A.M., Zheng B. Phytochemical contents and antioxidant and antiproliferative activities of selected black and white sesame seeds. BioMed Res Int. 2016:2016. doi: 10.1155/2016/8495630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bopitiya D., Madhujith T. Antioxidant activity and total phenolic content of sesame (Sesamum indicum L.) seed oil. Trop Agric Res. 2014;24:296–302. [Google Scholar]
- 15.Blois M.S. Antioxidant determination by the use of stable free radicals. Nature. 1958;181:1199–2000. [Google Scholar]
- 16.Li X.C., Wang X.Z., Chen D.F., Chen S.Z. Antioxidant activity and mechanism of protocatechuic acid in vitro. J Funct Food Health Dis. 2011;1:232–244. [Google Scholar]
- 17.Benzi I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 18.Pourmorad F., Hosseinimehr S.J., Shahabimajd N. Antioxidant activity, phenol and flavonoid content of some selected Iranian medicinal plants. Afr J Biotechnol. 2006;5(11):1142–1145. [Google Scholar]
- 19.Chang C.C., Yang M.H., Wen H.M., Chern J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- 20.Apak R., Gorinstein S., Böhm V.K., Schaich M.K. Methods of measurement and evaluation of natural antioxidant capacity/activity: IUPAC technical report. Pure Appl Chem. 2013;85:957–998. [Google Scholar]
- 21.Barreira J.C.M., Ferreira I.C.F.R., Oliveira M.B.P.P., Pereira J.A. Effects of different phenols extraction conditions on antioxidant activity of almond (Prunus dulcis) fruits. J Food Biochem. 2009;33:763–776. [Google Scholar]
- 22.Miliauskas G., Venskutonis P.R., van Beek T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004;85:231–237. [Google Scholar]
- 23.Rashad M.M., Mahmoud A.E., Ali M.M., Nooman M.U., Al-Kashef A.S. Antioxidant and anticancer agents produced from pineapple waste by solid state fermentation. Int J Toxicol Pharmacol Res. 2015;7(6):287–296. [Google Scholar]
- 24.Emmanuel E.U., Onagbonfeoana E.S., Adanma O.C., Precious O.C., Faith A.I., Ndukaku O.Y. In vivo and in vitro antioxidant and hypolipidemic activity of methanol extract of pineapple peels in Wistar rats. Int J Biosci. 2016;8(6):64–72. [Google Scholar]
- 25.Vishwanath H.S., Anilakumar K.R., Harsha S.N., Khanum F., Bawa A.S. In vitro antioxidant activity of Sesamum indicum seeds. Asian J Pharm Clin Res. 2012;5:56–60. [Google Scholar]
- 26.Xu J., Chen S., Hu Q. Antioxidant activity of brown pigment and extracts from black sesame seed (Sesamum indicum L.) Food Chem. 2005;91:79–83. doi: 10.1021/jf034485x. [DOI] [PubMed] [Google Scholar]
- 27.Fidrianny I., Windyaswari A.S., Wirasutisna K.R. Antioxidant capacities of various leaves extract from five colors varieties of sweet potatoes tubers using ABTS, DPPH assays and correlation with total flavonoid, phenolic, carotenoid content. Res J Med Plant. 2013;7(3):130–140. [Google Scholar]
- 28.Sani I., Okpalaoka C.C., Bello F., Warra A.A., Abdulhamid A. Flavonoid content and antioxidant potential of white and brown sesame seed oils. Eur J Biomed Pharm Sci. 2014;1:56–63. [Google Scholar]
- 29.Nigam D., Singh C., Tiwari U. Evaluation of in vitro study of antioxidant and antibacterial activities of methanolic seed extract of Sesamum indicum. J Pharmacogn Phytochem. 2015;3:88–92. [Google Scholar]
- 30.Heim K.E., Tagliaferro A.R., Bobilya D.J. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13:572–584. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 31.Fidrianny I., Johan Y., Sukrasno Antioxidant activities of different polarity extracts from three organs of makrut lime (Citrus hystrix DC) and correlation with total flavonoid, phenolic, carotenoid content. Asian J Pharm Clin Res. 2015;8(4):239–243. [Google Scholar]
- 32.Thaipong K., Boonprakob U., Crosby K., Zevallos L.C., Byrne D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Comp Anal. 2006;19:669–675. [Google Scholar]