Abstract
Objectives
In this study, we aimed to investigate the effects of oral administration of Myrmeleon formicarius (antlion) extract on liver and kidney histology in streptozotocin-induced diabetic mice.
Methods
Twenty-four mice received a single intraperitoneal injection of streptozotocin and were then divided into six groups. Untreated diabetic mice served as the negative control group, and glibenclamide-treated mice served as the positive control group. Mice in the other four groups, namely T1, T2, T3, and T4 groups, received M. formicarius (antlion) extract at 2.5, 5, 7.5, and 10 mg/kg, respectively. Permanent thin sections were used to examine liver and kidney histology.
Results
The most appropriate antihyperglycaemic dosage of the M. formicarius extract was 10 mg/kg for 2 days. Histological examination of the liver and kidneys showed that the antlion extract at 10 and 5 mg/kg exhibited significant tissue-repair effects.
Conclusion
M. formicarius (antlion) extract can not only reduce blood glucose levels but also repair hyperglycaemia-induced tissue damage.
Keywords: Antlion, Histology, Hyperglycaemia, Kidney, Liver
الملخص
أهداف البحث
تهدف هذه الدراسة إلى دراسة تأثير أخذ المستخلص مايرميليون فورميكاريوس (ليث عفرين) عن طريق الفم على أنسجة الكبد والكلى في الفئران المصابة بالسكري الناجم عن الستربتوزوتوزين.
طرق البحث
تم تحضير ٢٤ فأرا عن طريق حقنهم بجرعة واحدة من الستربتوزوتوزين داخل الصفاق وتم تقسيمهم إلى ست مجموعات. مجموعة المراقبة السلبية، ومجموعة المراقبة الإيجابية التي عولجت بغليبينكلاميد. تلقت أربع مجموعات علاجية مستخلص مايرميليون فورميكاريوس (ليث عفرين) بجرعة ٢.٥، و٥، و٧.٥، و١٠ مجم/كجم من وزن الجسم، على التوالي. ثم استخدمت مقاطع رقيقة دائمة لدراسة أنسجة الكبد والكلى للفئران.
النتائج
أظهرت النتائج أن الجرعة الأنسب لخفض مستويات الجلوكوز في الدم بعد يومين من العلاج كانت ١٠ مجم/ كجم من وزن الجسم لمستخلص مايرميليون فورميكاريوس (ليث عفرين). أظهر الفحص النسيجي للكبد والكلى أن مستخلص ليث عفرين ١٠ مجم/كجم من وزن الجسم و٥ مجم/كجم من وزن الجسم ينتج تأثيرات إصلاحية أكثر على الأنسجة التالفة.
الاستنتاجات
مستخلص مايرميليون فورميكاريوس (ليث عفرين) كانت له القدرة على خفض مستوى السكر في الدم كما كانت له أيضا القدرة على إصلاح تلف الأعضاء الناجم عن ارتفاع السكر في الدم.
الكلمات المفتاحية: ليث عفرين, علم الأنسجة, ارتفاع السكر في الدم, الكلى, الكبد
Introduction
Diabetes mellitus (DM), a degenerative disease characterised by high blood glucose levels (hyperglycaemia) owing to metabolic disruption in the body,1, 2 is associated with long-term complications in various organs, particularly the eyes, kidneys, heart, nerves, and blood vessels.3 In 2015, the International Diabetes Federation (IDF) reported that 415 million people are affected by DM worldwide, and it is expected to increase to 642 million patients in 2040. In addition, the prevalence of DM among people aged 20–79 years in Indonesia reached 6.2%.4 Furthermore, the World Health Organisation (WHO) showed that DM was the 6th leading cause of mortality in Indonesia in 2016.5 The lifestyle and eating behaviours of Indonesian people contribute to the rapidly increasing prevalence of DM in Indonesia.6 DM result from either improper response to insulin or insufficient insulin production.7
Sulphonylureas have been widely used for DM management to stimulate insulin production and reduce insulin resistance for approximately 50 years.8, 9, 10 They are derivatives of sulphonamides; however, they do not exhibit antibacterial activity.11 Sulphonylureas stimulate pancreatic β cells to produce more insulin and restore glucose homeostasis12; thus, they are most effective in the management of type 2 DM,13 when the pancreatic β cell function is still somewhat preserved.10, 14 Therefore, certain extent of β cell viability is required for their effectiveness.15, 16
Since “back to nature” has become a trend, natural medicines have gained much interest as generally safe and effective therapeutic agents.17, 18 Many medicinal plants, such as Allium cepa L. (onion), Allium sativum L. (garlic), and Annona squamosa L. (sugar-apple), are used to lower blood glucose levels.19 However, the mechanisms underlying the antihyperglycaemic actions of these medicinal plants are different. For example, Ocimum sanctum inhibits α-glucosidase activity and reduces inflammation and oxidative stress.20 Besides plants, animals present a wide source of useful natural products. In Muncar (Banyuwangi, Indonesia) and Jember Lor (Jember, Indonesia) districts, people consume Myrmeleon formicarius (antlion) larvae as a natural medicine for DM.21 The antihyperglycaemic effects of M. formicarius (antlion) have been verified in various studies. Compounds isolated from M. formicarius (antlion) have been shown to decrease blood glucose levels via a sulphonylurea-like mode of action21, 22; however, further studies are required to confirm these findings. A previous study showed that M. formicarius (antlion) extract at 7.5 mg/kg was effective in lowering blood glucose levels in streptozotocin-treated mice. Another study showed that antlion larvae contained a compound with α-glucosidase inhibitory activity.23 DM could result in cardiovascular complications.24
In this study, we aimed to investigate the effects of the oral administration of M. formicarius (antlion) extract on liver and kidney histology in streptozotocin-treated mice.
Materials and Methods
Drugs and M. formicarius (antlion) extract
Glibenclamide was obtained from Jember pharmacy. Antlion extract was prepared as previously described by Rahma et al.25 Antlions were collected from Mayang village, Jember, Indonesia and identified at the Laboratory of Zoology, Department of Biology Education, University of Jember. They were freshly dried at −80 °C. The antlion extract was prepared by cold maceration. Antlions were soaked in 70% (v/v) ethanol for 72 h at room temperature, followed by filtration. The filtrate was evaporated in a rotary evaporator at 40–50 °C under reduced pressure to remove the solvent. Then, the extract was kept in a refrigerator at 4 °C for future use. Sulphonylureas present in the antlion extract were detected by thin layer chromatography (TLC).26
Animals and experimental design
Male Balb/C mice weighing 20–30 g were selected for the current study. The animal experiments were carried out at the Biomedical Laboratory of Faculty of Dentistry, University of Jember. All animals were kept under standard laboratory conditions of temperature (25 ± 2 °C) and humidity with 12 h:12 h light/dark cycle. Twenty-four mice were randomly divided into six experimental groups (n = 4/group). Mice were acclimatised for 7 days prior to the experiment. Mice were given standard pellet diet and water ad libitum during the whole experiment.
All mice were rendered diabetic by a single intraperitoneal injection of streptozotocin (65 mg/kg, dissolved in 0.1 M citrate buffer, pH 4.5). Diabetes was allowed to develop and stabilise in streptozotocin-injected mice over 1 week. On day 7 post-streptozotocin injection, initial blood glucose levels were measured after 3–4 h fasting using a blood glucose meter. Mice were divided into six groups, as follows:
-
▪
Negative control (NC) group: Mice received normal water.
-
▪
Positive control (PC) group: Mice received glibenclamide.
-
▪
Group T1: Mice received M. formicarius (antlion) extract at 2.5 mg/kg.
-
▪
Group T2: Mice received M. formicarius (antlion) extract at 5 mg/kg.
-
▪
Group T3: Mice received M. formicarius (antlion) extract at 7.5 mg/kg.
-
▪
Group T4: Mice received M. formicarius (antlion) extract at 10 mg/kg.
M. formicarius (antlion) extract was orally administered once daily (volume, 0.5 mL/day) for 5 days. Fasting blood samples were collected at 7, 14, 16, and 20 days post-treatment (dpt) for blood glucose measurement.
Histological examination
Histology specimens were prepared as previously described by Carleton and Drurry.27 After 20 days, mice were sacrificed, and kidneys (±4 gr) and liver (±1.3 gr) were removed, fixed in Bouin's solution for at least 24 h, then and dehydrated in an alcohol series (70, 80, 90, 95, and 100%) for 30 min. They were cleared with xylene for 1.5 h until the colour of xylene turned pale, and then cleared with xylene/paraffin substitute (3:1, 1:1, and 1:3, v/v, respectively) each for 30 min in an oven at 45–50 °C. Then, they were infiltrated with hard paraffin twice daily and embedded in paraffin. Sections were cut and stained with eosin.
Data analysis
All data are presented as the means ± standard deviation (SD). Data were analysed using SPSS software version 20. The effects of treatments on blood glucose levels were analysed using one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test. A P-value < 0.05 indicated statistical significance. Data regarding tissue-repair effects are presented as percentages.
Results
Antlion extract contained sulphonylureas and decreased blood glucose levels
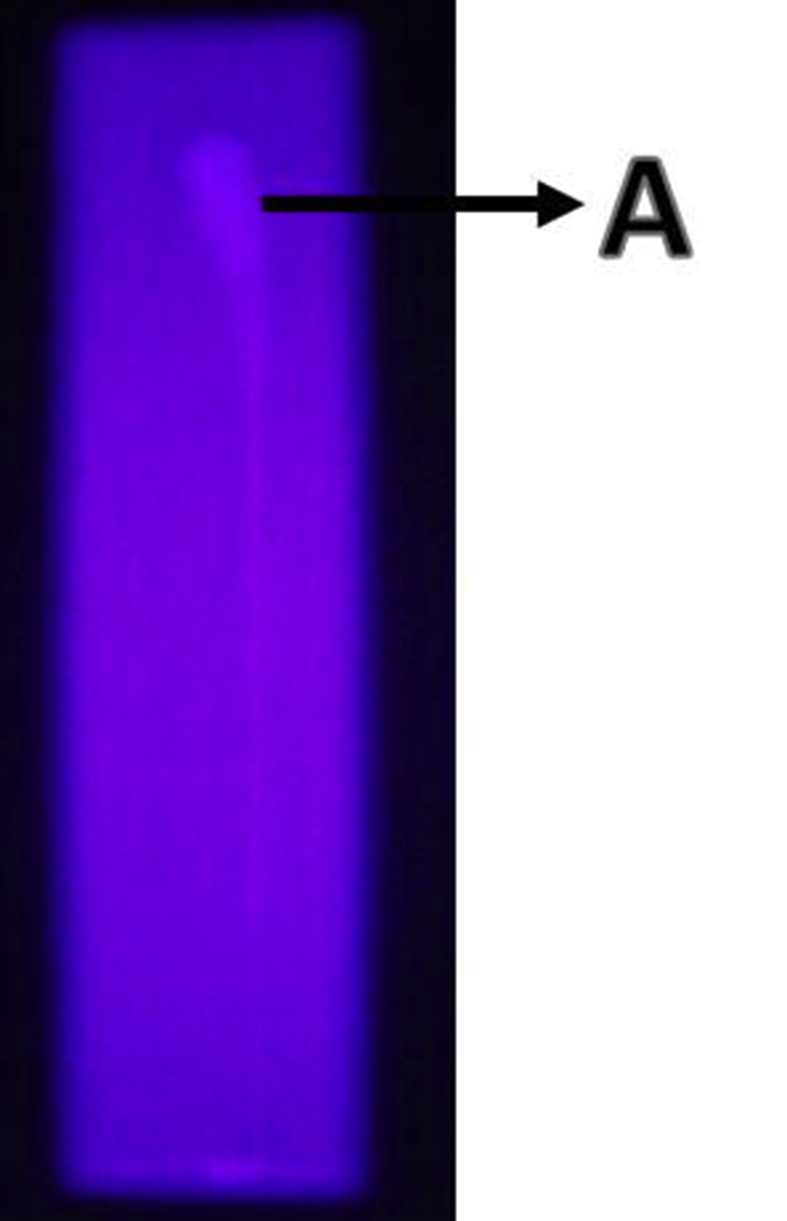
In this study, TLC was used for determination of sulphonylurea content in M. formicarius (antlion) extract. TLC plates were developed in a twin trough glass chamber containing a mixture of EtOAc + EtOH + H2O (10:2:1, v/v) as a mobile phase. The plates were removed from the chamber when the solvent moved to the top of the plates and were subsequently allowed to evaporate. Then, spots on the plates were visualised under visible light, or fluorescent bands were visualised under ultraviolet (UV) light at 366 nm. Result showed that M. formicarius (antlion) extract contained sulphonylureas, indicated by yellow stain (Figure 1).
Figure 1.
Results of TLC of antlion extract. (a) Sulphonylureas TLC stain.
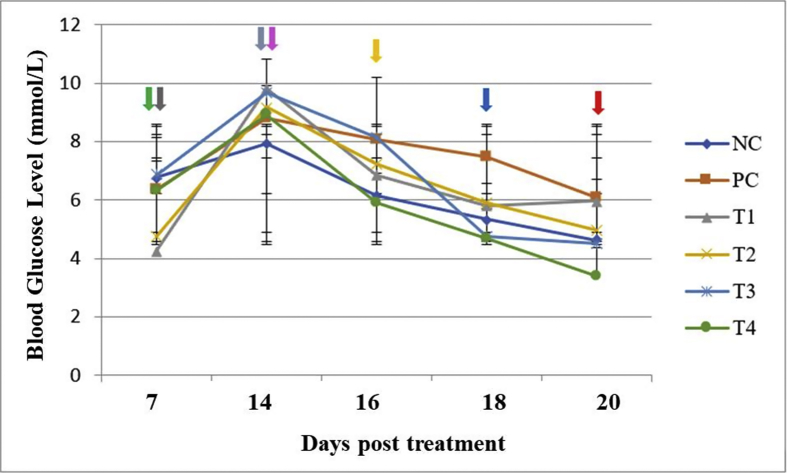
As shown in Figure 2 and Table 1, oral administration of the antlion extract at 2.5, 7.5, and 10 mg/kg for 2 days significantly decreased blood glucose levels, without significant differences among different doses. The effects of antlion extract at these doses were comparable to those of glibenclamide. On the last day (day 6 after antlion extract treatment), blood glucose levels in mice treated with antlion extract at 7.5 and 10 mg/kg were not significantly different from those in mice treated with glibenclamide. Additionally, the results showed that the most appropriate antihyperglycaemic dose of antlion extract was 10 mg/kg.
Figure 2.
Effects of antlion extract on blood glucose levels.  , end of acclimatisation stage;
, end of acclimatisation stage;  , induction by streptozotocin;
, induction by streptozotocin;  , end of diabetic stage;
, end of diabetic stage;  , recovery stage;
, recovery stage;  , collection of blood at 16 days post treatment (dpt);
, collection of blood at 16 days post treatment (dpt);  , collection of blood at 18 dpt;
, collection of blood at 18 dpt;  , collection of blood at 20 dpt; NC, negative control (normal water); PC, positive control (glibenclamide, 0.45 mg/kg); T1, antlion extract at 2.5 mg/kg; T2, antlion extract at 5 mg/kg; T3, antlion extract at 7.5 mg/kg; and T4, antlion extract at 10 mg/kg.
, collection of blood at 20 dpt; NC, negative control (normal water); PC, positive control (glibenclamide, 0.45 mg/kg); T1, antlion extract at 2.5 mg/kg; T2, antlion extract at 5 mg/kg; T3, antlion extract at 7.5 mg/kg; and T4, antlion extract at 10 mg/kg.
Table 1.
Average blood glucose levels.
| Treatment | Average blood glucose level (mg/dL) |
||||
|---|---|---|---|---|---|
| 7 dpt | 14 dpt | 16 dpt | 18 dpt | 20 dpt | |
| NC | 114.75 ± 15.94 | 158.25 ± 11.95 | 145.5 ± 8.1b | 134.75 ± 8.53b | 109.25 ± 7.27c |
| PC | 121.75 ± 15.39 | 142.75 ± 8.42 | 110.75 ± 23.5a | 96 ± 22.16a | 83.25 ± 11.35a |
| T1 | 76.5 ± 11 | 176.5 ± 15.5 | 123.25 ± 8.99a | 104.25 ± 20.1a | 107.25 ± 25.55b |
| T2 | 85.5 ± 15.24 | 165.25 ± 20.61 | 130.25 ± 16.56a | 106.5 ± 18.57a | 89.25 ± 24.14b |
| T3 | 123.5 ± 14.88 | 174.25 ± 19.43 | 146.5 ± 13.22b | 85.5 ± 13.82a | 81 ± 17.53a |
| T4 | 113.75 ± 11.32 | 160.5 ± 15.24 | 106.25 ± 21.06a | 84.25 ± 8.46a | 61 ± 14.02a |
NC, negative control; PC, positive control; T1, dosage of 2.5 mg/kg; T2, dosage of 5 mg/kg; T3, dosage of 7.5 mg/kg; T4, dosage of 10 mg/kg; dpt, days post treatment. Values with similar superscripts within a column are not significantly different at 0.05 probably level.
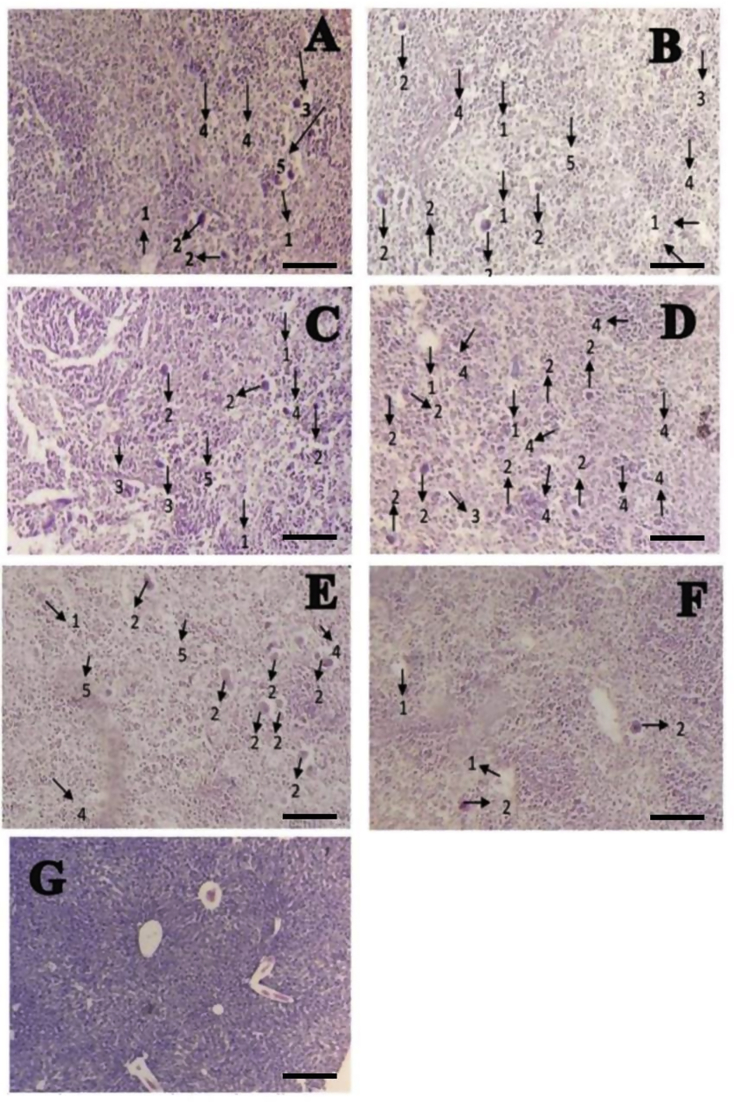
Antlion extract affected liver and kidney histological architecture in streptozotocin-induced diabetic mice
Streptozotocin-induced diabetic mice showed hepatocellular injury, as manifested by presence of inflammatory cells (IC), congestion (C), pyknosis (P), vacuole degeneration (VD), and necrosis (N) (Table 1). Representative images of liver histological examination are shown in Figure 3A–G Liver sections from untreated streptozotocin-induced diabetic mice showed the most severe histological damages (Figure 3A). Oral administration of antlion extract at 10 mg/kg resulted in minimal liver histological deficits in streptozotocin-induced diabetic mice (Figure 3F).
Figure 3.
Histological structure of the liver in male mice (Mus musculus). (A) Negative control group (normal water), (B) positive control group, (C) T1 group, (D) T2 group, (E) T3 group, (F) T4 group, and (G) normal liver. 1, vacuole degeneration; 2, necrosis; 3, congestion; 4, inflammatory cells; and 5, pyknosis. Bar = 1 μm.
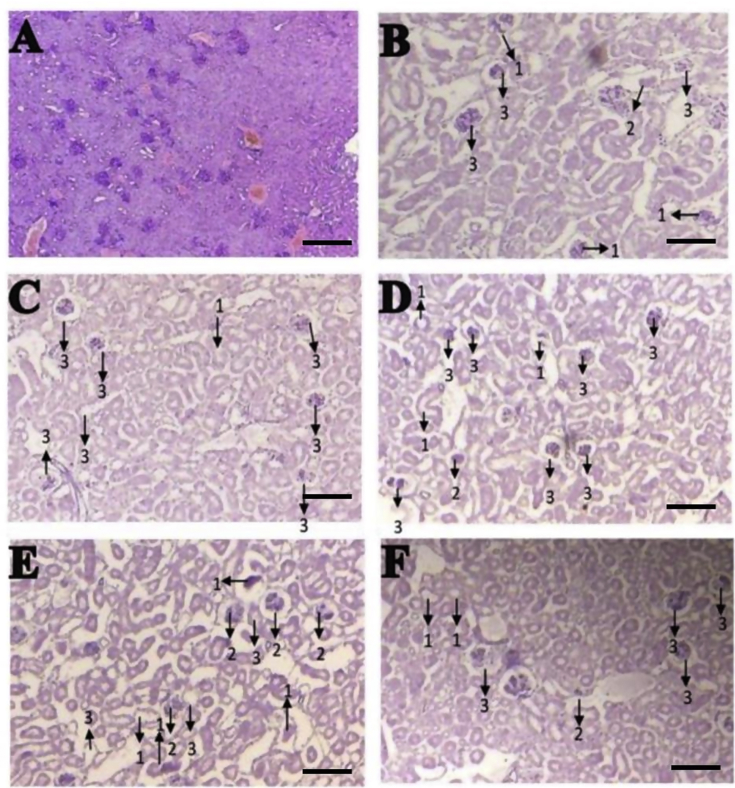
Additionally, streptozotocin-induced diabetic mice exhibited altered renal histological structure, including proximal convoluted tubule degeneration (PD), proximal convoluted tubule necrosis (NP), and glomerular damage (GD) (Table 2). Representative images of the histological examination of the kidneys are shown in Figure 4A–F. Figure 4B represents kidney sections isolated from untreated streptozotocin-induced diabetic mice showing severe damage, whereas Figure 4C represents kidney sections from glibenclamide-treated diabetic mice, showing moderate damage in necrotic tubular cells. The results indicate that oral administration of M. formicarius (antlion) extract at 5 mg/kg resulted in minimal kidney histological deficits in streptozotocin-induced diabetic mice (see Table 3).
Table 2.
Average liver damage.
| Treatment | The average liver damage (%) |
||||
|---|---|---|---|---|---|
| IC | C | P | VD | N | |
| Normal | 18.03 ± 0.32a | 26 ± 0.2a | 0 ± 0a | 50.03 ± 0.06a | 0 ± 0a |
| NC | 50.1 ± 0.25b | 50.13 ± 0.06b | 83.17 ± 0.49f | 85.47 ± 0.25d | 78.27 ± 0.12d |
| PC | 50.03 ± 0.16b | 50.1 ± 0.36b | 50.17 ± 0.35c | 50.13 ± 0.12ab | 50.43 ± 0.31b |
| T1 | 50.23 ± 0.25b | 50.23 ± 0.25b | 50.37 ± 0.06c | 50.37 ± 0.15b | 50.47 ± 0.31b |
| T2 | 50.1 ± 0.17b | 50.07 ± 0.16b | 62.13 ± 0.15d | 50.4 ± 0.1b | 66.5 ± 0.3c |
| T3 | 50 ± 0.25b | 50.07 ± 0.42b | 71.1 ± 0.17e | 69.13 ± 0.15c | 66.4 ± 0.3c |
| T4 | 50.03 ± 0.06b | 50.03 ± 0.21b | 45.17 ± 0.29b | 50.2 ± 0.23ab | 50.4 ± 0.2b |
NC, negative control; PC, positive control; T1, dosage of 2.5 mg/kg; T2, dosage of 5 mg/kg; T3, dosage of 7.5 mg/kg; T4, dosage of 10 mg/kg; IC, inflammatory cells; C, congestion; P, pyknosis; VD, vacuole degeneration; N, necrosis. Values with similar superscripts within a column are not significantly different at 0.05 probably level.
Figure 4.
Histological structure of the kidneys in male mice (Mus musculus). (A) Normal kidney, (B) negative control group, (C) positive control group, (D) T1 group, (E) T2 group, and (F) T4 group. 1, proximal convoluted tubule degeneration; 2, proximal convoluted tubule necrosis; and 3, glomerular damage. Bar = 1 μm.
Table 3.
Average kidney damage.
| Treatment | The average kidney damage (%) |
||
|---|---|---|---|
| Proximal convoluted tubules degeneration | Proximal convoluted tubule necrosis | Glomerular damage | |
| Normal | 0 ± 0a | 0 ± 0a | 14.27 ± 0.23a |
| NC | 50.4 ± 0.26c | 50.33 ± 0.25c | 76.47 ± 0.31d |
| PC | 50.27 ± 0.31c | 48.2 ± 0.2c | 50.43 ± 0.31b |
| T1 | 50.4 ± 0.3c | 50.33 ± 0.35c | 76.3 ± 0.3d |
| T2 | 40.37 ± 0.38b | 45.37 ± 0.21b | 50.23 ± 0.38b |
| T3 | 50.13 ± 0.06c | 50.37 ± 0.26d | 50.27 ± 0.57b |
| T4 | 50.12 ± 0.31c | 50.27 ± 0.21c | 55.43 ± 0.3c |
NC, negative control; PC, positive control; T1, dosage of 2.5 mg/kg; T2, dosage of 5 mg/kg; T3, dosage of 7.5 mg/kg; T4, dosage of 10 mg/kg. Values with similar superscripts within a column are not significantly different at 0.05 probably level.
Discussion
Results of this study showed that M. formicarius (antlion) extract exhibited glucose-lowering effects in streptozotocin-induced diabetic mice. This effect could be attributed to the presence of sulphonylurea- and metformin-like compounds in M. formicarius (antlion) larvae.21, 22, 28, 29 The presence of sulphonylurea compound was verified by the observation of a yellow band on the TLC plate, which is in line with the findings of a previous study.26 Sulphonylureas are insulin secretagogues that stimulate endogenous insulin secretion by binding to sulphonylurea receptor protein on β cells, resulting in inhibition of ATP-dependent potassium channels and K+ efflux from β cells, which depolarises the cell membrane and allows Ca2+ influx. Increased intracellular calcium concentration stimulates the exocytosis of insulin secretory granules.10, 33 Metformin reduces blood glucose levels by various mechanisms,30 mainly via reduction of hepatic glucose production (gluconeogenesis) and improvement of insulin sensitivity in muscle and liver cells.31, 32
Another compound present in antlion larvae extract is a chaperone protein, which was shown to stimulate cell regeneration. A study reported that the saliva of antlion larvae contained a chaperone protein that could induce the secretion of proinflammatory cytokines, where it was shown to play a role in the adaptation of inflammatory cells to stress-induced damage.34 Heat shock proteins (HSPs) constitute a group of chaperone proteins implicated in insulin resistance.35 HSP70 is the most common HSP in humans. It confers resistance against cell death induced by tumour necrosis factor-α (TNF-α), where it could inhibit TNF-α-induced free radical formation, lipid peroxidation, and apoptosis.36 Thus, the chaperone protein present in antlion extract might be responsible for its tissue-repair effects, whereas the antihyperglycaemic effects might be attributed to the presence of sulphonylureas in antlion larvae extract.
The most severe liver damage was observed in untreated streptozotocin-induced diabetic mice (receiving normal water). However, glibenclamide-treated streptozotocin-induced diabetic mice showed repair of cell damage. Glibenclamide belongs to sulphonylureas,37 increasing endogenous insulin secretion. Therefore, it could normalise blood glucose levels and prevent further cell damage.21, 22
Similarly, the most severe kidney damage was also observed in untreated streptozotocin-induced diabetic mice (receiving normal water). Mice treated with glibenclamide showed moderate damage with tubular cell necrosis. Blood reaching the kidneys firstly passes through the glomeruli then the tubules. Thus, hyperglycaemia-induced damage mainly affects the glomeruli. The insulin secretagogue activity of glibenclamide could prevent kidney damage until the tubules.
Results of this study showed that the doses of antlion extract required for repairing hyperglycaemia-induced liver and kidney damage were different. Histological examination of liver and kidney sections showed that antlion extract treatment at 5 and 10 mg/kg resulted in minimal damage in the kidneys and liver, respectively. This difference in the dose required for repairing tissue damage in the liver and kidneys could be explained that the liver is responsible for detoxification of toxic substances reaching it via blood vessels from all organs. Thus, the liver required a higher concentration of antlion extract for repairing hyperglycaemia-induced cell damage than that required for the kidneys. Since the kidney is the second highly vascularised organ after the liver; therefore, it needed a low dose of antlion extract for repairing cell damage.
Conclusions
Mice treated with M. formicarius (antlion) extract at 10 mg/kg showed a significant decrease in blood glucose levels. The doses of M. formicarius (antlion) extract required for repairing liver and kidney damage were different, where a dose of 10 mg/kg was required for repairing liver damage, whereas only 5 mg/kg was sufficient for repairing kidney damage. Further studies are needed to confirm the compounds responsible for these favourable effects and the molecular mechanisms of sulphonylurea and chaperone protein present in antlion larvae.
Source of funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The author(s) have no conflict of interest to declare.
Ethical approval
The experimental protocol was approved by the Animal Ethics Committee of the Medical Faculty University of Jember, Indonesia. Animal experiments were carried out in accordance with the ethical norms of the National Ethics Committee for Research in Health (NECRH), Government of Indonesia.
Authors' contributions
JP, EN, LM, AK, DW, and SH conceived and designed the study, conducted the experiments, provided research materials, and collected and organised the data. EN and JP analysed and interpreted the data. EN and SH wrote the initial and final drafts of the manuscript. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Acknowledgments
The authors would like to thank Tamyis and Ratna Sari Dewi for assisting with the oral administration of antlion extract and permanent thin section processing, respectively.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Riaz S. Study of protein biomarkers of diabetes mellitus type 2 and therapy with vitamin B1. J Diabetes Res. 2015:1–10. doi: 10.1155/2015/150176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mamun A., Islam S., Alam A.K., Rahman M.A., Rashid M. Effects of ethanolic extract of Hibiscus rosa-sinensis leaves on alloxan-induced diabetes with dyslipidemia in rats. Bangl Pharm J. 2013;16:27–31. [Google Scholar]
- 3.Soumya D., Srilatha Late stage complications of diabetes and insulin resistance. J Diabetes Metab. 2011;2:167. [Google Scholar]
- 4.International Diabetes Federation . 2015. Diabetes in Indonesia.http://www.idf.org/membership/wp/indonesia/ [cited 2017 Sep 5] [Google Scholar]
- 5.World Health Organization . 2016. Diabetes country profiles in Indonesia.http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf [cited 2017 Sep 5] [Google Scholar]
- 6.Sami W., Ansari T., Butt N.S., Hamid M.R.A. Effect of diet on type 2 diabetes mellitus. Int J Health Sci. 2017;11(2):65–71. [PMC free article] [PubMed] [Google Scholar]
- 7.Haghvirizadeh P., Haghvirdizadeh P., Mohamed Z., Haerian M.S., Abdullah N.A., Haerian B.S. KCNJ11: genetic polymorphisms and risk of diabetes mellitus. J Diabetes Res. 2015:1–9. doi: 10.1155/2015/908152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Fronzo R.A., Goodman A.M. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The multicenter metformin study group. N Engl J Med. 1995;333:541–549. doi: 10.1056/NEJM199508313330902. [DOI] [PubMed] [Google Scholar]
- 9.Nathan D.M., Buse J.B., Davidson M.B., Ferrannini E. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetes Care. 2009;31(1):193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sola D., Rossi L., Schianca G.P.C., Maffioli P., Bigliocca M., Mella R., Corliano F., Fra G.P., Bartoli E., Derosa G. Sulfonylureas and their use in clinical practice. Arch Med Sci. 2015;11(4):840–848. doi: 10.5114/aoms.2015.53304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zessel K., Mohring S., Hamscher G., Kietzmann M., Stahl J. Biocompatibility and antibacterial activity of photolytic products of sulphonamides. Chemosphere. 2014;100:167–174. doi: 10.1016/j.chemosphere.2013.11.038. [DOI] [PubMed] [Google Scholar]
- 12.Broichhagen J., Schönberger M., Cork S.C., Frank J.A., Marchetti P., Bugliani M., Shapiro A.M.J., Trapp S., Rutter G.A., Hodson D.J., Trauner D. Optical control of insulin release using a photoswitchable sulfonylurea. J Nat Commun. 2014:1–11. doi: 10.1038/ncomms6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibrahim R. Diabetes mellitus type II: review of oral treatment options. Int J Pharm Pharmaceut Sci. 2010;2(1):21–30. [Google Scholar]
- 14.Mkele G. What's the latest on sulfonylureas in the management of type 2 diabetes? S Afr Pharm J. 2013;80(5):12–16. [Google Scholar]
- 15.Fowler M.J. Diabetes treatment: oral agents. Clin Diabetes. 2010;28(3):132–136. [Google Scholar]
- 16.Hanefeld M. Pioglitazone and sulfonylureas: effectively treating type 2 diabetes. Int J Clin Pract. 2007;61(153):20–27. doi: 10.1111/j.1742-1241.2007.01361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunle, Folashade O., Egharevba, Omoregie H., Ahmadu, Ochogu P. Standardization of herbal medicines. Int J Biodivers Conserv. 2012;4(3):101–112. [Google Scholar]
- 18.Nasry H., Shirzad H. Toxicity and safety of medicinal plants. J Herb Med Pharmacol. 2013;2(2):21–22. [Google Scholar]
- 19.Osadebe P.O., Odoh E.U., Uzor P.F. Natural products as potential sources of antidiabetic drugs. Br J Pharm Res. 2014;4(17):2075–2095. [Google Scholar]
- 20.Mandare P.A., Neelam D., Smita V.P., Gaikwad P.B. Study of total cholesterol, triacylglycerols, high-density lipoprotein cholesterol in type 2 diabetes mellitus. Asian J Pharmaceut Clin Res. 2017;10(2):116–118. [Google Scholar]
- 21.Narulita E. Uji Efektifitas Undur-undur Darat terhadap Penurunan Gula Darah Mencit (Mus musculus) [Affectivity of antlion in decreasing blood glucose level of mice] J Sains Teknol. 2008;7(1):6–11. [Google Scholar]
- 22.Kurniasih T., Isma'il M., Susilowati F., Lestari S.P. Faculty of Biology, Universitas Gadjah Mada; Yogyakarta: 2006. Kajian potensi undur-undur darat (Myrmeleon sp.) sebagai antidiabetes [Study of antlion as antidiabetic agent] [Google Scholar]
- 23.Tjay T.H., Rahardja K. 7th ed. PT Elex Media Komputindo; Jakarta: 2007. Obat-Obat Penting: Khasiat, Penggunaan, dan Efek-Efek Sampingnya [Important medicine: benefit, dosage and side effect. [Google Scholar]
- 24.Mehta V., Arum S., Pallavi K., Udayabanu, Malairaman Antioxidant, anti-inflammatory, and antidiabetic activity of hydroalcoholic extract of Ocimun sanctum : an in-vitro and in-silico study. Asian J Pharmaceut Clin Res. 2016;9(5):44–49. [Google Scholar]
- 25.Rahma H.H., Sundhani E., Nurulita N.A. Proceeding of the ICMHS. 2016. Antidiabetic activity of powder and ethanolic extract of antlion (Myrmeleon sp.) on Wistar strain white male rats with glucose preload. Jan 7–8; Singapore. [Google Scholar]
- 26.Lestari I. 2011. Identifikasi Komponen Kimia Undur-undur Darat (Myrmeleon sp.) secara Kromatografi Lapis Tipis [Identification of chemical compound of Myrmeleon sp using thin layer chromatography]http://2009/03/kencing-manis-diabetes-mellitus.html [cited 2017 Sep 5] [Google Scholar]
- 27.Carleton H.M., Drurry R.A.B. 3rd ed. Oxford University Press; London: 1957. Histological technique for normal and pathological tissue and the identification of parasites. [Google Scholar]
- 28.Mujahid M.Z., Agistia D.D., Sa'adah M., Nugroho A.E. A combination of bitter gourd ethanolic extract with ant lion larvae aqueous extract for a blood glucose-lowering agent. Int Food Res J. 2013;20(2):851–855. [Google Scholar]
- 29.Muadifah A., Sulistyarti H., Prasetyawan S. Liquid chromatography for analysis of metformin in Myrmeleon sp. J Pur App Chem Res. 2017;6(3):196–206. [Google Scholar]
- 30.Tandiawan F. Pengaruh varian organic cation transporter 1 (OCT-1) terhadap bioavailabilitas dan intoleransi metformin [Effect of organic cation transporter 1 (OCT-1) variant on bioavailability and metformin intolerance] CDK-254. 2017;44(7):512–515. [Google Scholar]
- 31.Giannarelli R., Aragona Coppelli, Prato Del. Reducing insulin resistance with metformin: the evidence today. Diabetes Metab. 2003;29(4):6S28–6S35. doi: 10.1016/s1262-3636(03)72785-2. [DOI] [PubMed] [Google Scholar]
- 32.Viollet B., Guigas B., Garcia N.S., Leclerc J., Foretz M., Andreelli F. Cellular and molecular mechanism of metformin: an overview. Clin Sci. 2012;122(6):253–270. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kramer W., Muller G., Geisen K. Characterization of the molecular mode of action of the sulfonylurea, glimeripiride, at beta-cells. Horm Metab Res. 1996;28(9):464–468. doi: 10.1055/s-2007-979838. [DOI] [PubMed] [Google Scholar]
- 34.Kumar V., Cotran R.S., Robbins S.L. 7th ed. PA Saunders Elsevier Publisher; Philadelphia: 2005. Robbins & cotran pathologic basis of disease. [Google Scholar]
- 35.Widjaja F.F., Santoso L.A., Waspadji S. Peran heat shock protein terhadap resistensi insulin [Role of heat shock protein to insulin resistance] Majalah Kedokteran Indonesia. 2009;58(3):121–128. [Google Scholar]
- 36.Burkart V., Liu H., Bellmann K., Wissing D., Jaattela M., Cavallo M.G., Pozzilli P., Briviba K., Kolb H. Natural resistance of human beta cells toward nitric oxide is mediated by heat shock protein 70. J Biol Chem. 2000;276(26):19521–19528. doi: 10.1074/jbc.M002265200. [DOI] [PubMed] [Google Scholar]
- 37.Snyder K.C., Keegan C. 4th ed. Elsevier, Inc.; Missouri: 2017. Pharmacology for surgical technologist. [Google Scholar]