Abstract
Objectives
Serum magnesium (Mg) levels are often altered in dialysis patients. This study was conducted to ascertain the trends in Mg levels in patients on dialysis treatment.
Methods
A retrospective study was performed in the Dialysis Unit of King Khalid University Hospital, King Saud University, Riyadh, on patients undergoing regular dialysis. Patient demographic data, including body mass index (BMI), serum calcium (Ca), Mg, parathyroid hormone (PTH), cholesterol, and triglycerides were documented.
Results
Of a total of 115 patients, 70 (60.9%) were on haemodialysis (HD), and 45 (39.1%) were on peritoneal dialysis (PD). Of these, 10 patients (8.7%) had Mg levels of <0.7 mmol/L, 13 (11.3%) had 0.7 mmol/L, 24 (20.9%) had 0.8 mmol/L, 26 (22.6%) had 0.9 mmol/L, 16 (13.9%) 1.0 mmol/L, and 26 (23.9%) showed levels of ≥1.1 mmol/L. Approximately 93.0% had increased PTH levels, 43 (37.4%) had decreased serum Ca, 24 (20.9%) had low serum cholesterol, and 60 (52.2%) had low serum triglyceride. PD patients had significantly lower Mg and higher PTH levels compared to HD patients.
Conclusion
Patients with chronic kidney disease are generally considered at risk of developing hypermagnesaemia due to reduced renal excretion. However, a considerable number of dialysis patients in our unit had hypomagnesaemia (or low levels) instead. In addition to other factors, PTH secretion is affected by serum Mg levels. We found a significant correlation between serum Mg and Ca as well as PTH levels. Consequently, optimizing Mg concentration in patients on dialysate is essential to reduce risk of dyslipidaemia, arrhythmias, hyperparathyroidism, or adynamic bone disease.
Keywords: Calcium, Cholesterol, Chronic kidney disease, Dialysis, Magnesium, Parathyroid hormone
الملخص
أهداف البحث
غالبا ما يتغير مستوى المغنيسيوم في الدم عند مرضى غسيل الكلى. أجريت هذه الدراسة للتأكد من اختلاف مستويات المغنسيوم عند المرضى الذين يعالجون بغسيل الكلى.
طرق البحث
أجريت هذه الدراسة الاستعادية في وحدة غسيل الكلى بمستشفى الملك خالد الجامعي٬ جامعة الملك سعود بالرياض٬ على المرضى الذين يخضعون لغسيل الكلى بانتظام. تم توثيق البيانات الديموغرافية للمرضى٬ بما فيها مؤشر كتلة الجسم٬ ومستوى الكالسيوم بالدم٬ والمغنيسيوم٬ وهرمون الغدة الجار درقية٬ والكوليسترول٬ والدهون الثلاثية.
النتائج
من بين ما مجموعه ١١٥ مريضا٬٧٠ (٦٠.٩٪) كانوا على غسيل الكلى الدموي و ٤٥ (٣٩.١٪) على غسيل الكلى البريتوني. من هؤلاء؛ ١٠ مرضى (٨.٧٪) كان مستوى المغنيسيوم >٠.٧ ممول⁄ ل٬ وكان لدى ١٣ (١١.٣٪) ٧٬٠ممول⁄ ل٬ ولدى ٢٤ (٢٠.٩٪) ٠.٨ ممول⁄ ل٬ وعند ٢٦ (٢٢.٦٪) كان ٠.٩ ممول⁄ ل٬ وعند ١٦ (٩٬١٣٪) ٠٬١ ممول ⁄ ل٬ و ٢٦ (٢٣.٩٪) ظهرت المستويات ≥١٬١ممول⁄ ل. تقريبا كان لدى ٩٣٪ من العينة ارتفاع مستويات هرمون الغدة الجار درقية٬ و٤٣ (٣٧.٤٪) انخفاض مستوى الكالسيوم بالدم٬ و ٢٤ (٢٠.٩٪) انخفاض مستوى الكوليسترول و ٦٠ (٢٬٥٢٪) انخفاض الدهون الثلاثية بالدم. أظهر مرضى غسيل الكلى البيرتوني انخفاضا ملحوظا لمستويات المغنسيوم وارتفاعا لمستويات هرمون الغدة الجار درقية بالمقارنة بمرضى غسيل الكلى الدموي.
الاستنتاجات
يعتبر مرضى الكلى المزمن عموما معرضين لخطر الإصابة بزيادة مغنيسيوم الدم بسبب انخفاض تخلص الكلى. ولكن عددا كبيرا من مرضى غسيل الكلى في وحدتنا لديهم انخفاض في مستويات المغنسيوم بدلا من ذلك. بالإضافة إلى عوامل أخرى يتأثر إفراز هرمون الغدة الجار درقية بمستويات المغنسيوم في الدم. كما وجدنا علاقة مهمة بين مستويات المغنسيوم بالدم والكالسيوم وكذلك هرمون الغدة الجار درقية. بناء على ذلك٬ فإن تحسين تركيز المغنسيوم عند مرضى غسيل الكلى مهم لتقليل خطر اضطراب دهون الدم٬ وعدم انتظام ضربات القلب٬ وفرط إفراز هرمون الغدة الجار درقية أو أمراض انعدام الحركة العظمية.
الكلمات المفتاحية: المغنسيوم, هرمون الغدة الجار درقية, الكالسيوم, غسيل الكلى, مرض الكلى المزمن, الكوليسترول
Introduction
Magnesium (Mg++) is the fourth most abundant cation in the body and is involved in various cell functions. In health, the kidneys, gastrointestinal tract, and bones are responsible for maintaining Mg balance and keeping serum Mg++ concentrations in the normal range.1 Dietary intake of magnesium-rich foods is one of the factors responsible for variations in Mg levels among the general population and also among dialysis patients. The local Arab or Saudi diet contains a number of Mg-rich foods, including nuts, seeds, dried fruits, and dairy products. In chronic kidney disease (CKD), the reduced renal excretion of Mg++ and other ions leads to elevations in serum potassium, phosphate, and Mg++.2
Patients with chronic renal failure often have increased body Mg++ content because of reduced renal excretion of Mg leading to hypermagnesaemia.3 CKD, and particularly end-stage renal disease (ESRD), is the only clinical condition where sustained hypermagnesaemia may occur with a positive net Mg++ balance.1 In dialysis patients, hypermagnesaemia is frequent; usually mild (serum Mg++ < 1.5 mmol/L) and asymptomatic, severe and symptomatic hypermagnesaemia can be seen with exogenous Mg++ administration.4 A literature search for the effects of Mg balance in ESRD patients suggests that hypermagnesaemia may have a suppressive effect on PTH synthesis and/or secretion. Therefore, elevated serum Mg++ levels may play a role in the pathogenesis of adynamic bone disease.4
Conversely, hypomagnesaemia is also seen not infrequently in ESRD patients on dialysis. This can be attributed to dietary restrictions, use of loop and thiazide diuretics, and lower concentration of Mg in the dialysate, leading to loss of Mg from the body.5 Hypomagnesaemia is correlated to dyslipidaemia, thus predisposing the individual to vascular calcification, atherosclerosis, and cardiovascular mortality.6 Low Mg++ levels have been associated with impairment of myocardial contractility, intra-dialytic haemodynamic instability, hypertension, and thickening of the carotid inner wall linings.7 In addition, dietary limitations for ESRD patients limit the intake of Mg rich foods, thus increasing the potential for hypomagnesaemia. Mg++ studies in dialysis patients remain controversial with regard to their therapeutic applications.8, 9, 10 It has been reported that hypermagnesaemia can suppress PTH levels; however, it remains to be seen if the reverse is true; i.e., can Mg deficiency cause elevated serum PTH levels?
Both serum calcium and serum Mg++ levels are important in the regulation of serum parathyroid hormone (PTH) levels. Calcium, vitamin D, and phosphorus play a key role in the control of parathyroid gland function in uraemic patients. There is a significant inverse correlation between serum PTH level and serum Mg++ and calcium levels.11, 12 Mg++ can modulate PTH secretion in a way similar to calcium.13 Despite studies conducted on the relationship between Mg++ and PTH, the effect of Mg++ on PTH levels in dialysis patients is not well established. Whereas some studies have demonstrated that serum Mg++ levels did not affect PTH in dialysis patients, several studies have reported a statistically significant inverse correlation between serum Mg++ and PTH in dialysis patients. It is thought that early Mg++ deficiency in humans is characterized by high PTH, since Mg++ ions possess an effect on calcium-sensing receptors in the parathyroid glands similar to that of calcium.14 Conversely, with more severe Mg++ deficiency, PTH secretion has been reported to decrease.15 The influence of serum Mg++ levels on PTH secretion necessitates further investigation.
A number of our dialysis patients have hypomagnesaemia, or low normal Mg levels, rather than the expected hypermagnesaemia. To investigate this issue further, we designed this study to understand the prevalence of hypermagnesaemia and hypomagnesaemia in relation to serum parathyroid and calcium levels, diet, and lipid profile of our dialysis patients.
Materials and Methods
This is a retrospective study (It is a cross-sectional study) performed in the Dialysis Unit of King Khalid University Hospital, King Saud University, Riyadh, KSA, covering patients seen between January 2011 and December 2011. All patients above 16 years of age, who were on regular haemodialysis (HD) (the magnesium content in the dialysate fluid in haemodialysis is 0.5 Mmol/L) or on peritoneal dialysis (PD) (the magnesium content in the peritoneal solution is 0.25 Mmol/L, as magnesium chloride) for more than three months, were included in the study. Patients with active infection or bone malignancy were excluded from the study. The serum Mg++ was measured using standard kits®. The method used to measure serum magnesium is the Siemens Dimensions Assay, using FLEX® Reagent Cartridge. Intact serum PTH (iPTH) was measured by the RIA method using DSL-8000 of the USA. Other pertinent data included age, gender, weight and height for calculation of BMI, serum calcium, serum cholesterol, and serum triglyceride level. Normal values for serum Mg++, serum PTH, serum calcium, serum cholesterol and serum triglycerides were considered as follows: serum Mg++ (0.7–1.1 mmol/L), serum PTH (13–64 ng/L), serum corrected calcium (2.1–2.55 mmol/L), serum cholesterol (3.2–5.2 mmol/L) and serum triglycerides (0.4–1.48 mmol/L).
All HD patients used dialysate with Mg concentration of 0.5 mmol/L, while all PD patients used dialysate fluid with Mg concentration of 0.25 mmol/L. The blood samples were taken pre-dialysis.
Data analysis
For statistical analysis, descriptive data are expressed as the mean ± SD. Comparison between groups was performed using independent t-tests and chi-square tests. One-way ANOVA was performed to determine the relationship between groups of patients to PTH, calcium, cholesterol, and triglyceride levels. Post-hoc analysis was performed to determine the significance of normal, increased, and decreased Mg++ levels with PTH, calcium, cholesterol, and triglyceride levels. Statistical analysis was performed separately on the study group as a whole, females only, males only, or according to BMI or type of dialysis (PD or HD). All statistical analyses were performed using Predictive Analysis Software version 18.0 (PASW, SPSS, IBM, Chicago, Illinois, USA). Statistical significance was fixed at a p value < 0.05.
Results
One hundred and fifteen patients (54 males and 61 females) were studied. Of these, there were 70 (60.9%) on HD and 45 (39.1%) on PD. The mean ± SD age of all patients was 49.16 ± 18.25 years. The mean values of serum Mg++, serum PTH, calcium, cholesterol, and triglycerides were 0.90 ± 0.19 mmol/L, 43.73 ± 43.14 ng/L, 2.16 ± 0.23 mmol/L, 4.03 ± 0.98 mmol/L and 1.64 ± 0.85 mmol/L, respectively. Table 1 shows the demographic features and the levels of PTH, Mg++, Ca, cholesterol, and triglycerides. There were 14 patients (12.2%) who were underweight, 30 (26.1%) of normal weight, 42 (36.5%) who were overweight and 29 (25.2%) who were obese (Table 1).
Table 1.
Demographic characteristics of 115 dialysis patients.
| Variables | Values |
|---|---|
| Age in years, mean ± SD | 49.16 ± 18.25 |
| Mg++ in mmol/L, mean ± SD | 0.90 ± 0.19 |
| PTH in ng/L, mean ± SD | 43.73 ± 43.14 |
| Calcium in mmol/L, mean ± SD | 2.16 ± 0.23 |
| Triglycerides in mmol/L, mean ± SD | 1.64 ± 0.85 |
| BMI classification | |
| Underweight | 14 (12.2%) |
| Normal | 30 (26.1%) |
| Overweight | 42 (36.5%) |
| Obese | 29 (25.2%) |
Overall, 10 patients (8.7%) had Mg++ levels less than 0.7 mmol/L, 13 (11.3%) had Mg++ levels of 0.7 mmol/L, 24 (20.9%) had Mg++ levels of 0.8 mmol/L, 26 (22.6%) had Mg++ levels of 0.9 mmol/L, 16 (13.9%) had Mg++ levels of 1.0 mmol/L, and 26 (23.9%) had Mg++ levels greater than 1.0 mmol/L. Table 2 also shows the relationship of Mg++ levels to serum levels of corrected calcium, PTH, triglycerides, and cholesterol levels. A majority of patients (n = 107, 93.0%) had increased PTH levels, while 7 (6.1%) had decreased PTH levels and 1 (0.9%) had normal PTH levels. 71 patients (61.7%) had normal serum calcium levels, 43 (37.4%) had decreased serum calcium levels, and 1 (0.9%) had increased serum corrected calcium levels. There were 24 patients (20.9%) with decreased serum cholesterol levels; the remaining 91 patients (79.1%) had normal serum cholesterol. There were 53 patients (46.1%) with normal serum triglycerides, 60 (52.2%) with increased serum triglycerides, and 2 (1.7%) with decreased serum triglyceride levels.
Table 2.
Magnesium levels in relation to levels of calcium, PTH, triglycerides and cholesterol.
| Mg++ levels | N (%) | Calcium | PTH | Triglycerides | Cholesterol |
|---|---|---|---|---|---|
| <0.7 | 10 (8.7) | 2.35 ± 0.14 | 19.3 ± 11.8 | 1.90 ± 0.77 | 3.39 ± 0.74 |
| 0.7 | 13 (11.3) | 2.13 ± 0.25 | 34.68 ± 18.5 | 1.83 ± 1.17 | 4.61 ± 1.01 |
| 0.8 | 24 (20.9) | 2.19 ± 0.23 | 40.18 ± 4.9 | 1.45 ± 0.54 | 4.10 ± 0.99 |
| 0.9 | 26 (22.6) | 2.11 ± 0.21 | 56.99 ± 52.2 | 1.61 ± 0.88 | 3.80 ± 0.98 |
| 1.0 | 16 (13.9) | 2.11 ± 0.19 | 64.69 ± 58.52 | 1.75 ± 0.88 | 3.97 ± 1.06 |
| 1.1 | 16 (13.9) | 2.13 ± 0.29 | 37.7 ± 35.77 | 1.36 ± 0.77 | 4.14 ± 0.90 |
| >1.1 | 10 (8.7) | 2.13 ± 0.25 | 32.43 ± 28.88 | 1.94 ± 1.08 | 4.18 ± 0.85 |
Effect of dialysis modality
There were 70 patients on haemodialysis, of which 31 were male and 39 female. The mean age was 52.07 years (16–85 ± 16.004). The average BMI was 25.75 ± 5.36 kg/m2. 10 (14.28%) patients had BMI < 18 (underweight), 20 (28.57%) had BMI between 18.5 and 25 kg/m2 (normal), 27 (38.57%) had BMI between 25 and 30 kg/m2 (overweight), and 13 (18.57%) had BMI > 30 kg/m2 (obese).
There were 45 patients on peritoneal dialysis, of which 23 were male and 22 female. The mean age was 43.77 years (17–83 ± 19.92). The average BMI was 28.62 ± 6.79 kg/m2. 4 (8.89%) patients had BMI < 18 (underweight), 10 (22.22%) had BMI between 18.5 and 25 kg/m2 (normal), 16 (35.55%) had BMI between 25 and 30 kg/m2 (overweight), and 15 (33.33%) had BMI > 30 kg/m2 (obese). The biochemical parameters of both HD and PD patients are summarized in Table 3, along with results of Students' T Test.
Table 3.
Comparison of different lab parameters between Hd and Pd patients.
| HD (n = 70) |
PD (n = 45) |
p Value | |||||
|---|---|---|---|---|---|---|---|
| Mean | Range | St Dev (±) | Mean | Range | St Dev | ||
| Mg (mmol/L) | 0.993 | 0.7–1.3 | 0.162 | 0.802 | 0.5–1.3 | 0.95 | 0.000 |
| PTH (pmol/L) | 39.21 | 0.52–184.4 | 40.458 | 51.57 | 1.67–249 | 46.72 | 0.027 |
| Corr Ca (mmol/L) | 2.07 | 1.72–2.44 | 0.187 | 2.203 | 1.5–2.7 | 0.29 | 0.037 |
| Cholesterol (mmol/L) | 3.79 | 2.8–5.6 | 0.85 | 4.26 | 2.4–6.6 | 1.09 | 0.047 |
| Triglycerides (mmol/L) | 1.62 | 0.3–4.4 | 0.88 | 1.69 | 0.17–4.4 | 0.84 | 0.746 |
HD – haemodialysis, PD – peritoneal dialysis, n – number of patients, St Dev – standard deviation, Mg – serum magnesium level, PTH – parathyroid hormone, Corr Ca – serum corrected calcium.
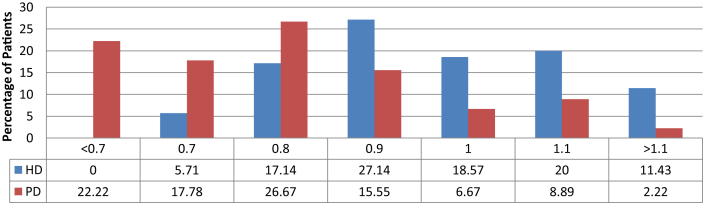
We found that PD patients had lower levels of serum Mg compared to HD patients (Table 3). Among the 45 PD patients, 10 patients (22.22%) had Mg levels below the recommended normal levels, while none of the patients on HD had low magnesium levels. However, 23% of HD patients and 44.45% of PD patients had serum Mg levels in the low to normal range (<0.9 mmol/L). The distribution of Mg levels in both dialysis modalities is shown in Table 4, Figure 1.
Table 4.
Magnesium levels in relation to dialysis modality.
| Mg++ levels | HD |
PD |
||
|---|---|---|---|---|
| No of patients | Percentage (%) | No of patients | Percentage (%) | |
| <0.7 | 0 | 0 | 10 | 22.22 |
| 0.7 | 4 | 5.71 | 8 | 17.78 |
| 0.8 | 12 | 17.14 | 12 | 26.67 |
| 0.9 | 19 | 27.14 | 7 | 15.55 |
| 1.0 | 13 | 18.57 | 3 | 6.67 |
| 1.1 | 14 | 20.00 | 4 | 8.89 |
| >1.1 | 8 | 11.43 | 1 | 2.22 |
Figure 1.
Mg level in HD and PD patients.
We did not find any statistically significant correlations between Mg++ levels and an increase or decrease in BMI, cholesterol, and triglycerides. However, there were significant correlations between serum Mg++ levels and serum parathyroid and corrected calcium levels. A decrease in serum Mg++ was significantly associated with an increase in serum calcium (p = 0.046, 95% CI = 0.003–0.460). Serum Mg++ was also found to be inversely correlated to PTH levels (p = 0.01, 95% CI = −37.846–21.453).
Discussion
The imbalances in Mg++ levels among chronic kidney disease patients may be due to decreased dietary intake and impaired intestinal absorption, versus reduced renal excretion of Mg by the kidneys. Impaired absorption of Mg++ seems to be related to deficient synthesis of the active metabolite of vitamin D by the nonfunctioning kidney. Bone concentrations and total body Mg++, however, frequently appear to be increased.15
Biochemical measurement of serum Mg++ remains the most frequently used test to assess the body's Mg++ status. Although this measurement is informative regarding Mg++ levels in the circulation, nevertheless a normal serum Mg++ level must be interpreted with caution. This is due to the nature of Mg++ distribution in the human body. Even when tissue and cellular Mg++ is depleted by as much as 20%, serum Mg++ levels (which represent only 1% of the body's total content of Mg++) may remain normal.16 Renal patients cannot undergo the Mg++ loading test that can detect latent Mg++ deficiency. Biochemical measurement of intracellular Mg++, the fraction that constitutes >99% of the total Mg++ content in humans, is not commonly reported, due to both technical difficulties and poor correlation of the measured levels with other clinical and biochemical parameters. In addition, some of the methods used to measure intracellular Mg++ are research tools beyond the scope of clinical laboratories.
Despite the above-mentioned limitations of Mg++ measurement, renal patients undergoing regular HD or PD are generally expected to develop hypermagnesaemia due to inefficient renal excretion of Mg++. Therefore, they are recommended to undergo frequent assessment of serum Mg++ levels because assessing the rate of rise in serum Mg++ levels is of the utmost importance. A rapid rise in serum Mg++ is clinically more harmful than a slow rate of increase.17 Conversely, recent studies suggest that hypomagnesaemia occurs more frequently among dialysis patients than previously realized.18 This can be attributed to dietary restrictions for ESRD patients who limit the intake of Mg-rich foods and concomitant use of loop and thiazide diuretics. Nonetheless, following the initiation of dialysis treatment, the major determinant of Mg++ balance is the concentration gradient of Mg++ between serum Mg levels and the dialysate, leading to an increased loss of Mg++ during dialysis.
Hypomagnesaemia in dialysis patients can lead to a number of symptoms, including anorexia, fatigue, and muscle weakness. As the deficiency worsens, muscle cramps, arrhythmias, and abnormalities in cardiac motility might develop. These data show that PD patients are more vulnerable to hypomagnesaemia and its consequences. This can be due to decreased dietary intake, loss of Mg during dialysis due to low Mg dialysis fluids, and effects of elevated PTH.18
In this study, we found no significant correlation between serum Mg++ levels and an increase or decrease in BMI, cholesterol, and serum triglyceride levels. However, we found a significant correlation between serum Mg++ and serum PTH and calcium levels. Our findings are similar to the study conducted by Gohda et al. on 86 HD patients not treated with vitamin D, where serum iPTH levels correlated negatively with serum Mg++ and where the authors concluded that serum Mg++ levels could predict serum iPTH levels.19 Still, other studies, including one by Gonella et al., found no significant changes in serum PTH levels, and serum Mg++ levels had no appreciable influence on PTH secretion in uraemic patients on regular HD.20 In addition, a number of studies emphasize that serum Mg++ is valuable in improving patient wellbeing and limiting cardiovascular morbidity.21
Nevertheless, analysing our data on the basis of dialysis modality indicates that patients on PD had overall lower Mg levels compared to HD patients. A significant number of patients on PD (>20%) had hypomagnesaemia (levels <0.7 mmol/L), while the majority of the remaining patients had Mg levels in the lower normal range (<0.9 mmol/L). On the other hand, none of the HD patients had hypomagnesaemia and 11% of HD patients had Mg levels above the normal range, compared to only 2% of PD patients. However, almost 23% of the HD patients had serum Mg levels in the low to normal range (<0.9 mmol/L). This can be attributed to the difference in dialysate Mg concentrations as well as dietary restrictions between PD and HD patients. Still, the laboratory results do not reveal the exact state of Mg in the body, and serum Mg levels can be normal even as cellular Mg is depleted.22 Therefore, Mg deficiency in the dialysis population might be more widespread than is commonly realized. The higher prevalence of hypomagnesaemia seen in PD patients can be ascribed to the lower concentration of Mg in PD fluids compared to the HD dialysate used in our unit, and also to the relatively greater use of diuretics in PD patients, since more PD patients have residual renal function, especially at the start of dialysis.23
Conclusion
In view of the findings of the current study, we can conclude that hypomagnesaemia and Mg deficiency might be more prevalent among ESRD patients using renal replacement therapy (HD and PD) than is commonly realized. We found a significant correlation between serum Mg±± and PTH levels as well as to serum calcium levels. It is important to take into account the fact that serum Mg±± may be normal even though a state of intracellular depletion exists.24 Consequently, optimizing the Mg±± concentration in dialysate to maintain serum levels in an acceptable range is essential to improving patient wellbeing and avoiding the complications of hypomagnesaemia, which include cardiac arrhythmias, hyperparathyroidism, and adynamic bone disease.
Conflict of interest
The author has no conflict of interest to declare.
Author's contribution
This paper is a single author. AHM it is my work, no plagiarism. AHM approved the draft and final version and I allow to publish it. I agree to stand accountable for all aspects of the work.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Spiegel D.M. Magnesium in chronic kidney disease: unanswered questions. Blood Purif. 2011;31:172–176. doi: 10.1159/000321837. [DOI] [PubMed] [Google Scholar]
- 2.Kanbay M., Goldsmith D., Uyar M.E., Turgut F., Covic A. Magnesium in chronic kidney disease: challenges and opportunities. Blood Purif. 2010;29:280–292. doi: 10.1159/000276665. [DOI] [PubMed] [Google Scholar]
- 3.Musso C.G. Magnesium metabolism in health and disease. Int Urol Nephrol. 2009;41:357–362. doi: 10.1007/s11255-009-9548-7. [DOI] [PubMed] [Google Scholar]
- 4.Navarro-González J.F. Magnesium in dialysis patients: serum levels and clinical implications. Clin Nephrol. 1998;49:373–378. [PubMed] [Google Scholar]
- 5.Navarro-Gonzalez J.F., Mora-Fernandez C., Garcia-Perez J. Clinical implications of disordered magnesium homeostasis in chronic renal failure and dialysis. Semin Dial. 2009;22:37–44. doi: 10.1111/j.1525-139X.2008.00530.x. [DOI] [PubMed] [Google Scholar]
- 6.Ansari M.R., Maheshwari N., Shaikh M.A., Laghari M.S., Darshana Lal K., Ahmad K. Correlation of serum magnesium with dyslipidemia in patients on maintenance hemodialysis. Saudi J Kidney Dis Transpl. 2012;23:21–25. [PubMed] [Google Scholar]
- 7.Kelepouris E., Agus Z.S. Hypomagnesemia: real magnesium handling. Semin Nephrol. 1998;18:58–73. [PubMed] [Google Scholar]
- 8.Galli D., Staffolani E., Miani N., Morosetti M., Di Daniele N. Treatment of electrolyte disorders by hemodialysis. G Ital Nefrol. 2011;28:408–415. [PubMed] [Google Scholar]
- 9.Tzanakis I.P., Oreopoulos D.G. Beneficial effects of magnesium in chronic renal failure: a foe no longer. Int Urol Nephrol. 2009;41:363–371. doi: 10.1007/s11255-008-9510-0. [DOI] [PubMed] [Google Scholar]
- 10.Cho M.S., Lee K.S., Lee Y.K., Ma S.K., Ko J.H., Kim S.W., Kim N.H., Choi K.C. Relationship between the serum parathyroid hormone and magnesium levels in continuous ambulatory peritoneal dialysis (CAPD) patients using low-magnesium peritoneal dialysate. Korean J Intern Med. 2002;17:114–121. doi: 10.3904/kjim.2002.17.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baradaran A., Nasri H. Correlation of serum magnesium with serum parathormone levels in patients on regular hemodialysis. Saudi J Kidney Dis Transpl. 2006;17:344–350. [PubMed] [Google Scholar]
- 12.Navarro J.F., Mora C., Macia M., Garcia J. Serum magnesium concentration is an independent predictor of parathyroid hormone levels in peritoneal dialysis patients. Perit Dial Int. 1999;19:455–461. [PubMed] [Google Scholar]
- 13.Rude R.K., Siger F.R., Gruber H.E. Skeletal and hormonal effects of magnesium deficiency. J Am Coll Nutr. 2009;28(2):131–141. doi: 10.1080/07315724.2009.10719764. [DOI] [PubMed] [Google Scholar]
- 14.Tahara H., Nishizawa Y. Hypomagnesemia and hypoparathyroidism. Clin Calcium. 2007;17:1200–1204. [PubMed] [Google Scholar]
- 15.Cunningham John, Rodríguez Mariano, Messa Piergiorgio. Magnesium in chronic kidney disease Stages 3 and 4 and in dialysis patients. Clin Kidney J. 2012;5(Suppl. 1):i39–i51. doi: 10.1093/ndtplus/sfr166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindeman R.D. Chronic renal failure and magnesium metabolism. Magnesium. 1986;5(5–6):293–300. [PubMed] [Google Scholar]
- 17.Nasri H., Baradaran A. Correlation of serum magnesium with dyslipidemia in maintenance hemodialysis patients. Acta Medica (Hradec Kralove) 2004;47(4):263–265. [PubMed] [Google Scholar]
- 18.Wei M., Esbaei K., Bargman J.M., Oreopoulos D.G. Inverse correlation between serum magnesium and parathyroid hormone in peritoneal dialysis patients: a contributing factor to adynamic bone disease? Int Urol Nephrol. 2006;38(2):317–322. doi: 10.1007/s11255-006-0082-6. [DOI] [PubMed] [Google Scholar]
- 19.Gohda T., Shou I., Fukui M. Parathyroid hormone gene polymorphism and secondary hyperparathyroidism in hemodialysis patients. Am J Kidney Dis. 2002;39(6):1255–1260. doi: 10.1053/ajkd.2002.33399. [DOI] [PubMed] [Google Scholar]
- 20.Gonella M., Bonaguidi F., Buzzigoli G., Bartolini v, Mariani G. On the effect of magnesium on the PTH secretion in uremic patients on maintenance hemodialysis. Nephron. 1981;27(1):40–42. doi: 10.1159/000182018. [DOI] [PubMed] [Google Scholar]
- 21.Massy Ziad A., Tilman B. Drüeke Magnesium and outcomes in patients with chronic kidney disease: focus on vascular calcification, atherosclerosis and survival. Clin Kidney J. 2012;5(Suppl. 1) doi: 10.1093/ndtplus/sfr167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eddington H., Hurst H., Ramli M.T., Speake M., Hutchison A.J. Calcium and magnesium flux in automated peritoneal dialysis. Perit Dial Int. 2009 Sep–Oct;29(5):536–541. [PubMed] [Google Scholar]
- 23.Kanbay M., Yilmaz M.I., Apetrii M. Relationship between serum magnesium levels and cardiovascular events in chronic kidney disease patients. Am J Nephrol. 2012;36:228–237. doi: 10.1159/000341868. [DOI] [PubMed] [Google Scholar]
- 24.Jahnen-Dechent Wilhelm, Ketteler Markus. Magnesium basics. Clin Kidney J. 2012;5(Suppl. 1):i3–i14. doi: 10.1093/ndtplus/sfr163. [DOI] [PMC free article] [PubMed] [Google Scholar]