Abstract
Objectives
KSA has the highest prevalence of diabetes mellitus among Middle Eastern countries with a prevalence range of 21%–24%. Gestational diabetes (GDM) is a well-known risk factor for type 2 diabetes mellitus (T2DM). GDM is associated with a 7-fold increased risk of T2DM. Thus, this research assessed the prevalence and risk factors associated with the development T2DM in a cohort of patients with GDM in KSA.
Methods
The medical records of patients with GDM who visited the outpatient clinics of a tertiary care hospital from 2011 to 2014 were included in this study. Patients with a prior diagnosis of diabetes mellitus before pregnancy and those with GDM who did not have postpartum diabetes screening were excluded.
Results
A total of 123 women with GDM and underwent postpartum diabetes screening, 82 (67%) developed T2DM based on follow-up records. Approximately 45% (37/82) of patients who developed T2DM were screened ≤6 months after delivery, whereas 55%(45/82) were screened >6 months after delivery. Older patients, patients who had a higher number of pregnancies (gravidity and parity), and patients with previous GDM were more likely to develop T2DM.
Conclusion
In KSA, women who developed GDM, particularly those who are older, multigravid, and multiparous and who have a prior history of GDM, are at an increased risk of developing T2DM. Postpartum diabetes screening of patients with GDM within the recommended period need to be improved.
Keywords: Gestational diabetes, Postpartum, Risk factors, Screening, Type 2 diabetes
Introduction
The prevalence of type 2 diabetes mellitus (T2DM) is approximately 8.5% among adults worldwide.1 A study performed in 2013 has shown that KSA has the highest prevalence (23.87%) of T2DM among Middle Eastern countries.2 Another study has shown that the prevalence of T2DM in KSA ranged from 21% to 24% between 1997 and 2011.3
Several risk factors are associated with the development of T2DM.4 Women who have a history of gestational diabetes mellitus (GDM) are at high risk of developing T2DM later in life.5 GDM refers to glucose intolerance of various degrees during pregnancy. Multiple risk factors are associated with GDM, including body mass index (BMI) > 30 kg/m2, history of a baby weighing 4.5 kg or more at birth, previous history of GDM, family history of first-degree relatives with T2DM, and high-risk ethnicity, such as Middle Eastern descent.6 All pregnant women should be screened for the development of GDM during 24–28 weeks of pregnancy. Women with multiple risk factors for GDM must be screened earlier in their pregnancy.7 The diagnosis of GDM is confirmed when one of the following parameters is present: fasting plasma glucose level ≥5.1 mmol/L, 1-h postprandial plasma glucose ≥10.0 mmol/L, or 2-h postprandial plasma glucose ≥8.5 mmol/L.8 Typically most patients with GDM are asymptomatic, and their dysglycaemia is resolved postpartum.9 However, a subset of patients with GDM will develop T2DM after delivery.
The American Diabetes Association recommends screening women with GDM for the persistence of diabetes at 4–12 weeks postpartum via an oral glucose tolerance test (OGTT) and performing subsequent lifelong screening tests at least every 3 years using the clinically appropriate diagnostic criteria for non-pregnant women.8 In contrast, the Canadian Diabetes Association recommends screening women with GDM for the persistence of diabetes within 6 weeks to 6 months after delivery.10
Several studies that measured the prevalence of T2DM in women with a history of GDM have been conducted. A systematic review performed in 2009 has shown that the prevalence of T2DM among women with a history of GDM ranged from 3.16% to 47.25%. Moreover, women with high blood glucose levels during pregnancy have at least a 7-fold increased risk of developing T2DM than those with normal blood sugar level.11
To the best of our knowledge, none of these studies were performed in KSA or in the Gulf region. Thus, this study aimed to assess the prevalence of T2DM in women with a history of GDM. Risk factors, such as age, body mass index (BMI), gravidity, parity, and family history of GDM, were examined to assess their association with the development of T2DM.
Materials and Methods
Study design and sampling technique
We conducted a retrospective chart review to examine the prevalence of T2DM in women with a history of GDM. Data were collected from the medical records of patients who had a diagnosis of GDM and underwent postpartum diabetes screening using structured data collection forms. The study was approved by the institutional review board of King Abdullah International Medical Research Center in Riyadh.
Study setting and participants
This study involved a retrospective chart review of patients who went to the GDM clinics at a tertiary care center in Riyadh. All women diagnosed with GDM between 2011 and 2014 were included in the study. Women who were diagnosed with T2DM or T1DM before gestation and those who presented with GDM but did not undergo postpartum diabetes screening were excluded from the study.
Data collection
Multiple variables, including age, BMI, number of pregnancies (gravida), number of children (para), family history of T2DM, and previous history of GDM, were assessed. The screening status for diabetes during and after pregnancy was identified in all patients. All available screening variables included fasting blood sugar (FBS) level, oral glucose tolerance test (OGTT) result, and glycated haemoglobin (HbA1C) level were collected. The main outcome variable was the development of T2DM after GDM. Patients were categorised into two categories: those who underwent any diabetes screening test within 6 months after delivery versus those who were screened >6 months postpartum. In this study, another category included women who developed T2DM versus those who did not develop T2DM after delivery.
Data management and analysis
The Statistical Package for the Social Sciences software version 21 was used to analyze the data. Numerical variables were presented as means and standard deviation, whereas categorical variables were presented as frequency, percentages, and interquartile range. Chi-square test, Mann–Whitney test, and independent samples t-test were used to test the association between the development of T2DM and several clinical variables, e.g., history of GDM, age, BMI, gravidity, parity, and a family history of T2DM. A p-value <0.05 was considered statistically significant.
Results
This study included 123 female patients with GDM who were diagnosed between 2011 and 2014 and underwent postpartum diabetes screening. The mean age of the patients was 34 ± 4.7 years, with a minimum age of 22 years and a maximum age of 44 years. The characteristics of patients are summarised in Table 1. Majority of patients were obese according to their BMI during the first antenatal clinic visit, and their mean BMI was 35.6 ± 5.2 kg/m2.. Moreover, the median values for gravidity was 6 (4–8), and that for parity was 4 (2–6). In total, 69 (56%) patients had a family history of T2DM, and 87 (71%) had a previous history of GDM. All patients underwent either two fasting glucose tests or fasting glucose and haemoglobin A1C tests as postpartum screening tests for diabetes.
Table 1.
Characteristics of patients with gestational diabetes mellitus (N = 123).
| Characteristics | |
|---|---|
| Age (years), mean ± sd | 34 ± 4.7 |
| BMI (kg/m2) mean ± sd | 35.6 ± 5.2 |
| Gravida (number of pregnancies), median (IQR) | 6 (4–8) |
| Para (number of children), median (IQR) | 4 (2–6) |
| Family history of T2DM, N (%) | 69 (56%) |
| GDM management with insulin, N (%) | 101 (82%) |
| Previous history of GDM, N (%) | 87 (71%) |
| Number of individuals who developed T2DM, N (%) | 82 (67%) |
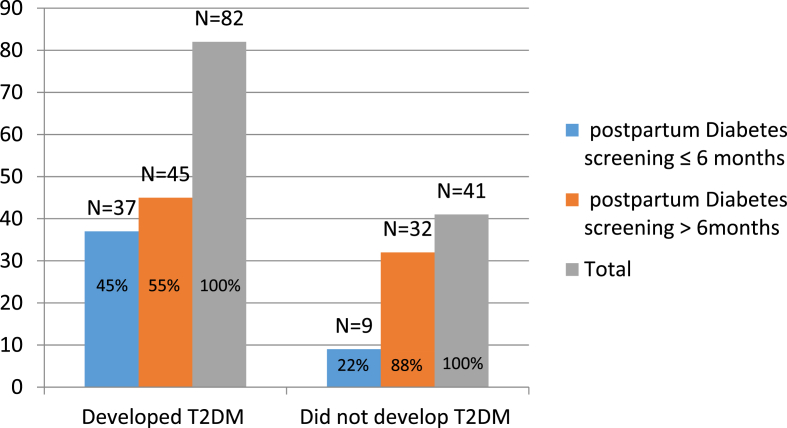
Out of 123 participants, 82 (67%) developed T2DM based on the follow-up records and available results of the postpartum diabetes screening tests. The diagnosis of postpartum T2DM was confirmed by either two fasting glucose readings or one fasting glucose reading and haemoglobin A1C level that are higher than the normal range. In total, 37 (45%) out of 82 patients who developed T2DM were screened during the recommended period (i.e. ≤6 months after delivery), whereas 45 (55%) were screened for T2DM >6 months after delivery (Figure 1).
Figure 1.
Screening practices and development of T2DM (N = 123).
Table 2 shows the risk factors associated with development of T2DM in women with GDM. Participants who developed T2DM were more likely to be older (p = 0.004) and have a higher number of pregnancies (p = 0.003) and children (p = 0.008) than those who did not develop T2DM. Approximately 82% of patients who developed T2DM had a prior history of GDM compared with 49% of those who did not, and this difference was statistically significant (p < 0.001).
Table 2.
Factors associated with the development of diabetes mellitus in patients with gestational diabetes mellitus.
| Developed T2DM (n = 82) | Did not develop T2DM (n = 41) | p-Value | |
|---|---|---|---|
| Age in years (mean ± sd) | 35.1 ± 4.1 | 32.5 ± 5.4 | 0.004a |
| Gravida (number of pregnancies), median (IQR) | 6 (4–8) | 4 (3–7) | 0.003b |
| Para (number of children), median (IQR) | 4 (2–6) | 3 (1–5) | 0.008b |
| Previous GDM (n, %) | 67 (82%) | 20 (49%) | <0.001c |
Student t-test.
Mann–Whitney test.
Chi-square test.
Discussion
KSA is among the countries with the highest prevalence of T2DM due to the high rates of obesity, physical inactivity, and unhealthy dietary habits.12 Another important risk factor for T2DM is a history of GDM. Up to 50% of women who develop GDM will later present with T2DM worldwide; therefore, all patients with GDM must be screened for T2DM within 1–6 months after delivery.10 In this study, we aimed to examine the practice of diabetes screening after GDM and the prevelence of T2DM among women who had GDM in KSA. Moreover, some of the risk factors associated with the development of T2DM in this population were assessed. We conducted a retrospective chart review of the records of patients who were newly diagnosed with GDM during pregnancy and underwent postpartum diabetes screening over 3 years. We found that the proportion of patients who developed T2DM after GDM (67%) was higher than the proportion that was reported in a large systematic review involving 20 cohorts from different populations with different ethnic backgrounds.11 In addition, the prevalence in this study was slightly higher than that reported in a large cohort study in Sri Lanka.13 Moreover, only 25% of women with GDM developed T2DM in a 2015 cohort study in Scotland.14
An important finding of this study showed that diabetes screening within the first 6 months after delivery was only performed in approximately one-third of the patients. This low screening rate is a universal finding, which highlights the need to develop a proper T2DM screening practice for patients with GDM.15 This is especially important because most of these patients are young and still of childbearing age. Periodic reminders for postpartum screening and HbA1c sampling of mothers with GDM during their children's vaccination appointments are some of the suggested strategies. These approaches must be adopted at a national level.16, 17
In addition to GDM, other risk factors associated with the development of T2DM must be determined, and interventions, such as lifestyle modification, should be advocated to reduce the risk of developing T2DM.8 Several studies have attempted to identify factors associated with the risk of developing T2DM in women with GDM via different analyses, such as univariate and multivariate analyses.13 In this study, older patients who are multigravid and multiparous and those who had GDM during prior pregnancies were more likely to develop T2DM later in life. These findings are not in accordance with that described in other studies, which reported no significant association between the risk of developing T2DM and a history of GDM in a previous pregnancy and multiple parity.13, 14 Moreover, multiple studies have reported that increasing age is not associated with the risk of developing T2DM in women with GDM.14, 18 In this study, BMI was not significantly different between women who developed T2DM and those who did not. However, in this study, BMI was during the first antenatal clinic visit, which was during the early first trimester. Data about changes in BMI during pregnancy and, more importantly, after delivery were not available. Therefore, the lack of association between BMI and the development of T2DM is not a valid finding. A BMI higher than the normal range is a known risk factor of T2DM particularly if associated with GDM.
The recommended screening test for diabetes after GDM is OGTT since it is more sensitive in identifying significant dysglycaemia than other diabetes screening tests.8 In our study, all GDM diagnoses were based on OGTT results during 24–28 week gestational period. However, none of the patients underwent OGTT during the postpartum period, and this is likely due to the lack of established evidence-based postpartum diabetes screening protocol and due to the extra steps involved in performing OGTT compared to other diabetes screening test. Fasting glucose and haemoglobin A1C tests were the tests conducted to screen for diabetes after GDM in our study, both tests are relatively easy to conduct than OGTT.
This study is among the few studies in the Gulf region that addressed the development of T2DM after GDM, and it revealed a number of important findings, as discussed above. The limitations of this study include the relatively small number of patients and its retrospective nature. Moreover, the study was conducted only in a single center. Therefore, further large-scale, prospective, multicenter studies must be conducted.
Conclusion/recommendation
In KSA, women with GDM during pregnancy are at high risk of developing T2DM. Older age, multigravidity and multiparity, and prior history of GDM are the risk factors associated with the development of T2DM in this study group. Screening of patients with GDM must be improved through innovative postpartum diabetes screening programs.
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
This study conducted in alignment with King Abdullah international medical research center IRB standards. Patient confidentiality was maintained throughout the study. The research team states that this study is ethically conducted with no conflict of interest.
Authors' contributions
MM, FA, and BA conceived and designed the study. MM, FA, BA, TA, HA, and AO conducted the research, provided research materials, and collected and organised the data. MM, FA, BA, TA, HA, and AO analysed and interpreted the data. FA, BA, MM, AO, and HA wrote the initial and final draft of the article and provided logistic support. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Organization W.H. World Health Organization; 2016. Global report on diabetes. [Google Scholar]
- 2.Majeed A., El-Sayed A.A., Khoja T., Alshamsan R., Millett C., Rawaf S. Diabetes in the Middle-East and North Africa: an update. Diabetes Res Clin Pract. 2014;103(2):218–222. doi: 10.1016/j.diabres.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Al-Daghri N.M., Al-Attas O.S., Alokail M.S., Alkharfy K.M., Yousef M., Sabico S.L. Diabetes mellitus type 2 and other chronic non-communicable diseases in the central region, Saudi Arabia (Riyadh cohort 2): a decade of an epidemic. BMC Med. 2011;9(1):76. doi: 10.1186/1741-7015-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan J.C., Malik V., Jia W., Kadowaki T., Yajnik C.S., Yoon K.-H. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301(20):2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 5.Kim C., Newton K.M., Knopp R.H. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25(10):1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 6.Penman L., Lees C. 22nd ed. Elsevier Limited; London: 2014. Alimentary tract and pancreatic disease. U: Walker BR, Colledge NR, Halston SR, Penman ID. Davidson's principles and practice of medicine. [Google Scholar]
- 7.Buchanan T.A., Xiang A.H. Gestational diabetes mellitus. J Clin Investig. 2005;115(3):485–491. doi: 10.1172/JCI24531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Association A.D. Standards of medical care in diabetes—2017 Diabetes care. [Internet] Diabetes Care. Jan 2017;40(Suppl. 1) [citado 2017 Feb 23] [Google Scholar]
- 9.Kumar P., Clark M.L. Elsevier Health Sciences; 2012. Kumar and Clark's clinical medicine E-Book. [Google Scholar]
- 10.Robinson D., Luthra M., Vallis M. Canadian diabetes association clinical practice guidelines expert committee. Canadian diabetes association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2013;37(Suppl. 1):S1–S212. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Bellamy L., Casas J.-P., Hingorani A.D., Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 12.Memish Z.A., El Bcheraoui C., Tuffaha M., Robinson M., Daoud F., Jaber S. Peer reviewed: obesity and associated factors—Kingdom of Saudi Arabia, 2013. Prev Chronic Dis. 2014:11. doi: 10.5888/pcd11.140236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herath H., Herath R., Wickremasinghe R. Gestational diabetes mellitus and risk of type 2 diabetes 10 years after the index pregnancy in Sri Lankan women—a community based retrospective cohort study. PLoS One. 2017;12(6):e0179647. doi: 10.1371/journal.pone.0179647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eades C.E., Styles M., Leese G.P., Cheyne H., Evans J.M. Progression from gestational diabetes to type 2 diabetes in one region of Scotland: an observational follow-up study. BMC Pregnancy Childbirth. 2015;15(1):11. doi: 10.1186/s12884-015-0457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tovar A., Chasan-Taber L., Eggleston E., Oken E. Peer reviewed: postpartum screening for diabetes among women with a history of gestational diabetes mellitus. Prev Chronic Dis. 2011;8(6) [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta Y., Gupta A. Post-partum screening after gestational diabetes. Lancet Diabetes Endocrinol. 2013;1(2):90–91. doi: 10.1016/S2213-8587(13)70066-4. [DOI] [PubMed] [Google Scholar]
- 17.Noctor E., Crowe C., Carmody L., Avalos G., Kirwan B., Infanti J. ATLANTIC DIP: simplifying the follow-up of women with previous gestational diabetes. Eur J Endocrinol. 2013;169(5):681–687. doi: 10.1530/EJE-13-0491. [DOI] [PubMed] [Google Scholar]
- 18.Rayanagoudar G., Hashi A.A., Zamora J., Khan K.S., Hitman G.A., Thangaratinam S. Springer; 2016. Quantification of the type 2 diabetes risk in women with gestational diabetes: a systematic review and meta-analysis of 95,750 women. [DOI] [PMC free article] [PubMed] [Google Scholar]