Abstract
Objectives
Chitosan, the N-deacetylated derivative of chitin, has useful biological properties that promote haemostasis, analgesia, wound healing, and scar reduction; chitosan is bacteriostatic, biocompatible, and biodegradable. This study determined the efficacy of chitosan derivative film as a superficial wound dressing.
Methods
This multicentre randomised controlled trial included 244 patients, of whom 86 were treated with chitosan derivative film and 84 with hydrocolloid. The percentage of epithelisation, as well as patient comfort, clinical signs, and patient convenience in application and removal of the dressings were assessed.
Results
The primary outcome of this study was the percentage of epithelisation. Except for race (p = 0.04), there were no significant differences between groups in sex, age, antibiotic usage, or initial wound size (p > 0.05). There was no significant difference in the mean epithelisation percentage between groups (p = 0.29). Patients using chitosan derivative film experienced more pain during removal of dressing than those in the hydrocolloid group (p = 0.007). The chitosan derivative film group showed less exudate (p = 0.036) and less odour (p = 0.024) than the control group. Furthermore, there were no significant differences between groups in terms of adherence, ease of removal, wound drainage, erythema, itchiness, pain, and tenderness. No oedema or localised warmth was observed during the study.
Conclusion
This study concluded that chitosan derivative film is equivalent to hydrocolloid dressing and can be an option in the management of superficial and abrasion wounds.
Clinical trial No.
NMRR-11-948-10565.
Keywords: Abrasion, Chitosan derivative, Chitosan film, Hydrocolloid dressing, Superficial wounds
الملخص
أهداف البحث
الشيتوزان هو من مشتقات الكيتين، التي تحتوي على خصائص بيولوجية مفيدة مثل التخثر، ومسكن الألم، والتئام الجروح، وتقليل الندبات، ووقف نمو الجراثيم، والتوافق مع الحياة والتحلل البيولوجي. تحدد هذه الدراسة فعالية الغشاء المشتق من الشيتوزان كضماد للجروح السطحية.
طرق البحث
أدرج في هذه التجربة العشوائية المحكمة متعددة المراكز ٢٤٤ مريضا. تم علاج ٨٦ مريضا بالغشاء المشتق من الشيتوزان و٨٤ تم علاجهم بغرواني مائي. وتم تقييم النسبة المئوية من تشكل النسيج الظهاري، وراحة المرضى والعلامات السريرية بالإضافة إلى راحة المريض في وضع وإزالة الضمادات.
النتائج
النتائج الأولية لهذه الدراسة هي النسبة المئوية من تشكل النسيج الظهاري. لم يكن هناك اختلافات كبيرة بين الجنس، والعمر، واستخدام المضادات الحيوية وحجم الجرح الأولي باستثناء العرق. لم يكن هناك اختلافا ملحوظا في متوسط النسبة المئوية لتشكل النسيج الظهاري بين المجموعتين. عانى المرضى الذين يستخدمون الغشاء المشتق من الشيتوزان من الألم أثناء إزالة الضماد بالمقارنة بمجموعة الغرواني المائي. أظهرت مجموعة الغشاء المشتق من الشيتوزان إفرازات أقل ورائحة أقل بالمقارنة بمجموعة التحكم. بالإضافة إلى ذلك، لم يكن هناك اختلافات كبيرة بين المجموعتين من حيث الالتزام، وسهولة الإزالة، وتصريف الجرح، والالتهاب الجلدي، والحكة، والألم والضغط. لم يلاحظ أي تورم وحرارة موضعية خلال الدراسة.
الاستنتاجات
تستنتج هذه الدراسة أن الغشاء المشتق من الشيتوزان يعادل الضماد الغرواني المائي ويمكن أن يكون خيارا لعلاج الجروح السطحية والتآكل.
الكلمات المفتاحية: غشاء الشيتوزان, مشتقات الشيتوزان, تضميد غرواني مائي, الجروح السطحية, تآكل
Introduction
Chitosan is a natural biopolymer derived from chitin, a cationic polysaccharide composed of glucosamine and N-acetylglucosamine residues. It is a biodegradable, nontoxic, complex carbohydrate derivative of chitin, a major component of crustacean exoskeletons and cell walls of fungi.1, 2
In general, the term chitosan is applied when the extent of deacetylation is >70% and the term chitin is used when <20%. These various grades of chitosan, differing in the degree of deacetylation, have varying effects on its clinical applications.3
Chitosan bioderivatives are chemical modifications of chitosan. These modifications are important for the association between bioactive molecules and polymers and control the drug–release profile. Various chitosan derivatives based on chemical modification include N-(aminoalkyl) chitosan; succinyl, quateraminated, and octanoyl chitosan; mitomycin C-conjugated N-succinyl-chitosan; N-alkyl and acylated chitosan; chitosan hydrochloride; thiolated chitosan, and others.4, 5 This study used chitosan as poly-(1,4-β-d-glucopyranosamine). This N-deacetylated polysaccharide derived from chitin has valuable properties for biomedical application.6, 7, 8, 9, 10, 11
Chitosan exhibits antibacterial, antifungal, haemostatic,12 analgesic, and wound-healing properties13 and promotes scar reduction. Chitosan is bacteriostatic, biocompatible, and biodegradable, with potential use as a wound dressing material.14
The need for an ideal wound dressing has long been recognised. In 1963, Scales wrote that ideal dressings should have high porosity to water vapour and non-adherence to blood clot or a granulating surface, and should permit penetration of capillary loops, absorb free blood exudate, act as a barrier to the passage of microorganism, and follow the wound contour. Dressings should not produce tissue reactions, should not be inflammable, and should be capable of being sealed to the skin. They should be sterilisable and available at a low cost.15
In recent studies, a wide variety of derivatives such as chitosan hydrogel and chitosan/sodium alginate revealed different swelling behaviours. Chitosan hydrogel containing lignin nanoparticles had a synergic antimicrobial effect, making it useful in wound dressing, food packing, and drug delivery applications.50, 51 A variety of dressings are available, suggesting that no one dressing material can be considered suitable for all types of wounds. Wound dressings are generally classified as passive, interactive, or bioactive products with active compounds that aid in wound healing. Chitosan is one such biomaterial.
A collaboration with SIRIM Malaysia and the Malaysian Nuclear Agency developed several wound dressing products based on chitosan bioderivatives in the form of a film, sheet, and paste for various clinical applications on wounds. The study materials were produced by SIRIM Malaysia at their pilot plant in Sepang (SIRIM incubation centre), which is a Good Manufacturing Practices (GMP)-compliant facility. The chitosan derivative produced for this clinical trial consisted of 70% chitosan and 30% glycerol.
Extensive preclinical study in a Good Laboratory Practices-certified facility (RMIT Lab) established the biocompatibility of these products in vitro and in vivo and confirmed they were acceptable for human use.6, 8, 10, 16, 17, 18
Few randomised clinical trials have assessed wound dressings. A few prospective randomised controlled trials (RCTs) have been performed on graft skin used to manage noninfected neuropathic diabetic foot ulcers,19 Vacuum-assisted closure versus modern wound dressings,20 negative pressure therapy after partial foot amputation,21 vacuum-assisted closure with advanced moist wound therapy for diabetic foot,22 and a few studies have examined hydrocolloid dressings.23, 24, 25, 26 To the best of our knowledge, this is the first prospective study on chitosan derivative film in comparison with hydrocolloid for use on superficial wounds. A previous RCT on bioactive dressings containing hydrophilic mucopolysaccharide used chitosan to treat diabetic foot ulcers, pressure ulcers, and leg ulcer.27 Chitosan derivatives have also been studied extensively in vitro and in vivo (using animal models) and have been used as haemostatic agents.6, 7, 8, 9, 10, 16, 17, 18, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41
The aim of this study was to evaluate the efficacy of a chitosan derivative film dressing in the treatment of superficial wounds, compared with that of a commercially available hydrocolloid wound dressing material.
Materials and Methods
This prospective, single-blind, randomised controlled clinical trial compared the efficacy of chitosan derivative film versus hydrocolloid for the treatment of superficial wounds/abrasions in patients attending the Reconstructive Sciences Unit and Emergency Department at the Hospital Universiti Sains Malaysia and Hospital Universiti Kebangsaan Malaysia.
Chitosan derivative film dressing preparation
This dressing was manufactured and supplied by SIRIM Malaysia. Their pilot plant in Sepang (SIRIM incubation centre) is a GMP-compliant facility. Preparation of film was produced as described by Ujang and colleagues (2014-in press).52
Patient selection
Patient of both sexes aged between 16 and 70 years who presented to the Accident and Emergency Department of two large medical centres in Malaysia with superficial or abrasion wounds were screened for the study. Patients with severely contaminated or infected wounds, allergy to seafood, uncontrolled diabetes (random blood glucose >10 mmol/L), noncompliance, pregnancy, and any skin pathology (such as eczema) were excluded from the study.
Patients who fulfilled the inclusion criteria were enrolled in the study upon signing a consent form. They were then randomised to 1 of 2 dressing groups by using a random sequence generated with web-based software (http://www.randomization.com).
Dressing and application
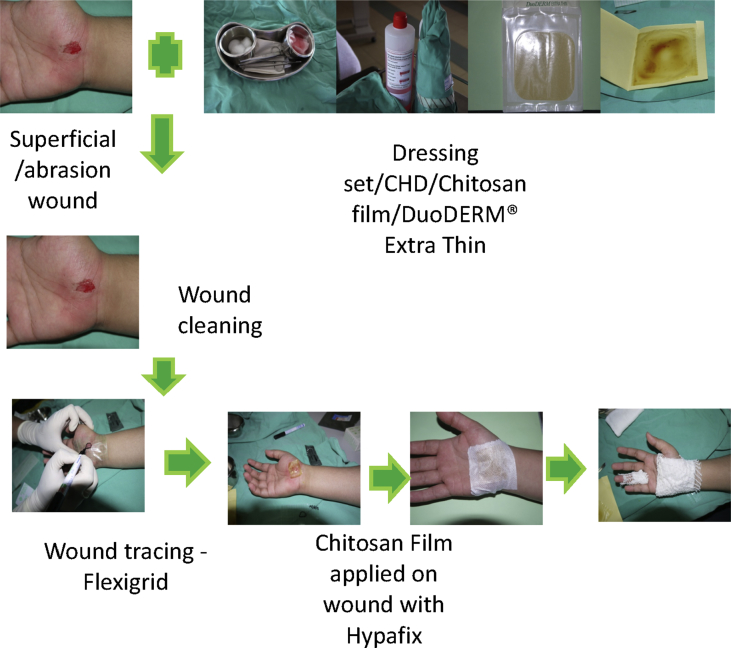
The wounds were treated according to usual departmental practice. Once a patient signed the consent form, the wound was cleaned with 0.9% normal saline and 0.5% chlorhexidine. The chitosan derivative film was cut according to wound size with a 1 cm overlap. This dressing was applied to the wound with Hypafix®, since it was not self-adhesive. A Hydrocolloid® Extra Thin dressing was selected, cut into desired size, and applied directly to the wound in accordance with the manufacturer's instructions.
The dressing was first inspected and changed on days 5 and 7, when the wound was fully epithelised. The wounds were photographed during every wound inspection and before new dressings were applied. Application of chitosan derivative film dressing is shown in Figure 4.
Figure 4.
Application of dressing.
Measurement of outcomes
Data on patient details and all clinical characteristics were recorded on a case report form. Time until healing (epithelisation percentage), patient comfort (pain and itching), clinical appearance (wound drainage, erythema, localised warmth, and oedema), and convenience in application and removal of dressing (pain upon removal, exudate, adherence, ease of removal, and odour) were all noted. In addition, notes were made regarding allergy and complications caused by the dressings.
Statistical methods
Data were analysed using IBM SPSS Statistics version 20. Numerical variables were summarised as mean and standard deviation (SD) or median and interquartile range (IQR), depending upon the normality of data distribution. The categorical variables were presented as frequency and percentage. Differences of means between groups at any particular time were analysed using an independent t-test, and differences of proportion were analysed using the Pearson chi-square test. A repeated-measures independent t-test, and differences in means of epithelisation percentage between chitosan derivative film and Hydrocolloid® Extra Thin were determined. The level of significance was set at p value 0.05.
Results
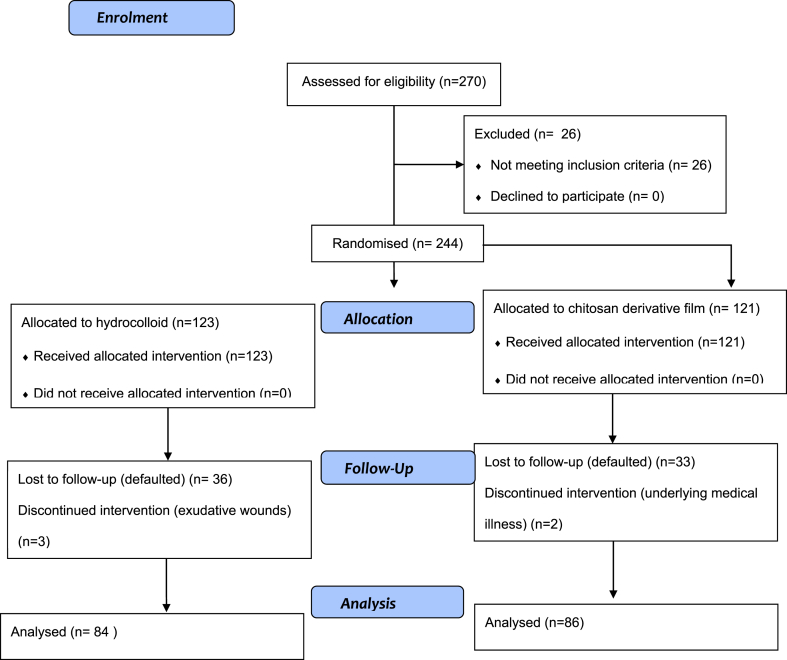
A total of 244 patients were enrolled in this randomised control trial study from May until October 2012. Of these, 121 (49.6%) received chitosan derivative film (treatment group) and 123 (50.4%) received hydrocolloid (control group) dressings. Figure 1 shows the allocation of subjects into groups. Thirty-five (28.9%) patients from the treatment group and 39 (31.7%) from the control group were not included in the final analysis. There was no significant difference in the proportion of drop-outs between groups (p value 0.636). Reasons for drop-out in the treatment group were failure to follow up (33 patients) and underlying medical illness (2 patients). In the control group, 36 patients failed to follow up and 3 discontinued participation due to adverse events such as development of an exudative wound. A total of 170 patients completed this study. The data are summarised in Figure 1.
Figure 1.
Flow chart of the trial for completed samples.
Table 1 shows sociodemographic and baseline data of the subjects. The mean age was 29.79 (SD 13.72) years in the treatment group and 26.12 (SD 11.67) years in the control group. There were 74 (52.1%) males and 12 (42.9%) females in the treatment group and 68 (47.9%) males and 16 (57.1%) females in the control group. Except for race, there were no statistically significant differences between the groups in terms of demographic data and wound size at baseline (p = 0.269).
Table 1.
Comparison of sociodemographic and baseline data in chitosan derivative film and hydrocolloid groups.
| Chitosan derivative film n = 86 | Hydrocolloid n = 84 | p value | |
|---|---|---|---|
| Agec (years) | 29.79 (13.72) | 26.12 (11.67) | 0.062a |
| Sexd | |||
| Male | 74 (52.1) | 68 (47.9) | |
| Female | 12 (42.9) | 16 (57.1) | 0.371b |
| Raced | |||
| Malay | 74 (48.1) | 80 (51.9) | |
| Non-Malay | 12 (75.0) | 4 (25.0) | 0.040b |
| Use of antibioticd | 12 (44.4) | 15 (55.6) | 0.486b |
| Initial wound sizec (cm) | 14.73 (18.18) | 11.89 (15.03) | 0.269a |
Independent t test.
Pearson's chi-square test.
mean (standard deviation).
frequency (%).
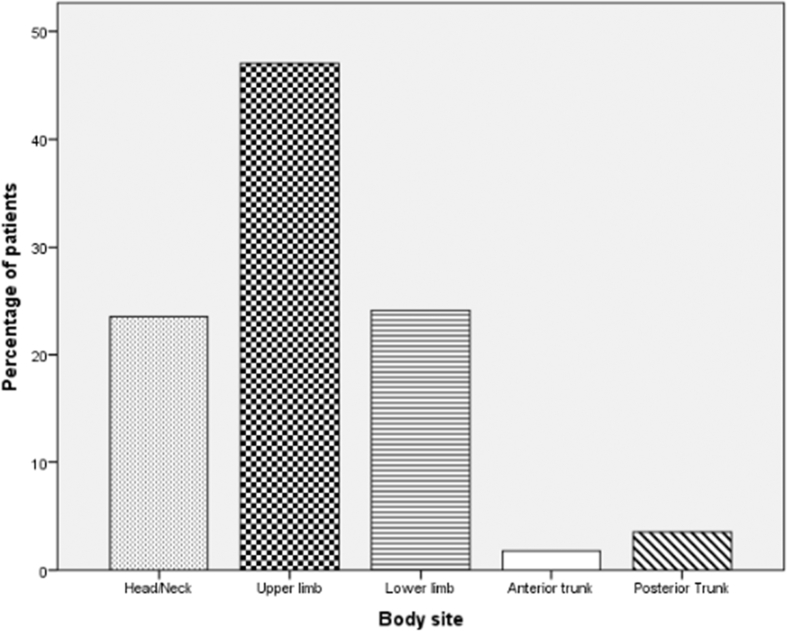
Figure 2 shows that most wounds in both groups occurred on the upper limbs (47.1%), followed by the lower limbs (24.1%), head/neck (23.5%), posterior trunk (3.5%), and anterior trunk (1.8%).
Figure 2.
Wound sites in both groups.
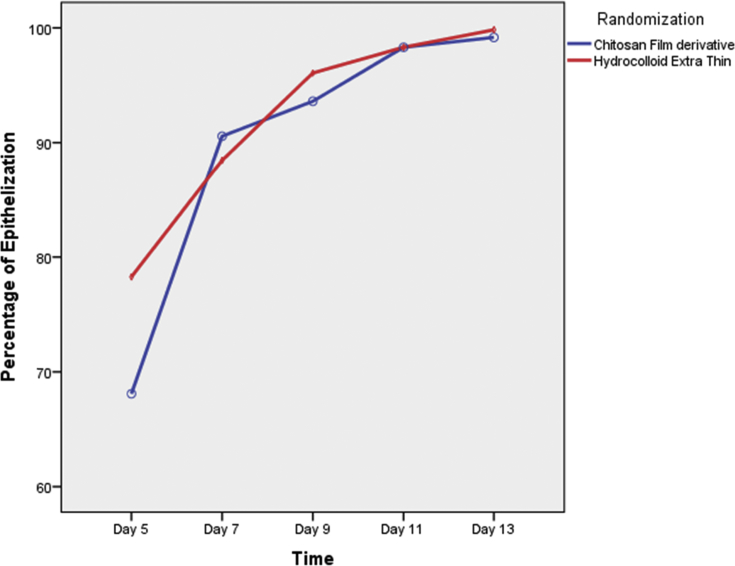
Table 2 and Figure 3 show that there was no significant difference in the mean wound epithelisation percentage between groups [F test (df) = 1.18 (1), p value 0.290]. The inter-observer agreement among 3 observers for measurement of epithelisation on day 5 (n = 104) was 0.582 (95% confidence interval 0.474, 0.679), 0.349 (95% confidence interval 0.129, 0.574) on day 7 (n = 31), and 0.197 (95% confidence interval −0.067, 0.525) on day 9 (n = 17).
Table 2.
Comparison of mean wound epithelisation percentage over time between the chitosan derivative film and hydrocolloid groups.
| Epithelisation Percentage Mean (95% CI) |
p valuea | |||||
|---|---|---|---|---|---|---|
| Day 5 | Day 7 | Day 9 | Day 11 | Day 13 | ||
| Chitosan derivative film | 68.09 (60.83,75.36) | 90.56 (85.18,95.95) | 93.61 (89.92,97.29) | 98.31 (96.06,100.55) | 99.17 (97.99,100.36) | 0.290 |
| Hydrocolloid | 78.27 (70.93,85.62) | 88.46 (83.00,93.91) | 96.07 (92.34,99.80) | 98.31 (96.03,100.58) | 99.84 (98.64,101,04) | |
| p valueb | 0.053 | 0.588 | 0.355 | 0.999 | 0.437 | |
Repeated-measures analysis of variance (ANOVA).
Independent t-test for each time point.
Figure 3.
Comparison of mean wound epithelisation percentage over time between the chitosan derivative film and hydrocolloid groups.
Assessment of patient comfort with the dressing showed satisfaction with both types. Table 3 compares both groups for patient comfort and clinical signs on follow-up days 5, 7, 9, 11, and 13. There was no significant difference in the mean score for itchiness, pain/tenderness, wound drainage, and erythema between both groups. No patients had localised warmth or oedema/induration.
Table 3.
Comparison of patient comfort and clinical signs between both groups on follow-up days 5, 7, 9, 11, and 13.
| Patient symptoms and wound assessment | Scores Mean (95% CI) |
p value* | ||||
|---|---|---|---|---|---|---|
| Day 5 | Day 7 | Day 9 | Day 11 | Day 13 | ||
| Itchiness | 0.686 | |||||
| Treatment | 0.93 (0.54, 1.32) | 0.06 (−0.05, 0.16) | 0.07 (−0.05, 0.19) | 0.02 (−0.02, 0.07) | 0.00 (−0.03, 0.03) | |
| Control | 0.52 (0.13, 0.92) | 0.18 (0.07, 0.29) | 0.16 (0.03, 0.28) | 0.05 (0.00, 0.09) | 0.04 (0.0, 0.06) | |
| p value | 0.147 | 0.117 | 0.341 | 0.461 | 0.083 | |
| Pain/tenderness | 0.337 | |||||
| Treatment | 1.02 (0.59, 1.46) | 0.15 (−0.00, 0.30) | 0.14 (−0.04, 0.32) | 0 (0) | 0 (0) | |
| Control | 0.71 (0.27, 1.15) | 0.12 (−0.04, 0.27) | 0.10 (−0.09, 0.28) | 0 (0) | 0 (0) | |
| p value | 0.324 | 0.770 | 0.735 | NA | NA | |
| Wound drainage | 0.719 | |||||
| Treatment | 0.30 (0.21, 0.40) | 0.15 (0.08, 0.23) | 0.08 (0.03, 0.13) | 0.06 (0.01, 0.11) | 0.01 (−0.02, 0.04) | |
| Control | 0.29 (0.19, 0.38) | 0.14 (0.07, 0.22) | 0.05 (−0.01, 0.10) | 0.05 (−0.00, 0.10) | 0.02 (−0.01, 0.05) | |
| p value | 0.813 | 0.879 | 0.374 | 0.761 | 0.549 | |
| Erythema | 0.767 | |||||
| Treatment | 0.02 (−0.02,0.06) | 0.02 (−0.00,0.06) | 0 (0) | 0.01 (0.01,0.03) | 0 (0) | |
| Control | 0.05 (0.01,0.09) | 0.02 (−0.01, 0.06) | 0 (0) | 0 (0) | 0 (0) | |
| p value | 0.394 | 0.981 | 0.324 | NA | NA | |
| Localised warmth | NA | |||||
| Treatment | All absent | |||||
| Control | All absent | |||||
| p value | ||||||
| Oedema/induration | NA | |||||
| Treatment | All Absent | |||||
| Control | All absent | |||||
| p value | ||||||
*p value for repeated-measures ANOVA between groups.
NA – not applicable.
Table 4 compares both groups for convenience of application and removal of dressings. Pain upon removal was significantly greater in the chitosan group on day 5 due to use of Hypafix to attach the chitosan derivative dressing to the wound, not because of the film itself. In contrast, assessment for exudate and odour showed significantly higher scores in the hydrocolloid group compared to the chitosan group. Slow-healing wounds were more exudative on day 13 and odour was stronger on day 5 in the hydrocolloid group.
Table 4.
Comparisons of patient convenience in application and removal of dressing between both groups on follow-up days 5, 7, 9, 11, and 13.
| Dressing assessments | Scores Mean (95% CI) |
p value* | ||||
|---|---|---|---|---|---|---|
| Day 5 | Day 7 | Day 9 | Day 11 | Day 13 | ||
| Pain Upon Removal | ||||||
| Treatment | 0.85 (0.54, 1.16) | 0.26 (0.08, 0.43) | 0.02 (−0.02, 0.07) | 0 (0) | 0 (0) | 0.007 |
| Control | 0.25 (−0.07, 0.57) | 0.06 (−0.12, 0.24) | 0.05 (0.00, 0.1) | 0 (0) | 0 (0) | |
| p value** | 0.008 | 0.115 | 0.461 | – | – | |
| Exudate | 0.036 | |||||
| Treatment | 0.12 (0.01, 0.23) | 0.06 (−0.01, 0.13) | 0.01 (−0.04, 0.06) | 0.00 (−0.04, 0.04) | 0 (−0.03, 0.03) | |
| Control | 0.25 (0.14, 0.36) | 0.12 (0.05, 0.19) | 0.07 (0.02, 0.12) | 0.06 (0.02, 0.10) | 0.05 (0.02, 0.08) | |
| p value** | 0.095 | 0.224 | 0.090 | 0.058 | 0.045 | |
| Adherence | 0.553 | |||||
| Treatment | 0.14 (0.06, 0.22) | 0.01 (−0.03, 0.05) | 0.04 (−0.00, 0.07) | 0 (−0.02, 0.02) | 0 (−0.02, 0.02) | |
| Control | 0.12 (0.04, 0.20) | 0.06 (0.02, 0.10) | 0.02 (−0.01, 0.06) | 0.02 (0.00, 0.05) | 0.02 (0.00, 0.05) | |
| Ease of removal | 0.466 | |||||
| Treatment | 1.06 (1.02, 1.10) | 0.25 (0.15, 0.35) | 0.17 (0.10, 0.24) | 0.06 (0.01, 0.11) | 0.01 (−0.03, 0.05) | |
| Control | 1.00 (0.96, 1.04) | 0.25 (0.15, 0.35) | 0.08 (0.01, 0.16) | 0.05 (−0.00, 0.10) | 0.05 (0.01, 0.08) | |
| Odour | 0.024 | |||||
| Treatment | 0.06 (−0.02, 0.13) | 0.02 (−0.02, 0.07) | 0.00 (−0.02, 0.02) | 0.00 (−0.02, 0.02) | 0.00 (−0.02, 0.02) | |
| Control | 0.18 (0.10, 0.26) | 0.06 (0.02, 0.10) | 0.01 (−0.01, 0.03) | 0.01 (−0.01, 0.03) | 0.01 (−0.01, 0.03) | |
| p value** | 0.029 | 0.239 | 0.320 | 0.320 | 0.320 | |
*p value for repeated-measures ANOVA between groups.
**p value for independent t-test at each time point.
Discussion
This report showed the effectiveness of chitosan derivative film in comparison with conventional hydrocolloid dressing for treating superficial/abrasion wounds. The findings support evidence-based use of chitosan derivatives in clinical practice. The use of film dressings on superficial wounds was supported by Harding et al. This type of dressing is able to transmit moisture vapour from the wound to outside the dressing.42
The sociodemographic and baseline patient data in this trial showed no differences except for race. The simple explanation is that Malays comprise the largest population group in Malaysia.
Our sample also showed that most injuries were on the upper limb (47.1%), despite the absence of adverse factors affecting wound healing such as infection and uncontrolled diabetes. Most wounds in this study were caused by motor vehicle accidents.
To evaluate the efficacy of chitosan derivative film dressing in treating superficial/abrasion wounds, this clinical trial focused on wound epithelisation as well as patient comfort, clinical appearance, and convenience in application and removal of dressings. This clinical trial suggested the effectiveness of chitosan derivative film dressings in the epithelisation of superficial wounds. However, it was difficult to prove that one material was superior to the other in terms of wound healing. This is because incisions and abrasions tend to heal in 5–10 days, depending on the site. The overall wound dimensions and other background data showed a reasonable degree of balance between the groups. Our results demonstrated clearly that there were no problems with healing. Almost all wounds were completely epithelised on day 5 in both treated and control groups (n = 104).
Epithelisation is very important in wound healing.43 The first phase takes place as epithelial cells migrate across new tissue to form a barrier between the wound and environment. This step is initiated by a cascade of inflammatory cytokines, including interleukin (IL)-1 and tumour necrosis factor-α, which upregulate keratinocyte growth factor (KGF) in fibroblasts. This in turn stimulates fibroblasts to secrete KGF-1, KGF-2 and IL-6. These chemicals stimulate and initiate neighbouring keratinocytes to migrate into the wound, resulting in proliferation and differentiation into the epidermis.44, 45, 46 Therefore, the time of onset of migration is variable and may begin about 1 day after injury. Cells at the wound margins proliferate on the second and third day after injury and provide more cells for migration. Therefore, it is important to cover the wound with an effective dressing, not just to prevent contamination or infection but also to secure the epithelisation process. Falanga (1988) proposed that faster epithelisation would occur if the wound remained under occlusive dressing, with fluid kept in contact with the wound and not lost to gauze.47
The main advantage of both dressings was patient comfort. Once applied, the dressing could remain in place and would not interfere with normal activities. Dressings used in this study could be kept in place for 5–7 days without changing. This is in line with the review by Harding et al.,42 suggesting that dressings should be durable and not require changing for 4–5 days. Observation on chitosan derivative film in our study also revealed that it will peel off by itself once the wound is healed because chitosan is a biodegradable polymer and does not injure cells.6, 14, 17, 35 Earlier studies on the effect of chitosan derivative film on proliferation of human skin fibroblasts supported our findings. Chitosan dressing aids the wound healing process in several ways.6, 8, 10 The advantages of chitosan as a dressing include its antimicrobial, analgesic, haemostatic, and wound-healing properties, as well as biocompatibality.14, 35, 48, 50, 51 Chitosan also accelerates epidermal regeneration and stimulates granulation and tissue formation.29 Application of chitosan on an open wound was found to induce significant contraction, thereby accelerating wound closure and the healing process.33
From our observation in an earlier dressing assessment, chitosan membrane was found to adhere uniformly to fresh and wet wounds. However, it needed to be secured with Hypafix®, since it is not self-adhesive, whereas the dressing in the control group had self-adhesive properties. The chitosan derivative film dressing remained dry at the first inspection, with minimal exudate. Wound dryness in both groups gradually increased with time. The wound improved from wet to finally dry when healing was near completion. There was a statistically significant difference in the control group in exudate and odour on day 13 compared to that in the treatment group. The presence of exudate on the wound is critical as it could delay healing. These conditions occur when hydrocolloid produces a viscous mobile gel in the presence of wound exudate.49 Since the dressing was changed for the first time on day 5, the accumulation of viscous material caused a strong odour and maceration of surrounding skin.
The application of a secondary dressing (Hypafix®) for chitosan derivative film resulted in significant pain upon removal on day 5, but not because of the film itself. In our analysis, the only significant finding was wound exudation and pain upon dressing removal. Other modalities of wound and dressing assessment such as the scoring of pain, itchiness, wound drainage, and erythema, as well as sociodemographic findings, revealed no statistically significant differences between the two groups according to baseline demographic data and wound size (p = 0.269).
The evolution of modern dressings has been stimulated by the understanding of phases of wound healing and factors contributing to enhance wound healing. Hydrocolloid, hydrofiber, and silicone-coated dressings, and many other non-stick dressings have been produced with advances in technology. These materials may be used alone or combination with others in the search for an ideal dressing product.
This prospective randomised study assessed various parameters that are useful in determining the practical aspects of wound dressings in actual clinical settings.
Conclusion
This prospective randomised controlled study showed that a film dressing manufactured from a deacetylated chitosan bioderivative is equivalent to hydrocolloid in terms of epithelisation, oedema, and ease of removal. The chitosan derivative film, however, produced less odour and exudate. These attributes represent attractive aspects of this new dressing.
Source of funding
This work was supported by a grant (No.: (304/PPSP/6150110/s105) from the Ministry of Science, Technology and Innovation (MOSTI) of Malaysia combined with SIRIM to support pilot production of wound management products from water soluble chitosan derivatives for preclinical and market evaluation.
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
The Research Ethics Committee (Human) of Universiti Sains Malaysia (FWA Reg. No. 00007718; IRB Reg. No: 00004494) and Research Ethics Committee of Universiti Kebangsaan Malaysia (JEPUKM) (No: FF-283-2012) approved this research. This study was also approved by Medical Research and Ethic Committee, Ministry of Health Malaysia (NMRR-11-948-10565).
Authors' contributions
Funding from AS Halim, Ethics approval for USM AZ Mat Saad, JEPUKM and NMRR FM Nor. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Acknowledgment
This work was supported by a grant (No.: 304/PPSP/6150110/s105) from the Ministry of Science, Technology and Innovation (MOSTI) of Malaysia combined with SIRIM to support pilot production of wound management products from water soluble chitosan derivatives for preclinical and market evaluation.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Barbosa M.A., Pego A.P., Amaral I.F. Chitosan. In: Ducheyne P., editor. Comprehensive biomaterials. Elsevier; Oxford: 2011. [Google Scholar]
- 2.M P Introductory remarks. Carb Eur. 1997;19(1):9–15. [Google Scholar]
- 3.Yang J., Tian F., Wang Z., Wang Q., Zeng Y.J., Chen S.Q. Effect of chitosan molecular weight and deacetylation degree on hemostasis. J Biomed Mater Res B Appl Biomater. 2008;84(1):131–137. doi: 10.1002/jbm.b.30853. [DOI] [PubMed] [Google Scholar]
- 4.Kumar M.N.V.R. A review of chitin and chitosan applications. React Funct Polym. 2000;46(1):1–27. [Google Scholar]
- 5.Prabaharan M. Chitosan derivatives as promising materials for controlled drug delivery. J Biomater Appl. 2008;23(1):5–36. doi: 10.1177/0885328208091562. [DOI] [PubMed] [Google Scholar]
- 6.Abdull Rasad M.S.B., Halim A.S., Hashim K., Rashid A.H.A., Yusof N., Shamsuddin S. In vitro evaluation of novel chitosan derivatives sheet and paste cytocompatibility on human dermal fibroblasts. Carbohydr Polym. 2010;79(4):1094–1100. [Google Scholar]
- 7.Halim A.S., Stone C.A., Devaraj V.S. The Hyphecan cap: a biological fingertip dressing. Injury. 1998;29(4):261–263. doi: 10.1016/s0020-1383(97)00194-0. [DOI] [PubMed] [Google Scholar]
- 8.Lim C.K., Halim A.S. Biomedical-grade chitosan in wound management and its biocompatibility in vitro. In: Elnashar M., editor. Biopolymers. Croatia. InTech; 2010. [Google Scholar]
- 9.Miyatake K., Okamoto Y., Shigemasa Y., Tokura S., Minami S. Anti-inflammatory effect of chemically modified chitin. Carbohydr Polym. 2003;53(4):417–423. [Google Scholar]
- 10.Muhamad Nor N.A., Halim A.S., Shamsuddin S., Che Hussin C.M., Ujang Z., Abdul Rashid A.H. The effect of chitosan derivatives film on the proliferation of human skin fibroblast: an-in vitro study. J Sustain Sci Manag. 2013;8(2):212–219. [Google Scholar]
- 11.Park S.Y., Lee B.I., Jung S.T., Park H.J. Biopolymer composite films based on κ-carrageenan and chitosan. Mater Res Bull. 2001;36(3–4):511–519. [Google Scholar]
- 12.Periayah M.H., Halim A.S., Hussein A.R., Mat Saad A.Z., Abdul Rashid A.H., Noorsal K. In vitro capacity of different grades of chitosan derivatives to induce platelet adhesion and aggregation. Int J Biol Macromol. 2013;52:244–249. doi: 10.1016/j.ijbiomac.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Singla A., Chawla M. Chitosan: some pharmaceutical and biological aspects-an update. J Pharm Pharmacol. 2001;53(8):1047–1067. doi: 10.1211/0022357011776441. [DOI] [PubMed] [Google Scholar]
- 14.Deng C.-M., He L.-Z., Zhao M., Yang D., Liu Y. Biological properties of the chitosan-gelatin sponge wound dressing. Carbohydr Polym. 2007;69(3):583–589. [Google Scholar]
- 15.Scales J.T. Wound healing and the dressing. Occup Environ Med. 1963;20(2):82–94. doi: 10.1136/oem.20.2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halim A.S., Keong L.C., Zainol I., Abdul Rashid A.H. Biocompatibility and biodegradation of chitosan and derivatives. In: Sarmento B., Neves Jd, editors. vol. 1. John Wiley & Sons; West Sussex: 2012. pp. 57–73. (Chitosan-based systems for biopharmaceuticals: delivery, targeting and polymer therapeutics). [Google Scholar]
- 17.Keong L.C., Halim A.S. In vitro models in biocompatibility assessment for biomedical-grade chitosan derivatives in wound management. Int J Mol Sci. 2009;10(3):1300–1313. doi: 10.3390/ijms10031300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim C.K., Yaacob N.S., Ismail Z., Halim A.S. In vitro biocompatibility of chitosan porous skin regenerating templates (PSRTs) using primary human skin keratinocytes. Toxicol In Vitro. 2010;24(3):721–727. doi: 10.1016/j.tiv.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Veves A., Falanga V., Armstrong D.G., Sabolinski M.L. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care. 2001;24(2):290–295. doi: 10.2337/diacare.24.2.290. [DOI] [PubMed] [Google Scholar]
- 20.Vuerstaek J.D.D., Vainas T., Wuite J., Nelemans P., Neumann M.H.A., Veraart J.C.J.M. State-of-the-art treatment of chronic leg ulcers: a randomized controlled trial comparing vacuum-assisted closure (VAC) with modern wound dressings. J Vasc Surg. 2006;44(5):1029–1037. doi: 10.1016/j.jvs.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong D.G., Lavery L.A., Diabetic Foot Study Consortium Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet. 2005;366(9498):1704–1710. doi: 10.1016/S0140-6736(05)67695-7. [DOI] [PubMed] [Google Scholar]
- 22.Blume P.A., Walters J., Payne W., Ayala J., Lantis J. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care. 2008;31(4):631–636. doi: 10.2337/dc07-2196. [DOI] [PubMed] [Google Scholar]
- 23.Cordts P.R., Hanrahan L.M., Rodriguez A.A., Woodson J., LaMorte W.W., Menzoian J.O. A prospective, randomized trial of Unna's boot versus Duoderm CGF hydroactive dressing plus compression in the management of venous leg ulcers. J Vasc Surg. 1992;15(3):480–486. [PubMed] [Google Scholar]
- 24.Feldman D.L., Rogers A., Karpinski R.H. A prospective trial comparing Biobrane, Duoderm and xeroform for skin graft donor sites. Surg Gynecol Obstet. 1991;173(1):1–5. [PubMed] [Google Scholar]
- 25.Nikoletti S., Leslie G., Gandossi S., Coombs G., Wilson R. A prospective, randomized, controlled trial comparing transparent polyurethane and hydrocolloid dressings for central venous catheters. Am J Infect Control. 1999;27(6):488–496. doi: 10.1016/s0196-6553(99)70026-x. [DOI] [PubMed] [Google Scholar]
- 26.Phillips T.J., Gerstein A.D., Lordan V. A randomized controlled trial of hydrocolloid dressing in the treatment of hypertrophic scars and keloids. Dermatol Surg. 1996;22(9):775–778. doi: 10.1111/j.1524-4725.1996.tb00728.x. [DOI] [PubMed] [Google Scholar]
- 27.Kordestani S., Shahrezaee M., Tahmasebi M.N., Hajimahmodi H., Ghasemali D.H., Abyaneh M.S. A randomised controlled trial on the effectiveness of an advanced wound dressing used in Iran. J Wound Care. 2008;17(7):323–327. doi: 10.12968/jowc.2008.17.7.30525. [DOI] [PubMed] [Google Scholar]
- 28.Aksungur P., Sungur A., Ünal S., Iskit A.B., Squier C.A., Şenel S. Chitosan delivery systems for the treatment of oral mucositis: in vitro and in vivo studies. J Contr Release. 2004;98(2):269–279. doi: 10.1016/j.jconrel.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Alemdaroğlu C., Değim Z., Çelebi N., Zor F., Öztürk S., Erdoğan D. An investigation on burn wound healing in rats with chitosan gel formulation containing epidermal growth factor. Burns. 2006;32(3):319–327. doi: 10.1016/j.burns.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 30.Aoyagi S., Onishi H., Machida Y. Novel chitosan wound dressing loaded with minocycline for the treatment of severe burn wounds. Int J Pharm. 2007;330(1–2):138–145. doi: 10.1016/j.ijpharm.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Azad A.K., Sermsintham N., Chandrkrachang S., Stevens W.F. Chitosan membrane as a wound-healing dressing: characterization and clinical application. J Biomed Mater Res B Appl Biomater. 2004;69(2):216–222. doi: 10.1002/jbm.b.30000. [DOI] [PubMed] [Google Scholar]
- 32.Brown M.A., Daya M.R., Worley J.A. Experience with chitosan dressings in a civilian EMS system. J Emerg Med. 2009;37(1):1–7. doi: 10.1016/j.jemermed.2007.05.043. [DOI] [PubMed] [Google Scholar]
- 33.Conti B., Giunchedi P., Genta I., Conte U. The preparation and in vivo evaluation of the wound-healing properties of chitosan microspheres. STP Pharma Sci. 2000;10(1):101–104. [Google Scholar]
- 34.Jayakumar R., Prabaharan M., Kumar P.T.S., Nair S.V., Tamura H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv. 2011;29(3):322–337. doi: 10.1016/j.biotechadv.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Jayakumar R., Prabaharan M., Nair S.V., Tamura H. Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol Adv. 2010;28(1):142–150. doi: 10.1016/j.biotechadv.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Khan T.A., Peh K.K. A preliminary investigation of chitosan film as dressing for punch biopsy wounds in rats. J Pharm Pharmaceut Sci. 2003;6(1):20–26. [PubMed] [Google Scholar]
- 37.Lu G., Ling K., Zhao P., Xu Z., Deng C., Zheng H. A novel in situ-formed hydrogel wound dressing by the photocross-linking of a chitosan derivative. Wound Repair Regen. 2010;18(1):70–79. doi: 10.1111/j.1524-475X.2009.00557.x. [DOI] [PubMed] [Google Scholar]
- 38.Ma J., Wang H., He B., Chen J. A preliminary in vitro study on the fabrication and tissue engineering applications of a novel chitosan bilayer material as a scaffold of human neofetal dermal fibroblasts. Biomaterials. 2001;22(4):331–336. doi: 10.1016/s0142-9612(00)00188-5. [DOI] [PubMed] [Google Scholar]
- 39.Sezer A.D., Hatipoglu F., Cevher E., Oğurtan Z., Bas A.L., Akbuğa J. Chitosan film containing fucoidan as a wound dressing for dermal burn healing: preparation and in vitro/in vivo evaluation. AAPS PharmSciTech. 2007;8(2):E94–E101. doi: 10.1208/pt0802039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stone C.A., Wright H., Devaraj V.S., Clarke T., Powell R. Healing at skin graft donor sites dressed with chitosan. Br J Plast Surg. 2000;53(7):601–606. doi: 10.1054/bjps.2000.3412. [DOI] [PubMed] [Google Scholar]
- 41.Wang W., Lin S., Xiao Y., Huang Y., Tan Y., Cai L. Acceleration of diabetic wound healing with chitosan-crosslinked collagen sponge containing recombinant human acidic fibroblast growth factor in healing-impaired STZ diabetic rats. Life Sci. 2008;82(3–4):190–204. doi: 10.1016/j.lfs.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Harding K.G., Jones V., Price P. Topical treatment: which dressing to choose. Diabetes Metab Res Rev. 2000;16(Suppl):S47–S50. doi: 10.1002/1520-7560(200009/10)16:1+<::aid-dmrr133>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 43.Winter G.D., Scales J.T. Effect of air drying and dressings on the surface of a wound. Nature. 1963;197:91–92. doi: 10.1038/197091b0. [DOI] [PubMed] [Google Scholar]
- 44.Broughton G.I., Janis J.E., Attinger C.E. Wound healing: an overview. Plast Reconstr Surg. 2006;117(7 Suppl):1e-S–32e-S. doi: 10.1097/01.prs.0000222562.60260.f9. [DOI] [PubMed] [Google Scholar]
- 45.Broughton G.I., Janis J.E., Attinger C.E. The basic science of wound healing. Plast Reconstr Surg. 2006;117(7S):12S–34S. doi: 10.1097/01.prs.0000225430.42531.c2. [DOI] [PubMed] [Google Scholar]
- 46.Martin P. Wound healing: aiming for perfect skin regeneration. Science. 1997;276(5309):75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 47.Falanga V. Occlusive wound dressings: why, when, which? Arch Dermatol. 1988;124(6):872–877. [PubMed] [Google Scholar]
- 48.Chen Y.-M., Chung Y.-C., Woan Wang L., Chen K.-T., Li S.-Y. Antibacterial properties of chitosan in waterborne pathogen. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2002;37(7):1379–1390. doi: 10.1081/ese-120005993. [DOI] [PubMed] [Google Scholar]
- 49.Thomas S. Hydrocolloid dressings in the management of acute wounds: a review of the literature. Int Wound J. 2008;5(5):602–613. doi: 10.1111/j.1742-481X.2008.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang W., Fortunati E., Bertoglio F., Owszarek J.S., Bruni G., Kozanecki M., Kenny J.M., Torre L., Visai L., Puglia D. Polyvinyl alcohol/chitosan hydrogels with enhanced antioxidant and antibacterial properties induced by lignin nanoparticles. Carbohydr Polym. 2018:275–284. doi: 10.1016/j.carbpol.2017.10.084. [DOI] [PubMed] [Google Scholar]
- 51.Wu Tiantian, Huang Jiaqi, Yang Yang, Hu Yaqin, Ye Xinggian, Liu Donghong, Chen Juanchu. Formation of hydrogels based on chitosan/alginate for the delivery of lysozyme and their antibacterial activity. Food Chem. 2018:361–369. doi: 10.1016/j.foodchem.2017.07.052. [DOI] [PubMed] [Google Scholar]
- 52.Zanariah U., Ahmad Hazri A.R., Siti Kasmarizawaty S., Ahmad Sukari H., Lim C.K. Physical properties and biocompatibility of oligochitosan membrane film as wound dressing. J Appl Biomater Funct Mater. 2014;12(3):155–162. doi: 10.5301/jabfm.5000190. [DOI] [PubMed] [Google Scholar]